Abstract
1–2% of live births are to very low birth weight, premature infants that often show a developmental trajectory plagued with neurological sequelae including ventriculomegaly and significant decreases in cortical volume. We are able to recapitulate these sequelae using a mouse model of hypoxia where early postnatal pups are exposed to chronic hypoxia for one week. However, because the timing of hypoxic exposure is so early in development, dams and pups are housed together in the hypoxic chamber, and therefore dams are also subjected to the same hypoxic conditions as the pups. To understand the relative contribution of hypoxia directly on the pups as opposed to the indirect contribution mediated by the effects of hypoxia and potential alterations in the dam’s care of the pups, we examined whether reducing the dams exposure to hypoxia may significantly increase pup outcomes on measures that we have found consistently changed immediately following chronic hypoxia exposure. To achieve this, we rotated dams between normoxic and hypoxic conditions, leaving the litters untouched in their respective conditions and compared gross anatomical measures of normoxic and hypoxic pups with non-rotating or rotating mothers. As we expected, hypoxic-rearing decreased pup body weight, brain weight and cortical volume. Reducing the dam’s exposure to hypoxic conditions actually amplified the effects of hypoxia on body weight, such that hypoxic pups with rotating mothers showed significantly less growth. Interestingly, rotation of hypoxic mothers did not have the same deleterious effect on brain weight, suggesting the presence of compensatory mechanisms conserving brain weight and development even under extremely low body weight conditions. The factors that potentially contribute to these compensatory changes remain to be determined, however, nutrition, pup feeding/metabolism, or changes in maternal care are important candidates, acting either together or independently to change pup body and brain development.
Keywords: hypoxia, brain development, mouse, maternal care
Introduction
1–2% of all live births are to very low birth weight (VLBW), premature infants, typically less than 32 weeks gestational age and weighing under 1kg. VLBW infants face a difficult developmental trajectory, plagued with psychological and neurological sequelae, including decreased cortical volume, ventriculomegaly, significant developmental delays and an increased incidence of psychiatric disorders such as schizophrenia, autism and anxiety disorders [1, 3, 17, 18, 21, 24–27, 38–40]. It is thought that the pathophysiology of these sequelae is, at least in part, due to chronic hypoxia experienced in VLBW infants during the early neonatal period as a consequence of poor oxygen exchange due to immature lung development [41]. There is also a documented deleterious effect of chronic hypoxia or simulated high altitude conditions on fetal and perinatal growth [10, 34, 35]. This strengthens the importance of oxygenation in achieving optimal fetal and perinatal growth and highlights the importance of chronic metabolic adaptations. Several rodent models have been developed that induce postnatal hypoxia to mimic both the neurological and psychological findings of children exposed to sub-optimal oxygenation conditions, documenting effects on the brain that include decreased brain weight, cortical volume and ventriculomegaly [33]. Using a model in which mice are reared in 10% oxygen during the early postnatal period, we have examined recovery from chronic hypoxic injury and the factors that may mediate recovery, including environmental enrichment and growth factor signaling [7, 12–14, 16, 19, 30]. We have demonstrated that, similar to the findings in VLBW children, there is heterogeneity in recovery among brain regions and cell types. In this model, cortical volume and excitatory neuron number recover by adulthood, whereas cortical interneurons fail to achieve complete maturation of their protein markers by adulthood. Indeed, we believe there is a delay in cortical maturation following chronic postnatal hypoxia, perhaps linked to an extended period of plasticity [29, 31]. While delayed maturation may increase the potential for recovery, it may also increase the likelihood of missing critical developmental windows. Despite the considerable evidence generated from the use of chronic postnatal hypoxia, a potential difficulty in the mechanistic interpretation of these studies is the inseparable interaction between the pups and dam during the period of low oxygen exposure (i.e., from postnatal day 3 (P3) to P11). During hypoxic exposure, dams and pups (typically in a C57/B6 genetic background) are housed together in the hypoxic chamber and therefore the dams are also subjected to the same hypoxic conditions as the pups. In order to minimize the adverse effects of maternal stress due to hypoxic exposure on the pups, we include a CD1 foster dam along with each C57 dam and her pups. These litters are culled to eight – ten pups to achieve homogeneity of total “maternal workload”. Nevertheless, the question remains as to the relative contribution of hypoxia directly on the pups as opposed to the indirect contribution mediated by the effects of hypoxia and potential alterations in the dam’s care of the pups. To disentangle these effects, we examined whether reducing the dams exposure to hypoxia may significantly increase pup outcomes on measures that we have found consistently changed immediately following chronic hypoxia exposure (i.e., body weight, brain weight, cortical volume and cortical neuronal number). In order to achieve this, we rotated dams between normoxic and hypoxic conditions, leaving the litters untouched in their respective conditions and compared gross anatomical measures of normoxic and hypoxic pups with non-rotating or rotating mothers.
Methods
Mice
Ten C57/B6 mice pregnant dams and ten CD1 pregnant fosters were ordered from Charles River and housed in single cages until birth. All litters were fostered to CD1 mothers, culling litters to a total of 8 pups for all dams on Postnatal Day 2 (P2). Dams and litters were housed in hypoxic (approx. 10% O2) or normoxic control conditions from P3–P11 as previously described [17–18, 20–21]. The mean O2 level during a typical 8 day hypoxic exposure period was 10%, with a range from 10.2 to 9.9% and a standard error of 0.0. Two litters remained with their dams in normoxic conditions for the entire period (P3–P11), while two litters remained with their dams in hypoxia for this period. In addition, two cohorts of three litters (2 normoxic and 1 hypoxic litter in each cohort) had the dams rotated every 12 hours among the normoxic litters and one hypoxic litter. We choose 12 hours based upon pilot experiments using rotation periods of 8 and 12 hours. The 12 hour period allowed each dam to appropriately acclimate to each new litter ensuring that the majority of time with each litter was spent in appropriate nursing behavior and care of the pups. For example, a dam for one of the normoxic litters would be with it’s normoxic litter for 12 hours, then be rotated to the second normoxic litter for 12 hours and then to a hypoxic litter for 12 hours after which it would start again with the original litter. Each dam during this cycle was exposed to 24 hours in normoxia and 12 hours in hypoxia. All experimental groups were comprised of pups from at least 2 different litters in order to control for any litter effects.
Body and Brain weights
Body weights for all pups were recorded just prior to placement of the hypoxia litters in the hypoxia chamber on P1. All pups were again weighed on P7 and on removal from the hypoxia chamber on P11. The day following removal from the chamber (P12) mice were perfused as described below. The spinal cord was removed and the brain weights were recorded.
Histology
Mice were overdosed using xylazine/ketamine and transcardially perfused with phosphate buffered saline and 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde for 24 hours and then cryoprotected in 30% sucrose for 48 hours before storing at −80. 20 μm sagittal sections were collected using a cryostat, placed on Superfrost++ slides and stored at −80 °C until staining. Sister sections (1 in 30) were pre-blocked at room temperature for one hour in 10% Normal Donkey Serum diluted in 0.3%Triton in 1XPBS (NDS-PBST) and subsequently stained for NeuN (mouse anti-Neuronal Nuclei, Chemicon) at a dilution of 1:500 in NDS-PBST overnight at room temperature. Sections were washed 3×10minutes with PBS and then incubated in anti-mouse 488 (Jackson Laboratories) at 1:1000 for 1.5 hours at room temperature. Slides were coverslipped with Vectashield Hard Set (Vector Laboratories) contacting DAPI counterstain and images taken using Zeiss ApoTome and cortical volume and cell number were assessed using Microbrightfield StereoInvestigator software as in our previous studies [7, 19]. The number of mice in each group that were used for statistical analyses are shown in Table 1.
Table 1.
Number of mice in each experimental group that were used for statistical analyses
| Norm-NR | Norm-Rot | Hyp-NR | Hyp-Rot | |
|---|---|---|---|---|
| Body weight | n=9 | n=31 | n=8 | n=25 |
| Brain Weight | n=9 | n=24 | n=8 | n=12 |
| Histology | n=3 | n=4 | n=4 | n=3 |
Statistical analysis
All dependent variables were analyzed by factorial analysis of variance, with oxygenic state, maternal status, and age (for body weights only) included as independent variables, where appropriate. Simple analysis and comparisons were conducted only when a significant interaction or main effect was observed (p<0.05) and non-orthogonal comparisons were controlled for by Bonferroni correction.
Results
Two litters of C57/B6 normoxic control pups were weighed at P3, P7 and P11, and compared to two litters of pups weighed prior to starting hypoxic exposure on P3, during exposure on P7 and immediately after being removed from hypoxia on P11. In order to understand the additional impact, if any, of maternal exposure to hypoxic conditions on pup outcome, we examined two additional cohorts of 3 litters (6 litters total) in which 3 dams were sequentially rotated every 12 hours between two normoxic cages (litters of pups) and a hypoxically exposed cage of pups. Thus in each 36 hour period, each dam would be exposed to 12 hours of hypoxia while with the hypoxic litter of pups, and 24 hours of normoxia while rotating between the two normoxic litters of pups. Each litter had equal time with all three dams. Four groups were examined in total: (1) normoxic, non-rotating controls (Norm-NR), (2) hypoxic, non-rotating (Hyp-NR), (3) normoxic, rotating mothers (Norm-Rot), and (4) hypoxic, rotating mothers (Hyp-Rot). Differences in body weights (Figure 1, top panel) were analyzed by a mixed 3-way ANOVA with normoxia/hypoxia, rotating/non-rotating, and time as variables/levels. An overall, significant, 3-way interaction was found (p<0.01). Post-hoc analysis revealed no significant differences in body weight between all groups prior to treatment at P3, a significant decrease in body weight in all hypoxic groups as compared to normoxic controls at P7 and P11, irrespective of rotation status, and that rotating, hypoxic pups weighed significantly less than all other groups at P7 and P11. No effect of rotation was seen on body weight of normoxic pups (see Figure 1, top panel).
Figure 1.
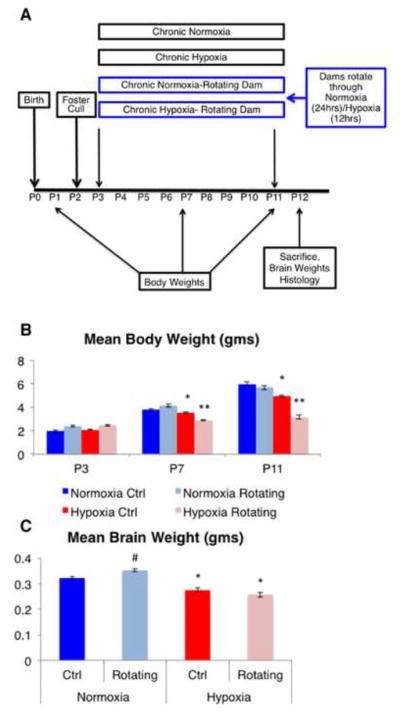
A, schematic drawing of the experimental protocol. B, mean body weight in grams of pups weighed prior to (P3), during (P7) and at the end of chronic hypoxic condition (P11). C, mean brain weight in grams at P12, one day following hypoxia (# indicates a significant increase from all other groups; * denotes a significant decrease from normoxic groups; **denotes a significant decrease from all other groups). Error bars represent SEM.
Although body weight can be related to and predictive of brain weight, there are conditions under which the two may dissociate, particularly when body weight is challenged; in addition, we have also noted that brain weight is highly predictive of outcome following chronic hypoxic injury, therefore, we also measured brain weights of all mice following sacrifice, one day after the cessation of hypoxia, at P12. Results showed a significant interaction between hypoxic status and rotation (p=0.01), such that the brains of hypoxic pups weighed less than their normoxic counterparts overall. Rotation of dams increased brain weights of normoxic pups (p<0.01), however no effects of rotation were seen on the brain weights of hypoxic pups (Figure 1, bottom panel).
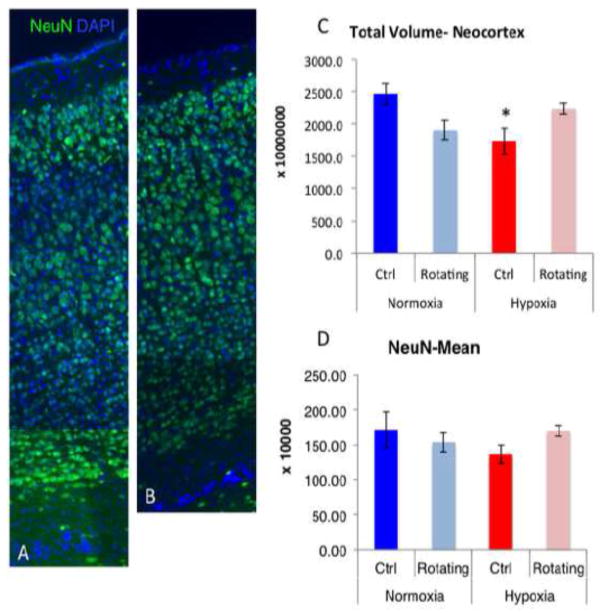
We next assessed the volume of the neocortex, a structure consistently and robustly affected by chronic postnatal hypoxia, by unbiased stereological analyses. The results (Figure 2, C) showed a significant interaction (p=0.01) of hypoxia with total neocortical volume, such that hypoxic mice showed the expected decrease in cortical volume (p=0.047), however there were no additional significant effects of rotation in hypoxic mice. Interestingly, there were statistical trends suggesting opposite effects of rotation on neocortical volume of normoxic (decreased volume, p=0.055) and hypoxic (increased volume, p=0.10) mice. While hypoxic rotating mice were not significantly different from the hypoxic non-rotating group (p=0.10), the hypoxic rotating group also did not show a significant decrease from normoxic control mice, suggesting that rotation causes a mild attenuation of the effects of hypoxia on cortical volume. Finally, we also assessed the total number of neurons stereological analysis using NeuN+ immunostaining and found no main effects of hypoxia or rotation nor overall significant interactions, although there was a non-significant 21% decrease in the number of NeuN+ cells in non-rotating, hypoxic mice as compared to normoxic controls, which is comparable to the decrease we have observed in previous studies [13] (Figure 2, D). Qualitative analysis of cortical lamination in these sections suggested that despite this decrease in NeuN+ cells (albeit non-significant), this seems to reflect an overall decrease in cortical volume rather than a layer specific effect per se. However, further studies that quantify layer specific markers and markers for elements of the neuropil would be needed in order to fully ascertain layer integrity following hypoxia and changes in maternal care states.
Figure 2.
Panel A and B show a representative composite of immunostaining for NeuN and Dapi counterstain in the cerebral cortex of normoxic controls (A) and hypoxic controls (B). Panel C shows the total column of the neocortex and panel D shows the mean of the total number of NeuN+ cells in the neocortex of all groups (* denotes a significant decrease from the normoxic control group). Error bars represent SEM.
Discussion
In the current analyses we find that, corresponding with our previous studies, hypoxic-rearing decreased pup body weight, brain weight and cortical volume [7, 9, 14, 19]. However, no significant decrease was seen in the total number of NeuN+ cortical neurons. Importantly, rotating mothers to reduce the dam’s exposure to hypoxic conditions not only did not reverse the effects of hypoxia on body and brain weight, but actually amplified the effects of hypoxia on body weight, such that hypoxic pups with rotating mothers showed significantly less growth as the hypoxic period continued. Together these data suggest that postnatal chronic hypoxic injury induces an important effect on body and brain weight, regardless of maternal hypoxic status.
Interestingly, rotation of hypoxic mothers did not have the same deleterious effect on brain weight, suggesting the presence of compensatory mechanisms conserving brain weight and development even under extremely low body weight conditions. Overall, the additive effect of hypoxia and rotation on body weight may be explained by 1) a decreased nutritional output to the pups due to rotation, 2) stress induced by changes in feeding behavior and/or metabolism and 3) changes in maternal care due to stressful effects of rotation on the mother, none of which are mutually exclusive and may all contribute partially or completely to decreased pup body weight. Certainly, handling, removing dams from pups (albeit typically for extended periods of time), changes in nutrition and maternal care, have all been shown to individually and collectively influence pup growth and brain development [4, 6, 8, 11, 15, 28, 32, 36, 42]. However, the relative contribution of these factors to changes observed in body and brain weight remains to be determined.
Although no additional effects of maternal rotation were observed on brain weights in hypoxic-reared pups, paradoxical effects on neocortical volume were observed between rotating normoxic and rotating hypoxic groups, such that maternal rotation decreased brain volume in normoxic pups whereas maternal rotation caused a mild non-significant increase in the neocortical volume of hypoxic pups. Because no overall significant differences were observed in total numbers of neurons in the cortex, the decrease in neocortical volume observed in normoxic pups as well as the increase observed in hypoxic pups with rotating mothers may represent a change in synaptic connectivity and arborization or a change in non-neuronal elements such as glial cells. Indeed, changes in synaptic connectivity are amongst the most significant changes observed in very premature infants [2, 20, 22]. Furthermore, maternal care, early life stress and nutritional conditions during early postnatal development have all been shown to influence cortical synaptic connectivity of the rodent brain [5, 23, 37, 43], any and all of which may be changed in rotating mother conditions. It is also possible that the mother’s rotation has distinct cellular consequences in normoxic and hypoxic pups, perhaps underlying the opposite effect on volume in the two conditions. Further studies that characterize cortical connectivity following hypoxia and in response to changes in maternal care during hypoxia are needed.
Taken together, these results suggest that exposure of dams to hypoxic conditions influences both the brain and body development of pups reared under normoxic conditions. However, decreasing the dam’s exposure to hypoxic conditions is not sufficient to uniformly improve the outcome of hypoxic-reared pups, suggesting chronic postnatal hypoxia is the critical factor for deleterious growth, at least on these gross anatomical measures employed in the current studies.
Highlights.
Perinatal hypoxia models the effects of bioenergetics deficiency on brain development
Rotating dams between normoxia and hypoxia further decreased pups’ body weight
Dam’s rotation did not significantly impact the brain weight loss due to hypoxia
Dam’s rotation caused a paradoxical mild increase in cortical volume
Acknowledgments
Supported by USPHS grant PO1- P01 NS062686 and a Canadian Institute of Health Research Fellowship to N.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Natalina Salmaso, Email: natalina.salmaso@yale.edu.
Moises Dominguez, Email: moises.dominguez@yale.edu.
Mila Komitova, Email: mila.komitova@yale.edu.
Flora M. Vaccarino, Email: flora.vaccarino@yale.edu.
Michael L. Schwartz, Email: Michael.schwartz@yale.edu.
References
- 1.Allin M, Walshe M, Fern A, Nosarti C, Cuddy M, Rifkin L, Murray R, Rushe T, Wyatt J. Cognitive maturation in preterm and term born adolescents. Journal of neurology, neurosurgery, and psychiatry. 2008;79:381–386. doi: 10.1136/jnnp.2006.110858. [DOI] [PubMed] [Google Scholar]
- 2.Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ. The influence of preterm birth on the developing thalamocortical connectome. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:1711–1721. doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 2011;22:1016–1024. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Sale A, Maffei L. Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ. 2010;17:1092–1103. doi: 10.1038/cdd.2009.193. [DOI] [PubMed] [Google Scholar]
- 5.Bedi KS, Massey RF, Smart JL. Neuronal and synaptic measurements in the visual cortex of adult rats after undernutrition during normal or artificial rearing. The Journal of comparative neurology. 1989;289:89–98. doi: 10.1002/cne.902890107. [DOI] [PubMed] [Google Scholar]
- 6.Belluscio LM, Berardino BG, Ferroni NM, Ceruti JM, Canepa ET. Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiology & behavior. 2014;129:237–254. doi: 10.1016/j.physbeh.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Bi B, Salmaso N, Komitova M, Simonini MV, Silbereis J, Cheng E, Kim J, Luft S, Ment LR, Horvath TL, Schwartz ML, Vaccarino FM. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J Neurosci. 2011;31:9205–9221. doi: 10.1523/JNEUROSCI.0518-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Laye S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–2100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- 9.Chahboune H, Ment LR, Stewart WB, Rothman DL, Vaccarino FM, Hyder F, Schwartz ML. Hypoxic Injury during Neonatal Development in Murine Brain: Correlation between In Vivo DTI Findings and Behavioral Assessment. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JH, Rutledge JC, Stoops D, Abbe R. Hypobaric hypoxia-induced intrauterine growth retardation. Biology of the neonate. 1984;46:10–13. doi: 10.1159/000242026. [DOI] [PubMed] [Google Scholar]
- 11.Embleton ND. Early nutrition and later outcomes in preterm infants. World review of nutrition and dietetics. 2013;106:26–32. doi: 10.1159/000342553. [DOI] [PubMed] [Google Scholar]
- 12.Fagel DM, Ganat Y, Cheng E, Silbereis J, Ohkubo Y, Ment LR, Vaccarino FM. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia up-regulates Fibroblast Growth Factor ligands in the perinatal brain and induces Fibroblast Growth Factor -responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112:977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 15.Guzzetta A, Baldini S, Bancale A, Baroncelli L, Ciucci F, Ghirri P, Putignano E, Sale A, Viegi A, Berardi N, Boldrini A, Cioni G, Maffei L. Massage accelerates brain development and the maturation of visual function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6042–6051. doi: 10.1523/JNEUROSCI.5548-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jablonska B, Scafidi J, Aguirre A, Vaccarino F, Nguyen V, Borok E, Horvath TL, Rowitch DH, Gallo V. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. The Journal of pediatrics. 2010;156:525–531. e522. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. e451. [PubMed] [Google Scholar]
- 19.Komitova M, Xenos D, Salmaso N, May Tran K, Brand T, Schwartz ML, Ment L, Vaccarino FM. Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. J Neurosci. 2013;33:13375–13387. doi: 10.1523/JNEUROSCI.5286-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubsen J, Vohr B, Myers E, Hampson M, Lacadie C, Schneider KC, Katz KH, Constable RT, Ment LR. Microstructural and functional connectivity in the developing preterm brain. Seminars in perinatology. 2011;35:34–43. doi: 10.1053/j.semperi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, Duncan CC, Makuch RW. Change in cognitive function over time in very low-birth-weight infants. Jama. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 22.Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage. 2011;54:2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy KD, Desiraju T. Synapses in developing cingulate and hippocampal cortices in undernourished rats. Neuroreport. 1991;2:433–436. doi: 10.1097/00001756-199108000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SC, Murray RM. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain: a journal of neurology. 2008;131:205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 25.Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM. Impaired executive functioning in young adults born very preterm. Journal of the International Neuropsychological Society: JINS. 2007;13:571–581. doi: 10.1017/S1355617707070725. [DOI] [PubMed] [Google Scholar]
- 26.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants.[comment] Jama. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 27.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 28.Sale A, Berardi N, Maffei L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiological reviews. 2014;94:189–234. doi: 10.1152/physrev.00036.2012. [DOI] [PubMed] [Google Scholar]
- 29.Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmaso N, Silbereis J, Komitova M, Mitchell P, Chapman K, Ment LR, Schwartz ML, Vaccarino FM. Environmental Enrichment Increases the GFAP+ Stem Cell Pool and Reverses Hypoxia-Induced Cognitive Deficits in Juvenile Mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:8930–8939. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmaso N, Tomasi S, Vaccarino FM. Neurogenesis and maturation in neonatal brain injury. Clinics in perinatology. 2014;41:229–239. doi: 10.1016/j.clp.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato S, Nakagawasai O, Tan-No K, Niijima F, Suzuki T, Tadano T. Executive functions of postweaning protein malnutrition in mice. Biological & pharmaceutical bulletin. 2011;34:1413–1417. doi: 10.1248/bpb.34.1413. [DOI] [PubMed] [Google Scholar]
- 33.Scafidi J, Fagel DM, Ment LR, Vaccarino FM. Modeling premature brain injury and recovery. Int J Dev Neurosci. 2009;27:863–871. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz J, Cioffi-Ragan D, Wilson MJ, Julian CG, Beatty B, Moore LG, Galan HL. Little effect of gestation at 3,100 m on fetal fat accretion or the fetal circulation. American journal of human biology: the official journal of the Human Biology Council. 2013;25:544–549. doi: 10.1002/ajhb.22407. [DOI] [PubMed] [Google Scholar]
- 35.Soria R, Julian CG, Vargas E, Moore LG, Giussani DA. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res. 2013;74:633–638. doi: 10.1038/pr.2013.150. [DOI] [PubMed] [Google Scholar]
- 36.Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reproductive toxicology. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ. Stress and excitatory synapses: from health to disease. Neuroscience. 2013;248:626–636. doi: 10.1016/j.neuroscience.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 38.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 40.Volpe JJ. Cognitive deficits in premature infants. N Engl J Med. 1991;325:276–278. doi: 10.1056/NEJM199107253250409. [DOI] [PubMed] [Google Scholar]
- 41.Volpe JJ. Neurologic outcome of prematurity. Archives of neurology. 1998;55:297–300. doi: 10.1001/archneur.55.3.297. [DOI] [PubMed] [Google Scholar]
- 42.Walker CD. Maternal touch and feed as critical regulators of behavioral and stress responses in the offspring. Developmental psychobiology. 2010;52:638–650. doi: 10.1002/dev.20492. [DOI] [PubMed] [Google Scholar]
- 43.Yucel F, Warren MA, Gumusburun E. The effects of undernutrition on connectivity in the cerebellar cortex of adult rats. J Anat. 1994;184(Pt 1):59–64. [PMC free article] [PubMed] [Google Scholar]