Abstract
BACKGROUND
Controversy surrounds the question of whether clinical trial participants have better outcomes than comparable patients who are not treated on a trial. We explored this question using a recent large, randomized, multi-center study comparing peripheral blood (PB) with bone marrow (BM) transplantation from unrelated donors (URD), conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN).
METHODS AND FINDINGS
We compared characteristics and outcomes of study participants (n=494) and non-participants (n=1384) who appeared eligible and received similar treatment without enrolling on the BMT CTN trial at participating centers during the study time-period. Data were obtained from the Center for International Blood and Marrow Transplant Research. Outcomes were compared between the two groups using Cox proportional hazards regression models.
No significant differences in age, sex and disease distribution, race/ ethnicity, HLA matching, comorbidities and interval from diagnosis to HCT were seen between the participants and non-participants. Non-participants were more likely to have lower performance status, lower-risk disease, and older donors, and to receive myeloablative conditioning and anti-thymocyte globulin. Non-participants were also more likely to receive PB grafts, the intervention tested in the trial (66% vs. 50% p<0.001). Overall survival, transplant-related mortality, and incidences of acute or chronic GVHD were comparable between the two groups though relapse was higher (HR 1.22, 95% CI 1.02–1.46, p=0.028) in non-participants.
CONCLUSION
Despite differences in certain baseline characteristics, survival was comparable between study participants and non-participants. The results of the BMT CTN trial appear generalizable to the population of trial-eligible patients.
Introduction
Randomized clinical trials (RCT) are considered the gold standard in clinical research. However, their applicability to larger populations may be limited because trial patients may not be representative of most patients due to selection bias.(1) Despite this potential limitation, very few trials have the generalizability of their results assessed, even though discussion about generalizability is a quality indicator for RCT reporting within the CONSORT guidelines.(2) It is also controversial whether patients enrolled in trials have better outcomes than those not enrolled in trials, controlling for biological characteristics. While some studies show improved outcomes in trial participants as compared to non-participants,(3–6) others report no trial effect.(7, 8) Peppercorn et al reported that most studies comparing outcomes between trial and non-trial participants failed to control for potential confounding factors between the groups, and therefore, available evidence does not support a trial effect on outcomes.(9)
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducts multicenter trials to improve treatment approaches in hematopoietic cell transplantation (HCT). A phase III randomized, multicenter study conducted by BMT CTN (BMT CTN 0201) between March 2004 and September 2009 compared outcomes after bone marrow (BM) and filgrastim mobilized peripheral blood (PB) HCT from unrelated donors (URD).(10) The trial found no significant difference in survival between the two groups, but a significant increase in the risk of chronic graft vs. host disease (GVHD) with PB. Its practice changing potential is based on the fact that the study supports the use of BM grafts with decreased chronic GVHD, in the current era where PB is used in 70% of URD transplants.
Before applying the study results to clinical practice, it is important to understand their generalizability to the universe of potential patients. To do so, we compared the characteristics and outcomes of participants in BMT CTN protocol 0201 with those of patients receiving unrelated donor HCTs at the same centers during the study time-period but who were not study participants, using data from the Center for International Blood and Marrow Transplant Research (CIBMTR). We restricted the analysis to centers participating in BMT CTN and to patients receiving similar treatment off-protocol in order to minimize confounding variables while assessing for a trial effect.
Patients and Methods
Data Source
The CIBMTR is a research collaboration of the Medical College of Wisconsin and the National Marrow Donor Program (NMDP)/Be The Match. More than 350 transplantation centers worldwide contribute detailed data on consecutive allogeneic and autologous HCT to the CIBMTR’s outcomes registry. The CIBMTR also leads the data coordinating center for the BMT CTN. Patients are followed longitudinally with yearly follow-up. Compliance is monitored by on-site audits. Observational studies by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority and with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Board of the NMDP.
The current study included two main cohorts of patients for whom information was retrieved from the CIBMTR database: patients treated on the BMT CTN 0201 protocol and patients who underwent URD transplants during the study time period at 38 participating centers but not on the BMT CTN study. The eligibility criteria for the BMT CTN protocol included age <66 years and HCT for acute leukemia, myelodysplasia, chronic myeloid or myelomonocytic leukemia or myelofibrosis. Exclusion criteria are included in supplementary table 1.
The comparator group of interest was patients who, based on information from the CIBMTR database, appeared eligible per the inclusion and exclusion criteria of the BMT CTN study and received URD HCT at the participating centers during the time the trial was open, but did not enroll in the study. Since not all information needed to determine eligibility for the protocol (organ function requirements) was available from the CIBMTR database, we selected patients treated with similar regimens to identify a group as close as possible in clinical profile to the trial participants. The assumption was that patients able to receive regimens used in the clinical trial were likely to have organ function consistent with eligibility criteria for the trial.
Study outcomes
We estimated the proportion of all potentially eligible URD transplants that were enrolled on the protocol. Survival, relapse, transplant-related mortality (TRM) and occurrence of acute and chronic GVHD were compared between the participants and non-participants. TRM was defined as death while in complete remission. TRM and relapse were considered competing risks where occurrence of one of them prevents occurrence of the other one. Overall survival (OS) was calculated as time from transplant to death. Death from any cause was considered as an event and surviving patients were censored at the time of last follow-up. Disease free survival (DFS) was defined as time from transplant to treatment failure (death or relapse). Patients alive in remission were censored at the time of last follow-up.
Statistical analysis
The characteristics of the participants and non-participants in the entire cohort and the separate PB and BM subgroups were compared using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Multivariate analyses of acute and chronic GVHD, TRM, relapse, DFS and OS were performed using Cox proportional hazards regression, using participation in the BMT CTN study as the main effect. Variables considered in the multivariate analysis are described in Supplementary Table 2. The assumption of proportional hazards was tested for each variable using a time-dependent covariate, and appropriate adjustments were performed where needed. Multivariate models were built using a forward variable selection method. In addition, stratified analysis of BM and PB recipients was performed to compare the outcomes of BMT CTN 0201 participants versus non-participants within each graft source subgroup because the proportion of graft source was significantly different between the study participants and non-participants. All P values are 2-sided, and a level of significance (alpha) of 0.05 was used throughout. All analyses were performed using SAS Version 9.3 statistical software (SAS Institute, Cary, NC).
Results
Patient characteristics
Figure 1 shows the selection of patients for this study. A total of 5716 patients received a first allogeneic HCT for diseases specified by the BMT CTN 0201 protocol at participating transplant centers during the study period. Among these, 2708 were excluded for not meeting the trial eligibility criteria. We also excluded 32 participants and 109 non-participants from 10 centers that had no eligible non-participants for analysis. An additional 6 patients were excluded because they had not consented for CIBMTR research or had a followup <100 days. From the remaining 2893 patients, 1046 received a regimen different from what was specified by the BMT CTN 0201 protocol; these patients were excluded from further analysis. Five hundred fifty one patients were enrolled on the BMT CTN 0201 study. In addition to excluding the 32 patients from centers where comparable non-trial patients could not be identified, we excluded 25 patients who did not undergo HCT. The final study population consisted of 494 patients who participated in the BMT CTN 0201 study and 1353 patients who appeared to be eligible based on the trial’s inclusion/exclusion criteria, did not enroll, and yet received the same conditioning and GVHD prophylaxis regimens as trial participants, suggesting that they could have enrolled on the trial. Thus, approximately 27% (494/ 494+1353) of apparently eligible patients at participating centers were enrolled on the trial. If we instead estimate the participation rate based on all non-participants without regard to conditioning regimens and GVHD prophylaxis, the trial participation rate would be 17% (494/494+1353+1046).
Figure 1.
Patient selection flowchart
There were no significant differences in the age, sex and disease distribution, race/ ethnicity, HLA matching and interval from diagnosis to HCT between the participants and non-participants. A pretransplant HCT comorbidity index, available for 64% patients, was comparable between participants and non-participants. A higher proportion of non-participants had a Karnofsky performance score (KPS) <90, lower risk disease, and received antithymocyte globulin (ATG). PB was used more commonly as the graft source in the non-participants as compared to the study participants (66% vs. 50%; p<0.001). (Table 1) Characteristics of participants and non-participants were also evaluated separately in the groups receiving PB and BM. Among those receiving PB grafts, non-participants were older, had more myeloid diseases but comparable disease risk, higher proportion of ≤7/8 HLA match donors and more frequent use of ATG than the study participants. In the BM group, non-participants were younger, more likely to be Hispanic, have low risk disease, receive myeloablative conditioning and ATG and less likely to have a KPS ≥90.
Table 1.
Characteristics of patients who received a BM or PB transplant between 2004 and 2009 from participating centers, according to enrollment status and regimen received a
| Characteristics | Patients enrolled and transplanted on BMT CTN 0201 study |
Likely eligible but not enrolled, transplanted with same regimen |
P value b |
|---|---|---|---|
| Number of patients | 494 | 1353 | |
| Age at transplant, years | 0.73 | ||
| 0–9 | 17 (3) | 52 (4) | |
| 10–19 | 34 (7) | 107 (8) | |
| 20–49 | 252 (51) | 704 (52) | |
| >50 | 191 (39) | 490 (36) | |
| Patient gender | 0.18 | ||
| Male | 276 (56) | 708 (52) | |
| Female | 218 (44) | 645 (48) | |
| Recipient race | 0.79 | ||
| Caucasian | 446 (90) | 1232 (91) | |
| African-American | 20 (4) | 55 (4) | |
| Other | 28 (6) | 66 (5) | |
| Recipient ethnicity | 0.06 | ||
| Hispanic | 19 (4) | 92 (7) | |
| Non-Hispanic | 470 (95) | 1250 (92) | |
| Unknown | 5 (1) | 11 (<1) | |
| KPS | 0.03 | ||
| >=90 | 313 (63) | 766 (57) | |
| <90 | 138 (28) | 441 (33) | |
| Unknown | 43 (9) | 146 (11) | |
| Sorror comorbidity index | 0.48 | ||
| 0 | 184 (37) | 512 (38) | |
| 1–2 | 68 (14) | 158 (12) | |
| >=3 | 62 (13) | 198 (15) | |
| Unknown (info not collected on patients reported prior to 2008) | 180 (36) | 485 (36) | |
| Disease | 0.24 | ||
| AML | 233 (47) | 673 (50) | |
| ALL | 106 (21) | 245 (18) | |
| CML | 62 (13) | 150 (11) | |
| MDS/Myelofibrosis/CMML | 93 (19) | 285 (21) | |
| Disease risk prior to transplantc | 0.02 | ||
| Low risk | 360 (73) | 1063 (79) | |
| High risk | 134 (27) | 288 (21) | |
| Unknown | 0 | 2 (<1) | |
| Secondary leukemia/MDS with prior Auto (> 12 months from current transplant) | 0.86 | ||
| No | 489 (99) | 1338 (99) | |
| Yes | 5 (1) | 15 (1) | |
| Graft type | <0.001 | ||
| Bone Marrow | 247 (50) | 456 (34) | |
| Peripheral Blood | 247 (50) | 897 (66) | |
| Donor age, years | <0.001 | ||
| 18–29 | 200 (40) | 308 (23) | |
| 30–39 | 154 (31) | 347 (26) | |
| 40–49 | 114 (23) | 200 (15) | |
| 50–61 | 26 (5) | 47 (3) | |
| Unknown | 0 | 451 (33) | |
| Antigen match at A, B, DRB1 | 0.13 | ||
| 8/8 | 371 (75) | 954 (71) | |
| <=7/8 | 123 (25) | 398 (29) | |
| Missing | 0 | 1 (<1) | |
| Donor/recipient sex match | 0.03 | ||
| M/M | 208 (42) | 486 (36) | |
| M/F | 132 (27) | 399 (29) | |
| F/M | 68 (14) | 214 (16) | |
| F/F | 86 (17) | 239 (18) | |
| Unknown | 0 | 15 (1) | |
| Donor/recipient CMV match | <0.001 | ||
| −/− | 186 (38) | 370 (27) | |
| −/+ | 154 (31) | 450 (33) | |
| +/+ | 96 (19) | 280 (21) | |
| +/− | 57 (12) | 154 (11) | |
| Unknown | 1 (<1) | 99 (7) | |
| Conditioning regimen intensity | 0.04 | ||
| Myeloablative | 384 (78) | 1119 (83) | |
| Reduced Intensity | 110 (22) | 233 (17) | |
| Unknown | 0 | 1 (<1) | |
| Use of ATG or Campath | <0.001 | ||
| ATG or Campath | 126 (26) | 430 (32) | |
| No ATG or Campath | 362 (73) | 923 (68) | |
| Unknown | 6 (1) | 0 | |
| Interval from diagnosis to transplant months, median(range) | 8 (<1–168) | 9 (<1–357) | 0.49 |
| Year of transplant | <0.001 | ||
| 2004 | 30 (6) | 155 (11) | |
| 2005 | 73 (15) | 269 (20) | |
| 2006 | 93 (19) | 275 (20) | |
| 2007 | 134 (27) | 248 (18) | |
| 2008 | 103 (21) | 215 (16) | |
| 2009 | 61 (12) | 191 (14) |
Patients receiving any of the four conditioning regimens (Cyclophosphamide+TBI, Cyclophosphamide+Busulfan, Fludarabine+Busulfan+ATG, Fludarabine+Melphalan) and cyclosporine/ tacrolimus+Methotrexate GVHD prophylaxis were classified as receiving the same regimen as specified by the BMT CTN 0201 protocol; patients receiving any other conditioning regimen or any other GVHD prophylaxis were excluded
Chi-square test (for categorical variables) or Wilcoxon rank-sum test (for continuous variables) p-values between BMT CTN 0201 patients (column 1) and non-BMT CTN 0201 patients with same conditioning regimen (column 2)
High-risk disease includes acute myeloid leukemia in third or subsequent remission or not in remission, acute lymphoblastic leukemia not in remission, the myelodysplastic syndrome with excess blasts in transformation, chronic myeloid leukemia in blast phase, and chronic myelomonocytic leukemia in any stage. All others were considered low risk.
Abbreviations: AML, Acute myelogenous leukemia; ALL, Acute lymphoblastic leukemia; CML, Chronic myeloid leukemia; MDS/ CMML, Myelodysplastic syndrome/ Chronic myelomonocytic leukemia; BM, bone marrow; PB, peripheral blood; KPS, Karnofsky performance score; CMV, cytomegalovirus; ATG, antithymocyte globulin
Reasons for non-participation
To gain insight into the reasons for non-participation, we reviewed study coordinator tracking logs. We identified a group of patients (n=713) who consented to the study, but didn’t proceed on the protocol. Among these, 344 patients (48%) had a transplant off protocol reported to CIBMTR. Removal from the protocol was mainly for donor related issues (n=237, 69%), for example, the donor did not provide consent or was ineligible for the study or due to donor center decision. Among transplanted patients removed from the trial for a donor reason, 72% received PB grafts. The remaining patients (n=107, 31%) were removed because of patient related issues (e.g., patient found to be ineligible or withdrew consent). Among these, 84% received PB grafts.
Outcomes of participants vs. non-participants
Compared to the BMT CTN participants, non-participants did not have a significantly increased risk of mortality both in the unadjusted analysis (p=0.46) and after adjusting for all the clinical variables (HR 1.08, 95% CI 0.94–1.24, p=0.26). (Figure 2A) While TRM was comparable between the two groups, relapse was higher (HR 1.22, 95% CI 1.02–1.46, p=0.028) resulting in a trend towards lower disease free survival in non-participants (HR 1.14, 95% CI 1.0–1.3 p=0.05). Center effect was examined and was not found to be significant in the multivariate model (p=0.11). No statistically significant differences in acute (HR 0.96, 95% CI 0.82–1.12, p=0.57) and chronic GVHD (HR 1.06, 95% CI 0.92–1.23, p=0.41) were noted between study participants and non-participants. (Table 2)
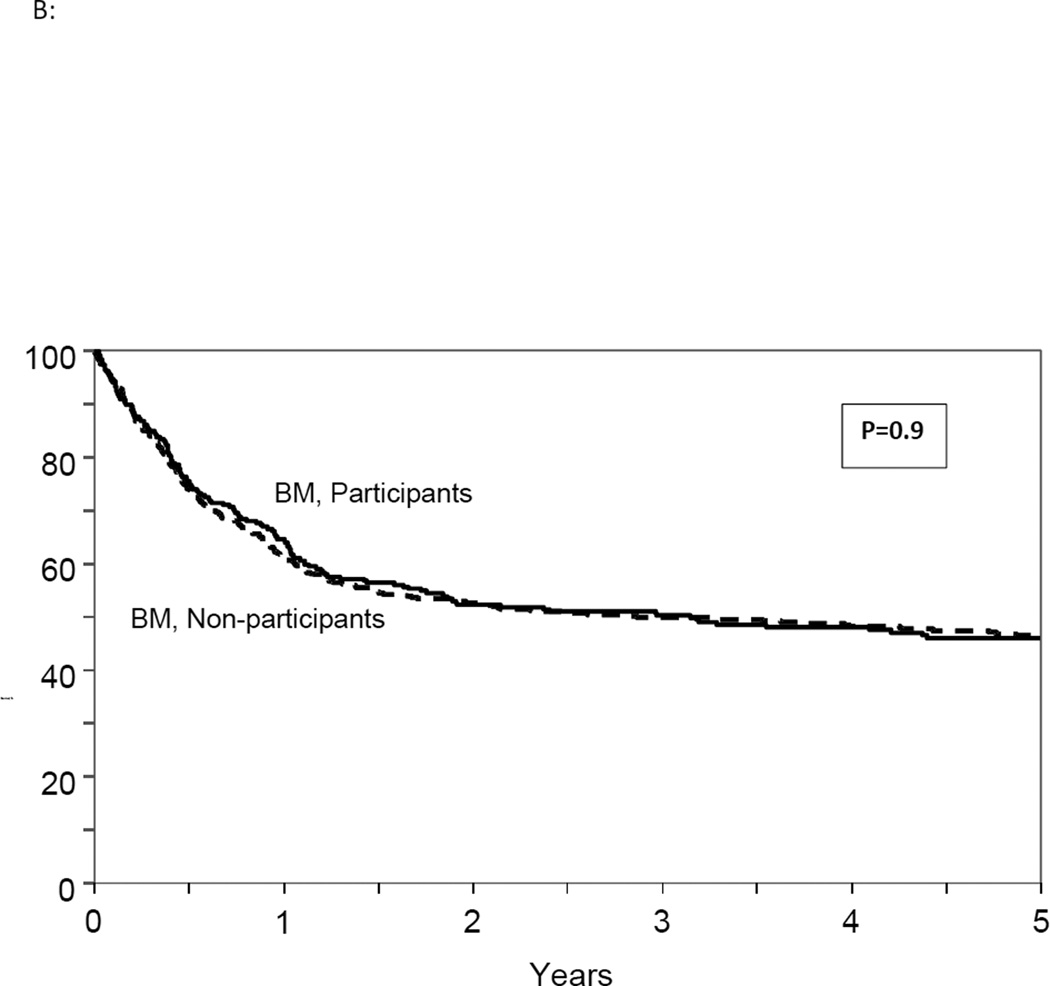
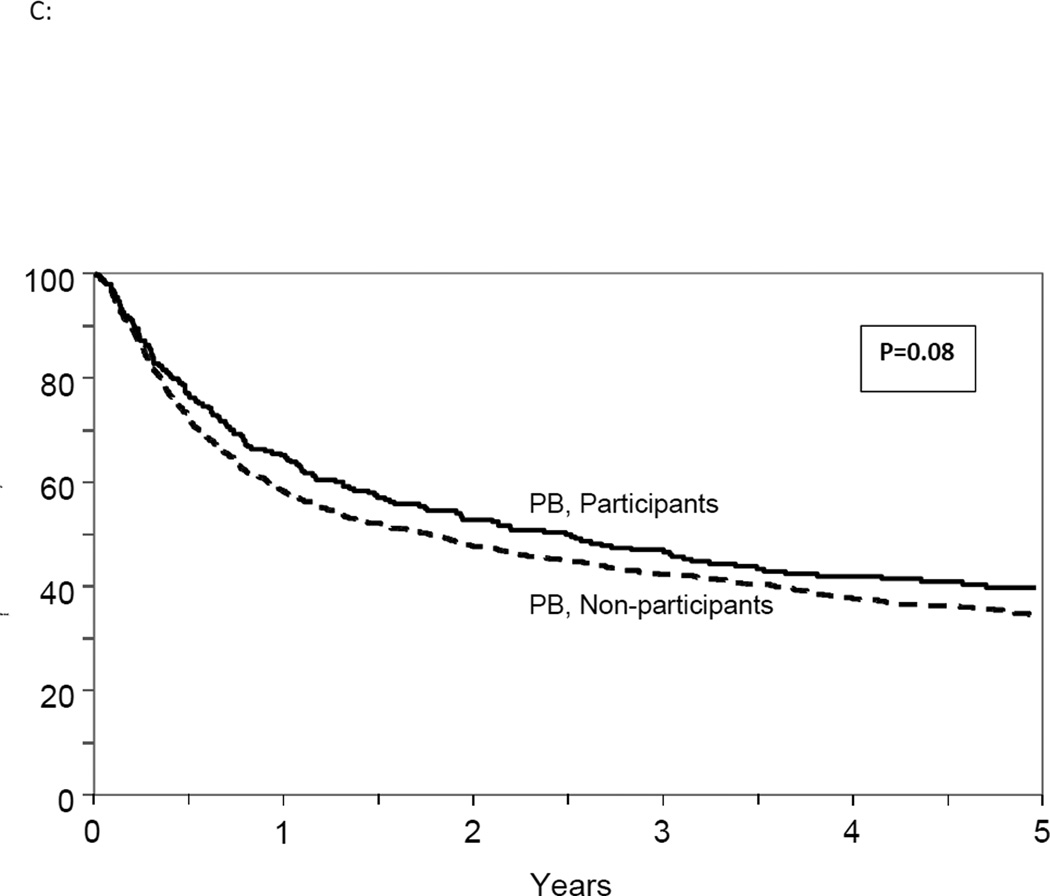
Figure 2.
(A): Adjusted overall survival in all study participants and non-participants
(B): Adjusted overall survival in study participants and non-participants receiving bone marrow graft
(C): Adjusted overall survival in study participants and non-participants receiving PB graft
Table 2.
Multivariable models for Overall survival, Transplant related mortality, Relapse, Disease free survival, and GVHD
| HR (95% CI) for non-participants vs. study participants |
P value | |
|---|---|---|
| Overall survival1 | 1.08 (0.94–1.24) | 0.27 |
| Transplant related mortality2 | 1.08 (0.8–1.29) | 0.43 |
| Relapse3 | 1.22 (1.02–1.46) | 0.03 |
| Disease free survival4 | 1.14 (1.00–1.30) | 0.05 |
| Acute GVHD5 | 0.96 (0.82–1.12) | 0.58 |
| Chronic GVHD6 | 1.07(0.92–1.2) | 0.4 |
Other significant variables include: patient age, diagnosis, disease risk, graft source, interval between diagnosis and HCT, KPS, HLA match and donor-recipient sex match
Other significant variables include: patient age, disease risk, HLA match and donor-recipient sex match, GVHD prophylaxis and use of ATG/ alemtuzumab
Other significant variables include: disease risk, donor-recipient sex match, interval between diagnosis and HCT, KPS and regimen intensity
Other significant variables include: patient age, disease risk, HLA match, donor-recipient sex match, interval between diagnosis and HCT and KPS
Other significant variables include graft source, HLA match, regimen intensity and use of ATG/ alemtuzumab
Other significant variables include: graft source, diagnosis, ethnicity, donor-recipient sex match and use of ATG/ alemtuzumab
Although the interaction between trial participation and graft source was not significant (p=0.23), we compared OS between study and non-study participants receiving PB and BM separately in a secondary analysis. The risk of mortality was not significantly higher for BM recipients (HR 1.0, 95% CI 0.80–1.23, p=0.94) or for PB recipients (HR 1.2, 95% CI 0.98–1.41, p=0.07) in non-participants compared to trial participants. (Figure 2B and 2C) A stratified analysis for other clinical outcomes also showed comparable results in BM and PB group separately in the participants and non-participants. (Table 3)
Table 3.
Stratified analysis for Overall survival, Transplant related mortality, Relapse, Disease free survival, and GVHD
| HR (95% CI) for non-participants vs. study participants ; p value |
||
|---|---|---|
| Outcomes | Peripheral Blood | Bone Marrow |
| Overall survival | 1.2 (0.9–1.4); 0.08 | 1.0 (0.8–1.2); 0.9 |
| Transplant related mortality | 1.2 (0.9–1.5); 0.2 | 0.9 (0.7–1.3); 0.8 |
| Relapse | 1.3 (0.9–1.6); 0.06 | 1.14 (0.8–1.5); 0.3 |
| Disease free survival | 1.2 ( 0.9–1.4); 0.1 | 1.06 (0.8–1.3); 0.6 |
| Acute GVHD | 0.9 (0.8–1.2); 0.9 | 0.9 (0.7–1.8); 0.6 |
| Chronic GVHD | 1.2 (0.9–1.4); 0.1 | 0.9 (0.7–1.2); 0.6 |
Impact of graft source
In the multivariate analysis of OS for the entire cohort with a median follow up of 60 months for survivors, graft source emerged as a significant predictor with PB associated with increased mortality (HR 1.16, 95% CI 1.01–1.32, p=0.02). However when the analysis was limited to 2 years post HCT similar to the original report, no difference was observed (HR 1.03, 95% CI 0.89–1.19, p=0.64).(10) Similar to the original report, PB was associated with a higher incidence of chronic GVHD than BM (HR 1.38, 95% CI 1.2–1.6, p<0.001).
Discussion
Concerns have been raised about the generalizability of RCT results due to the questionable representativeness of patients enrolled in the trials.(5, 11, 12) Differences in the outcomes of patients treated on versus off trials may be due to inclusion of a highly selected patient population (based on medical status, disease status or compliance), differences in care due to clinical trial participation (e.g., closer follow-up or more precise application of therapies), or due to true biological effects.(7, 13, 14) Interestingly, a recent study that compared patients on the standard arms of a series of South West Oncology Group phase III cancer clinical trials to non-trial control subjects selected from the Surveillance, Epidemiology, and End Results program found that the improved OS with trial participation was only seen for the first year after diagnosis and evened out in the long- term.(15) In HCT, there is a paucity of studies evaluating if the results from RCTs are likely to translate to the general transplant population.
In one of the first such studies in HCT, we found that approximately a quarter of the potentially eligible patients participated in the randomized study. This is likely an over-estimate since some potentially eligible patients may have received alternative conditioning regimens due to competing protocols or center practices and, thus, were not included in the denominator because of our patient selection criteria. Even with the most conservative estimate of the participation rate (17%), this is still much better than <10 % participation rate that has been reported in other studies.(16, 17) We observed higher proportion of high risk disease in the trial participants than the non-participants and comparable distribution of age, comorbidities and race/ethnicity which is also different from the pattern reported in the literature. Usually, patients from racial/ ethnic minorities or those who are higher risk, older and with comorbidities are less represented in clinical trials.(14, 18–20) The fact that we did not observe this may be attributed to either the relatively broad inclusion criteria of the clinical trial, or to our selection of comparator patients that were healthy enough to be potential trial participants based on age and comorbidities. A difference between the participants and non-participants was the greater use of ATG in the non-trial participants reflecting the standard practice of some centers to use it outside of a clinical trial to decrease GVHD with URD transplants.(21, 22)
Despite some differences in the clinical characteristics of patients enrolled on the trial compared to those not enrolled, there was no difference in the OS. This may be due to similar baseline characteristics between the two groups such as age, disease, interval from diagnosis to treatment and HLA match that were associated with lower OS. While a higher proportion of the trial non-participants had lower KPS, which was associated with worse OS, this may have been counterbalanced by a lower proportion of high risk disease. Our results are consistent with what has been reported in systemic reviews that there is no definitive evidence for superior outcomes in patients participating in a clinical trial as compared to non-participants treated in a similar fashion when adjusted for the confounding factors.(9, 23) In addition, it is also possible that HCT patients are treated and followed in specialized settings with aggressive supportive care regardless of trial participation leading to similar outcomes between participants and non-participants.
We did observe that relapse was lower and DFS marginally better in trial participants. Analyses stratified by graft type showed that this observation is primarily due to increased relapse in non-participants compared to participants when PB is used. It is possible that more non-participants had unmeasured high risk disease (such as high risk cytogenetics or FLT3 mutation positive) not appropriately captured in the risk categorization used for the BMT CTN study or the current study. While a higher proportion of non-participants received ATG, receipt of ATG was not associated with relapse in our multivariate analysis.
Poorer survival in patients receiving PB grafts as compared to BM grafts in our multivariate analysis is different from the results of the BMT CTN study as well as a prior observational study which reported comparable OS between PB and BM groups except for the good risk CML patients.(24) Since this difference was not detected when the observations were limited to 2 years, our findings may be explained by a longer period of follow-up than the clinical study (BMT CTN 0201 is currently analyzing their 5 year follow-up data). This does, however, potentially add increased evidence that BM should be the default graft source.
Our study has some limitations. By applying the inclusion criteria and restricting the non-trial cohort to those with the same conditioning and GVHD prophylaxis, we aimed to create a group that was similar to the trial participants to enhance confidence in the analysis. However, it is possible that some ineligible patients on the basis of organ function were misclassified as potentially eligible since we did not have detailed information about organ function. Conversely, some patients may not have appeared potentially eligible because they received different conditioning or GVHD prophylaxis regimens or participated in different clinical trials, even though they could have tolerated the treatment specified by the trial. These biases would affect the estimate of the percentage of potentially eligible patients that ended up on the trial, but in opposite directions. It is also possible that other unmeasured differences, such as use of maintenance therapy post HCT or different dosing of busulfan were present, but these were not specified in the 0201 protocol either. We also did not study patients who received their URD transplants at non- study centers, thereby precluding a truly population-based comparison and focusing only on the ‘trial effect’ aspect of generalizability. Other facets of generalizability including the difference in outcomes between patients treated in a similar fashion at study centers vs. at non-study centers will be addressed in subsequent analyses. We do not know the reasons for low trial enrollment, such as refusal to participate, investigators not offering the trial to patients, or enrollment on competing protocols, due to lack of information for trial non-participation. By restricting the treatment regimens to those specified by the study, we were able to select non-trial participants who appeared the most comparable to trial participants, based on known characteristics.
The strength of our study is that we were able to perform a careful comparison of patients who did or did not participate in the trial at the same centers treated in a similar fashion during the same time period using a consistent data source. This analysis improves our confidence that treatment effects for this particular trial translate to the real-world setting and can be extrapolated to future patients who meet the disease and health requirements outlined in the trial.
While consideration of external validity in the design and reporting of randomized clinical trials is important, not all studies can do this. Our study was possible because of the availability of the CIBMTR database which provides a unique resource of observational data to address questions about the generalizability of HCT clinical trials. Such a database can also track real-world use of technologies to understand shifts in clinical practice. RCTs are unique in providing an unbiased comparison of therapies but are logistically difficult and expensive. Gathering the data to support the generalizability of clinical trial results and the actual effectiveness of study interventions in practice will help translate the investment in time- and resource-intensive studies into clinical practice changes.
Supplementary Material
Compared characteristics and outcomes of BMTCTN 0201participants and non-participants
Few differences in baseline characteristics were observed
Survival was comparable between study participants and non-participants
Acknowledgments
Acknowledgements of Research support:
Support for the clinical trial BMT CTN 0201 was provided by grant #U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, the Department of the Navy, Office of Naval Research, and the National Marrow Donor Program. Enrollment support was provided by DKMS Germany. Any views, opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not reflect the views or the official policy or position of the above mentioned parties.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented: In part at 2014 American Society of Hematology Meeting at San Francisco, USA
References
- 1.Gale RP, Eapen M, Logan B, Zhang MJ, Lazarus HM. Are there roles for observational database studies and structured quantification of expert opinion to answer therapy controversies in transplants? Bone marrow transplantation. 2009;43:435–446. doi: 10.1038/bmt.2008.447. [DOI] [PubMed] [Google Scholar]
- 2.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 3.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a "trial effect". J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 4.Davis S, Wright PW, Schulman SF, et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer. 1985;56:1710–1718. doi: 10.1002/1097-0142(19851001)56:7<1710::aid-cncr2820560741>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Fossa SD, Skovlund E. Selection of patients may limit the generalizability of results from cancer trials. Acta Oncol. 2002;41:131–137. doi: 10.1080/028418602753669490. [DOI] [PubMed] [Google Scholar]
- 6.Udell JA, Wang TY, Li S, et al. Clinical trial participation after myocardial infarction in a national cardiovascular data registry. JAMA. 2014;312:841–843. doi: 10.1001/jama.2014.6217. [DOI] [PubMed] [Google Scholar]
- 7.Mengis C, Aebi S, Tobler A, Dahler W, Fey MF. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol. 2003;21:3933–3939. doi: 10.1200/JCO.2003.03.186. [DOI] [PubMed] [Google Scholar]
- 8.Stiller CA, Benjamin S, Cartwright RA, et al. Patterns of care and survival for adolescents and young adults with acute leukaemia--a population-based study. Br J Cancer. 1999;79:658–665. doi: 10.1038/sj.bjc.6690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363:263–270. doi: 10.1016/S0140-6736(03)15383-4. [DOI] [PubMed] [Google Scholar]
- 10.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. The New England journal of medicine. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 12.Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106:2452–2458. doi: 10.1002/cncr.21907. [DOI] [PubMed] [Google Scholar]
- 13.Jha P, Deboer D, Sykora K, Naylor CD. Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: a population-based comparison. Journal of the American College of Cardiology. 1996;27:1335–1342. doi: 10.1016/0735-1097(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 14.Steg PG, Lopez-Sendon J, Lopez de Sa E, et al. External validity of clinical trials in acute myocardial infarction. Archives of internal medicine. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Unger JM, Barlow WE, Martin DP, et al. Comparison of Survival Outcomes Among Cancer Patients Treated In and Out of Clinical Trials. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herland K, Akselsen JP, Skjonsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99:11–19. doi: 10.1016/j.rmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 18.Bahit MC, Cannon CP, Antman EM, et al. Direct comparison of characteristics, treatment, and outcomes of patients enrolled versus patients not enrolled in a clinical trial at centers participating in the TIMI 9 Trial and TIMI 9 Registry. Am Heart J. 2003;145:109–117. doi: 10.1067/mhj.2003.43. [DOI] [PubMed] [Google Scholar]
- 19.van de Water W, Kiderlen M, Bastiaannet E, et al. External Validity of a Trial Comprised of Elderly Patients With Hormone Receptor–Positive Breast Cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju051. [DOI] [PubMed] [Google Scholar]
- 20.Sateren WB, Trimble EL, Abrams J, et al. How Sociodemographics, Presence of Oncology Specialists, and Hospital Cancer Programs Affect Accrual to Cancer Treatment Trials. Journal of Clinical Oncology. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 21.Finke J, Schmoor C, Lang H, Potthoff K, Bertz H. Matched and mismatched allogeneic stem-cell transplantation from unrelated donors using combined graft-versus-host disease prophylaxis including rabbit anti-T lymphocyte globulin. Journal of clinical oncology : official journal of the American Society of C linical Oncology. 2003;21:506–513. doi: 10.1200/JCO.2003.03.129. [DOI] [PubMed] [Google Scholar]
- 22.Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database Syst Rev. 2008:MR000009. doi: 10.1002/14651858.MR000009.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.