Abstract
Background
There are no established guidelines for pathologic diagnosis/reporting of IPMNs.
Design
An international multidisciplinary group brought together by the Verona Pancreas Group in Italy-2013, was tasked to devise recommendations.
Results
1) Crucial to rule out invasive carcinoma with extensive (if not complete) sampling. 2) Invasive component is to be documented in a full synoptic report including its size, type, grade, stage. 3) The term “minimally invasive” should be avoided; instead, invasion size with stage and substaging of T1 (1a, b, c; ≤0.5, >0.5–≤1, >1 cm), is to be documented. 4) Largest diameter of the invasion, not the distance from the nearest duct, is to be used. 5) A category of “indeterminate/(suspicious) for invasion” is acceptable for rare cases. 6) The term “malignant” IPMN should be avoided. 7) The highest grade of dysplasia in the non-invasive component is to be documented separately. 8) Lesion size is to be correlated with imaging findings in cysts with rupture. 9) The main duct diameter, and if possible, its involvement is to be documented; however, it is not required to provide main vs branch duct classification in the resected tumor. 10) Subtyping as gastric/intestinal/pancreatobiliary/oncocytic/mixed is of value. 11) Frozen section is to be performed highly selectively, with appreciation of its shortcomings. 12) These principles also apply to other similar tumoral intraepithelial neoplasms (mucinous cystic neoplasms, intra-ampullary, intra-biliary/cholecystic).
Conclusion
These recommendations will ensure proper communication of salient tumor characteristics to the management teams, accurate comparison of data between analyses, and development of more effective management algorithms.
INTRODUCTION
The term IPMN was created in 1994 by Klöppel et al1 to embrace entities previously reported in the literature under a plethora of designations including duct-ectatic mucinous cystic neoplasms,2, 3 mucin-producing tumors,4–6 intraductal papillary neoplasms,7 papillary adenocarcinomas, villous adenomas8 and others. Since then much has been learned about the nature and behavior of these distinctive neoplasms; however, many of the diagnostic criteria and management protocols remain challenging to apply and some are highly controversial.
The challenges also extend to, and often stem from, a lack of uniform criteria in the pathologic evaluation, terminology and parameters to be reported. In fact, the absence of a uniform approach in pathology may be partly responsible for the obstacles to better characterization of these tumors. For example, the terms “malignant IPMN”9–28 and “minimally invasive IPMN”29–39 have been highly variably defined in the literature creating great confusion in understanding the behavior of different stages of this entity and leading to major contradictions in establishing uniform management protocols for patients with this tumor.
In April of 2013, an international meeting was convened in Verona, Italy, under the leadership and organization of Verona Pancreas Group. The Pathology team of this consensus meeting was tasked with assessing the current state and possible improvements in the pathologic evaluation of terminology and documentation of IPMNs, as well as determination of clinically relevant parameters for the management of these tumors. Accordingly, through literature analysis as well as interdisciplinary discussions with various experts both among the participants of the meeting and outside of the meeting, this group developed consensus on refined definitions and basic guidelines for the pathologic evaluation and reporting of IPMNs, which are discussed in detail in this manuscript.
METHODS
A pathology task group was created in 2012 in preparation for the consensus meeting to be held in Verona, Italy, in April 2013, under the leadership of Verona Pancreas Group. This task group consisted of an interdisciplinary team of participants with invested interest in the pathologic evaluation of branch duct IPMNs including pathologists, surgeons, radiologists, and gastroenterologist (VA, MMK, TF, GZ, CLW, HM, JO, MA, MJB). The team was tasked with analyzing the state of pathologic diagnosis of IPMNs.
Pre-meeting analysis of the literature was performed especially focusing on the pathologic assessment of IPMNs, and a list of discussion points was created by participation of all the team members. During this preparation, it was determined that wide disparities in pathologic evaluation, terminology and reporting of IPMNs existed. These disparities were presented to the participants of the Consensus Meeting in Verona in a general session, followed by active discussions conducted with the participation of the entire consensus meeting membership Further discussions were held in break-out sessions. The results of these discussions were collated and presented to the participants at the last day of the meeting.
After the meeting, further analyses were performed as tasked during the consensus meeting. These included the analysis of institutional databases for the parameters in question and investigation of pathology reports on IPMN (performed in the community as well as in expert institutions). Evaluation of these pathology reports revealed that crucial pieces of information were missing from the vast majority of these reports, even in institutions where IPMN surgery is performed routinely.
In establishing the clinically relevant pathologic parameters, consultation with other experts from various disciplines who had not participated in the consensus meeting was also sought.
Separately, another international consensus meeting was held on the topic of pancreatic intraepithelial neoplasia (including IPMNs) at The Johns Hopkins University School of Medicine, led by Drs. Ralph Hruban and David Klimstra, under the auspices of the Sol Goldman Pancreatic Cancer Research Center of The Johns Hopkins University in June 2014. Some of the parameters and salient issues were also discussed during this meeting. Although the proceedings of this meeting are the subject of another manuscript, the conclusions presented below are in accordance with the conclusions of that latter meeting.
RESULTS/DISCUSSION
Definition
Intraductal papillary mucinous neoplasms40–43 are pathologically defined as mass-forming pre-invasive neoplasms (tumoral intraepithelial neoplasms) that grow within the ducts of the pancreas (Table 1)40–43. In the WHO-2010 classification,44 in order to distinguish IPMNs from incidental microcysts and large pancreatic intraepithelial neoplasms (PanINs), IPMNs were required to be > 1 cm in diameter.44
Table 1.
PANCREATIC RESECTION FOR INVASIVE CARCINOMAS ARISING IN ASSOCIATION WITH INTRADUCTAL NEOPLASMS
Tumor type
|
Invasive carcinoma type
|
Grade of invasive carcinoma
|
Location of invasive carcinoma
|
Size of invasive carcinoma
|
Extrapancreatic invasion
|
Vascular invasion
|
Perineural invasion
|
Connectivity of preinvasive and invasive components (if documentable)
|
Size of intraductal/preinvasive neoplasm
|
Maximum diameter of the main duct (if identified)
|
Gross papillary nodules (in the preinvasive component)
|
Intraductal/preinvasive neoplasm cell type
|
Grade of intraductal/preinvasive neoplasm
|
Adjacent pancreas
|
Neck (pancreatic duct) margin
|
Retroperitoneal (SMA/uncinate) margin
|
CBD margin
|
Other organs
|
Lymph nodes
|
| Staging (AJCC 2010) | |
|---|---|
| pT1 | Invasive carcinoma is limited to the pancreas and ≤ 2 cm in greatest dimension |
| T1a: ≤ 0.5 cm | |
| T1b: > 0.5 cm; ≤ 1 cm | |
| T1c: > 1 cm; ≤ 2 cm | |
| pT2 | > 2 Invasive carcinoma is limited to the pancreas and cm in greatest dimension |
| pT3 | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery |
| pT4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
| pN0 | No regional lymph node metastasis |
| pN1 | Regional lymph node metastasis |
With the current definitions,40, 41, 43, 44 IPMNs encompass a spectrum of lesions ranging from those that are very innocuous-appearing (what used to be called “hyperplasia”) to those that are full-blown intramucosal carcinomatous lesions (those used to be designated as “papillary adenocarcinoma”).40, 41, 43, 44 This spectrum, previously classified45 as “hyperplasia → adenoma → borderline → in-situ carcinoma → invasive carcinoma” was later re-categorized42 as “low-grade dysplasia → intermediate-grade dysplasia → and high-grade dysplasia → invasive carcinoma” (Table 2).45 As such, IPMNs represent adenoma-carcinoma sequence (tumoral intraepithelial neoplasia). Invasive carcinoma arising in or concomitant with IPMN can be of various types (especially colloid or tubular), and can be very limited or extensive.
Table 2.
PANCREATIC RESECTION FOR PREINVASIVE NEOPLASMS
Intraductal/preinvasive neoplasm type
|
Grade of intraductal/preinvasive neoplasm
|
Extent of high grade dysplasia (Tis)
|
Invasion
|
Predominant cell type
|
Location
|
Size of preinvasive neoplasm
|
Gross papillary nodules
|
Multifocality
|
Maximum diameter of the main duct (if identified)
|
Adjacent pancreas
|
Neck (pancreatic duct) margin
|
Retroperitoneal (SMA/uncinate) margin
|
CBD margin
|
Other organs/findings
|
Lymph nodes
|
Tumor sampling:
|
Overall sampling:
|
IPMNs have different cytologic types classified as gastric, intestinal, pancreatobiliary, and oncocytic and it has been shown that these cell types not only have different morphologic and immunohistochemical phenotypes, but also some have different genetic drivers46–55 (see below, the subtypes section).
IPMNs can progress to invasive carcinoma of different types (ductal/tubular44, 56–61 and colloid/muconodular51, 62–65) and of variable extent. The invasive component of these tumors ought to be regarded and documented separately (see below invasive carcinoma section for details).
Branch vs Main duct IPMN: Documentation of main pancreatic duct findings pathologically
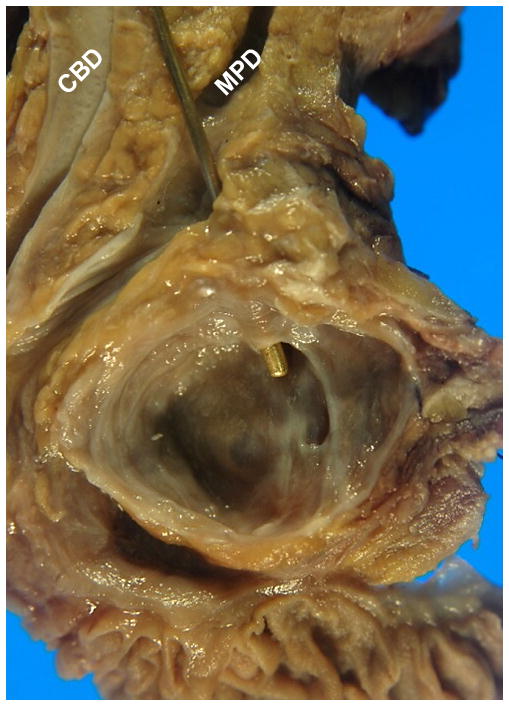
The classification of IPMNs as branch (Fig. 1) versus main duct (Fig. 2) type is of utmost importance in assessing the risk of carcinoma during the pre-operative management of patients with an IPMN;40, 41, 66–74 however, once the tumor is resected, naturally, this separation loses virtually all of its clinical value, and is superseded at that point by the absence or presence of an associated invasive carcinoma.40, 41, 75, 76 Furthermore, recent pathology studies have shown that the main duct not infrequently shows some degree of involvement even in the IPMNs that were classical branch duct type by imaging, and more importantly, those IPMNs with minimal involvement of the main duct, defined as “non-circumferential main duct involvement limited in a few histologic sections”, were very similar to branch duct cases and were different from main duct IPMNs, both by clinicopathologic features and clinical outcome.77 Consequently, for pathologic reporting, it is advised to make an attempt to document the widest diameter of the main duct, and also the extent of involvement of main duct by abnormal epithelium, if possible; however, it is not required for pathologists to make a specific distinction between the two groups.78–84
Figure 1.
A branch duct IPMN manifesting as a cyst without any significant dilation of the main pancreatic duct. Papilla formation is not evident grossly.
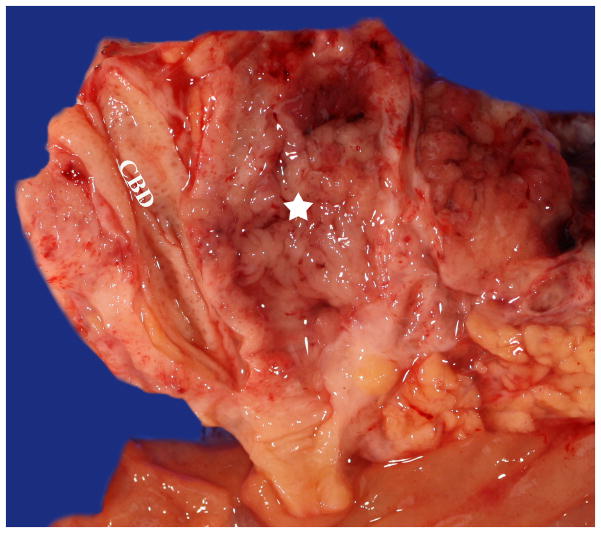
Figure 2.
Main duct IPMN involving the entire pancreatic duct (star), filled with friable papillary projections and sticky mucin. Note the adjacent normal common bile duct (CBD).
Measuring overall tumor size
Size of an IPMN at the time of diagnosis and its growth over time is an important criterion in the pre-operative management of IPMNs12, 40, 41, 69, 85–88 although its significance becomes less in resected specimens. Regardless, every attempt should be made to accurately determine the size of the lesion in the pathology report. This may be important not only for assessment of future risk but also serves as a feedback for radiologists to hone their skills.
There are, however, challenges in measuring the size of cystic lesions. Some IPMNs have very thin walled cysts that can rupture between the time of specimen resection and measurement in the gross room due to manipulation, dissection or for tumor banking purposes, which leads to shrinkage of the lesion. Therefore, for such thin-walled cystic IPMNs the true size needs to be determined by close correlation with the imaging findings, and in fact, if the size measured in the gross room is smaller, then, the clinical measurement is to be included in the pathology report. For these reasons, it is recommended that the size of the lesion be documented along with the mode of measurement as, for example: “Overall size of IPMN: 3 cm, as measured clinically (cyst ruptured during processing)”.
Documenting the gross size of any solid component can be useful as invasive carcinomas are often solid and the size is prognostically significant for invasive cancers. These also often correspond to “mural nodules” detected by imaging pre-operatively. For cases with a solid component, florid papillary nodules or invasive areas, imaging studies may under-estimate or mis-calculate the overall tumor size, and thus gross and microscopic evaluation is crucial for determining the size in such cases. If there is a discrepancy with the clinically measured size, an attempt can be made to explain this discrepancy in a comment.
In the physical measurement of the cysts in the gross room, certain issues ought to be kept in mind. For unifocal but multilocular lesions, it is preferable to document the overall size of the collection of locules along with the size of the largest locule. If a papillary lesion is present, it too can be measured. For multifocal lesions, the size of the largest focus should be documented, as can the sizes of the smaller lesions if it is clinically indicated.
Every attempt should be made to also measure the diameter of the main pancreatic duct. This requires proper identification of the main duct, which can be altered due to tumor, and may prove difficult to trace in a significant proportion of the cases. However, if the main duct is identifiable, then its diameter in the widest focus ought to be documented. Most importantly, the size of the invasive component, if present, should be carefully and separately documented. This will be discussed in detail in the invasive carcinoma section below.
Invasive carcinoma: Evaluation and reporting
Significance
The most important determinant of outcome in the management of patients with IPMN is whether an associated invasive carcinoma is present or not.42, 44, 59, 87, 89–92 Recent studies have uniformly shown that the vast majority of completely resected non-invasive IPMNs have a very positive outcome, with a 5-year survival of >90%,59, 87, 93 whereas half of those with an associated invasive carcinoma die from their disease.32, 33, 57, 76, 94–97
Sampling
An invasive carcinoma arising in association with an IPMN can only be definitely excluded with thorough evaluation of not only the entire lesion but also the uninvolved pancreas as well, since the neighboring pancreas often shows peri-tumoral abnormalities and may harbor subtle invasive carcinomas. In fact, the aggressive behavior of tumor reported in some patients with a “non-invasive IPMN,” may be due to “missed” invasive carcinomas in under-sampled IPMNs.42 Some invasive carcinomas also arise away from the lesion, and that is why it is important to regard the entire specimen as suspect for invasive carcinoma, perform thorough gross examination, as well as liberal sampling of even seemingly normal pancreas.
Typing of associated invasive carcinoma
Different histologic types of invasive carcinoma can arise from IPMNs, and these histologic types are associated with markedly different prognostic and biologic properties.51, 62, 64, 65, 98–100 About half of the invasive carcinomas that arise in association with an IPMN are ordinary tubular (ductal) adenocarcinoma (Fig. 3A), characterized by infiltrating small to medium tubular units separated by abundant stroma.44, 60 This type often arises from either gastric or pancreatobiliary type IPMN, and appears to have an aggressive behavior, although its prognosis may not be as poor as for ordinary PDACs, either due to early stage detection, and/or different molecular/biologic characteristics (although it is often morphologically indistinguishable from ordinary PDACs).32, 33, 57, 94–97
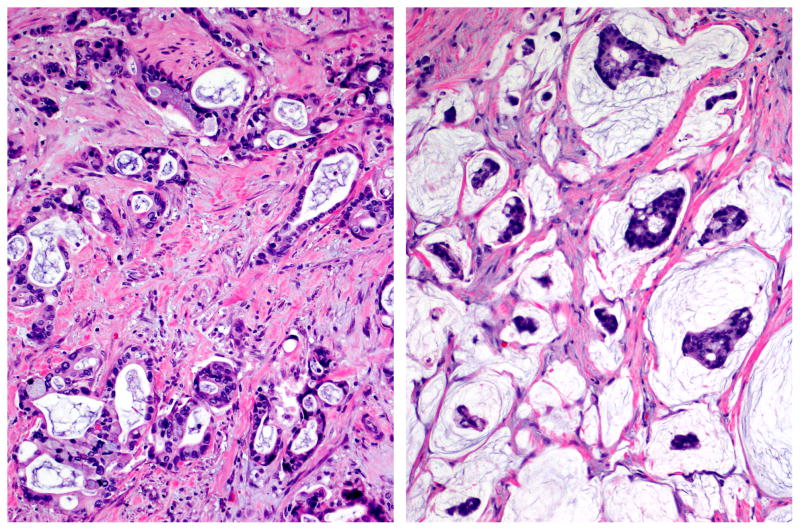
Figure 3.
Ductal (tubular)-type invasive carcinoma arising in IPMN (left) is virtually indistinguishable from ordinary pancreatic ductal adenocarcinomas, characterized by atypical cells forming irregular, often complex, tubular or glandular structures, usually accompanied by dense stroma. In contrasts, colloid carcinoma (right) is characterized by the muconodular pattern composed of distinct pools of mucin that contain scanty clusters of carcinoma cells, floating within the mucin (hematoxylin and eosin stain).
The other half of the invasive carcinomas that arise in association with IPMNs are colloid carcinomas (Fig. 3B), characterized by muconodular type invasion (nodules of stromal mucin that contain relatively scant clusters of carcinoma cells). Colloid carcinomas typically arise from the intestinal type IPMN, and are seldom, if ever, seen in association with other IPMN subtypes or with mucinous cystic neoplasms.65, 101, 102 Patients with colloid carcinomas have a significantly better prognosis than do patients with tubular invasive carcinomas.51, 62, 63, 65, 98, 100 Further, both colloid carcinoma and its common precursor, intestinal type IPMN, are characterized by intestinal type differentiation markers - diffuse and strong expression of the intestinal epithelial marker, MUC2, and, the intestinal differentiation marker, CDX2, - neither of which are expressed substantially in any other tumor type in the pancreas.50–52, 103–105 These render colloid carcinomas a potential candidate for a different management approach, and thus accentuate the importance of its recognition and proper documentation in the pathology reports.
Rarely, other invasive carcinoma types can arise in association with IPMNs, such as oncocytic,55 sarcomatoid/undifferentiated (some with osteoclast-like giant cells), medullary, or neuroendocrine carcinomas.106, 107 These also ought to be recognized and reported accordingly. Some carcinomas are of mixed type.108, 109
Grading of invasive carcinoma
The histologic grade of invasive carcinoma ought to be given separately from the grading of the non-invasive component. For tubular/ductal carcinomas, the principles employed in grading of ordinary PDACs can be employed.110 There are different grading schemes with their corresponding challenges, which are beyond the scope of this manuscript. Readers are referred to appropriate literature.111–114
For the grading of colloid type carcinoma, there are currently no guidelines. Most colloid carcinomas are by default well differentiated. However, some mucinous carcinomas in the pancreas exhibit significantly more prominent cellularity, cytologic atypia and cells clinging to the stroma. These tend to be more aggressive neoplasms. In such cases, the possibility of a non-colloid type mucinous carcinoma arising from the ampulla such as mucinous signet ring carcinoma or mixed mucinous-intestinal carcinoma, or a pancreatic ductal adenocarcinoma with excessive mucin production ought to be considered.108, 109, 115
Size of invasive carcinoma
As in any other cancer types, the size (and stage) of the invasive carcinoma is one of the most important prognostic parameters in IPMNs.32, 37, 56, 97 Unfortunately, many studies have failed to analyze this as a separate parameter. In fact, our review of the literature highlighted the unfortunate fact that the size of overall tumor (with non-invasive and invasive components lumped together) is often used interchangeably with the size of the invasive carcinoma.
The consensus at the Verona meeting was that it is imperative to measure the size of invasive carcinoma as accurately as possible, and, if this cannot be achieved (due to fragmented sampling or multifocality), then, to at least give an estimation of the invasion size. The invasive component should be staged, independent of the non-invasive component, as in any other cancer (see discussion below on staging).
If the invasive carcinoma is unifocal, the recommendation is to measure the largest diameter of the invasive focus, as is done for any invasive carcinoma in this organ and in other solid organs. An alternate possibility of measuring the “depth” from the nearest duct was discussed but dismissed because it is often impossible to determine where the nearest duct is located in 2-dimensional histologic sections.
For multifocal tumors,116 it is recommended that both the diameter of the largest one as well as the overall estimated size of all foci in aggregate be provided in the comment of the report (Synoptic 1). As in other organs such as the breast, it is not yet clear as to which one of these better reflects the tumor burden and thus is to be taken as the main tumor size.114, 117, 118
Indeterminate (suspicious) for invasion
Due to the complexity of the intraductal lesions and their common extension into the neighboring smaller ductules, non-invasive IPMNs often show remarkable architectural complexity with a pseudo-invasive appearance. This is especially striking in main duct lesions (of the intestinal type) where atrophy and fibrosis of the surrounding pancreatic parenchyma can be prominent, further accentuating the pseudo-invasive pattern. Prior biopsy site changes and inflammation also contribute to and accentuate this challenge. Separately, rupture of the ducts can lead to extrusion of mucin into the stroma. This acellular mucin is not invasive carcinoma, but in some instances it can be very difficult to distinguish from true invasive colloid carcinoma (Fig. 4). As a result, in some cases, it may be extremely difficult to determine whether a focus is invasive or pseudo-invasive. For such cases, the diagnosis of “indeterminate for invasion” may have to be rendered. This would correspond to the “suspicious for invasion” category of the Vienna classification of reporting GI malignancies.119 It is not yet known the clinical implications and behavior of such cases of IPMNs; however, it is certain that some cases cannot be classified definitively as invasive versus non-invasive and thus this category is a necessary one. In such cases, the estimated size of a suspect focus should be provided in a comment.
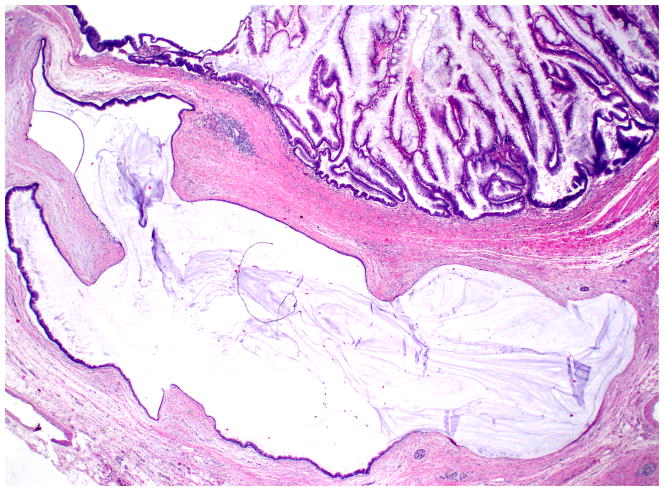
Figure 4.
In IPMNs, rupture of the ducts can lead to extrusion of mucin into the stroma, which can be difficult to distinguish from true invasive colloid carcinoma (hematoxylin and eosin stain).
Staging of invasive carcinoma; proposed refined definition of “minimally invasive” as pT1 and T1a, b and c
As for any invasive cancer, it is required to stage invasive carcinomas arising from (or in association) with IPMNs.44 A significant proportion of the invasive carcinomas discovered in IPMNs is small, and have been termed “minimally invasive”. However, this term is vague, non-specific and potentially misleading since it has been used highly variably in the literature: In some studies, also including “indeterminate” cases,29–39, 120–122 in others,32–37 the term minimally invasive was reserved for cases with minute invasive foci, while in others,120–122 the term was defined as a carcinoma having invaded slightly beyond the duct wall, and yet in still others, as T1 cancers, and in yet others, for invasion not appreciated grossly but discovered on microscopic examination. Thus it is recommended that the term “minimally invasive” be abandoned and replaced by proper documentation of the size of invasive carcinoma and its proper staging. It has been proposed to sub-stage these small carcinomas as pT1a, if ≤ 0.5 cm; pT1b, if >0.5 and ≤1.0, and as pT1c, if > 1.0 and ≤ 2.0 cm, although this classification needs to be validated in large-scale (multiinstitutional) studies.40
In staging of IPMNs with more overt invasive carcinomas, it is recommended that the UICC/AJCC staging protocol be employed.114 Unfortunately, numerous studies have disclosed that the current T-stage protocol lack linear prognostic correlation due to the irreproducibility and pathologic inapplicability of ill defined parameters like “peripancreatic soft tissue involvement,”123–125 or “common bile duct involvement” (as to which part of the common bile duct is to be taken into consideration).126, 127 For these reasons, a protocol that replaces these parameters with tumor size as the main defining parameter for also pT3 (in the current system, pT1 and T2 are already defined by size) is being considered to replace the current failed protocol.128 Until this change is formally adopted, however, it is recommended that both the size of invasive carcinoma as well as the current AJCC/UICC stage be documented.114
Invasive carcinoma “derived from” or “concomitant with” IPMN
There is emerging evidence that there may be some biologic and prognostic differences between carcinomas “derived from” (arising in the area of) IPMN versus those that are “concomitant” (not contiguous with) IPMN.97, 129 Therefore, it is recommended that every attempt be made to document the relationship of invasive carcinoma to the IPMN. For this purpose, it is recommended that sections be taken to verify the continuity (or discontinuity) of the invasive carcinoma with the IPMN. Considering that tumors and carcinomas can grow in a dumb-bell fashion or with skip areas, examination of the complete, full-thickness of the tissue and assurance of the discontinuity between the invasion and IPMN have to be documented unequivocally before a case can be classified as “concomitant” rather than primarily arising in IPMN. Many current grossing/sampling protocols used in the West may fall short in being convincing in this regard. Therefore, this distinction may not be achievable in every case.
Evaluation of invasive carcinomas for adjunct parameters
Invasive carcinomas in IPMN cases ought to be evaluated, in addition to staging, for adjunct parameters that are typically required in cancer synoptic reporting such as those recommended by the College of American Pathologists. These include grade, perineural invasion, vascular invasion, and optionally also for budding.130 Invasion to neighboring organs ought to be documented as well.
N-stage
It is imperative that the lymph node status be documented properly in IPMNs, regardless of whether invasion is detected or not. There have been cases in which the identification of lymph node metastasis has led to a more careful investigation of the IPMN and ultimate detection of invasive carcinoma in an otherwise seemingly non-invasive IPMN.131 It is recommended that a minimum of 12 lymph nodes be identified in a pancreatoduodenectomy specimen as recommended by American College of Surgeons,132, 133 and if this is not achieved, additional sampling should be performed in an attempt to identify more lymph nodes, and this should be clarified in a comment. Certain grossing approaches such as the “orange-peeling” method may facilitate a more complete harvesting of lymph nodes.78
For assessment and documentation of lymph nodes, routine protocols in the AJCC/UICC guidelines114 are to be followed. Accordingly, a direct invasion to a lymph node is regarded as lymph node metastasis, although the prognostic134 and scientific merit of this is being debated. For pancreatic cancers, the number of lymph nodes involved also appears to be a significant prognosticator and for this reason sub-staging of node-positive cases as N1 and N2 has been proposed.135 Similarly, lymph node ratio may also have prognostic significance but is currently not applicable in daily practice. For these reasons, it is recommended to document the number of lymph nodes involved, as in any other oncologic pathology report.
Definition of “malignant IPMN”
In the literature, the term “malignant” has been used highly variably, and this has led to major confusion in assessing the behavior and significance of IPMNs: In some studies, the term malignant IPMN was used in reference to clinical behavior with the term “malignant” used to refer only to those cases with metastasis and mortality. In other studies, both high-grade dysplasia (carcinoma in-situ) and invasive carcinomas were regarded as “malignant”,9–20 whereas, in other studies, only the cases with an associated invasive carcinoma were classified as “malignant”.21–28 Considering that only invasive cases have the ability to exhibit true malignant behavior (uncontrollable growth, destruction and metastasis), the latter definition was determined at the Verona meeting to be the most accurate. Nevertheless, in order to avoid confusion, and in accordance with routine oncologic pathology practice in other organs, it was recommended that the term “malignant IPMN” should not be used, and instead, clarification is provided as to whether it is invasive versus non-invasive.
Grading of IPMN
The degree of cytoarchitectural atypia (dysplasia) in IPMNs is graded as low, intermediate and high.44 The criteria for this is established in previous publications and are now widely in use.40, 41, 43, 50, 51, 136 In the WHO-2010, it was advocated to avoid the term carcinoma in-situ or intramucosal carcinoma, to be replaced by “high-grade dysplasia” for all duct-confined (non-invasive) neoplastic changes. The main reason for this was to avoid over-treatment. However, it should be noted here that, in many parts of the world the word “carcinoma” (in-situ) is still employed for the most advanced forms of high-grade dysplasia. If this is preferred, then it is also crucial that this is clarified explicitly as “non-invasive carcinoma”.
In a given case, the final grade of IPMN is based on the highest-grade focus, no matter how small. In cases with high-grade dysplasia (“carcinoma in-situ”), it is advisable to document the extent of this as focal (< 25% of the tumor), substantial (25–75%) or diffuse (> 75%) (Synoptic 2), although the clinical and behavioral relevance of this has not yet been established.
IPMN subtypes
The category of IPMN had been created as an umbrella entity to encompass all tumoral intraepithelial neoplasms (intraductal mass-forming neoplasms) with a predominantly papillary architecture involving the large ducts of the pancreas.43, 44, 51 It has now become clear that the four cell lineages (Fig. 5) that occur in the papillary components of IPMNs may represent different carcinogenetic pathways with significant differences in their clinicopathologic associations, cancer progression rates, immunophenotype and molecular characteristics.33, 36, 44, 46, 62, 87, 137–139
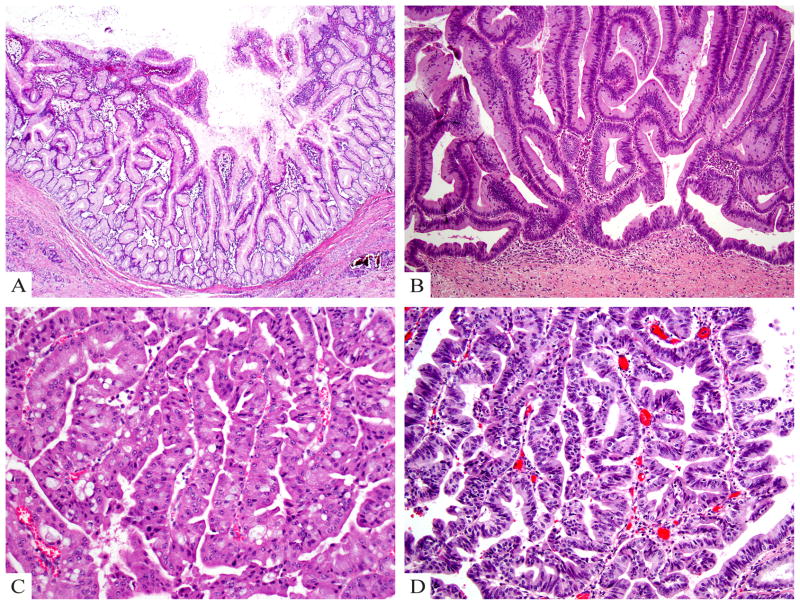
Figure 5.
A) Gastric type IPMN shows relatively simple and typically short papillae and often have pyloric-like glandular elements at their base in the cyst wall. The epithelial lining is highly similar to gastric foveolar epithelium. B) Intestinal type IPMN typically has a villous growth pattern and reveals pseudostratified columnar cells with a basophilic appearance and apical mucin. C) Oncocytic type IPMN reveals arborizing papillae lined by 2–5 layers of cuboidal cells with oncocytic cytoplasm and prominent, eccentric nucleoli as well as intraepithelial lumina. D) Pancreatobiliary type IPMN has complex arborizing and interconnecting papillary configurations with delicate fibrovascular cores and is composed of cuboidal cells with enlarged nuclei and little mucin production (hematoxylin and eosin stain).
Most branch duct IPMNs prove to be of gastric type,49, 50 characterized by papilla lining that is highly similar to gastric foveolar epithelium. Although invasive carcinoma is less commonly associated with gastric IPMNs than it is with the other IPMN types, once it occurs, it is typically of the tubular/ductal type with aggressive behavior akin to the ordinary PDAC.62, 140, 141,136 Intestinal type IPMNs are similar to colonic adenomas, distinguished by villous architecture and pale basophilic mucin that is typically diffusely positive for MUC2, in addition to expression of CDX2 (transcription factor of intestinal programming) 50, 51, 142 Intestinal type IPMNs are typically main duct type, and fairly extensive.50–52, 103–105, 143 and are more likely to harbor GNAS mutations than are the other types of IPMN.144–146 Invasive carcinoma is identified in a third of intestinal IPMNs resected, and these invasive foci often prove to be of colloid type51, 65 with surprisingly protracted clinical course.51, 62, 63, 65, 100
Oncocytic IPMNs, previously called intraductal oncocytic papillary neoplasms,53, 55 typically present as complex, large multilocular cystic lesions rather than the typical IPMN presentation of ductal dilatation or mucin extrusion from the ampulla. They are characteristically complex, arborizing, with florid proliferation of oncocytic cells with a relatively monotonous appearance.53–55 Despite their large size and striking complexity, invasive carcinoma is surprisingly rare in these tumors, and if seen, is usually very limited in quantity. The prognosis seems to be relatively indolent as well. Mutations in KRAS and GNAS, which are otherwise common in IPMNs, are seldom detected in this group.147, 148
IPMNs that have cuboidal cells with high-grade cytology and that do not have the characteristics of the intestinal or oncocytic types are designated as pancreatobiliary.51 Many of these may represent high-grade transformation of gastric type IPMN; however, some have a distinctive pattern and morphology that supports a separate lineage. It is also this pattern that has overlapping features with intraductal tubulopapillary neoplasms.149–155
It is acknowledged that in some cases (about 15%), there are overlapping features, and it is not possible to place a given tumor into any one category, in which case the term “mixed” is to be employed, with an explanatory note in the comment section.
Definition and delineation of IPMN from its mimickers
Large PanINs
Gastric type IPMNs have significant morphologic and molecular overlaps with PanINs. IPMNs are defined by their “tumoral” nature (formation of clinically or grossly visible cystic and papillary nodules that are typically > 1 cm) and PanINs are incidental/microscopic (< 0.5 cm) forms of dysplasia (intraepithelial neoplasia), but otherwise they have very similar pathologic characteristics.43, 44 In order to provide a numerical value to this distinction, the size criterion of 1 cm is generally employed. For cysts < 1 cm and > 0.5 cm that are lined by gastric-like mucinous epithelium, the term “incipient IPMN” has been proposed as an option.156, 157 For intestinal and oncocytic lesions, this differential is largely a moot point, because these are classified as IPMN irrespective of size, although it is exceedingly uncommon for them to be discovered when less than 1 cm.156
Simple mucinous cyst (cystic mucinous duct lesion)
Cysts larger than 1 cm lined by mucinous epithelium but without overt papillary configuration and without any ovarian type stroma create a challenging entity to classify. Previously, the term “mucinous non-neoplastic cyst” has been recommended for such lesions,158–160 but considering that mucinous change in the pancreas is now equated with the earliest form of neoplastic transformation, this name is no longer practical. Since many of these lesions do not show any signs of obstruction or any other abnormality in the remaining pancreas, they cannot be exactly regarded as retention cysts either. Of note, these appear to be mega-cystic versions of PanIN-1A (also known as “mucinous duct lesion”), and thus, the term cystic mucinous duct lesion was also proposed.161 However, the true identity and the best name to assign to this entity require further analysis and discussions.
Retention cysts; secondary dilatation of the ducts
Mass lesions (and other obstructive factors) can lead to secondary dilatation of the pancreatic ducts, and when these acquire mucinous epithelium, they can be difficult to distinguish from IPMNs. Lack of florid papillary elements and their round open lumina as well as the location of the lesions (commonly located in the periphery of the main mass) may help differentiate these from true IPMNs. Additionally, they often have at least focal cuboidal lining.42
Occasionally, the main pancreatic duct is also dilated significantly due to an obstructive process. Recently, minute neuroendocrine tumors arising on the duct wall (especially from serotonin cells) are coming to clinical attention, and may clinically mimic an IPMN as they can cause significant ductal dilatation.162, 163 More importantly, on rare occasions, small PDACs can lead to marked dilatation of the main duct, again clinically mimicking an IPMN. In these cases the obstructive carcinoma can be missed.
Similar tumoral intraepithelial neoplasms
Intraductal tubular/tubulopapillary neoplasms are very close kindreds of IPMNs, to an extent that some have proposed to regard them as a variant of IPMN. However, since they lack two of the characteristic features of IPMN that even have been incorporated into the name,152 i.e., significant mucin production and papilla formation, this entity was kept separate from IPMN in the WHO-2010 classification.44 ITPNs are composed of non-mucinous (or minimally mucinous) cells, which grow in a predominantly tubular pattern with minimal papilla formation.44, 149, 154, 155
In the areas lacking the ovarian type stroma, mucinous cystic neoplasms can appear identical to branch duct IPMNs, and in the areas with florid papilla formation, they are very similar to pancreatobiliary type IPMNs.101, 164, 165
Adenomas and non-invasive papillary neoplasms growing within the ampulla, which have recently been proposed to be unified under the heading of intra-ampullary papillary tubular neoplasms can also be virtually identical to IPMNs, especially when they show extension into the pancreatic ducts.166 If taken out of context, intraductal papillary neoplasms of the bile ducts are also indistinguishable from IPMNs.167 Proper grossing, dissection and sampling (with identifiers of the location of each tissue sampled) are crucial in making the distinction between these entities, because without knowledge of the specific location of a given lesion, these can be indistinguishable at microscopic level. For the appropriate grossing protocols, the readers are referred to other detailed texts.78 It should also be noted here that some intestinal type IPMN show fistulous extension into adjacent organs168 which can mimic tumors of those secondary sites.
Large duct type invasive adenocarcinomas
Some invasive ductal adenocarcinomas of the pancreas are composed of larger dilated ducts, rather than the more classical small tubular pattern. These are often well-differentiated and may exhibit abortive papillary elements, such that, when taken in isolation, many of the invasive glandular units can closely mimic IPMNs. These large duct type invasive adenocarcinomas can be distinguished from IPMNs by the highly irregular contours of the ducts, relatively flat epithelial lining, open, round lumen formation (rather than the compressed ducts with undulating contours), and the presence of necrotic, granular debris in their lumen.169–171 The distinction of these invasive carcinomas from non-invasive IPMNs is of utmost significance since both the outcome and pathogenesis are vastly different. In fact, some of the rapidly progressive IPMNs in some studies may belong to this category.
Congenital, duplication, enteric and paraduodenal wall cysts
A variety of cysts close to duodenal wall can clinically mimic IPMNs. In fact, when these cysts develop papillary carcinomatous changes, they can be indistinguishable from IPMNs.172 Some congenital, duplication or enteric cysts can be recognized by the presence of a muscular wall, which can be very helpful. Some also have focal ciliated epithelium that is not seen in IPMNs.
Para-ampullary duodenal wall cysts that occur as a consequence of paraduodenal (groove) pancreatitis often have partial mucinous lining, because they arise from preferential injury of pancreatic tissue that occurs on the wall of the duodenum (“cystic dystrophy of heterotopic pancreas”).173–176 These can be confused with IPMNs.
Co-incidental tumors and uninvolved pancreas
In a patient with IPMN, it is important to analyze the uninvolved parts of the specimen. As discussed previously, it is now well established that IPMNs can be multifocal,177,116 and furthermore, concomitant invasive carcinomas can be encountered far away from the main IPMN lesions.97, 178–181 Although neuroendocrine tumors can occur synchronously with IPMNs, it is much more common to see a non-neoplastic aggregation of the islets of Langerhans secondary to long-standing duct obstruction.182–185 This latter finding should not be over-diagnosed as “islet cell hyperplasia,” or as a neuroendocrine neoplasm.
It should also be kept in mind that many patients with IPMNs have metachronous and synchronous tumors in other organs particularly the colon, and some of these can be present in the resection specimen, including the ampulla and duodenum.186–191
It is also advisable to document the changes in the uninvolved portions of the pancreas. Peri-tumoral pancreatitis is very commonly encountered in main duct IPMNs as a consequence of the local effects of the tumor, and it would be preferable to not to designate these as chronic pancreatitis, since chronic pancreatitis is a specific disease entity, defined by a constellation of findings at the clinical level.192–194 It may be more appropriate to be descriptive about these chronic changes as atrophy, inflammation and others.
Sampling of seemingly uninvolved pancreas can also reveal subtle, grossly-unapparent obstructive lesions that lead to “pseudo-IPMN” 162.
Frozen section evaluation
After discussions at both the Verona and Johns Hopkins consensus meetings, where different opinions were raised initially, it was finally widely agreed that if routine frozen section of the primary tumor is performed, it should be done with the understanding that: 1) Both high-grade dysplasia and invasive carcinoma, which are the main targets of a frozen section, are typically focal and often grossly not distinguishable from lower grade portions of the lesion, and therefore, cannot be confidently excluded in a frozen section setting. Thus, discrepancy is commonly observed between frozen section and permanent findings. 2) Even if such a focus of high-grade dysplasia and/or carcinoma is present, histologic interpretation can be difficult due to the freezing artifact, the small size of the focus, and considering that the pathologic assessment of IPMNs is already difficult even in properly obtained sections. 3) Freezing of tissue often leads to adverse alterations in the specimen that may hamper the final diagnostic evaluation in permanent sections, especially considering that the focus of concern is often small and may disappear as well.195–198
Evaluation of margins by frozen sections may be indicated in some cases of IPMNs.93, 199–202 When evaluating the margins, the concerns described above would have to be taken into consideration. The main task for the pathologist is to determine whether there is high-grade dysplasia or invasive carcinoma, because it is believed that these require more aggressive management with consideration of the performance of more extensive surgery, including total pancreatectomy. The current data indicate that the presence of low-grade dysplasia or intermediate-grade dysplasia do not require any further surgery provided that there are no other lesions clinically (and intra-operatively) in the remaining pancreas.203 This approach also pertains to incidental low-grade PanINs at a margin. Low-grade PanINs are often encountered in general population 204 and thus also in pancreata with IPMNs (see below for mimickers of IPMN). While intestinal and oncocytic type IPMNs are easy to distinguish from PanINs, gastric IPMNs (and cystic components of intestinal and oncocytic IPMNs which also often have gastric type epithelium) are virtually indistinguishable from PanINs. For this reason, it is recommended that low-grade gastric like epithelium in pancreatic margins be reported as “No high-grade dysplasia or invasive carcinoma is identified; low-grade mucinous epithelium is present (low-grade PanIN or low-grade IPMN)”. Current data indicate that these findings by themselves do not justify further resection.205, 206
One challenging finding in frozen sections for margins is the inflamed ducts with denuded epithelium, especially if the duct is dilated.207 These should be reported as “denuded epithelium, cannot assess for neoplastic process”. At this point, clinical and operative judgment may have to be used in determining the course of action, and the presence or absence of a lesion in the remaining pancreas may be an important factor in this decision.
In terms of indications to obtain frozen sections, it should also be kept in mind that a frozen section should only be performed if it might change the course of the operation. Since such criteria are mostly surgical, the specific situations that it might be needed are beyond the scope of this manuscript and require further discussions.
Reporting of cytology specimens
There are different opinions on the value of fine needle aspiration in the diagnosis of IPMNs. In Japan, this is mostly avoided because of concerns about tumor seeding or biopsy-related complications. In the United States, however, endoscopic-ultrasound-guided FNA biopsy is widely utilized in the diagnostic algorithm of IPMNs,208–210 especially those with undetermined risk, such as branch duct IPMNs with indeterminate features, or in patients in whom surgery is contraindicated
The findings of FNA biopsy ought to be interpreted in the context of the clinical findings, and with close communication between the radiologist, clinical management team and cytopathologists.211–216 Otherwise, results can be misleading. For example, in the right context, the presence of thick mucin alone may be sufficient for a diagnosis of IPMN, even if it is relatively acellular.
Most branch duct IPMNs (and the cystic component of other IPMN types) have a lining that is indistinguishable from that of normal gastric epithelium.44 For this reason, the presence of gastric type epithelium on FNA smears from a pancreatic cyst should be reported as “gastric type epithelium, cannot exclude gastric contamination or a low-grade gastric type IPMN”. If present, parietal cells may help make this distinction.
It is important to note that foci of invasive carcinoma are often small and the “invasive/carcinomatous” cells are less likely to be shed into the cyst fluid. Therefore, unless the solid areas containing these invasive elements are sampled separately, invasive carcinoma can easily be missed by FNA. The same is also true for microfocal high-grade dysplasia. Conversely, reactive ductal epithelium can be mistaken as “carcinomatous” changes. For these reasons, the results of FNA should be evaluated with the clinical findings in mind.
It is also often impossible to distinguish the cells of high-grade dysplasia from those of invasive carcinoma. For these cases, the diagnosis of “high-grade atypia, possible carcinomatous change in IPMN” is recommended, with the understanding that “high-grade atypia” is an all-encompassing term that incorporates features of both “high-grade dysplasia” as well as “invasive carcinoma,” which are cytologically inseparable and both require resection. It should be noted here that, the diagnosis of “high-grade atypia” is to be used only in FNA specimens (not in surgical biopsies or resections), and even in FNAs, it must be followed by a comment indicating that this diagnosis conveys a suspicion of carcinomatous transformation, but cannot distinguish between in-situ or invasive carcinoma, since this distinction is typically not possible in cytologic specimens.
IPMNs and MCNs are indistinguishable on FNA.215 This is because the subepithelial ovarian stroma of MCN is often not sampled or recognizable on smears or cell block. For this reason, in cytologic specimens deemed to represent one of these entities, the term “mucinous neoplastic cyst” is preferred, with a qualifying comment indicating that both these two differentials are included. Once a cyst is determined to be a neoplastic mucinous cyst it is important to determine the presence, and grade of cytologic atypia (low- versus high-grade atypia) in all cases, so as to better stratify risk of invasion.
Reporting principles for IPMN are also applicable for other tumoral intraepithelial neoplasms of the pancreatobiliary tract
Although the main task of the consensus group was to focus on pancreatic IPMNs, further detailed elaborations have led to the conclusion that IPMNs are indeed the prototype of a group of closely related lesions that occur in various aspects of the pancreatobiliary tract, and that many of the principles discussed above regarding pathologic evaluation and reporting would be highly applicable to these similar tumors, namely tumoral intraepithelial neoplasms, as well.
Mucinous cystic neoplasms (of the pancreas and hepatobiliary tract),40, 41, 217, 218 intraductal tubular/tubulopapillary neoplasms (of the pancreas and bile ducts),149–155 “adenomas” and “papillary neoplasms” growing within the ampulla (recently proposed to be collected under the heading of intra-ampullary papillary tubular neoplasm with an approach similar to IPMNs),166 and “adenomas” and “intrcystic papillary neoplasms” of the gallbladder (recently proposed to be unified under one title of intracholecystic papillary tubular neoplasms),219 all share various important characteristics with IPMN. All are mass-forming non-invasive neoplasms that show a spectrum of neoplastic transformation; i.e., adenoma-carcinoma sequence, with the intramucosal/intraepithelial spectrum ranging from hyperplastic-like gastric epithelium to full-blown intramucosal/intraepithelial carcinomatous changes that used to be referred as “papillary adenocarcinomas”. All of these entities also exhibit cell lineages identified in IPMNs (gastric, intestinal, pancreatobiliary, oncocytic and mixed). The grading scheme and terminology for these tumors are mostly adopted from those used for IPMNs. Furthermore, they can be associated with invasive carcinoma of various types. All the reporting principles discussed above (grading, staging, typing, separate reporting of invasive carcinoma, and staging of invasive carcinoma) are also applicable to these tumors.
CONCLUSION
Proper, consistent evaluation and thorough documentation of pathologic characteristics of the neoplasms and application of uniform terminology are crucial for both the management of patients with IPMNs and further unraveling of many puzzles of this entity as well as other similar tumoral intraepithelial neoplasms of the pancreatobiliary tract.
Acknowledgments
The authors thank Rhonda Everett for her assistance in the preparation of the manuscript Allyne Manzo and Lorraine Biedrzycki for assistance with the figures.
References
- 1.Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa A, Ohashi K, Hori M, et al. Ductectatic-type mucinous cystadenoma and cystadenocarcinoma of the human pancreas: a novel clinicopathological entity. Jpn J Cancer Res. 1993;84:474–9. doi: 10.1111/j.1349-7006.1993.tb00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itai Y, Ohhashi K, Nagai H, et al. “Ductectatic” mucinous cystadenoma and cystadenocarcinoma of the pancreas. Radiology. 1986;161:697–700. doi: 10.1148/radiology.161.3.3786719. [DOI] [PubMed] [Google Scholar]
- 4.Yamada M, Kozuka S, Yamao K, et al. Mucin-producing tumor of the pancreas. Cancer. 1991;68:159–68. doi: 10.1002/1097-0142(19910701)68:1<159::aid-cncr2820680129>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Tanaka M. Mucin-hypersecreting tumor of the pancreas with mucin extrusion through an enlarged papilla. Am J Gastroenterol. 1991;86:835–9. [PubMed] [Google Scholar]
- 6.Rickaert F, Cremer M, Deviere J, et al. Intraductal mucin-hypersecreting neoplasms of the pancreas. A clinicopathologic study of eight patients. Gastroenterology. 1991;101:512–9. doi: 10.1016/0016-5085(91)90032-g. [DOI] [PubMed] [Google Scholar]
- 7.Morohoshi T, Kanda M, Asanuma K, et al. Intraductal papillary neoplasms of the pancreas. A clinicopathologic study of six patients. Cancer. 1989;64:1329–35. doi: 10.1002/1097-0142(19890915)64:6<1329::aid-cncr2820640627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Payan MJ, Xerri L, Moncada K, et al. Villous adenoma of the main pancreatic duct: a potentially malignant tumor? Am J Gastroenterol. 1990;85:459–63. [PubMed] [Google Scholar]
- 9.Ammori JB, Do RK, Brennan MF, et al. Uncinate duct dilation in intraductal papillary mucinous neoplasms of the pancreas: a radiographic finding with potentially increased malignant potential. J Gastrointest Surg. 2014;18:911–6. doi: 10.1007/s11605-014-2449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42:883–8. doi: 10.1097/MPA.0b013e31827a7b84. [DOI] [PubMed] [Google Scholar]
- 11.Ohno E, Itoh A, Kawashima H, et al. Malignant transformation of branch duct-type intraductal papillary mucinous neoplasms of the pancreas based on contrast-enhanced endoscopic ultrasonography morphological changes: focus on malignant transformation of intraductal papillary mucinous neoplasm itself. Pancreas. 2012;41:855–62. doi: 10.1097/MPA.0b013e3182480c44. [DOI] [PubMed] [Google Scholar]
- 12.Hwang DW, Jang JY, Lim CS, et al. Determination of malignant and invasive predictors in branch duct type intraductal papillary mucinous neoplasms of the pancreas: a suggested scoring formula. J Korean Med Sci. 2011;26:740–6. doi: 10.3346/jkms.2011.26.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomimaru Y, Takeda Y, Tatsumi M, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep. 2010;24:613–20. doi: 10.3892/or_00000899. [DOI] [PubMed] [Google Scholar]
- 14.Mimura T, Masuda A, Matsumoto I, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2010;44:e224–9. doi: 10.1097/MCG.0b013e3181d8fb91. [DOI] [PubMed] [Google Scholar]
- 15.Shimamoto T, Tani M, Kawai M, et al. MUC1 is a useful molecular marker for malignant intraductal papillary mucinous neoplasms in pancreatic juice obtained from endoscopic retrograde pancreatography. Pancreas. 2010;39:879–83. doi: 10.1097/MPA.0b013e3181d6ba04. [DOI] [PubMed] [Google Scholar]
- 16.Turrini O, Waters JA, Schnelldorfer T, et al. Invasive intraductal papillary mucinous neoplasm: predictors of survival and role of adjuvant therapy. HPB (Oxford) 2010;12:447–55. doi: 10.1111/j.1477-2574.2010.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bausch D, Mino-Kenudson M, Fernandez-Del Castillo C, et al. Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2009;13:1948–54. doi: 10.1007/s11605-009-1001-9. discussion 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manfredi R, Graziani R, Motton M, et al. Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology. 2009;253:106–15. doi: 10.1148/radiol.2531080604. [DOI] [PubMed] [Google Scholar]
- 19.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 20.Le H, Ziogas A, Rhee JM, et al. A population-based, descriptive analysis of malignant intraductal papillary mucinous neoplasms of the pancreas. Cancer Epidemiol Biomarkers Prev. 2008;17:2737–41. doi: 10.1158/1055-9965.EPI-08-0417. [DOI] [PubMed] [Google Scholar]
- 21.He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–65. doi: 10.1016/j.jamcollsurg.2012.12.026. discussion 665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cone MM, Rea JD, Diggs BS, et al. Predicting malignant intraductal papillary mucinous neoplasm: a single-center review. Am J Surg. 2011;201:575–9. doi: 10.1016/j.amjsurg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Fritz S, Hackert T, Hinz U, et al. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg. 2011;98:104–10. doi: 10.1002/bjs.7280. [DOI] [PubMed] [Google Scholar]
- 24.Simons JP, Ng SC, Shah SA, et al. Malignant intraductal papillary mucinous neoplasm: are we doing the right thing? J Surg Res. 2011;167:251–7. doi: 10.1016/j.jss.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Bassi C, Sarr MG, Lillemoe KD, et al. Natural history of intraductal papillary mucinous neoplasms (IPMN): current evidence and implications for management. J Gastrointest Surg. 2008;12:645–50. doi: 10.1007/s11605-007-0447-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–42. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto S, Horton KM, Lawler LP, et al. Intraductal papillary mucinous neoplasm of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics. 2005;25:1451–68. doi: 10.1148/rg.256055036. discussion 1468–70. [DOI] [PubMed] [Google Scholar]
- 28.Niedergethmann M, Grutzmann R, Hildenbrand R, et al. Outcome of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas (IPMN): a 10-year experience. World J Surg. 2008;32:2253–60. doi: 10.1007/s00268-008-9692-8. [DOI] [PubMed] [Google Scholar]
- 29.Oguro S, Esaki M. A case of minimally invasive intraductal papillary mucinous carcinoma resected after 17-year follow-up. Jpn J Clin Oncol. 2011;41:1152. doi: 10.1093/jjco/hyr125. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Nakamori S, Nakahira S, et al. Surgical outcomes of noninvasive and minimally invasive intraductal papillary-mucinous neoplasms of the pancreas. Ann Surg Oncol. 2006;13:955–960. doi: 10.1245/ASO.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 31.Nakagohri T, Asano T, Kenmochi T, et al. Long-term surgical outcome of noninvasive and minimally invasive intraductal papillary mucinous adenocarcinoma of the pancreas. World J Surg. 2002;26:1166–9. doi: 10.1007/s00268-002-6254-3. [DOI] [PubMed] [Google Scholar]
- 32.Marchegiani G, Mino-Kenudson M, Sahora K, et al. IPMN Involving the Main Pancreatic Duct: Biology, Epidemiology, and Long-Term Outcomes Following Resection. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada S, Fujii T, Shimoyama Y, et al. Clinical implication of morphological subtypes in management of intraductal papillary mucinous neoplasm. Ann Surg Oncol. 2014;21:2444–52. doi: 10.1245/s10434-014-3565-1. [DOI] [PubMed] [Google Scholar]
- 34.Kang MJ, Lee KB, Jang JY, et al. Disease spectrum of intraductal papillary mucinous neoplasm with an associated invasive carcinoma invasive IPMN versus pancreatic ductal adenocarcinoma-associated IPMN. Pancreas. 2013;42:1267–74. doi: 10.1097/mpa.0b013e3182954137. [DOI] [PubMed] [Google Scholar]
- 35.Nakata K, Ohuchida K, Aishima S, et al. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas. 2011;40:581–7. doi: 10.1097/MPA.0b013e318214fa86. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Jang KT, Mo Park S, et al. Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol. 2011;32:535–42. doi: 10.1007/s13277-010-0148-z. [DOI] [PubMed] [Google Scholar]
- 37.Nara S, Shimada K, Kosuge T, et al. Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2008;32:243–55. doi: 10.1097/PAS.0b013e3181484f1e. [DOI] [PubMed] [Google Scholar]
- 38.Karasaki H, Mizukami Y, Tokusashi Y, et al. Localization of the most severely dysplastic/invasive lesions and mucin phenotypes in intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2011;40:588–94. doi: 10.1097/MPA.0b013e31820d1a03. [DOI] [PubMed] [Google Scholar]
- 39.Fukushima N, Mukai K, Sakamoto M, et al. Invasive carcinoma derived from intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic and immunohistochemical study of eight cases. Virchows Arch. 2001;439:6–13. doi: 10.1007/s004280100438. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 42.Hruban R, Pitman MB, Klimstra DS. Tumors of the Pancreas. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 43.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 44.Adsay NV, Kloeppel G, Fukushima N, et al. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors. Lyon: WHO Press; 2010. pp. 304–313. [Google Scholar]
- 45.Longnecker DS, Hruban RH, Adler G, et al. Intraductal papillary-mucinous neoplasm. In: Hamilton SR, Aaltonen LA, editors. WHO classification of tumors. Lyon: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 46.Kang MJ, Lee KB, Jang JY, et al. Evaluation of clinical meaning of histological subtypes of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42:959–66. doi: 10.1097/MPA.0b013e31827cddbc. [DOI] [PubMed] [Google Scholar]
- 47.Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–16. doi: 10.1136/gut.2010.210567. [DOI] [PubMed] [Google Scholar]
- 48.Ishida M, Egawa S, Aoki T, et al. Characteristic clinicopathological features of the types of intraductal papillary-mucinous neoplasms of the pancreas. Pancreas. 2007;35:348–52. doi: 10.1097/mpa.0b013e31806da090. [DOI] [PubMed] [Google Scholar]
- 49.Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561–1569. doi: 10.1097/01.pas.0000213305.98187.d4. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa T, Kloppel G, Volkan AN, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 51.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–48. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–8. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol. 2000;17:16–30. [PubMed] [Google Scholar]
- 54.Jyotheeswaran S, Zotalis G, Penmetsa P, et al. A newly recognized entity: intraductal “oncocytic” papillary neoplasm of the pancreas. Am J Gastroenterol. 1998;93:2539–43. doi: 10.1111/j.1572-0241.1998.00714.x. [DOI] [PubMed] [Google Scholar]
- 55.Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–994. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–46. doi: 10.1001/archsurg.143.7.639. discussion 646. [DOI] [PubMed] [Google Scholar]
- 57.Shimada K, Sakamoto Y, Sano T, et al. Invasive carcinoma originating in an intraductal papillary mucinous neoplasm of the pancreas: a clinicopathologic comparison with a common type of invasive ductal carcinoma. Pancreas. 2006;32:281–287. doi: 10.1097/01.mpa.0000202955.33483.e2. [DOI] [PubMed] [Google Scholar]
- 58.Lai EC, Lau WY. Intraductal papillary mucinous neoplasms of the pancreas. Surgeon. 2005;3:317–24. doi: 10.1016/s1479-666x(05)80110-6. [DOI] [PubMed] [Google Scholar]
- 59.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 61.Falconi M, Salvia R, Bassi C, et al. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg. 2001;88:376–81. doi: 10.1046/j.1365-2168.2001.01720.x. [DOI] [PubMed] [Google Scholar]
- 62.Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–20. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–6. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seidel G, Zahurak M, Iacobuzio-Donahue C, et al. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56–63. doi: 10.1097/00000478-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Jang JY, Park T, Lee S, et al. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014;101:686–92. doi: 10.1002/bjs.9491. [DOI] [PubMed] [Google Scholar]
- 67.Goh BK, Tan DM, Ho MM, et al. Utility of the sendai consensus guidelines for branch-duct intraductal papillary mucinous neoplasms: a systematic review. J Gastrointest Surg. 2014;18:1350–7. doi: 10.1007/s11605-014-2510-8. [DOI] [PubMed] [Google Scholar]
- 68.Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703–11. doi: 10.1016/j.dld.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–75. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 70.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–90. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–51. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651–4. [DOI] [PubMed] [Google Scholar]
- 72.Kawamoto S, Lawler LP, Horton KM, et al. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186:687–95. doi: 10.2214/AJR.04.1820. [DOI] [PubMed] [Google Scholar]
- 73.Jang JY, Kim SW, Ahn YJ, et al. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005;12:124–132. doi: 10.1245/ASO.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 74.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–9. doi: 10.1053/j.gastro.2007.05.010. quiz 309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahora K, Fernandez-del Castillo C, Dong F, et al. Not all mixed-type intraductal papillary mucinous neoplasms behave like main-duct lesions: implications of minimal involvement of the main pancreatic duct. Surgery. 2014 doi: 10.1016/j.surg.2014.04.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adsay V, Basturk O, Saka B, et al. Whipple made simple for surgical pathologists: orientation, dissection and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct and ampullary tumors. American Journal of Surgical Pathology. 2014 doi: 10.1097/PAS.0000000000000165. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maksymov V, Hogan M, Khalifa MA. An anatomical-based mapping analysis of the pancreaticoduodenectomy retroperitoneal margin highlights the urgent need for standardized assessment. HPB (Oxford) 2013;15:218–23. doi: 10.1111/j.1477-2574.2012.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg. 2012;99:1036–49. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- 81.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11:282–9. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–7. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 83.Chatelain D, Flejou JF. Pancreatectomy for adenocarcinoma: prognostic factors, recommendations for pathological reports. Ann Pathol. 2002;22:422–31. [PubMed] [Google Scholar]
- 84.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–6. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]
- 85.Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:913–21. doi: 10.1016/j.cgh.2013.02.010. quiz e59–60. [DOI] [PubMed] [Google Scholar]
- 86.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 87.Grutzmann R, Niedergethmann M, Pilarsky C, et al. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Oncologist. 2010;15:1294–309. doi: 10.1634/theoncologist.2010-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas. 2010;39:232–6. doi: 10.1097/MPA.0b013e3181bab60e. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi K, Sadakari Y, Ohtsuka T, et al. Factors in intraductal papillary mucinous neoplasms of the pancreas predictive of lymph node metastasis. Pancreatology. 2010;10:720–5. doi: 10.1159/000320709. [DOI] [PubMed] [Google Scholar]
- 90.Nagai K, Doi R, Kida A, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg. 2008;32:271–8. doi: 10.1007/s00268-007-9281-2. discussion 279–80. [DOI] [PubMed] [Google Scholar]
- 91.Raut CP, Cleary KR, Staerkel GA, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 93.White R, D’Angelica M, Katabi N, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–93. doi: 10.1016/j.jamcollsurg.2006.12.040. discussion 993–5. [DOI] [PubMed] [Google Scholar]
- 94.Koh YX, Chok AY, Zheng HL, et al. Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21:2782–800. doi: 10.1245/s10434-014-3639-0. [DOI] [PubMed] [Google Scholar]
- 95.De Moor V, Arvanitakis M, Nagy N, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathological features and long term outcome related to histopathological group. Hepatogastroenterology. 2012;59:565–9. doi: 10.5754/hge10343. [DOI] [PubMed] [Google Scholar]
- 96.Waters JA, Schnelldorfer T, Aguilar-Saavedra JR, et al. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: a multi-institutional comparison according to American Joint Committee on Cancer Stage. J Am Coll Surg. 2011;213:275–83. doi: 10.1016/j.jamcollsurg.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–80. doi: 10.1097/MPA.0b013e318215010c. [DOI] [PubMed] [Google Scholar]
- 98.Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253:968–74. doi: 10.1097/SLA.0b013e318214bcb4. [DOI] [PubMed] [Google Scholar]
- 99.Sadakari Y, Ohuchida K, Nakata K, et al. Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery. 2010;147:812–7. doi: 10.1016/j.surg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Yopp AC, Allen PJ. Prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:359–62. doi: 10.4240/wjgs.v2.i10.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jang K, Park S, Bagci P, et al. Invasive Carcinomas Arising from Pancreatic Mucinous Cystic Neoplasm With Ovarian-Type Stroma - A Clinicopathologic Analysis of 29 Invasive Carcinomas from 178 Pancreatic Mucinous Cystic Neoplasms. American Journal of Surgical Pathology. 2014 doi: 10.1097/PAS.0000000000000357. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adsay NV, Merati K, Nassar H, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003;27:571–578. doi: 10.1097/00000478-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 104.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201–210. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]
- 105.Yonezawa S, Sueyoshi K, Nomoto M, et al. MUC2 gene expression is found in noninvasive tumors but not in invasive tumors of the pancreas and liver: its close relationship with prognosis of the patients. Hum Pathol. 1997;28:344–52. doi: 10.1016/s0046-8177(97)90134-9. [DOI] [PubMed] [Google Scholar]
- 106.Abue M, Suzuki M, Onodera H, et al. A case of pancreatic endocrine tumor developing from intraductal papillary mucinous neoplasm (IPMN) Nihon Shokakibyo Gakkai Zasshi. 2009;106:1070–7. [PubMed] [Google Scholar]
- 107.Hashimoto Y, Murakami Y, Uemura K, et al. Mixed ductal-endocrine carcinoma derived from intraductal papillary mucinous neoplasm (IPMN) of the pancreas identified by human telomerase reverse transcriptase (hTERT) expression. J Surg Oncol. 2008;97:469–75. doi: 10.1002/jso.20959. [DOI] [PubMed] [Google Scholar]
- 108.Masuda A, Arisaka Y, Hara S, et al. MUC2 expression and prevalence of high-grade dysplasia and invasive carcinoma in mixed-type intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2013;13:583–8. doi: 10.1016/j.pan.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Khanani F, Basturk O, Khayyata S, et al. The distinction of mixed mucinous carcinomas of ampullo-pancreatobiliary region from colloid carcinoma: a clinicopathologic analysis of 25 cases. Mod Pathol. 2005;18:282A. doi: 10.1038/modpathol.3800460. Abstract. [DOI] [PubMed] [Google Scholar]
- 110.Hruban R, Kloppel G, Boffetta P, et al. Ductal adenocarcioma of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors. Lyon: WHO Press; 2010. pp. 281–291. [Google Scholar]
- 111.Adsay NV, Basturk O, Bonnett M, et al. A proposal for a new and more practical grading scheme for pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2005;29:724–33. doi: 10.1097/01.pas.0000163360.40357.f1. [DOI] [PubMed] [Google Scholar]
- 112.Luttges J, Schemm S, Vogel I, et al. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol. 2000;191:154–161. doi: 10.1002/(SICI)1096-9896(200006)191:2<154::AID-PATH603>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 113.Klöppel G, Lingenthal G, von Bülow M, et al. Histological and fine structural features of pancreatic ductal adenocarcinomas in relation to growth and prognosis: studies in xenografted tumours and clinico-histopathological correlation in a series of 75 cases. Histopathology. 1985;9:841–856. doi: 10.1111/j.1365-2559.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 114.Edge SB, Byrd DR, Campton CC, et al. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 115.Sung CO, Seo JW, Kim KM, et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol. 2008;21:1533–41. doi: 10.1038/modpathol.2008.170. [DOI] [PubMed] [Google Scholar]
- 116.Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326–33. doi: 10.1097/SLA.0b013e3182378a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andea AA, Wallis T, Newman LA, et al. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002;94:1383–90. doi: 10.1002/cncr.10331. [DOI] [PubMed] [Google Scholar]
- 118.Andea AA, Bouwman D, Wallis T, et al. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer. 2004;100:20–7. doi: 10.1002/cncr.11880. [DOI] [PubMed] [Google Scholar]
- 119.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakagohri T, Kinoshita T, Konishi M, et al. Surgical outcome of intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2007;14:3174–80. doi: 10.1245/s10434-007-9546-x. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241–246. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 122.Japan Pancreatic Society. Classification of Pancreatic Carcinoma. 2. Tokyo: Kanehara; 2003. [Google Scholar]
- 123.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–9. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–9. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 125.Weber A, Kehl V, Mittermeyer T, et al. Prognostic factors for survival in patients with unresectable pancreatic cancer. Pancreas. 2010;39:1247–53. doi: 10.1097/MPA.0b013e3181e21b1b. [DOI] [PubMed] [Google Scholar]
- 126.Saka B, Oliva I, Bandyopadhyay S, et al. Will the pT1 and pT2 Pancreas Cancer Please Stand Up? “Peripancreatic Soft Tissue” Is Involved in Most Pancreatic Ductal Adenocarcinomas (PDAC), Negating Its Value as a Staging Parameter and Necessitating a New Staging Scheme. Mod Pathol. 2014;27:454A. [Google Scholar]
- 127.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–41. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 128.Saka B, Balci S, Bagci P, et al. Proposal For a New and Prognostically Valuable Tumor Size Based T-Stage For Pancreatic Adenocarcinoma. Mod Pathol. 2014;27:453A. [Google Scholar]
- 129.Yamaguchi K, Ohuchida J, Ohtsuka T, et al. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484–90. doi: 10.1159/000064716. [DOI] [PubMed] [Google Scholar]
- 130.College of American Pathologists. [Accessed 12/29/2013];Pancreas (exocrine) 2012 Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2013/PancreasExo_13protocol_3201.pdf Posted: October, 2013.
- 131.Nagai K, Doi R, Koizumi M, et al. Noninvasive intraductal papillary mucinous neoplasm with para-aortic lymph node metastasis: report of a case. Surg Today. 2011;41:147–52. doi: 10.1007/s00595-009-4210-7. [DOI] [PubMed] [Google Scholar]
- 132.House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–55. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 133.Berger AC, Watson JC, Ross EA, et al. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–40. discussion 240. [PubMed] [Google Scholar]
- 134.Pai RK, Beck AH, Mitchem J, et al. Pattern of lymph node involvement and prognosis in pancreatic adenocarcinoma: direct lymph node invasion has similar survival to node-negative disease. Am J Surg Pathol. 2011;35:228–34. doi: 10.1097/PAS.0b013e318206c37a. [DOI] [PubMed] [Google Scholar]
- 135.Choi H, Saka B, Balci S, et al. Proposal for a Revised N-Stage for Pancreatic Ductal Adenocarcinoma as N1(<3) and N2 (≥ 3) with Strong Prognostic Correlation. Mod Pathol. 2014;27:448A. [Google Scholar]
- 136.Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364–70. doi: 10.1097/PAS.0b013e3181cf8bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Distler M, Kersting S, Niedergethmann M, et al. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013;258:324–30. doi: 10.1097/SLA.0b013e318287ab73. [DOI] [PubMed] [Google Scholar]
- 138.Mohri D, Asaoka Y, Ijichi H, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol. 2012;47:203–13. doi: 10.1007/s00535-011-0482-y. [DOI] [PubMed] [Google Scholar]
- 139.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43:1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 140.Ideno N, Ohtsuka T, Kono H, et al. Intraductal papillary mucinous neoplasms of the pancreas with distinct pancreatic ductal adenocarcinomas are frequently of gastric subtype. Ann Surg. 2013;258:141–51. doi: 10.1097/SLA.0b013e31828cd008. [DOI] [PubMed] [Google Scholar]
- 141.Okabayashi T, Shima Y, Kosaki T, et al. Invasive carcinoma derived from branch duct-type IPMN may be a more aggressive neoplasm than that derived from main duct-type IPMN. Oncol Lett. 2013;5:1819–1825. doi: 10.3892/ol.2013.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802–8. doi: 10.1245/s10434-013-3096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maker AV, Katabi N, Gonen M, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–27. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. doi: 10.1038/srep00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiao HD, Yamaguchi H, Dias-Santagata D, et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J Pathol. 2011;224:508–16. doi: 10.1002/path.2875. [DOI] [PubMed] [Google Scholar]
- 148.Basturk O, Bhanot UB, Berger MF, et al. Intraductal Oncocytic Papillary Neoplasm of the Pancreas Have Distinct Molecular Characteristics than Intraductal Papillary Mucinous Neoplasm. Mod Pathol. 2015 in press. [Google Scholar]
- 149.Kasugai H, Tajiri T, Takehara Y, et al. Intraductal tubulopapillary neoplasms of the pancreas: case report and review of the literature. J Nippon Med Sch. 2013;80:224–9. doi: 10.1272/jnms.80.224. [DOI] [PubMed] [Google Scholar]
- 150.Yamaguchi H, Kuboki Y, Hatori T, et al. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J Pathol. 2013;231:335–41. doi: 10.1002/path.4242. [DOI] [PubMed] [Google Scholar]
- 151.Furukawa T, Hatori T, Fukuda A, et al. Intraductal tubular carcinoma of the pancreas: case report with molecular analysis. Pancreas. 2009;38:235–7. doi: 10.1097/MPA.0b013e318175e3e0. [DOI] [PubMed] [Google Scholar]
- 152.Yamaguchi H, Shimizu M, Ban S, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164–72. doi: 10.1097/PAS.0b013e3181a162e5. [DOI] [PubMed] [Google Scholar]
- 153.Klimstra DS, Adsay NV, Dhall D, et al. Intraductal Tubular Carcinoma of the Pancreas: Clinicopathologic and Immunohistochemical Analysis of 18 Cases (Abstract) Mod Pathol. 2007;20:285A. [Google Scholar]
- 154.Tajiri T, Tate G, Inagaki T, et al. Intraductal tubular neoplasms of the pancreas: histogenesis and differentiation. Pancreas. 2005;30:115–21. doi: 10.1097/01.mpa.0000148513.69873.4b. [DOI] [PubMed] [Google Scholar]
- 155.Tajiri T, Tate G, Kunimura T, et al. Histologic and immunohistochemical comparison of intraductal tubular carcinoma, intraductal papillary-mucinous carcinoma, and ductal adenocarcinoma of the pancreas. Pancreas. 2004;29:116–22. doi: 10.1097/00006676-200408000-00006. [DOI] [PubMed] [Google Scholar]
- 156.Matthaei H, Wu J, Dal Molin M, et al. GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as “incipient IPMNs”. Am J Surg Pathol. 2014;38:360–3. doi: 10.1097/PAS.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi C, Klein AP, Goggins M, et al. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737–7743. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang JD, Song JS, Noh SJ, et al. Mucinous Non-neoplastic Cyst of the Pancreas. Korean J Pathol. 2013;47:188–90. doi: 10.4132/KoreanJPathol.2013.47.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu B, Lin X. Immunoprofile of mucinous non-neoplastic cyst of the pancreas. Appl Immunohistochem Mol Morphol. 2013;21:265–70. doi: 10.1097/PAI.0b013e3182606f2d. [DOI] [PubMed] [Google Scholar]
- 160.Kosmahl M, Pauser U, Anlauf M, et al. Cystic pancreas tumors and their classification: features old and new. Pathologe. 2005;26:22–30. doi: 10.1007/s00292-004-0734-1. [DOI] [PubMed] [Google Scholar]
- 161.Krasinskas A, Oakley GJ, Bagci P, et al. Cystic Mucinous Duct Lesion of the Pancreas: A Clinicopathologic Analysis of 40 Examples of a Diagnostically Challenging and Terminologically Controversial Entity. Mod Pathol. 2012;25:445A. Abstract. [Google Scholar]
- 162.Kenney B, Singh G, Salem RR, et al. Pseudointraductal papillary mucinous neoplasia caused by microscopic periductal endocrine tumors of the pancreas: a report of 3 cases. Hum Pathol. 2011;42:1034–41. doi: 10.1016/j.humpath.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 163.Kawamoto S, Shi C, Hruban RH, et al. Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction. AJR Am J Roentgenol. 2011;197:W482–8. doi: 10.2214/AJR.10.5428. [DOI] [PubMed] [Google Scholar]
- 164.Jang KT, Choi YL, Hill C, et al. Immunphenotype and Molecular Alterations in the Carcinogenetic Progression of Mucinous Cystic Neoplasms of the Pancreas (Abstract) Mod Pathol. 2012;25:445A. [Google Scholar]
- 165.Jang KT, Choi YL, Hill C, et al. Carcinogenetic Progression of Pancreatic Mucinous Cystic Neoplasm Is of Aggressive Pancreatobiliary Lineage (Abstract) Mod Pathol. 2013;26:425A. [Google Scholar]
- 166.Ohike N, Kim GE, Tajiri T, et al. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731–48. doi: 10.1097/PAS.0b013e3181f8ff05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Katabi N, Torres J, Klimstra DS. Intraductal tubular neoplasms of the bile ducts. Am J Surg Pathol. 2012;36:1647–55. doi: 10.1097/PAS.0b013e3182684d4f. [DOI] [PubMed] [Google Scholar]
- 168.Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45:1080–9. doi: 10.1007/s00535-010-0263-z. [DOI] [PubMed] [Google Scholar]
- 169.Bagci P, Andea AA, Basturk O, et al. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol. 2012;25:439–48. doi: 10.1038/modpathol.2011.181. [DOI] [PubMed] [Google Scholar]
- 170.Kelly PJ, Shinagare S, Sainani N, et al. Cystic papillary pattern in pancreatic ductal adenocarcinoma: a heretofore undescribed morphologic pattern that mimics intraductal papillary mucinous carcinoma. Am J Surg Pathol. 2012;36:696–701. doi: 10.1097/PAS.0b013e318249ce1c. [DOI] [PubMed] [Google Scholar]
- 171.Klöppel G. Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology. 1998;45:1981–1985. [PubMed] [Google Scholar]
- 172.Shirahige Y, Watanabe T, Oki Y, et al. A case of cervical carcinoma of the uterus presenting with hyperosmolar non-ketotic coma as a manifestation of ectopic adrenocorticotropic hormone syndrome. Jpn J Cancer Res. 1991;82:710–5. doi: 10.1111/j.1349-7006.1991.tb01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging. 2008;33:342–8. doi: 10.1007/s00261-007-9245-x. [DOI] [PubMed] [Google Scholar]
- 174.Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004;21:247–54. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 175.Coban I, Basturk O, Martin D, et al. Paraduodenal Pancreatitis Is One of the Main Causes of Pseudotumor in the Pancreas and Periampullary Region: Features of a Distinct Entity Becoming Clearer (Abstract) Mod Pathol. 2009;22:309A. [Google Scholar]
- 176.Liu TH, Consorti ET. Inflammatory pseudotumor presenting as a cystic tumor of the pancreas. Am Surg. 2000;66:993–997. [PubMed] [Google Scholar]
- 177.Salvia R, Partelli S, Crippa S, et al. Intraductal papillary mucinous neoplasms of the pancreas with multifocal involvement of branch ducts. Am J Surg. 2009;198:709–14. doi: 10.1016/j.amjsurg.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 178.Mori Y, Ohtsuka T, Tamura K, et al. Intraoperative irrigation cytology of the remnant pancreas to detect remnant distinct pancreatic ductal adenocarcinoma in patients with intraductal papillary mucinous neoplasm undergoing partial pancreatectomy. Surgery. 2014;155:67–73. doi: 10.1016/j.surg.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 179.Ohtsuka T, Ideno N, Aso T, et al. Role of endoscopic retrograde pancreatography for early detection of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci. 2013;20:356–61. doi: 10.1007/s00534-012-0541-7. [DOI] [PubMed] [Google Scholar]
- 180.Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg. 2010;251:70–5. doi: 10.1097/SLA.0b013e3181c5ddc3. [DOI] [PubMed] [Google Scholar]
- 181.Tanno S, Nakano Y, Sugiyama Y, et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology. 2010;10:173–8. doi: 10.1159/000231982. [DOI] [PubMed] [Google Scholar]
- 182.Ishida M, Shiomi H, Naka S, et al. Concomitant intraductal papillary mucinous neoplasm and neuroendocrine tumor of the pancreas. Oncol Lett. 2013;5:63–67. doi: 10.3892/ol.2012.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kadota Y, Shinoda M, Tanabe M, et al. Concomitant pancreatic endocrine neoplasm and intraductal papillary mucinous neoplasm: a case report and literature review. World J Surg Oncol. 2013;11:75. doi: 10.1186/1477-7819-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tewari N, Zaitoun AM, Lindsay D, et al. Three cases of concomitant intraductal papillary mucinous neoplasm and pancreatic neuroendocrine tumour. JOP. 2013;14:423–7. doi: 10.6092/1590-8577/1491. [DOI] [PubMed] [Google Scholar]
- 185.Goh BK, Ooi LL, Kumarasinghe MP, et al. Clinicopathological features of patients with concomitant intraductal papillary mucinous neoplasm of the pancreas and pancreatic endocrine neoplasm. Pancreatology. 2006;6:520–6. doi: 10.1159/000097361. [DOI] [PubMed] [Google Scholar]
- 186.Larghi A, Panic N, Capurso G, et al. Prevalence and risk factors of extrapancreatic malignancies in a large cohort of patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Oncol. 2013;24:1907–11. doi: 10.1093/annonc/mdt184. [DOI] [PubMed] [Google Scholar]
- 187.Kawakubo K, Tada M, Isayama H, et al. Incidence of extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:1249–53. doi: 10.1136/gut.2010.227306. [DOI] [PubMed] [Google Scholar]
- 188.Benarroch-Gampel J, Riall TS. Extrapancreatic malignancies and intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:363–7. doi: 10.4240/wjgs.v2.i10.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ishida M, Egawa S, Kawaguchi K, et al. Synchronous and metachronous extrapancreatic malignant neoplasms in patients with intraductal papillary-mucinous neoplasm of the pancreas. Pancreatology. 2008;8:577–82. doi: 10.1159/000159844. [DOI] [PubMed] [Google Scholar]
- 190.Yoon WJ, Ryu JK, Lee JK, et al. Extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasm of the pancreas: prevalence, associated factors, and comparison with patients with other pancreatic cystic neoplasms. Ann Surg Oncol. 2008;15:3193–8. doi: 10.1245/s10434-008-0143-4. [DOI] [PubMed] [Google Scholar]
- 191.Choi MG, Kim SW, Han SS, et al. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–6. doi: 10.1001/archsurg.141.1.51. discussion 56. [DOI] [PubMed] [Google Scholar]
- 192.DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2006;22:487–497. doi: 10.1097/01.mog.0000239862.96833.89. [DOI] [PubMed] [Google Scholar]
- 193.Klöppel G. Chronic pancreatitis of alcoholic and nonalcoholic origin. Semin Diagn Pathol. 2004;21:227–236. doi: 10.1053/j.semdp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 194.Klöppel G, Maillet B. Chronic pancreatitis: evolution of the disease. Hepatogastroenterology. 1991;38:408–412. [PubMed] [Google Scholar]
- 195.Pang TC, Wilson O, Argueta MA, et al. Frozen section of the pancreatic neck margin in pancreatoduodenectomy for pancreatic adenocarcinoma is of limited utility. Pathology. 2014;46:188–92. doi: 10.1097/PAT.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 196.Mathur A, Ross SB, Luberice K, et al. Margin status impacts survival after pancreaticoduodenectomy but negative margins should not be pursued. Am Surg. 2014;80:353–60. [PubMed] [Google Scholar]
- 197.Hernandez J, Mullinax J, Clark W, et al. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg. 2009;250:76–80. doi: 10.1097/SLA.0b013e3181ad655e. [DOI] [PubMed] [Google Scholar]
- 198.Lad NL, Squires MH, Maithel SK, et al. Is it time to stop checking frozen section neck margins during pancreaticoduodenectomy? Ann Surg Oncol. 2013;20:3626–33. doi: 10.1245/s10434-013-3080-9. [DOI] [PubMed] [Google Scholar]
- 199.Sauvanet A, Gaujoux S, Blanc B, et al. Parenchyma-Sparing Pancreatectomy for Presumed Noninvasive Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 200.Frankel TL, LaFemina J, Bamboat ZM, et al. Dysplasia at the surgical margin is associated with recurrence after resection of non-invasive intraductal papillary mucinous neoplasms. HPB (Oxford) 2013;15:814–21. doi: 10.1111/hpb.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leng KM, Wang ZD, Zhao JB, et al. Impact of pancreatic margin status and lymph node metastases on recurrence after resection for invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Dig Surg. 2012;29:213–25. doi: 10.1159/000339334. [DOI] [PubMed] [Google Scholar]
- 202.Couvelard A, Sauvanet A, Kianmanesh R, et al. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242:774–8. doi: 10.1097/01.sla.0000188459.99624.a2. discussion 778–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nara S, Shimada K, Sakamoto Y, et al. Clinical significance of frozen section analysis during resection of intraductal papillary mucinous neoplasm: should a positive pancreatic margin for adenoma or borderline lesion be resected additionally? J Am Coll Surg. 2009;209:614–21. doi: 10.1016/j.jamcollsurg.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 204.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 205.Matthaei H, Hong SM, Mayo SC, et al. Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer. Ann Surg Oncol. 2011;18:3493–9. doi: 10.1245/s10434-011-1745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fujii T, Kato K, Kodera Y, et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery. 2010;148:285–90. doi: 10.1016/j.surg.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 207.Sauvanet A, Couvelard A, Belghiti J. Role of frozen section assessment for intraductal papillary and mucinous tumor of the pancreas. World J Gastrointest Surg. 2010;2:352–8. doi: 10.4240/wjgs.v2.i10.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42:717–21. doi: 10.1097/MPA.0b013e3182883a91. [DOI] [PubMed] [Google Scholar]
- 209.Pitman MB, Centeno BA, Daglilar ES, et al. Cytological criteria of high-grade epithelial atypia in the cyst fluid of pancreatic intraductal papillary mucinous neoplasms. Cancer Cytopathol. 2013 doi: 10.1002/cncy.21344. [DOI] [PubMed] [Google Scholar]
- 210.Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg. 2011;254:977–83. doi: 10.1097/SLA.0b013e3182383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fernandez-Esparrach G, Pellise M, Sole M, et al. EUS FNA in intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology. 2007;54:260–264. [PubMed] [Google Scholar]
- 212.Emerson RE, Randolph ML, Cramer HM. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of intraductal papillary mucinous neoplasm of the pancreas is highly predictive of pancreatic neoplasia. Diagn Cytopathol. 2006;34:457–462. doi: 10.1002/dc.20446. [DOI] [PubMed] [Google Scholar]
- 213.Layfield LJ, Cramer H. Fine-needle aspiration cytology of intraductal papillary-mucinous tumors: a retrospective analysis. Diagn Cytopathol. 2005;32:16–20. doi: 10.1002/dc.20149. [DOI] [PubMed] [Google Scholar]
- 214.Sole M, Iglesias C, Fernandez-Esparrach G, et al. Fine-needle aspiration cytology of intraductal papillary mucinous tumors of the pancreas. Cancer. 2005;105:298–303. doi: 10.1002/cncr.21242. [DOI] [PubMed] [Google Scholar]
- 215.Recine M, Kaw M, Evans DB, et al. Fine-needle aspiration cytology of mucinous tumors of the pancreas. Cancer. 2004;102:92–99. doi: 10.1002/cncr.20052. [DOI] [PubMed] [Google Scholar]
- 216.Stelow EB, Stanley MW, Bardales RH, et al. Intraductal papillary-mucinous neoplasm of the pancreas. The findings and limitations of cytologic samples obtained by endoscopic ultrasound-guided fine-needle aspiration. Am J Clin Pathol. 2003;120:398–404. doi: 10.1309/CEPK-542W-3885-2LP8. [DOI] [PubMed] [Google Scholar]
- 217.Zamboni G, Fukushima H, Hruban R, et al. Mucinous cystic neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors. Lyon: WHO Press; 2010. pp. 300–303. [Google Scholar]
- 218.Wilentz RE, Albores-Saavedra J, Hruban RH. Mucinous cystic neoplasms of the pancreas. Sem Diagn Pathol. 2000;17:31–42. [PubMed] [Google Scholar]
- 219.Adsay V, Jang KT, Roa JC, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are >/=1. 0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012;36:1279–301. doi: 10.1097/PAS.0b013e318262787c. [DOI] [PubMed] [Google Scholar]