Abstract
BACKGROUND
Health-related quality of life (HRQOL), functional status, and cardiac event-free survival are outcomes used to assess the effectiveness of interventions in patients with heart failure (HF). However, the nature of the relationships among HRQOL, functional status and cardiac event-free survival remains unclear.
OBJECTIVE
The purpose of this study was to examine the nature of the relationships among HRQOL, functional status, and cardiac event-free survival in patients with HF.
METHODS
This was a prospective, observational study of 313 patients with HF that was a secondary analysis from a registry. At baseline, patient demographic and clinical data were collected. HRQOL was assessed using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and functional status was measured using the Duke Activity Status Index (DASI). Cardiac event-free survival data were obtained by patient interview, hospital database and death certificate review. Multiple linear and Cox regressions were used to explore the relationships among HRQOL, functional status and cardiac event-free survival while adjusting for demographic and clinical factors.
RESULTS
Participants (n = 313) were male (69%), Caucasians (79%), and aged 62 ± 11 years. Mean left ventricular ejection fraction of (LVEF) was 35 ± 14%. Mean HRQOL score of 32.3 ± 20.6 indicated poor HRQOL. Mean DASI score of 16.2 ± 12.9 indicated poor functional status. Cardiac event-free survival was significantly worse in patients who had worse HRQOL or poorer functional status. Patients who had better functional status had better HRQOL (p <.001). HRQOL was not a significant predictor of cardiac event-free survival after entering functional status in the model (p = .54) demonstrating that it was a mediator of the relationship between HRQOL and outcome.
CONCLUSION
Functional status was a mediator between HRQOL and cardiac event-free survival. These data suggest intervention studies to improve functional status are needed.
Keywords: Health-related quality of life, Functional Status, Event-free Survival, Outcomes, Heart Failure
Introduction
Heart failure (HF) is a chronic and prevalent syndrome with a poor prognosis,1 and a multitude of symptoms that reduce activity level and result in psychological distress. Health-related quality of life (HRQOL) is a multidimensional construct referring to how a health condition affects total well-being, including physical, functional, emotional, and social dimensions;2 adults with HF have poorer HRQOL compared to those without HF.3–5 Health-related quality of life is a predictor of hospitalization and death in patients with HF.6–14 It is a subjective, patient-centered outcome that is recognized by researchers and clinicians as an important outcome for patients with HF.15 However, little is known about the potential mechanisms linking HRQOL with hospitalization and death.
Poor functional status is also associated with poorer HF outcomes (i.e., hospitalizations and death).16, 17 Functional status, the ability to perform activities of daily living, predicted survival in patients with HF, and was an indicator for selection of patients with HF for heart transplantation.18–20 Because of the association of HRQOL, functional status, and hospitalization/death, these variables have become important endpoints of HF care.21–25 Numerous investigators have tested interventions to improve HRQOL, functional status, and survival. A number of multidisciplinary disease management, and exercise intervention studies in patients with HF have led to improvement in HRQOL,21–24 functional status,22–24 hospitalization, and death.21, 25, 26 Moreover, many demographic (age, gender, ethnicity) 8, 17, 27–31 and clinical factors (ejection fraction, comorbidities, beta-blocker, ACE inhibitor) 5, 8, 9, 13, 16, 17, 27, 28, 30–33 have been reported to influence HRQOL, functional status, hospitalization or death. However, the relationships among HRQOL, functional status, and hospitalization and death are not clear. Therefore, the purpose of this study was to examine the nature of the relationships among HRQOL, functional status, and cardiac event-free survival in patients with HF by determining whether functional status was a mediator between HRQOL and cardiac event-free survival in this patient population with and without adjustment of some important covariates.
Methods
Design and Sample
This was a prospective, observational study conducted as a secondary analysis34 that included a sample of 313 patients enrolled from outpatient setting of multiple large community hospitals and academic medical centers in the United States. Patients were eligible for inclusion if they had a confirmed diagnosis of chronic HF, preserved or non-preserved systolic function, had been optimized on HF medications and on stable doses for three months, and were English-speaking. They were excluded for a myocardial infarction within 3 months or unstable angina, obvious cognitive impairment (i.e., not able to give informed consent or participate in an interview), discharge to a skilled nursing facility, or were diagnosed with severe psychiatric impairment other than depression or anxiety. For the purposes of this study, patients were only included if they had complete data on HRQOL, functional status and cardiac events.
Measurement
Cardiac event-free survival
Cardiac event-free survival was the composite end-point of time to the first occurrence of one of the following cardiac events from the enrollment date: cardiac emergency department (ED) visits, cardiac hospitalizations, or cardiac mortality. This composite end-point is commonly used in such research.9, 21, 25, 28, 35, 36 During data collection, the date and reasons for hospitalization and death were noted. Data were obtained by trained research assistants with expertise in cardiovascular nursing by patient/family interview, hospital database review and review of death certificates and records. All outcome assessment was blinded to patient HRQOL and functional status.
Health-related quality of life (HRQOL)
The Minnesota Living with Heart Failure questionnaire (MLHFQ) is a measure of HRQOL that is used to assess the patient’s perceptions of the influence of HF on physical and emotional aspects of life.2, 37 The 21 items are summed and ranged from 0–105 with higher scores indicating worse HRQOL. This instrument has been widely used to measure quality of life in this population.38–40 Researchers have demonstrated evidence for validity and reliability.2, 37 The Cronbach’s alpha in our study was 0.93, adding support for reliability.
Functional Status
Functional status was assessed using the Duke Activity Status Index (DASI).41 The DASI has been used in a variety of cardiac disease populations, including HF.29, 42, 43 The DASI consists of 12 items and each item has four response options ranging from 1 = “can perform activity with difficulty” to 4 = “cannot perform activity at all”. Each item is weighted based on the metabolic equivalent associated with the activity represented by that item. For example, the weight for walking indoors is 1.75, while that for running is 8. Only items that are rated 1 by the respondent receive a score. Items that are rated 2, 3, or 4, indicating that the activity can only be performed with difficulty or cannot be done at all, are scored as zero. The total score is calculated by adding the weighted score for each item. The total score can range from 0 to 58.2, with higher scores indicating better functional status. The activities in the DASI included personal care, ambulation, household tasks, sexual function and recreational activities which represent major aspects of physical function.41 The reliability and validity of the DASI have been demonstrated previously.31, 41, 44, 45 Cronbach’s α in the current study was 0.84.
Demographic and clinical characteristics
To describe the sample and obtain data about potential confounding variables, the following information was collected by patient interview and chart review: age, gender, ethnicity, highest education level attained, financial status, and living alone or with someone else. Financial status was measured by one question about whether patients “had more than enough, enough, or did not have enough to make ends meet”. The following clinical characteristics were collected by chart review: left ventricular ejection fraction (LVEF) within the past 3 months, and medications (e.g., taking ACE inhibitor, or β-blocker). Data about comorbidities were collected by chart review and patient interview using the Charlson Comorbidity Index (CCI).46, 47 The CCI is weighted for severity of comorbidity and is computed as a total score. A higher CCI score implies higher comorbidity burden.46, 47
Procedure
Institutional Review Board approval was obtained for each site and patients gave written informed consent. The review board at the primary author’s institution approved all secondary data analyses of this investigation as an exempt protocol. Patients were enrolled and recruited in cardiology clinics after referral from clinicians. These patients completed baseline assessment and were followed monthly by telephone to collect data about cardiac events and confirmed by hospital data base review and review of death certificates and records. Patients were followed for a median of 360 days to determine cardiac event-free survival.
Data analysis
SPSS version 22.0 (Chicago, IL) was used for data analysis; a p value of less than 0.05 was considered significant. Patients were categorized as better or worse HRQOL based on the median score of the MLHFQ in this sample (i.e., 30) and divided into better or poorer functional status groups based on the median score of the DASI in this sample (i.e., 10.7). The median score was used in this study because there are no standard cutpoints, and the median is the most commonly used cutpoint in the literature.8, 12 Differences in demographic and clinical variables between the groups formed by median MLHFQ and DASI scores were assessed with independent t-tests or chi-square tests of association based on the level of measurement.
Logistic/linear regressions, t-tests, Pearson correlation, Kaplan-Meier plots with log-rank test, and Cox regressions were used to explore the relationships among HRQOL, functional status, and cardiac event-free survival. In the linear regression models, we performed multicollinearity tests to evaluate this assumption of regression. There were no issues with multicollinearity as all variance inflation factors were < 8. The log-rank test was used to compare the time to cardiac event-free survival between patients with better and worse HRQOL, and with better and worse functional status. Kaplan-Meier plots were used to graphically depict group differences in cardiac event-free survival. Univariate and adjusted Cox proportional hazards regression modeling was used to assess the time to cardiac event between groups. Variables showing marginal association with cardiac events in univariate analyses with alpha set at < 0.10, as were those with prior evidence of association with one or more of the independent variables or dependent variable (e.g., age, gender, ethnicity, education level, financial status, and living status, comorbidity, LVEF, taking ACE inhibitor, and β-blocker) 5, 8, 9, 13, 16, 17, 27–33 were forced into the Cox regression analysis. We also conducted the analyses with HRQOL and functional status as continuous variables.
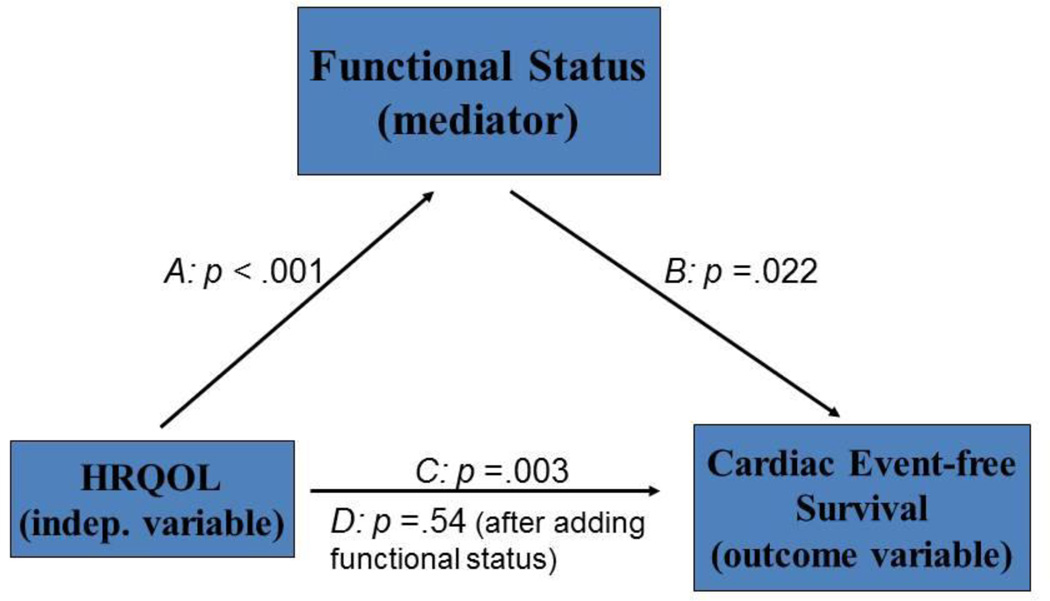
To further explore the relationships among HRQOL, functional status, and cardiac event-free survival, we conducted mediation analyses to test whether functional status was a mediator of the relationship between HRQOL and cardiac event-free survival using a series of regression models and Cox-survival analyses. The test for mediation followed the steps outlined by Baron and Kenny 48 and Bennett.49 Four regression models were performed to test for the mediator effect. The first model tested whether HRQOL (the independent variable) was a predictor of functional status (mediator). The second model tested whether HRQOL was a predictor of cardiac event-free survival (outcome variable). The third model tested whether functional status was a predictor of event-free survival. In the fourth model, both HRQOL and functional status (independent and mediator variables) were entered simultaneously as predictors of cardiac event-free survival (outcome variable). The following conditions must be met if a mediator effect is present: 1) the results of the first, second, and the third models should be significant, and 2) the significance level of the coefficient associated with the independent variable in the fourth model is less significant (partial mediator) or non-significant (full mediator) compared to the third model (Figure 1).48–50
Figure 1.
Functional Status is a mediator
Path A: Test of whether HRQOL is a predictor of functional status
Path B: Test of whether functional status is a predictor of cardiac event-free survival
Path C: Test of whether HRQOL is a predictor of cardiac event-free survival
Path D: Test of whether HRQOL and functional status together are predictors of cardiac event-free survival
Results
Sample Characteristics
Three hundred and thirteen patients who had complete data on HRQOL, functional status, and cardiac events were included in this study. There were no differences in characteristics between the 313 patients who had complete data and those who did not have complete data. The mean age of patients in the sample was 62 ± 11 years, and about one third of patients were female. The majority of the patients were Caucasian (79%) and living with someone (69%). Full sample characteristics and comparison of better and worse HRQOL, and better and worse functional status groups are presented in Table 1.
Table 1.
Sample characteristics and comparison of clinical and demographic characteristics by health-related quality of life and functional status classification
| Characteristics | Overall (N = 313) |
Better HRQOL (n = 154) |
Worse HRQOL (n = 159) |
p | Better Functional status (n = 181) |
Poorer Functional status (n = 132) |
p |
|---|---|---|---|---|---|---|---|
| Age, years | 62±11 | 64±11 | 60±11 | .006 | 62±11 | 62±11 | .961 |
| Male | 217 (69) | 111 (72) | 106 (67) | .328 | 136 (75) | 81 (61) | .013 |
| Living alone | 98 (31) | 48 (31) | 50 (31) | 1.00 | 55 (30) | 43 (33) | .712 |
| Caucasian | 247 (79) | 116 (75) | 131 (82) | .130 | 139 (77) | 108 (82) | .327 |
| Education, years | 13±3 | 14±3 | 12±3 | < .001 | 14±3 | 13±3 | .001 |
| Etiology, ischemic | 159 (52) | 78 (51) | 81 (52) | .467 | 89 (50) | 70 (54) | .636 |
| Body mass index | 30.6±7.5 | 29.7±7.5 | 31.5±7.4 | .062 | 29.8±7.5 | 31.6±7.5 | .071 |
| Comorbidity | 3.2±2.0 | 3.1±1.8 | 3.4±2.1 | .213 | 2.9±2.0 | 3.6±2.0 | .002 |
| Prior heart attack | 161 (52) | 79 (51) | 82 (53) | .820 | 88 (49) | 73 (56) | .249 |
| Hypertension | 224 (73) | 112 (74) | 112 (73) | .898 | 123 (70) | 101 (78) | .091 |
| LVEF, % | 35±14 | 34±14 | 35±15 | .938 | 34±14 | 35±14 | .552 |
| ACEI use | 227 (73) | 115 (75) | 112 (70) | .448 | 133 (74) | 94 (71) | .701 |
| Beta-blocker use | 278 (89) | 139 (90) | 139 (87) | .476 | 160 (88) | 118 (89) | .857 |
| HRQOL | 32.3±20.6 | 14.9±8.4 | 49.2±13.7 | < .001 | 23.7±17.1 | 44.2±19.1 | < .001 |
| Functional status | 16.2±12.9 | 22.5±14.4 | 10.2±7.2 | < .001 | 23.5±12.6 | 6.2±2.5 | < .001 |
Data are presented as means ± SD, or N (%), interval level data compared by independent t-test, categorical by Chi-square; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; ACEI =angiotensin-converting-enzyme inhibitor.
The mean HRQOL score as measured by MLHFQ was 32.3 ± 20.6 (median: 30). Of the total sample, 159 out of the 313 patients were classified as having poorer HRQOL. Patients with worse HRQOL were younger, had less education (p < .001), and lower functional status score (p < .001) than those with better HRQOL.
The mean functional status score as measured by the DASI was 16.2 ± 12.9 (median: 10.7). We had a greater percentage of female patients in the poorer functional status group than in the better functional status group The proportion of female patients with poorer functional status was significantly more than predicted and higher than the group with better functional status (higher functional status 39%, lower functional status 25%, p = .013). In addition, patients in the poorer functional status group had one year less education (poorer 13 ± 3 years, better 14 ± 3 years, p = 0.001), greater comorbidity burden (poorer 3.6 ± 2, better 2.9 ± 2, p = 0.002) and worse HRQOL scores (poorer 44.2 ± 19.1, better 23.7 ± 17.1, p < 0.001) compared with those in the better functional status group.
Association of health-related quality of life with cardiac event-free survival
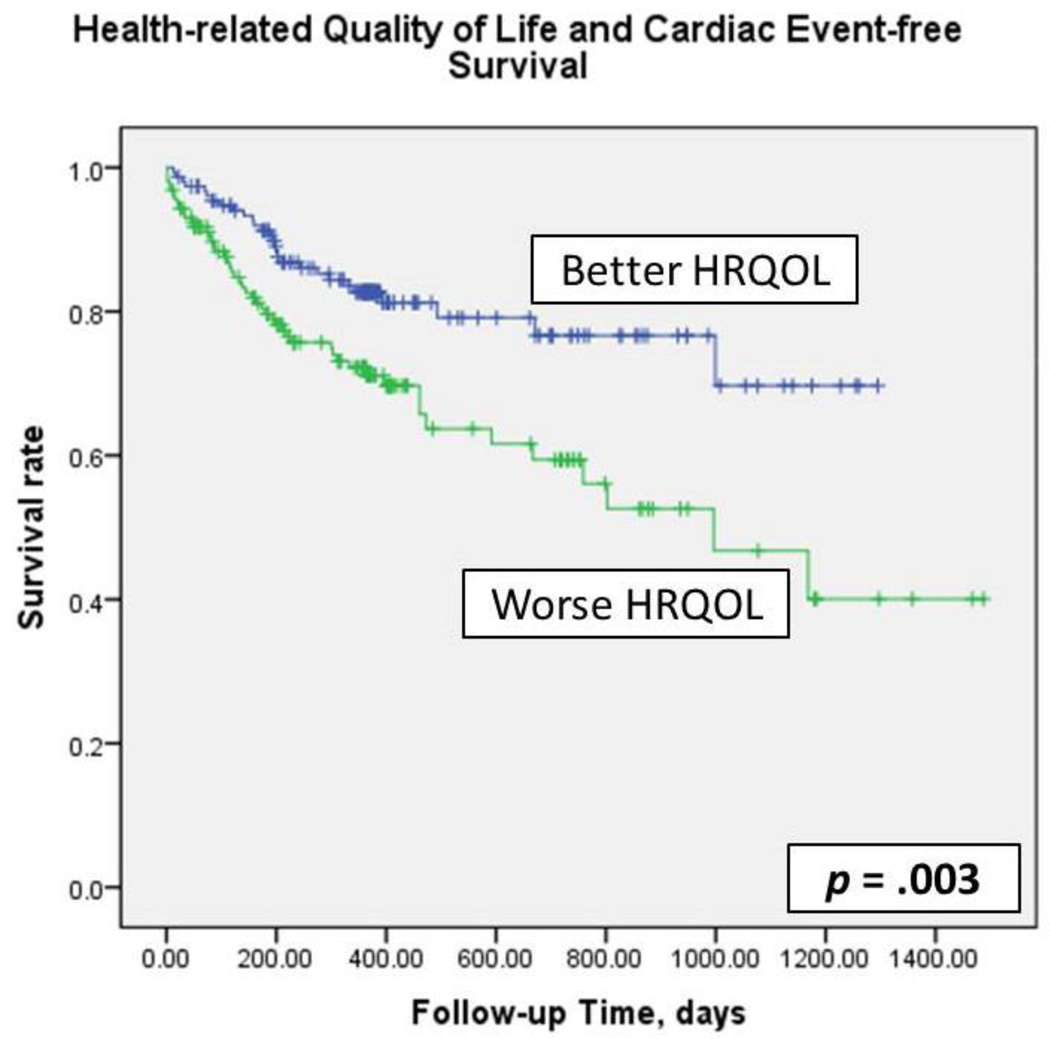
Kaplan-Meier plots with log-rank tests (Figure 2) demonstrated that cardiac event-free survival was significantly worse in patients who had worse HRQOL (p = .003). In simple Cox regression modeling, HRQOL predicted cardiac event-free survival (hazard ratio [HR] =2.01, p = .003). After controlling for age, gender, ethnicity, education level, financial status, living status, comorbidity, LVEF, ACE inhibitor use, and β-blocker use, patients who had worse HRQOL had 2.32 times the risk of experiencing a cardiac event compared to patients who had better HRQOL. Likewise, when HRQOL was analyzed as a continuous variable, HRQOL predicted cardiac event-free survival before and after adjusting for covariates (HR = 1.015 and 1.016, respectively, p = .008) (Table 2). For every one-point increase in MLHFQ score, the risk of a cardiac event during follow-up increased 1.5–1.6%.
Figure 2.
Kaplan-Meier plots and log-rank test: health-related quality of life and cardiac event –free survival
Table 2.
Cox Regression Modeling: Health-related Quality of Life on Cardiac Event-free Survival (N = 313)
| Variables | Hazard Ratio | Wald | Significance |
|---|---|---|---|
|
*Simple Cox Regression HRQOL (MLHFQ score) |
1.015 |
7.054 |
008 |
|
**Multiple Cox Regression Age |
1.018 |
2.594 |
107 |
| Gender | .555 | 3.876 | .049 |
| Ethnicity | 1.385 | 1.179 | .277 |
| Education | .983 | .242 | .622 |
| Living status | .764 | 1.266 | .261 |
| Financial status | .918 | .200 | .655 |
| LVEF | .985 | 2.715 | .099 |
| Comorbidity | 1.055 | .870 | .351 |
| Taking ACEI | .802 | .694 | .405 |
| Taking BB | .669 | 1.339 | .247 |
| HRQOL (MLHFQ score) | 1.016 | 6.995 | .008 |
χ2 = 7.148, p = 0.008;
χ2 = 23.977, p = 0.013
ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; HRQOL = health-related quality of life; LVEF = left ventricular ejection fraction; MLHFQ = Minnesota Living with Heart Failure Questionnaire
Association of functional status with cardiac event-free survival
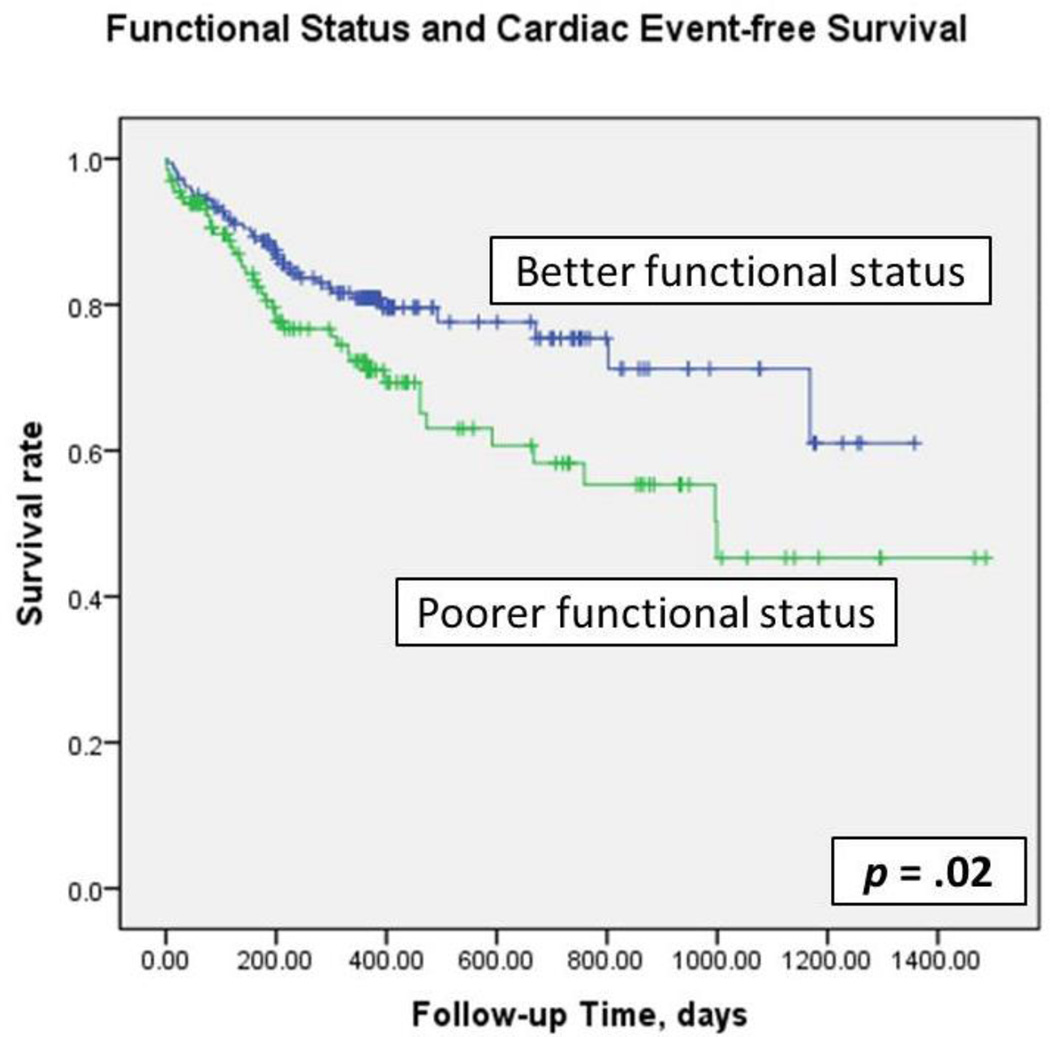
Kaplan-Meier plots with log-rank tests (Figure 3) demonstrated that cardiac event-free survival was significantly worse in patients who had poorer functional status (p =. 02). In simple Cox regression modeling, functional status predicted cardiac event-free survival (HR=1.69, p = .022). After controlling for age, gender, ethnicity, education level, financial status, living status, comorbidity, LVEF, ACE inhibitor use, and β-blocker use, patients who had poorer functional status had 1.63 times the risk of experiencing a cardiac event compared to patients with better functional status. When functional status was analyzed as a continuous variable, functional status predicted cardiac event-free survival before and after adjusting for covariates (HR = .956, p ≤ .001) (Table 3). For every one-point increase in DASI score, the risk of cardiac event during follow-up decreased 4.4%.
Figure 3.
Kaplan-Meier plots and log-rank test: functional status and cardiac event –free survival
Table 3.
Cox Regression Modeling: Functional Status on Cardiac Event-free Survival (N = 313)
| Variables | Hazard Ratio | Wald | Significance |
|---|---|---|---|
|
*Simple Cox Regression Functional status (DASI score) |
956 |
12.213 |
< .001 |
|
**Multiple Cox Regression Age |
1.010 |
868 |
352 |
| Gender | .512 | 5.067 | .024 |
| Ethnicity | 1.423 | 1.380 | .240 |
| Education | .983 | .234 | .628 |
| Living status | .735 | 1.613 | .204 |
| Financial status | .932 | .135 | .713 |
| LVEF | .987 | 2.280 | .131 |
| Comorbidity | 1.027 | .199 | .656 |
| Taking ACEI | .801 | .696 | .404 |
| Taking BB | .630 | 1.741 | .187 |
| Functional status (DASI score) | .956 | 11.726 | .001 |
χ2 = 12.876, p < .001;
χ2 = 29.015, p = 0.002
ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; DASI = Duke Activity Status Index; LVEF = left ventricular ejection fraction
HRQOL, functional status, cardiac event-free survival—Mediation analysis
HRQOL was moderately correlated with functional status (r = −.55, p < .001). When patients had better functional status (higher scores on DASI), they also had better HRQOL (lower scores on MLHFQ). In a series of regression models and Cox-survival analyses, functional status mediated the relationship between HRQOL and cardiac event-free survival based on the following sequence of regression analyses. First, in Path A (Figure 1), HRQOL independently predicted functional status (p < .001). Second, in Path B, functional status predicted cardiac event-free survival (p = .022). Third, in Path C, HRQOL was an independent predictor of cardiac event-free survival (p = .003). In the final Path D, HRQOL (p = .54) was no longer a significant predictor of cardiac event-free survival when functional status (p = .004) was entered into the model, indicating functional status mediated the relationship between HRQOL and cardiac event-free survival. When adding age, gender, ethnicity, education level, financial status, living status, comorbidity, LVEF, ACE inhibitor and β-blocker use to the model, functional status (p = .007) still mediated the relationship between HRQOL and cardiac event-free survival.
Additional Analysis
To examine the possibility that HRQOL was a mediator, we treated functional status as an independent variable. Functional status independently predicted HRQOL scores (p < .001) and cardiac event-free survival (p = .022). However, HRQOL scores did not predict cardiac event-free survival in combination with functional status (p > 0.05). Therefore, HRQOL did not mediate the relationship between functional status and cardiac event-free survival, providing further support for the hypothesis that it is functional status that mediates the relationship between HRQOL and cardiac event-free survival.
Discussion
Heart failure is characterized by worse HRQOL and functional status, and also by poor prognosis, including frequent hospitalizations, and high mortality. In this study, we examined the role of functional status in the link between HRQOL and cardiac events in patients with HF. We found that functional status was a mediator of the association of HRQOL with hospitalization and death. Patients with HF and better HRQOL have enhanced cardiac event-free survival because of superior functional status.
In the HF literature, HRQOL is a predictor of hospitalization and death in patients with HF,6–14, 37 and many investigators have found that worse physical HRQOL is an indication of worsening prognosis.7, 12 For example, in the EPICAL study,7 a worse score in the physical, but not the mental, component of the Duke Health Profile (a HRQOL questionnaire) was associated with a greater risk of HF hospitalization or death. Similarly, Rodriguez-Artalejo and colleagues12 also reported a relationship between mortality and the physical score on the MLHFQ and Short Form-36 (another generic HRQOL questionnaire), but no association with the emotional score. Our findings not only support the hypothesis, as we found that functional status, as measured by the DASI, predicted cardiac event-free survival, but also demonstrated that functional status mediated the relationship between HRQOL and cardiac event-free survival.
Patient-reported HRQOL has been demonstrated to be an independent predictor of hospitalization and mortality in patients with HF.6–14 Rector and colleagues2 reported that many patients with HF would accept some risk of medication-induced death in exchange for improved health-related quality of life, demonstrating the importance of HRQOL in this population. Our findings are consistent with prior studies.6–14 Patients who had worse self-reported HRQOL had more than twice the risk of experiencing a cardiac event compared to patients with better HRQOL before and after controlling for potential confounders. Lupon and colleagues8 assessed HRQOL at baseline,1, 3, and 5 years and found that baseline and follow-up HRQOL assessments were independently associated with death.8 Patients who died in the one-year period after any HRQOL assessment reported significantly worse HRQOL compared with those who survived.8 Moser and colleagues37 found that HRQOL remained impaired, but improved substantially within 1 month of hospital discharge for most patients. However, there was a 3.3 times increased risk of rehospitalization or death in those without improved 1-month HRQOL. Results from these studies and our study confirm the importance of assessing patient-reported HRQOL.
In addition to HRQOL, functional status was associated with hospitalization and mortality.16, 17 In the current study, patients with poorer functional status had about twice the risk of experiencing a cardiac event, compared to patients with better functional status before and after controlling for potential confounders. Parissis and colleagues16 found that functional status, as measured by the DASI, was independently associated with cardiac events after adjusting for demographic and clinical variables.16 Furthermore, Koch and colleagues reported a “dose-response” relationship between baseline and follow-up functional status and risk of long-term survival (median follow-up was 8.6 years).17 Our results, combined with findings from these investigators, support the need for regular assessment of patient-reported HRQOL and functional status to identify patients who are at risk for reduced event-free survival.
A number of investigators previously provided evidence that interventions like exercise training may improve HRQOL, and also reduce cardiac events including death in patients with HF.21–24, 51, 52 Our findings suggested that improving functional status may attenuate the association between poor HRQOL, and cardiac hospitalizations and mortality. Nishi and colleagues53 used exercise training in patients with HF and found that functional status was improved by 16 ± 15% in those in the exercise training group, while functional status remained unchanged in the control group (p < .001). Gary and colleagues22 tested the effects of a home-based exercise program, and found that the exercise group had improved HRQOL. In a subsequent aerobic and resistance exercise intervention study, Gary and colleagues23, 24 found that exercise participants had significant improvement in physical function, muscle strength, and HRQOL compared with those in the attention control group. Similarly, Servantes and colleagues54 found that a home-based exercise training intervention improved both HRQOL (as assessed by MLHFQ) and functional status (as measured by exercise testing, peak oxygen consumption) in patients with HF. In the HF-ACTION trial, the largest and longest exercise intervention trial in patients with HF published to date, the investigators found that exercise training improved functional status 51, HRQOL 52, and was also associated with reduction in hospitalizations and death, after adjusting for highly prognostic baseline characteristics like depressive symptoms.51 The findings and conclusions from numerous intervention trials support the importance of exercise for HRQOL, functional status, and reductions in hospitalizations and prolonged survival. Our findings further define the relationship between these variables.
Limitations
Our study has several limitations. First, the DASI is a self-report of functional status and not an objective measure. Use of an objective measure may increase accuracy of assessment. Our data, which demonstrated a strong relationship between functional status and outcomes, suggested that functional status was accurately reflected by the self-report measure in this study. Moreover, the DASI has been validated against the reference standard of peak oxygen uptake in younger patients,41 patients with HF,45 and patients with chronic obstructive pulmonary disease.44
Second, we measured HRQOL and functional status in a cross-sectional fashion, which limited our ability to determine a causal relationship. However, in our additional analyses, there was no evidence to support the mediating role of HRQOL on the relationship between functional status and cardiac event-free survival. Longitudinal studies are necessary to determine the causal relationship between HRQOL and functional status.
Third, HRQOL was measured by self-reported method and we only included English-speaking patients in this study. However, HRQOL is subjective and there is no other valid way to assess HRQOL than by self-report. The MLHFQ was specifically developed to measure HRQOL in patients with HF; the instrument has demonstrated good reliability and validity, and is widely used in studies with patients with HF.38–40
Fourth, the mean age of our sample was younger than patients in general, which might limit the generalizability of the study. The difference might have resulted from recruiting participants in the ambulatory care settings and not the hospital settings. The mean age of participants in many HF studies (range 54–64),25, 55–58 including a few large multi-center trials,56, 57 was similar to participant age in our study. Regardless, the findings from this study provide important information related to the nature of the relationships among functional status, HRQOL and cardiac events in patients with HF.
Conclusions
In this study, we found that functional status was a mediator between HRQOL and cardiac event-free survival. Because patients with HF tend to experience poorer HRQOL and poorer functional status, it is vital for clinicians to regularly assess HRQOL and functional status in these patients, and provide tailored, evidence-based interventions to improve HRQOL, functional status, and health outcomes.
Acknowledgements
This study was supported by funding from the National Institute Of Nursing Research of the National Institutes of Health under Award Number K23NR014489 (Jia-Rong Wu, principal investigator) and a Center grant to the University of Kentucky, College of Nursing from NIH, NINR, 1P20NR010679 (Debra Moser, principal investigator).
Footnotes
Conflict of Interest
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics-2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, Keeler CA, Silver MA. Use of the living with heart failure questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug-induced death. J. Card. Fail. 1995;1:201–206. doi: 10.1016/1071-9164(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 3.Heo S, Moser DK, Lennie TA, Zambroski CH, Chung ML. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart Lung. 2007;36:16–24. doi: 10.1016/j.hrtlng.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Lesman-Leegte I, Jaarsma T, Coyne JC, Hillege HL, Van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: A comparison between patients with heart failure and age- and gender-matched community controls. J. Card. Fail. 2009;15:17–23. doi: 10.1016/j.cardfail.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: A cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur. Heart J. 2002;23:1867–1876. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 6.Konstam V, Salem D, Pouleur H, Kostis J, Gorkin L, Shumaker S, Mottard I, Woods P, Konstam MA, Yusuf S. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. Solvd investigations. Studies of left ventricular dysfunction investigators. Am. J. Cardiol. 1996;78:890–895. doi: 10.1016/s0002-9149(96)00463-8. [DOI] [PubMed] [Google Scholar]
- 7.Alla F, Briancon S, Guillemin F, Juilliere Y, Mertes PM, Villemot JP, Zannad F. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the epical study. Eur J Heart Fail. 2002;4:337–343. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 8.Lupon J, Gastelurrutia P, de Antonio M, Gonzalez B, Cano L, Cabanes R, Urrutia A, Diez C, Coll R, Altimir S, Bayes-Genis A. Quality of life monitoring in ambulatory heart failure patients: Temporal changes and prognostic value. Eur J Heart Fail. 2013;15:103–109. doi: 10.1093/eurjhf/hfs133. [DOI] [PubMed] [Google Scholar]
- 9.Parissis JT, Nikolaou M, Farmakis D, Paraskevaidis IA, Bistola V, Venetsanou K, Katsaras D, Filippatos G, Kremastinos DT. Self-assessment of health status is associated with inflammatory activation and predicts long-term outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:163–169. doi: 10.1093/eurjhf/hfn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murberg TA, Bru E, Svebak S, Tveteras R, Aarsland T. Depressed mood and subjective health symptoms as predictors of mortality in patients with congestive heart failure: A two-years follow-up study. Int. J. Psychiatry Med. 1999;29:311–326. doi: 10.2190/0C1C-A63U-V5XQ-1DAL. [DOI] [PubMed] [Google Scholar]
- 11.Bennett SJ, Pressler ML, Hays L, Firestine LA, Huster GA. Psychosocial variables and hospitalization in persons with chronic heart failure. Prog. Cardiovasc. Nurs. 1997;12:4–11. [PubMed] [Google Scholar]
- 12.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, Otero CM, Montes AO, Garcia AN, Conthe P, Chiva MO, Banegas JR, Herrera MC. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch. Intern. Med. 2005;165:1274–1279. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 13.Stull DE, Clough LA, Van Dussen D. Self-report quality of life as a predictor of hospitalization for patients with lv dysfunction: A life course approach. Res. Nurs. Health. 2001;24:460–469. doi: 10.1002/nur.10006. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Health status identifies heart failure outpatients at risk for hospitalization or death. J. Am. Coll. Cardiol. 2006;47:752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan M. The new subjective medicine: Taking the patient's point of view on health care and health. Soc. Sci. Med. 2003;56:1595–1604. doi: 10.1016/s0277-9536(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 16.Parissis JT, Nikolaou M, Birmpa D, Farmakis D, Paraskevaidis I, Bistola V, Katsoulas T, Filippatos G, Kremastinos DT. Clinical and prognostic value of duke's activity status index along with plasma b-type natriuretic peptide levels in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2009;103:73–75. doi: 10.1016/j.amjcard.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Koch CG, Li L, Lauer M, Sabik J, Starr NJ, Blackstone EH. Effect of functional health-related quality of life on long-term survival after cardiac surgery. Circulation. 2007;115:692–699. doi: 10.1161/CIRCULATIONAHA.106.640573. [DOI] [PubMed] [Google Scholar]
- 18.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 19.Mancini D, LeJemtel T, Aaronson K. Peak vo(2): A simple yet enduring standard. Circulation. 2000;101:1080–1082. doi: 10.1161/01.cir.101.10.1080. [DOI] [PubMed] [Google Scholar]
- 20.Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, Fowler MB. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann. Intern. Med. 1998;129:286–293. doi: 10.7326/0003-4819-129-4-199808150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kasper EK, Gerstenblith G, Hefter G, Van Anden E, Brinker JA, Thiemann DR, Terrin M, Forman S, Gottlieb SH. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J. Am. Coll. Cardiol. 2002;39:471–480. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 22.Gary RA, Sueta CA, Dougherty M, Rosenberg B, Cheek D, Preisser J, Neelon V, McMurray R. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33:210–218. doi: 10.1016/j.hrtlng.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Gary RA, Cress ME, Higgins MK, Smith AL, Dunbar SB. Combined aerobic and resistance exercise program improves task performance in patients with heart failure. Arch. Phys. Med. Rehabil. 2011;92:1371–1381. doi: 10.1016/j.apmr.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gary RA, Cress ME, Higgins MK, Smith AL, Dunbar SB. A combined aerobic and resistance exercise program improves physical functional performance in patients with heart failure: A pilot study. J. Cardiovasc. Nurs. 2012;27:418–430. doi: 10.1097/JCN.0b013e31822ad3c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dracup K, Evangelista LS, Hamilton MA, Erickson V, Hage A, Moriguchi J, Canary C, MacLellan WR, Fonarow GC. Effects of a home-based exercise program on clinical outcomes in heart failure. Am. Heart J. 2007;154:877–883. doi: 10.1016/j.ahj.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch. Intern. Med. 2002;162:705–712. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 27.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J. Card. Fail. 2006;12:87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Heo S, Moser DK, Chung ML, Lennie TA. Social status, health-related quality of life, and event-free survival in patients with heart failure. Eur J Cardiovasc Nurs. 2012;11:141–149. doi: 10.1016/j.ejcnurse.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J. Card. Fail. 2006;12:439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Jaeger AA, Hlatky MA, Paul SM, Gortner SR. Functional capacity after cardiac surgery in elderly patients. J. Am. Coll. Cardiol. 1994;24:104–108. doi: 10.1016/0735-1097(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 31.Nelson CL, Herndon JE, Mark DB, Pryor DB, Califf RM, Hlatky MA. Relation of clinical and angiographic factors to functional capacity as measured by the duke activity status index. Am. J. Cardiol. 1991;68:973–975. doi: 10.1016/0002-9149(91)90423-i. [DOI] [PubMed] [Google Scholar]
- 32.Johnson G, Carson P, Francis GS, Cohn JN. Influence of prerandomization (baseline) variables on mortality and on the reduction of mortality by enalapril. Veterans affairs cooperative study on vasodilator therapy of heart failure (v-heft ii). V-heft va cooperative studies group. Circulation. 1993;87:VI32–VI39. [PubMed] [Google Scholar]
- 33.Investigators S. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 34.Riegel B, Moser DK, Rayens MK, Carlson B, Pressler SJ, Shively M, Albert NM, Armola RR, Evangelista L, Westlake C, Sethares K. Ethnic differences in quality of life in persons with heart failure. J. Card. Fail. 2008;14:41–47. doi: 10.1016/j.cardfail.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Wu JR, Moser DK, De Jong MJ, Rayens MK, Chung ML, Riegel B, Lennie TA. Defining an evidence-based cutpoint for medication adherence in heart failure. Am. Heart J. 2009;157:285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennie TA, Song EK, Wu JR, Chung ML, Dunbar SB, Pressler SJ, Moser DK. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J. Card. Fail. 2011;17:325–330. doi: 10.1016/j.cardfail.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser DK, Yamokoski L, Sun JL, Conway GA, Hartman KA, Graziano JA, Binanay C, Stevenson LW. Improvement in health-related quality of life after hospitalization predicts event-free survival in patients with advanced heart failure. J. Card. Fail. 2009;15:763–769. doi: 10.1016/j.cardfail.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing the psychometric properties of the minnesota living with heart failure questionnaire. Nurs. Res. 2005;54:265–272. doi: 10.1097/00006199-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. Jama. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 40.Rector TS, Kubo SH, Cohn JN. Validity of the minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am. J. Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 41.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the duke activity status index) Am. J. Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 42.Alonso J, Permanyer-Miralda G, Cascant P, Brotons C, Prieto L, Soler-Soler J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the duke activity status index (dasi) Eur. Heart J. 1997;18:414–419. doi: 10.1093/oxfordjournals.eurheartj.a015260. [DOI] [PubMed] [Google Scholar]
- 43.Bairey Merz CN, Olson M, McGorray S, Pakstis DL, Zell K, Rickens CR, Kelsey SF, Bittner V, Sharaf BL, Sopko G. Physical activity and functional capacity measurement in women: A report from the nhlbi-sponsored wise study. Journal of women's health & gender-based medicine. 2000;9:769–777. doi: 10.1089/15246090050147745. [DOI] [PubMed] [Google Scholar]
- 44.Carter R, Holiday DB, Grothues C, Nwasuruba C, Stocks J, Tiep B. Criterion validity of the duke activity status index for assessing functional capacity in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2002;22:298–308. doi: 10.1097/00008483-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Arena R, Humphrey R, Peberdy MA. Using the duke activity status index in heart failure. J Cardiopulm Rehabil. 2002;22:93–95. doi: 10.1097/00008483-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 47.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 49.Bennett JA. Mediator and moderator variables in nursing research: Conceptual and statistical differences. Res. Nurs. Health. 2000;23:415–420. doi: 10.1002/1098-240x(200010)23:5<415::aid-nur8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 50.Sonnentag S, Zijlstra FR. Job characteristics and off-job activities as predictors of need for recovery, well-being, and fatigue. J. Appl. Psychol. 2006;91:330–350. doi: 10.1037/0021-9010.91.2.330. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: Hf-action randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pina IL, Lin L, Weinfurt KP, Isitt JJ, Whellan DJ, Schulman KA, Flynn KE. Hemoglobin, exercise training, and health status in patients with chronic heart failure (from the hf-action randomized controlled trial) Am. J. Cardiol. 2013 doi: 10.1016/j.amjcard.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 53.Nishi I, Noguchi T, Iwanaga Y, Furuichi S, Aihara N, Takaki H, Goto Y. Effects of exercise training in patients with chronic heart failure and advanced left ventricular systolic dysfunction receiving beta-blockers. Circ J. 2011;75:1649–1655. doi: 10.1253/circj.cj-10-0899. [DOI] [PubMed] [Google Scholar]
- 54.Servantes DM, Pelcerman A, Salvetti XM, Salles AF, de Albuquerque PF, de Salles FC, Lopes C, de Mello MT, Almeida DR, Filho JA. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: A randomized comparison of two different programmes. Clin. Rehabil. 2012;26:45–57. doi: 10.1177/0269215511403941. [DOI] [PubMed] [Google Scholar]
- 55.Daullxhiu I, Haliti E, Poniku A, Ahmeti A, Hyseni V, Olloni R, Vela Z, Elezi S, Bajraktari G, Daullxhiu T. Predictors of exercise capacity in patients with chronic heart failure. Journal of cardiovascular medicine (Hagerstown, Md. 2011;12:223–225. doi: 10.2459/JCM.0b013e328343e950. [DOI] [PubMed] [Google Scholar]
- 56.De Marco T, Wolfel E, Feldman AM, Lowes B, Higginbotham MB, Ghali JK, Wagoner L, Kirlin PC, Kennett JD, Goel S, Saxon LA, Boehmer JP, Mann D, Galle E, Ecklund F, Yong P, Bristow MR. Impact of cardiac resynchronization therapy on exercise performance, functional capacity, and quality of life in systolic heart failure with qrs prolongation: Companion trial sub-study. J. Card. Fail. 2008;14:9–18. doi: 10.1016/j.cardfail.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Gottlieb SS, Kop WJ, Ellis SJ, Binkley P, Howlett J, O'Connor C, Blumenthal JA, Fletcher G, Swank AM, Cooper L. Relation of depression to severity of illness in heart failure (from heart failure and a controlled trial investigating outcomes of exercise training [hf-action]) Am. J. Cardiol. 2009;103:1285–1289. doi: 10.1016/j.amjcard.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maurer MS, Cuddihy P, Weisenberg J, Delisle S, Strong BM, Gao Q, Kachnowski S, Howell J. The prevalence and impact of anergia (lack of energy) in subjects with heart failure and its associations with actigraphy. J. Card. Fail. 2009;15:145–151. doi: 10.1016/j.cardfail.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]