Abstract
Background
Allergic reactions to walnut can be life threatening. While IgE epitopes of walnut have been studied, CD4+ T-cell specific epitopes for walnut remain uncharacterized. Particularly, the relationship of both phenotype and frequency of walnut specific T-cells to the disease have not been examined.
Objectives
We sought to provide a thorough phenotypic analysis for walnut reactive T-cells in allergic and non-allergic subjects. Particularly, the relationship of phenotypes and frequencies of walnut specific T-cells with the disease.
Methods
CD154 up-regulation assay was used to examine CD4+ T-cell reactivity towards walnut allergens.Jug r 1, Jug r 2 and Jug r 3. Tetramer-Guided epitope mapping approach was utilized to identify HLA-restricted CD4+ T-cells epitopes in Jug r 2. Direct ex vivo staining with peptide-major histocompatibility complex class II (pMHC-II) tetramers enabled the comparison of frequency and phenotype of Jug r 2-specific CD4+ T-cells between allergic and non-allergic subjects. Jug r 2-specific T-cell-clones were also generated and mRNA transcription factor levels were assessed by RT qPCR. Intracellular cytokine staining (ICS) assays were performed for further phenotypical analyses.
Results
Jug r 2 was identified as the major allergen that elicited CD4+ T-cell responses. Multiple Jug r 2 T-cell epitopes were identified. The majority of these T-cells in allergic subjects have a CCR4+ TCM (central memory) phenotype. A subset of these T-cells express CCR4+CCR6+ irrespectively of the asthmatic status of the allergic subjects. ICS confirmed these TH2, TH2/TH17 and TH17-like heterogenic profiles. Jug r 2-specific T-cell-clones from allergic subjects mainly expressed GATA3; nonetheless, a portion of T-cell clones expressed either GATA3 and RORC, or RORC, confirming the presence of TH2, TH2/TH17 and TH17 cells.
Conclusions
Jug r 2 specific responses dominate walnut T-cell responses in subjects with walnut allergy. Jug r 2 central memory CD4+ cells and terminal effector T-cells were detected in peripheral blood with the central memory phenotype as the most prevalent phenotype. In addition to conventional TH2-cells, TH2/TH17 and TH17 cells were also detected in non-asthmatic and asthmatic subjects with walnut allergy. Understanding this T-cell heterogeneity may render better understanding of the disease manifestation.
Keywords: Food allergy, walnut, Jug r 2, T-cells, MHC class II tetramers, epitopes
INTRODUCTION
Allergic reactions to tree nuts, including walnut, cashew and almond are common, affecting approximately 1.1% of children younger than 18 years and 0.5% of adults in the United States(1). Similar to peanut allergy, tree nut allergy generally has an onset in early childhood and persists throughout life. It is estimated that only 9% of patients outgrow tree nut allergy(2;3). In the United States, walnut allergy is the most frequent among tree nut allergic subjects (34%)(4) and both cashew and walnut accounted for the majority of life-threatening anaphylactic reactions due to tree nuts(2). Food avoidance is the only therapeutic option, however, the ubiquity of these foods in the diet makes avoidance difficult and accidental ingestion is a common occurrence(5;6).
The most common consumed walnut species is Juglans regia. Currently 5 allergens have been reported(7). Jug r 1 (2S albumin), Jug r 2 (7S vicilin-like protein) and Jug r 4 (11S legumin-like protein) have been described as important major allergens in the US(8-10). On the other hand, Jug r 3 (Lipid Transfer Protein) has been proposed as a major allergen in the Mediterranean area(11). Jug r 5 is a profilin and its role as a walnut allergen is limited(7).
While IgE epitopes of walnut allergens have been studied(12-14), CD4+ T-cell specific epitopes for walnut allergens remain uncharacterized. It is now established that allergen-specific T-cells play an important role in allergic inflammation(15;16). In this study, we examined T-cell reactivity towards Jug r 1 and Jug r 2, as their corresponding allergens in peanut, 2S albumin (Ara h 2) and 7S vicilin-like seed storage protein (Ara h 1) respectively, are highly immunogenic in peanut allergic subjects(17;18). Jug r 3 was also studied since we have a small cohort of samples from Spain, where LTP is the major plant food allergen(11). We initially investigated Jug r 1 Jug r 2 and Jug r 3-specific T-cell responses using CD154 activation assay(19). Jug r 2, but neither Jug r 1 nor Jug r 3, elicited dominant T-cell responses in allergic subjects. Several Jug r 2 derived epitopes were then identified by using tetramer-guided epitope mapping (TGEM)(20). The magnitude and phenotype of the response of Jug r 2-specific CD4+ T-cells in allergic and non-allergic subjects were determined directly ex-vivo. Results show that allergic subjects have a predominant TH2 phenotype, however, TH17 responses in some individuals were also observed. T-cells with CCR4+CD27+, CCR4+CD27−, CCR4+CCR6+ and CCR4+CCR6− surface phenotypes were detected in allergic subjects. T-cells from non-allergic subjects have a TH1 and TH1/TR1 phenotypes characterized by surface expression of CXCR3. Understanding this T-cell heterogeneity may improve our understanding of disease manifestation.
RESULTS
Jug r 2-reactive CD4+ T-cells dominate the immune response in walnut allergy
Up-regulation of CD154 in CD4+ T-cells after 3 h stimulation of PBMC with Jug r 1, Jug r 2 and Jug r 3 peptide libraries was used to evaluate frequencies of walnut allergen reactive CD4+ T-cells in allergic and non-allergic subjects. For non-allergic subjects, magnitudes of Jug r 1, Jug r 2 and Jug r 3 T-cell responses were low (average frequencies of 1.3 ± 0.6, 4.8 ± 1.7 and <1 per million CD4+ T-cells, respectively) (Figure 1A and Figure E1). In the allergic group, stimulation with Jug r 1 peptide libraries induced CD154+CD4+ T-cells (average frequency of 10.2 ± 3.4 per million CD4+ T-cells). Jug r 3 responses were nearly absent (average frequency of 0.8 ± 1.8 per million), even amongst the Spanish cohort. In contrast, Jug r 2 responses were strong (average 20.4± 3.6 per million CD4+ T-cells). The average frequency of Jug r 2 responses was 2 fold greater than Jug r 1 T-cell immune responses in allergic subjects and 4 fold greater than Jug r 2 T-cell immune responses in non-allergic subjects. Thus, Jug r 2-reactive CD4+ T-cells dominate the walnut allergen specific T-cell repertoire in subjects with walnut allergy. A correlation between Jug r 1, Jug r 2 and Jug r 3-specific IgE with their respective allergen-reactive T-cell frequencies was not observed (data not shown). Lack of correlation might be due to high cross-reactivity at the IgE level between tree nuts or unlinked cognate T-B cell cooperation, which the latter has been observed in other allergens(24).
Figure 1.
Frequencies of walnut allergen reactive CD4+ T-cells. A, Frequencies of Jug r 1-, Jug r 2- and Jug r 3-reactive T-cells in subjects with walnut allergy (n=11; filled squares) and non-allergic subjects (n=8; opened circles) with CD154 assays. Each data point represents the frequency of T-cells reactive to each allergen. An ANOVA test (with Bonferroni correction) was used to compare all columns in the statistical analysis. B, Frequencies of Jug r 2 epitope-reactive T-cells in subjects with walnut allergy (n=17; filled squares) and subjects without walnut allergy (n=19; opened circles) with tetramer assays. Each data point represents the frequency of T-cells specific to a combination of epitopes in Jug r 2. A Student t test was used in the statistical analysis. *P<0.05, **P<0.001, ***P<0.0001. NS. Not significant.
Identification of CD4+ T-cell epitopes in walnut allergen Jug r 2
The TGEM approach was used to identify CD4+ T-cell epitopes within the dominant walnut allergen Jug r 2 (Figure E2). A total of 11 immunogenic epitopes restricted to DRB1*01:01, DRB1*01:03, DRB1*03:01, DRB1*04:01, DRB1*04:02, DRB1*04:04, DRB1*07:01, DRB1*09:01, DRB1*11:01, DRB1*14:01 and DRB1*15:01 were identified (Table 2). Peptides Jug r 2152-171, Jug r 2184-203, Jug r 2224-243, Jug r 2392-411, Jug r 2456-475 and Jug r 2520-539 were presented by 3 or more different DRB1 alleles. Identical epitopes were identified in allergic and non-allergic subjects (data not shown).
TABLE 2.
Jug r 2 CD4+ T-cell epitopes
|
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA DRB1 Restriction | |||||||||||||
|
| |||||||||||||
| Jug r 2 | Amino acid sequence | 01:01 | 01:02 | 01:03 | 03:01 | 04:01 | 04:02 | 04:04 | 07:01 | 09:01 | 11:01 | 14:01 | 15:01 |
| Jug r 2 152-171 | EQQRHNPYYFHSQSIRSRH | • | • | • | • | • | |||||||
|
| |||||||||||||
| Jug r 2 184-203 | FTERTELLRGIENYRVVILD | • | • | • | • | • | |||||||
|
| |||||||||||||
| Jug r 2 224-243 | TRGRATLTLVSQETRESFNL | • | • | • | |||||||||
|
| |||||||||||||
| Jug r 2 280-299 | PGQFREYYAAGAKSPDQSYL | • | • | ||||||||||
|
| |||||||||||||
| Jug r 2 296-315 | QSYLRVFSNDILVAALNTPR | • | |||||||||||
|
| |||||||||||||
| Jug r 2 360-379 | SGGPISLKSESPSYSNQFGQ | • | |||||||||||
|
| |||||||||||||
| Jug r 2 392-411 | QEMDVLVNYAEIKRGAMMVP | • | • | • | • | ||||||||
|
| |||||||||||||
| Jug r 2 416-435 | KATVVVYVVEGTGRYEMACP | • | |||||||||||
|
| |||||||||||||
| Jug r 2 456-475 | TGRFQKVTARLARGDIFVIP | • | • | • | • | • | • | ||||||
|
| |||||||||||||
| Jug r 2 520-539 | EAKELSFNMPREEIEEIFES | • | • | • | |||||||||
|
| |||||||||||||
| Jug r 2 536-555 | IFESQMESYFVPTERQSRRG | • | |||||||||||
High frequencies of Jug r 2-reactive CD4+ T-cells in peripheral blood of allergic subjects
Frequency of Jug r 2-specific CD4+ T-cells was also examined by direct ex vivo staining with Jug r 2-tetramers (Figure 1B and Figure E3). Each subject was stained with a panel of tetramers corresponding to the HLA of the subject (Table E1). In non-allergic subjects, the frequency of Jug r 2-specific CD4+ T-cell responses was low with an average frequency of 6.3 ± 0.8 per 106 CD4+ T-cells. Within the memory compartment (CD45RA−), the average frequency was 2.9 ± 0.6 per 106 CD4+ T-cells. Conversely, the average frequency of Jug r 2- specific CD4+ T-cell in allergic subjects was 26.53 ± 2.26 per 106, which was at least 4-fold higher compared to non-allergic subjects. The average frequency within the CD45RA− compartment was 18.34 ± 1.72 reactive CD4+ T-cells per 106. This tetramer staining frequency data agree with the results from the CD154 assays and confirm that Jug r 2-reactive CD4+ T-cells are present in higher frequencies in PBMC of allergic compared to non-allergic subjects.
Surface phenotype of Jug r 2 specific CD4+ T-cells
The surface phenotypes of Jug r 2-specific T-cells were determined by direct ex vivo staining of PBMC (Figure 2A). A higher percentage of the tetramer positive cells in non-allergic group expressed CXCR3 (TH1 marker) compared to the allergic group (Figure 2B). However, because of the higher frequency of total Jug r 2-specific T-cells in the allergic group compared to the non-allergic group, the average frequency of TH1allergen specific T-cells in both groups was similar (Figure 2C). Conversely, a higher percentage of tetramer positive cells in the allergic group expressed CCR4 and CRTH2 (TH2 markers)(25;26) compared to the non-allergic group (Figure 2B). Significant difference in percentage of Jug r 2-specific T-cells that lost CD27 expression was also observed between the two groups, with CD27− Jug r 2-specific T-cells being present only in the allergic group. Thus in the allergic group, there were higher frequencies of CCR4+, CRTH2+ and CD27− Jug r 2-specific effector T-cells (Teff) compared to the non-allergic group (Figure 2C). Though CD27− Jug r 2-specific Teff were present, there were still higher percentages of CD27+ Jug r 2-reactive T-cells compared to CD27− Jug r 2-specific cells in the allergic group. The majority of these tetramer positive CD27+ T-cells also co-expressed CCR7 and CD62L, suggesting these CCR4+CD27+CCR7+ cells are central memory T-cells (TCM)(27-29) (Figure 2D and data not shown). It should also be noted that most Jug r 2-reactive T-cells in allergic subjects were CRTH2−. Though there was no difference in percentage of Jug r 2-specific T-cells that were CCR6+ (TH17 subset marker and gut homing marker)(30-32) between the 2 groups, the percentage of CCR4+CCR6+ (TH17 subset marker) Jug r 2-specific cells between the two groups was different (Figure 2E). On average, 32.6 % of the Jug r 2-specific T-cells from the allergic subjects had a TH17 phenotype and were essentially absent in non-allergic subjects. Also of interest, TH17 Jug r 2-reactive T-cells were detected in both non-asthmatic and asthmatic subjects with walnut allergy (Figure 2F), suggesting there is no link between asthmatic status and the appearance of this cell type. In contrast, 20.4 % of Jug r 2-reactive T-cells expressed CXCR3+CCR6+ (TH1/TH17 subset markers) in non-allergic subjects which was significantly higher compared to allergic subjects (Figure 2E). No difference was observed in total T-cell frequencies (27.5± 2.5 vs. 19.91 ± 3.4 per 106) and T-cell phenotypes (data not shown) between subjects with or without history of walnut ingestion. However, significant difference was observed in memory T-cell frequencies (19.46 ± 1.96 vs. 11.98 ± 1.67 per 106) among these two groups.
Figure 2.
Phenotypes of Jug r 2 reactive T-cells. A, First row, profile in a DRB1*15:01 non-allergic subject. Second row, profile in a DRB1*15:01 allergic subject. The percentages of surface markers expressed by Jug r 2-specific T-cells are as indicated. B, Ex vivo expression of CCR4, CRTH2, CCR6, CXCR3 and CD27 of Jug r 2-specific T-cells in subjects with walnut allergy (n=17; filled square) and subjects without walnut allergy (n=19; opened circle). Each data point represents the percentage of tetramer positive T-cells with marker expression. C, Ex vivo frequencies of CCR4, CRTH2, CCR6, CXCR3 and CD27 expressing Jug r 2-specific T-cells per million CD4+ T-cells in subjects with walnut allergy (n=17; filled square) and subjects without walnut allergy (n=19; opened circle). Each data point represents the frequency of T-cells specific to a combination of epitopes in Jug r 2. D, Tetramer positive CD45RA− T-cells were gated against CCR7 and CD27. Data are representative of 8 allergic subjects. E, Tetramer positive CD45RA− T-cells were gated against CCR4 and CCR6; CXCR3 and CCR6. Each data point represents results for surface expression in tetramer positive T-cells from 17 subjects with walnut allergy (filled square) and 19 subjects without walnut allergy (opened circle). F, Surface expression of CCR4 and CCR6 was analyzed on tetramer positive T-cells from non-asthmatic and asthmatic walnut allergic subjects. A Student t test was used in the statistical analysis for Figure 1B, C, E and F. An ANOVA test (with Bonferroni correction) was used to compare all columns in the statistical analysis for Figure 1D. *P<0.05, **P<0.001, ***P<0.0001. NS. Not significant.
Cytokine profiles of Jug r 2-specific CD4+ T-cells in non-allergic and allergic subjects
Jug r 2-specific T-cells were single cell sorted for generation of TCC. In non-allergic subjects, a total of 12 TCCs were generated. All TCCs from non-allergic subjects elicited a distinct protective TH1/TR1 (IFN-γ and IL-10) or TH1 (IFN-γ) response (Figure 3A and Figure E4 and E5). For subjects sensitized or allergic to walnut, a total of 59 TCCs were generated. All these clones were CCR4+CD27−. Three heterogenic profiles (TH2, TH2/TH17 and TH17-like) were observed (Figure 3B and Figure E4 and E5). The first profile is exemplified by production of TH2 cytokines (IL-4, IL-5 and IL-13). This TH2 profile was detected for all epitopes tested. TCCs with the second profile show the ability to produce both IL-4 and IL17A (TH2/TH17) and was detected for three specificities only, Jug r 2280-299, Jug r 2360-374 and Jug r 2536-555. The third profile is exemplified by the production of IL-17A only (TH17). In total 23.7% of the T-cell clones obtained from allergic subjects were capable of producing IL-17A. Transcript levels of 3 transcription factors of these clones were also assessed by RT quantitative PCR (Figure 3C). In accordance with cytokine production profile, TH1 clones derived from non-allergic subjects expressed the highest levels of T-bet (Tbx21). In allergic subjects, TH2 clones mainly expressed GATA-3. As expected, TH2/TH17 TCCs expressed GATA-3 and RORC, indicating their ability to produce IL-4 and IL17A. Finally, TH17 TCCs mainly expressed RORC.
Figure 3.
Jug r 2 T-cell subsets. A and B, Phenotype of T-cell clones derived from 6 non-allergic (Figure 3, A) and 8 allergic (Figure 3, B) subjects. Phenotype profiles based on surface marker expression and cytokine production in TH2= 45 TH2/TH17=6, TH17=8 TH1*=12 T-cell clones. *Derived from non-allergic subjects. The numbers of T-cell clones for each profile and specificity are as indicated. Percentages of clones for each specificity are presented as mean values from each group in pie charts. C, mRNA levels corresponding to GATA-3, TBX21, and RORC were assessed by quantitative PCR. Data were expressed as relative amounts of cytokine mRNA in Jug r 2 epitope-specific T-cell clones derived from 6 non-allergic and 8 allergic subjects. Data were normalized based on relative amounts of GTF2B mRNA.
The TH1 responses in non-allergic subjects and TH2 and TH17 responses in allergic subjects were also confirmed by direct ex vivo staining. For these experiments, PBMC were co-stained for cytokine expression and CD154 after 6 hours of peptide stimulation. Non-allergic Jug r 2-reactive CD4+ T-cell responses were dominated by production of IFN-ϒ; production of both IL-4 and IL-17A were absent in these individuals. On the other hand, TH2 and TH17 responses were detected in allergic subjects, confirming our in vitro observations with TCCs. Interestingly, we also observed CD154+ Jug r 2 T-cells that were incapable of producing cytokines (average 80.3% ± 3.84 and 52.5% ± 2.15 in non-allergic and allergic subjects, respectively). Examples of these ex vivo experiments are shown in Figure 4A. Amongst the TH2 producing cells, cells can also be classified as IL-4 producers, IL-4 and IL-13 producers and IL-4, IL-5 and IL-13 producers. We did not detect cells that produced IL-13 alone. Gating strategy for identifying double and triple producers is shown in Figure 4B. Results of experiments from 8 non-allergic subjects and 11 allergic subjects are summarized in Figure 4C and Figure 4D. Both TCM and Teff cells are capable of producing TH2 cytokines (Figure 5A). Interestingly, Teff cells are more capable of producing IL-4 and IL-5 compared to TCM cells (Figure 5B). These data are in agreement with earlier studies which suggest that loss of CD27 correlates with an increase of IL-4 production and Teff cells are more terminally differentiated cells(27;33;34). In contrast to the TCC data, we failed to observe a TH1/TR1 profile in non-allergic subjects, or a TH2/TH17 profile in allergic subjects. Different kinetics of cytokine production for these cell types, and the rarity of these cells, may account for these different outcomes by these two approaches.
Figure 4.
Cytokine profiles of Jug r 2-reactiveT-cells. A, First row, Cytokine profile in a DRB1*15:01 non-allergic subject. Second row, Cytokine profile in a DR15:01 allergic subject. The frequencies of Jug r 2-specific cytokine producing T-cells per million CD4+ T-cells are as indicated. B. Gating strategy for identifying IL-4, IL-4+IL-13 and IL-4+IL-13+1L-5 Jug r-2 reactive T-cells, in this subject IL-4= 40.4%, IL-4+IL-13=36.5%, IL4+IL13+IL-5=21.2%. C and D, Cytokine profiles of Jug r 2-reactive T-cells in non-allergic and allergic subjects. Data are representative of 11 subjects with walnut allergy and 8 non-allergic subjects and are presented as the mean frequency of cytokine producing T-cells from each group in pie charts.
Figure 5.
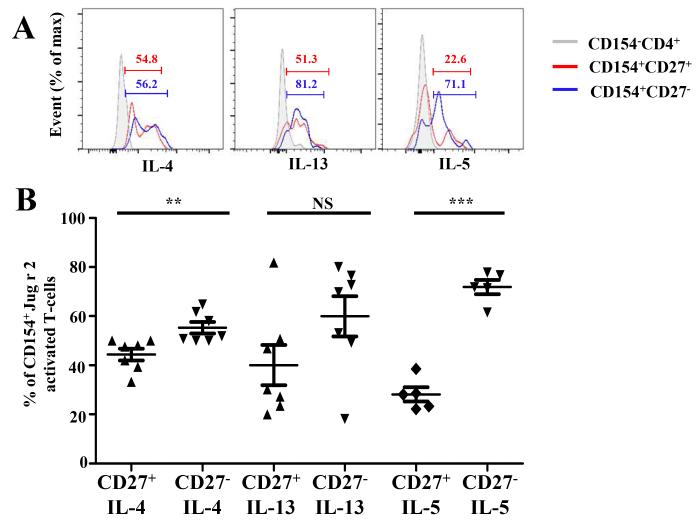
Ex vivo cytokine producing capacities of CD27+ and CD27− Jug r 2-reactive T-cells. A,IL-4, IL-13 and IL-5 expression by CD27+ (red histogram), CD27− (blue histogram) and CD154−CD4+ as control (grey histogram). B, Cytokine production by CD27+ and CD27− CD154+Jug r 2-reactive T-cells in allergic subjects. Data are representative of 7 allergic subjects. A Student t test was used in the statistical analysis. *P<0.05, **P<0.001, ***P<0.0001. NS. Not significant.
DISCUSSION
Allergic reactions to walnut account for the vast majority of severe reactions in tree-nut allergic subjects in the US(1). Contributions of CD4+ T-cells to this disease remain elusive. In particular, walnut specific CD4+ T-cell epitopes have not been identified. The frequency and the phenotype of walnut allergen specific T-cells have not been examined ex vivo. In this study, both class II tetramer assay and CD154 up-regulation assay were used to examine walnut allergen specific T-cells. Jug r 2 was identified as the major walnut allergen that elicits CD4+ T-cell responses. Hot spots with promiscuous Jug r 2 CD4+ T-cell peptides presented by multiple DRB1 alleles were identified. Six of the Jug r 2 peptides (20 aa each) can be presented by at least 3 different DRB1 alleles, including 3 peptides that can be presented by at least 5 different DRB1 alleles. These 6 promiscuous epitope regions should be good candidates for peptide vaccine to desensitize subjects with walnut allergy.
Consistent with previous studies (18;33-36), allergen-tolerant subjects and allergic subjects recognize identical allergen-derived epitopes. However, Jug r 2-reactive T-cells are present at substantially lower frequencies in non-allergic subjects (Figure 1). In addition, surface phenotypes and functional properties of these T-cells are distinct in non-allergic and allergic subjects. Through direct ex vivo analysis of PBMC and analysis of TCC, CXCR3+ Jug r 2-specific T-cells with predominant IFN-ϒ and low IL-10 production were observed in non-allergic subjects. On the other hand, CCR4+ T-cells that produced TH2 and TH17 cytokines were exclusively observed in allergic subjects. It remains a possibility that walnut allergen specific TH2 cells are also present in non-allergic subjects at a very low frequency which is below the threshold of the detection method. Results obtained from either the tetramer assay or CD154 up-regulation assays are compatible. Antigen specific T-cells which were cytokine non-producers were also detected with both assays. Different differentiation state and stage of cell cycle should be responsible for the heterogeneous cytokine production capacity(37;38).
We have previously demonstrated that only terminally differentiated (CD27−) allergen specific T-cells from pollen allergic subjects display a TH2 phenotype(33;34) and that lack of CD27 expression coincides with CRTH2 expression(34). In the present study, Jug r 2-specific terminally differentiated TH2 T-cells were present in allergic subjects and were essentially absent in non-allergic subjects. In addition, both CD27+CRTH2−CCR4+ TCM and CD27−CRTH2+CCR4+ Teff cells were present in allergic subjects with TCM as the most prevalent phenotype. We also demonstrated that both TCM and Teff were capable of producing TH2 cytokines. A previous report show CCR4+TCM in humans are capable of producing IL-4 even though they are not fully differentiated(29). Variable expressions of CRTH2 have been previously observed for Ara h 1-(18) and Pen m 2-(39)reactive T-cells, and these cells were capable to produce IL-4. Accumulating evidence proposes that CD27 is lost after repetitive antigenic stimulation (40;41) and loss of CD27 after TCC generation suggest that occasional antigen stimulation is essential for the expression of CRTH2 in food allergen specific T-cells(18). Food avoidance in walnut allergic subjects may have resulted in the accumulation of CRTH2− TCM in peripheral blood of allergic subjects. The results from this study do not contradict to our previous pollen studies(33;34), as subjects with pollen allergy are subjected to annual challenges of high doses of pollens during the pollen season. The presence of allergen specific TCM as consequence of food avoidance in food allergic subjects may complicate the treatment of food allergy, as TCM are less susceptible to deletion by allergen specific immunotherapy, as shown in a murine model(42).
It has been demonstrated that IL-17A can promote class switch to IgE(43) and IL-17A producing CD4+ T-cells are more frequent on allergic subjects(43-45). TH2/TH17 cells are also observed in subjects with allergic asthma(46-48). However, the involvement of TH17 cells in food allergy remains obscure. In the current study a sub-population of CCR4+CCR6+ Jug r 2 T-cells which produced IL-17A alone or IL-4 and IL-17A was detected in both asthmatic and non-asthmatic walnut allergic individuals. These results are consistent with previous studies with food allergens(18;39),where TH2/TH17 allergen-specific T-cells have been previously described. This data implicates a direct association of CCR4+CCR6+ antigen specific CD4+ TH2/TH17 cells with food allergy disregarding the asthmatic status. This is indeed a possibility as CCR6 is also a GALT-associated homing marker(30;31). A murine model also suggests that CCR6 plays a role in the development of gastrointestinal allergic disease(49). On the other hand, Dhuban et al(50) recently suggested that TH17 responses are impaired in food allergic children and that lack of TH17 responses may play a potential role in food tolerance. The presence of this population in allergic subjects raises important questions of the pathophysiological role of these CCR4+CCR6+ food allergen specific CD4+ T-cells in food allergy in general. Future effort should commit to examine whether CCR4+CCR6+ allergen specific cells are more prevalent in food allergy compared to airborne allergy.
METHODS ONLINE
Ex-vivo analysis of walnut-specific CD4+ T-cells
The frequency of Jug r 2-specific T-cells was measured as previously described (E1). Briefly, 30 million PBMC in 200 μL T-cell culture medium were stained with 20μg/mL PE-labeled tetramers (tetramers being used are shown in Table E1) for 100 minutes. Cells were then washed and incubated with anti-PE magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 20 minutes at 4°C and a 1/100 fraction was saved for analysis; the other fraction was passed through a magnetic column (Miltenyi Biotec, Bergisch Gladbach, Germany). Bound PE-labeled cells were flushed and collected. Cells in the bound and precolumn fractions were stained with a panel of antibodies of interest for 20 minutes at room temperature. After staining, cells were stained with Via-probe+ (BD Biosciences, San Jose, CA) for 10 minutes at 4°C before flow-cytometry. Data were analyzed utilizing FlowJo (Tree Star, Ashland, Ore) gating on forward scatter/side scatter and excluding CD14+, CD19+ and Viaprobe populations. Frequency was calculated as previously described (E1). For phenotyping studies, antibodies were used against markers of interest; CCR4 (R&D systems, Minneapolis, MN), CD45RA (eBioscience, San Diego, CA) and CD38 (eBioscience, San Diego, CA).
Intracellular cytokine staining
For ex-vivo intracellular cytokine staining (ICS) combined with CD154 activation assay, BD GolgiStop™ was added during stimulation (BD biosciences, East Rutherford, NJ) according to the manufacturer’s instructions. In vitro ICS combined with tetramer staining was performed as previously described(E2). Briefly, T-cell lines or TCC were restimulated with 50 ng/mL phorbol 12-myristate-13-acetate and 1 mg/mL ionomycin in the presence of 1× brefeldin-A (eBiosciences, San Diego, CA) for 5 hours at 37°C, 5% CO2. After 10 minutes at room temperature, cells were then fixed with fixation buffer (eBioscience, San Diego, CA) and washed twice with a permeabilization buffer (eBioscience, San Diego, CA). Cells were then stained with a panel of antibodies directed against cytokines (eBioscience, San Diego, CA and BD biosciences, East Rutherford, NJ) of interest for 20 minutes at room temperature; cells were washed and immediately analyzed in LSR-II flow cytometer.
T-cell cloning procedure
T-cell lines were generated by staining T-cells with tetramer directly ex-vivo and tetramer-positive CD4+ and CD45RA− cells were sorted by using a FACSAria (at single-cell purity). Cells were and expanded in a 96-well plate in the presence of 1.0 × 105 irradiated PBMC and 2 μg/ml of PHA (Remel, Lenexa, KS). T-cells were re-screened with tetramers loaded with antigenic epitopes to assess positivity for the corresponding specificity. Five profiles (TH2, TH2/TH17, TH17-like, TH1 and TR1) of TCCs were arbitrarily defined as follows (Figure 3 and Figure E4 and E5):TH2 profile is exemplified by CCR4+ with or without CRTH2 expression, production of IL-4 (≥10%), IL-5(≥10%), and IL-13 (≥10%), and expression of GATA3; TH2/TH17 profile is characterized by co-expression CCR4 and CCR6 and show the ability to produce both IL-4 (≥10%), IL-and IL17A (≥10%) but no IFN-ϒ and IL-5 and express both GATA3 and RORϒ. The TH17 profile is exemplified by co-expression of CCR4 and CCR6, production of IL-17A (≥10%) and sometimes low IFN-ϒ (=10%) and expression of RORϒ; the TH1 profile is exemplified by CXCR3 expression (some clones also co-expressed CD27), production of IFN-ϒ (≥10%) and expression of T-bet (TBX21); and the TR1 profile is exemplified by CXCR3 expression (some clones also co-expressed CD27), production of IFN-ϒ (≥10%) and IL-10 (≥10%) and expression of T-bet (TBX21).
RNA isolation, cDNA synthesis, and real-time quantitative RT-PCR
Total RNA was extracted from Jug r 2-specific T-cell-clones (TCC) derived from non-allergic and allergic subjects with Gene elute™ Mammalian total RNA miniprep kit (Sigma-aldrich,St. Louis, MO) and reverse transcribed as cDNAs using the TaqMan Reverse Transcription Reagent kit (Applied Biosystems, Foster city, CA) according to the manufacturer’s instructions. Messenger RNAs were assessed by quantitative PCR using pre-designed Taqman Gene Expression reagents (Applied Biosystems, Foster city, CA). Data were expressed as relative amounts of cytokine mRNA and were normalized based on relative amounts of GTF2B mRNA.
Supplementary Material
Key messages.
Jug r 2 is the dominant walnut allergen recognized by T-cells.
The predominant phenotype for Jug r 2 reactive T-cells is central memory phenotype.
Walnut-specific T-cells with TH2, TH17 and TH2/TH17 phenotypes could be detected in non-asthmatic and asthmatic walnut allergic subjects.
Table 1.
HLA and allergic status of recruited subjects
| ID | Age | Sex | HLA (DRB1*) |
sIgE walnut (f256) kU/L |
Skin Prick Test |
Jug r 1 ISAC ISU |
Jug r 2 ISAC ISU |
Jug r 3 ISAC ISU |
Symptoms to Walnut |
Asthma |
|---|---|---|---|---|---|---|---|---|---|---|
| Walnut allergies | ||||||||||
| 1 | 26 | F | 07:01 , 14:01 | 38.6 | Not tested | 12.46 | 6.82 | 0 | I, III, V, VII , VIII | yes |
| 2 | 9 | F | 07:01 , 15:01 | 26.1 | Not tested | 3.11 | 1.42 | 0 | I, II, V | no |
| 3 | 10 | M | 15:01 , 09:01 | 81.7 | 6 × 4 mm | 48.6 | 1.58 | 0 | II, III, V | no |
| 4 | 10 | M | 15:01 , 14:02 | 23.4 | 6 × 10 mm | 43.9 | 1.64 | 0.28 | II, IV, VI, VII | yes |
| 5 | 10 | M | 04:01 , 15:01 | 37 | 9 × 9 mm | 11.26 | 1.88 | 0 | I, III | yes |
| 6 | 34 | F | 04:04 , 11:01 | 2.29 | Not tested | 0.26 | 3.81 | 0 | I, II, III, VIII | no |
| 7 | 23 | F | 01:01 , 15:01 | 8.86 | Not tested | 3.69 | 0 | 0.06 | I, II, III, IV, VI | no |
| 8 | 10 | F | 01:01 , 08:01 | 97.3 | 15 × 9 mm | 81.5 | 1.46 | 0 | III, IV, V | no |
| 9 | 8 | F | 13:01 , 15:01 | 1.51 | 7 × 7 mm | 4.77 | 0 | 0 | I, II, III | no |
| 10 | 34 | F | 07:01 , 15:01 | 77.7 | 15×10 mm | 43.9 | 1.9 | 0.02 | I, II, III, IV, V | no |
| 11 | 27 | F | 15:01 , 16:01 | 1.91 | Not tested | 0 | 0 | 1.18 | II, III, IV, V, VII | no |
| 12 | 14 | F | 04:05 , 15:01 | 4.97 | Not tested | 0 | 4,36 | 7.3 | II, IV, V | yes |
| Walnut sensitized subjects | ||||||||||
| 13 | 36 | F | 01:01 , 04:04 | 0.39 | 12×12 mm | 0.34 | 0 | 0 | no | |
| 14 | 18 | F | 01:01, 13:01 | 0.58 | 5 × 5 mm | 0 | 0 | 0.30 | yes | |
| 15 | 15 | F | 11:01 , 13:01 | >100 | Not tested | — | — | — | yes | |
| 16 | 26 | M | 15:01 , 03:01 | 22.5 | Not tested | 0 | 0 | 5.5 | no | |
| 17 | 24 | M | 04:03 , 07:01 | 1.07 | Not tested | 0 | 0 | 0.95 | no | |
| Nonatopic subjects | ||||||||||
| 18 | 28 | M | 04:01 , 15:01 | 0 | Not tested | — | — | — | Absent | no |
| 19 | 29 | M | 01:01 , 03:01 | 0 | Not tested | — | — | — | Absent | no |
| 20 | 31 | F | 07:01 , 07:01 | 0 | Not tested | — | — | — | Absent | no |
| 21 | 37 | M | 03:01 , 0401 | 0 | Not tested | — | — | — | Absent | no |
| 22 | 26 | M | 04:03 , 15:01 | 0 | Not tested | — | — | — | Absent | no |
| 23 | 34 | M | 07:01 , 13:01 | 0 | Not tested | — | — | — | Absent | no |
| 24 | 32 | F | 15:01 , 15:01 | 0 | Not tested | — | — | — | Absent | no |
| 25 | 31 | F | 07:01 , 08:01 | 0 | Not tested | — | — | — | Absent | no |
| Atopic subjects without walnut allergy | ||||||||||
| 26 * | 26 | F | 04:01 , 15:01 | 0 | Not tested | — | — | — | Absent | yes |
| 27 | 56 | M | 11:01 , 15:01 | 0 | Not tested | — | — | — | Absent | no |
| 28 * | 20 | M | 13:02 , 07:01 | 0 | Not tested | — | — | — | Absent | yes |
| 29 | 41 | F | 09:01 , 15:01 | 0 | Not tested | — | — | — | Absent | yes |
| 30 | 28 | M | 01:01 , 07:01 | 0 | Not tested | — | — | — | Absent | yes |
| 31 | 47 | F | 09:01 , 15:01 | 0 | Not tested | — | — | — | Absent | yes |
| 32 * | 63 | M | 15:01 , 01:01 | 0 | Not tested | — | — | — | Absent | yes |
| 33 * | 56 | F | 07:01 , 03:01 | 0 | Not tested | — | — | — | Absent | yes |
| 34 | 53 | M | 11:01 , 15:01 | 0 | Not tested | — | — | — | Absent | no |
| 35 | 48 | F | 11:01 , 15:01 | 0 | Not tested | — | — | — | Absent | no |
I Itchy mouth, lips and / or pharynx
II Abdominal discomfort and / or diarrhea
III Nausea or vomiting
IV Severe skin itching or hives, acute or angioedema
V Rhinitis and / or conjunctivitis and / or respiratory compromise
VI Dizziness (feeling loss of consciousness)
VII Syncope (loss of consciousness)
VIII Desaturation with respiratory compromise
Subjects also had history of peanut and positive IgE ImmunoCAP for peanut
ACKNOWLEDGEMENTS
We thank Lisa Myers for help with subject recruitment. We also thank members of our laboratory, especially Amedee Renand and I-Ting Chow for discussions and suggestions.
Declaration of all sources of funding: NIH contract HHSN272200700046C
List of nonstandard abbreviations used
- HLA
Human histocompatibility leukocyte antigen
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cell
- Jug r
Juglans regia
- PE
Phycoerythrin
- pMHCII
Peptide/MHC class II
- TH
T helper
- CRTH2
Chemoattractant receptor-homologous molecule expressed on TH2 cells
- TGEM
Tetramer-guided epitope mapping
- ICS
Intracellular cytokine staining
- TCL
T-cell line
- TCC
T-cell clone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference List
- (1).Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 Jun;125(6):1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- (2).Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005 Nov;116(5):1087–93. doi: 10.1016/j.jaci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- (3).Fleischer DM. The natural history of peanut and tree nut allergy. Curr Allergy Asthma Rep. 2007 Jun;7(3):175–81. doi: 10.1007/s11882-007-0018-y. [DOI] [PubMed] [Google Scholar]
- (4).Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001 Jul;108(1):128–32. doi: 10.1067/mai.2001.115755. [DOI] [PubMed] [Google Scholar]
- (5).Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014 Feb;133(2):291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- (6).Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007 Aug;62(8):857–71. doi: 10.1111/j.1398-9995.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- (7).Costa J, Carrapatoso I, Oliveira MB, Mafra I. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2014 Mar;44(3):319–41. doi: 10.1111/cea.12267. [DOI] [PubMed] [Google Scholar]
- (8).Teuber SS, Dandekar AM, Peterson WR, Sellers CL. Cloning and sequencing of a gene encoding a 2S albumin seed storage protein precursor from English walnut (Juglans regia), a major food allergen. J Allergy Clin Immunol. 1998 Jun;101(6 Pt 1):807–14. doi: 10.1016/S0091-6749(98)70308-2. [DOI] [PubMed] [Google Scholar]
- (9).Teuber SS, Jarvis KC, Dandekar AM, Peterson WR, Ansari AA. Identification and cloning of a complementary DNA encoding a vicilin-like proprotein, jug r 2, from english walnut kernel (Juglans regia), a major food allergen. J Allergy Clin Immunol. 1999 Dec;104(6):1311–20. doi: 10.1016/s0091-6749(99)70029-1. [DOI] [PubMed] [Google Scholar]
- (10).Wallowitz M, Peterson WR, Uratsu S, Comstock SS, Dandekar AM, Teuber SS. Jug r 4, a legumin group food allergen from walnut (Juglans regia Cv. Chandler) J Agric Food Chem. 2006 Oct 18;54(21):8369–75. doi: 10.1021/jf061329s. [DOI] [PubMed] [Google Scholar]
- (11).Pastorello EA, Farioli L, Pravettoni V, Robino AM, Scibilia J, Fortunato D, et al. Lipid transfer protein and vicilin are important walnut allergens in patients not allergic to pollen. J Allergy Clin Immunol. 2004 Oct;114(4):908–14. doi: 10.1016/j.jaci.2004.06.020. [DOI] [PubMed] [Google Scholar]
- (12).Rosenfeld L, Shreffler W, Bardina L, Niggemann B, Wahn U, Sampson HA, et al. Walnut allergy in peanut-allergic patients: significance of sequential epitopes of walnut homologous to linear epitopes of Ara h 1, 2 and 3 in relation to clinical reactivity. Int Arch Allergy Immunol. 2012;157(3):238–45. doi: 10.1159/000327841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Barre A, Sordet C, Culerrier R, Rance F, Didier A, Rouge P. Vicilin allergens of peanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol Immunol. 2008 Mar;45(5):1231–40. doi: 10.1016/j.molimm.2007.09.014. [DOI] [PubMed] [Google Scholar]
- (14).Robotham JM, Teuber SS, Sathe SK, Roux KH. Linear IgE epitope mapping of the English walnut (Juglans regia) major food allergen, Jug r 1. J Allergy Clin Immunol. 2002 Jan;109(1):143–9. doi: 10.1067/mai.2002.120558. [DOI] [PubMed] [Google Scholar]
- (15).Woodfolk JA. T-cell responses to allergens. J Allergy Clin Immunol. 2007 Feb;119(2):280–94. doi: 10.1016/j.jaci.2006.11.008. [DOI] [PubMed] [Google Scholar]
- (16).Wambre E, James EA, Kwok WW. Characterization of CD4+ T cell subsets in allergy. Curr Opin Immunol. 2012 Dec;24(6):700–6. doi: 10.1016/j.coi.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Prickett SR, Voskamp AL, Dacumos-Hill A, Symons K, Rolland JM, O’Hehir RE. Ara h 2 peptides containing dominant CD4+ T-cell epitopes: candidates for a peanut allergy therapeutic. J Allergy Clin Immunol. 2011 Mar;127(3):608–15. doi: 10.1016/j.jaci.2010.09.027. [DOI] [PubMed] [Google Scholar]
- (18).Delong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011 May;127(5):1211–8. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bacher P, Schink C, Teutschbein J, Kniemeyer O, Assenmacher M, Brakhage AA, et al. Antigen-reactive T cell enrichment for direct, high-resolution analysis of the human naive and memory Th cell repertoire. J Immunol. 2013 Apr 15;190(8):3967–76. doi: 10.4049/jimmunol.1202221. [DOI] [PubMed] [Google Scholar]
- (20).Novak EJ, Liu AW, Gebe JA, Falk BA, Nepom GT, Koelle DM, et al. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 2001 Jun 1;166(11):6665–70. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- (21).Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010 Jun;125(6):1407–9. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999 Dec;104(12):R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kwok WW, Liu AW, Novak EJ, Gebe JA, Ettinger RA, Nepom GT, et al. HLA-DQ tetramers identify epitope-specific T cells in peripheral blood of herpes simplex virus type 2-infected individuals: direct detection of immunodominant antigen-responsive cells. J Immunol. 2000 Apr 15;164(8):4244–9. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- (24).Oseroff C, Sidney J, Tripple V, Grey H, Wood R, Broide DH, et al. Analysis of T Cell Responses to the Major Allergens from German Cockroach: Epitope Specificity and Relationship to IgE Production. J Immunol. 2012 Jul 15;189(2):679–88. doi: 10.4049/jimmunol.1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999 Feb 1;162(3):1278–86. [PubMed] [Google Scholar]
- (26).Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000 Oct;30(10):2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- (27).Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005 Nov 15;175(10):6489–97. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- (28).Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- (29).Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004 Sep 20;200(6):725–35. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ito T, Carson WF, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011 Mar 10;317(5):613–9. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003 Jun;10(3-4):313–23. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- (32).Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007 Jun;8(6):639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- (33).Wambre E, Delong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012 Feb;129(2):544–51. 551. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wambre E, Delong JH, James EA, Torres-Chinn N, Pfutzner W, Mobs C, et al. Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. J Allergy Clin Immunol. 2014 Mar;133(3):872–9. doi: 10.1016/j.jaci.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Carballido JM, Carballido-Perrig N, Terres G, Heusser CH, Blaser K. Bee venom phospholipase A2-specific T cell clones from human allergic and non-allergic individuals: cytokine patterns change in response to the antigen concentration. Eur J Immunol. 1992 Jun 22;(6):1357–63. doi: 10.1002/eji.1830220605. [DOI] [PubMed] [Google Scholar]
- (36).Ebner C, Schenk S, Najafian N, Siemann U, Steiner R, Fischer GW, et al. Nonallergic individuals recognize the same T cell epitopes of Bet v 1, the major birch pollen allergen, as atopic patients. J Immunol. 1995 Feb 15;154(4):1932–40. [PubMed] [Google Scholar]
- (37).Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9488–93. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Renand A, Newbrough S, Wambre E, Delong JH, Robinson D, Kwok WW. Arginine kinase Pen m 2 as an important shrimp allergen recognized by T2 cells. J Allergy Clin Immunol. 2014 Sep 12; doi: 10.1016/j.jaci.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hintzen RQ, de JR, Lens SM, Brouwer M, Baars P, van Lier RA. Regulation of CD27 expression on subsets of mature T-lymphocytes. J Immunol. 1993 Sep 1;151(5):2426–35. [PubMed] [Google Scholar]
- (41).Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997 Nov 3;186(9):1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Mackenzie KJ, Nowakowska DJ, Leech MD, McFarlane AJ, Wilson C, Fitch PM, et al. Effector and central memory T helper 2 cells respond differently to peptide immunotherapy. Proc Natl Acad Sci U S A. 2014 Feb 25;111(8):E784–E793. doi: 10.1073/pnas.1316178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Milovanovic M, Drozdenko G, Weise C, Babina M, Worm M. Interleukin-17A promotes IgE production in human B cells. J Invest Dermatol. 2010 Nov;130(11):2621–8. doi: 10.1038/jid.2010.175. [DOI] [PubMed] [Google Scholar]
- (44).Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151(4):297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- (45).Ciprandi G, Filaci G, Battaglia F, Fenoglio D. Peripheral Th-17 cells in allergic rhinitis: New evidence. Int Immunopharmacol. 2010 Feb;10(2):226–9. doi: 10.1016/j.intimp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- (46).Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010 Jan;125(1):222–30. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- (47).Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010 Oct 25;207(11):2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive T2/T17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014 Jul 18; doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Blazquez AB, Knight AK, Getachew H, Bromberg JS, Lira SA, Mayer L, et al. A functional role for CCR6 on proallergic T cells in the gastrointestinal tract. Gastroenterology. 2010 Jan;138(1):275–84. doi: 10.1053/j.gastro.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Dhuban KB, d’Hennezel E, Ben-Shoshan M, McCusker C, Clarke A, Fiset P, et al. Altered T helper 17 responses in children with food allergy. Int Arch Allergy Immunol. 2013;162(4):318–22. doi: 10.1159/000354028. [DOI] [PubMed] [Google Scholar]
- (E1).Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010 Jun;125(6):1407–9. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (E2).Archila LD, Delong JH, Wambre E, James EA, Robinson DM, Kwok WW. Grass-specific CD4(+) T-cells exhibit varying degrees of cross-reactivity, implications for allergen-specific immunotherapy. Clin Exp Allergy. 2014 Jul;44(7):986–98. doi: 10.1111/cea.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.