Abstract
PURPOSE OF REVIEW
Patients suffering from end-stage organ failure requiring organ transplantation face donor organ shortage and adverse effect of chronic immunosuppression. Recent progress in the field of organ bioengineering based on decellularized organ scaffolds and patient derived cells holds great promise to address these issues.
RECENT FINDINGS
Perfusion-decellularization is the most consistent method to obtain decellularized whole-organ scaffolds to serve as a platform for organ bioengineering. Important advances have occurred in organ bioengineering using decellularized scaffolds in small animal models. However, the function exhibited by bioengineered organs has been rudimentary. Pluripotent stem cells seem hold promise as the ideal regenerative cells to be used with this approach but the techniques to effectively and reliably manipulate their fate are still to be discovered. Finally, this technology needs to be scaled up to human size to be of clinical relevance.
SUMMARY
The search for alternatives to allogeneic organ transplantation continues. Important milestones have been achieved in organ bioengineering with the use of decellularized scaffolds. However, many challenges remain on the way to producing an autologous, fully functional organ that can be transplanted similar to a donor organ.
Keywords: Bioartificial organs, Tissue scaffolds, Stem cells, Regeneration, Transplantation
INTRODUCTION
According to the WHO, cardiovascular disease and diabetes are the leading cause of mortality in adults between the ages of 30-70 years, resulting in 245 deaths per 100000 population while chronic respiratory conditions come third after cancer at a rate of 52 deaths per 100000 population [1]. Incidence of these conditions is expected to increase, as the prevalence of risk factors (i.e. raised fasting blood glucose, high blood pressure, obesity and smoking) remains at alarming high numbers worldwide [1]. Additionally, life expectancy is growing, and it is anticipated that by the year 2050 at least 16% of the world's population will be at least 65 years old [2].
The common clinical pathway leading to mortality for the majority of patients is the irreversible process of end-stage organ failure. In the United States, more than 5 million adults suffer from heart failure, with approximately 670000 new cases diagnosed annually, resulting in more than 56000 deaths [3]; COPD affects nearly 13 million Americans, resulting in 135000 deaths annually [4]; an estimated 3.5-5.3 million Americans are chronically infected with hepatitis B or C [5]; additionally, ESRD is prevalent in nearly 600000 persons, with 65% of these cases needing hemodialysis and more than 100000 new cases reported annually [6]. End-stage organ failure greatly impacts quality of life and represents a huge burden to society as it impairs the performance of daily activities, affects employment and increases health care expenditure.
The only definitive treatment for patients suffering from end-stage organ failure is organ transplantation. As of 2013, more than 54000 patients are waiting for a kidney, 12000 for a liver, 2200 for a heart, and 1300 for a lung transplant [7]. The situation is worrisome given that donors are scarce: only over 17000 kidney, 6000 liver, 1900 heart and 1800 lung transplants are performed annually in the United States [7]. Additionally, current transplant recipients face life-long challenges with immunosuppression and rejection.
The quest for alternatives to allogeneic organ transplantation is gaining traction in an attempt to overcome donor shortage and problems related to chronic immunosuppression. Particularly, the fields of tissue engineering and regenerative medicine have increased their efforts to find the methods and techniques necessary to develop functional replacement tissues of clinical relevance. So far, success has been achieved regenerating tissue substitutes with rather “simple” architectures such as flat two-dimensional or hollow tubular and non-tubular structures using the extracellular matrix as a biological scaffold [8], some of which have reached clinical applications [9-11]. However, the regeneration of complex functional solid organs such as the heart, lungs, kidney, liver and pancreas remains the main challenge and goal. Recent years have seen progress and innovation in this area, as regeneration of functionally rudimentary solid organs has been achieved in small animal models using decellularized whole-organ scaffolds as a substrate [12-16]. Here, we attempt to summarize recent advances in the use of decellularized scaffolds to bioengineer functional organs.
THE USE OF PERFUSION-DECELLULARIZATION TO OBTAIN WHOLE ORGAN SCAFFOLDS
The process of decellularization entails the isolation of the ECM from any given tissue with minimal loss, damage or disruption, while maximizing the removal of cellular material. This can be achieved by the combined application of physical, chemical and enzymatic methods [17]. Specific methods include agitation in solution, thermal shock (i.e. freeze-thaw cycles), ultrasound, hydrostatic pressure, convective flow and manual disruption [17]. A very promising and consistent technique that can be applied to any cadaveric solid organ is perfusiondecellularization, which we first described in 2008 [12]. On the account of a preserved and relatively healthy vasculature, this method uses perfusion via the intrinsic vascular network as the most efficient way to deliver decellularizing agents, even to thick tissues, as it greatly decreases the diffusion distance of the decellularizing agent while preserving the three-dimensional macro- and microarchitecture (Figure 1). Applying this same concept, other plausible routes for the delivery of decellularizing agents are the airways in the lungs, biliary ducts in the liver and the ureter in the kidney [17]. Nonetheless, there exists a great variability in currently used perfusion-decellularization protocols, as most of them have been developed from anecdotal experience. Different types and combinations of decellularizing agents (e.g. detergents, osmotically active solutions, nucleases) with different exposure times, as well as widely varying perfusion pressure targets have been described, which in most cases make direct comparisons between methods difficult [18]. Even though alternative decellularization methods have been explored, no direct comparison to perfusion-decellularization has been performed. When translating the decellularization process to human scale whole organs, it appears that perfusion-based techniques may provide a more even distribution of decellularizing agents and avoid overexposure of outer layers, while deeper parts of the organ remain cellular. In our experience, we favor the use of perfusiondecellularization at constant low physiological pressures as it decreases the chances of ECM damage due to excessive mechanical forces while maximizing the delivery of decellularizing agents.
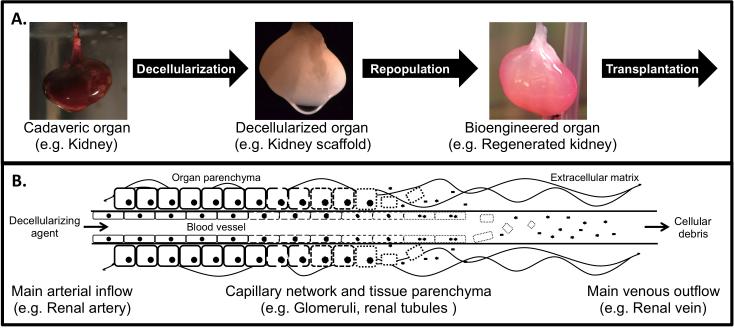
Figure 1.
Perfusion-decellularization is a valid vmethod to obtain whole-organ scaffolds as a platform to bioengineer functional organs. A. Concept of organ bioengineering based on decellularized whole-organ scaffolds. Organs are harvested from donors and undergo the process of decellularization yielding acellular scaffolds composed of extracellular matrix that preserve the macro- and micro-architecture of the original organ. Decellularized scaffolds are then repopulated with regenerative cells (e.g. stem cells) and placed under biomimetic in vitro culture conditions inside a bioreactor. After this period of culture, the bioengineered organ can then be transplanted to supplement or completely replace the function of a failing organ in the recipient. B. Schematic representation of the perfusion-decellularization process. The main artery supplying the organ is cannulated and decellularizing agents (e.g. detergents, enzymes) are delivered through the vasculature under pressure with the help of a pump system. The decellularizing agent reaches all the tissue with the minimal diffusion distance possible thanks to its distribution throughout the organ's capillary network. Decellularizing agents then provoke the lysis, detachment or digestion of cellular elements in the tissues. Cellular debris is then removed from the tissue via the venous system, leaving behind the extracellular matrix scaffold composed of collagen, elastin, laminin, fibronectin, glycosaminoglycans and other elements, that preserves key micro-architectural components such as basement membranes.
THE USE OF DECELLULARIZED SCAFFOLDS IN WHOLE ORGAN BIOENGINEERING
Research in the use of decellularized scaffolds for organ bioengineering has focused on organs such as the heart, lungs, kidney, liver and pancreas. Here, we present a review of some of the advances made over the recent years, emphasizing on the methods of decellularization, scaffold properties, recellularization techniques and functional results.
Advances in heart bioengineering
The first report on the use of perfusion-decellularized ECM scaffolds to bioengineer a whole heart was published in 2008 [12]. The use of antegrade coronary perfusion of SDS, by means of retrograde aortic cannulation, proved to be superior to other agents for the full removal of cellular components in a rat model. DNA content in the decellularized scaffolds was reduced to less than 4%, while GAGs, collagen I and III, laminin and fibronectin remained within the matrix [12] and vascular basal membranes were preserved. Detailed protocols for whole-heart decellularization have been published ever since in an attempt to standardize this process [19]. Recent years have seen the application of similar methods to hearts derived from large animals, especially pig hearts, in an attempt to scale up the decellularization process to obtain scaffolds of clinical relevance to humans [20-22].
A direct comparison of heart decellularization protocols was reported in 2011 using rats [23]. Results showed that protocols differ in the capacity to remove cellular debris and produce heterogeneous scaffolds in terms of architecture and retained ECM components [23]. However, in spite of these differences, there was no significant cytotoxicity or impact on scaffold repopulation between tested protocols.
Mechanical testing of decellularized heart tissue has shown increased stiffness consistent with densification, unchanged mechanical anisotropy and unchanged variation in tissue properties after decellularization [12, 24]. Furthermore, the use of multiphoton microscopy combined with image correlation spectroscopy has been proposed as a noninvasive method to predict the mechanical properties of decellularized heart tissue [25]. Other imaging techniques, such as transmission electron microscopy, cryo-electron tomography and atomic force microscopy are gaining attention in the evaluation of cardiac ECM [26].
In terms of regeneration of whole hearts, in the first publication [12], decellularized heart scaffolds were seeded with freshly isolated neonatal cardiomyocytes through intramural injection and placed in a bioreactor providing pulsatile antegrade heart perfusion and electrical stimulation. The heart constructs showed electric and contractile responses after 8 days in culture. Achieved contractile force was approximately 2% of the adult rat heart. The coronary vessels as well as the ventricular cavities were successfully re-endothelialized with rat aortic endothelial cells. Others [27], have repopulated scaffolds with hESCs and hMECs delivered through coronary perfusion. Cells differentiated from both hESCs and hMECs expressed cardiac markers such as cTnT and Nkx2.5 similarly, while there was differential expression of myosin heavy and light chains. Also, there were CD31+ cells, indicating that some stem cells had differentiated into vascular endothelial cells.
Recently, decellularized mouse hearts were repopulated with human iPSCs-derived multipotent cardiovascular progenitors [28]. Heart constructs exhibited spontaneous contractions at a rate of 40-50 beats per minute after 20 days. Electrocardiography showed irregular wave morphology, indicating the lack of a conduction system. However, the constructs showed intracellular calcium transients and responded to pharmacologic stimulation (i.e. beta-adrenergic agonism, selective blockade delayed rectifying K+ current and increased extracellular calcium concentrations). Mechanically, these constructs exhibited a contraction force of 0.18mN when paced at 1Hz.
The use of bioreactors that provide biomechanical stimulation during culture of repopulated heart constructs is desirable as it aids in cell proliferation, differentiation and electrical coupling [29]. The application of 3D left ventricular stretch with an inflatable latex balloon inserted into decellularized rodent hearts results in improved cellular 3D spatial orientation and alignment [30].
Advances in lung bioengineering
The first reports on lung bioengineering were published in 2010 [13,31], with follow up reports in 2011 and 2013 using decellularized rat lung scaffolds obtained by means of perfusion-decellularization. One group used a CHAPS-based protocol [31] while the other used 0.1% SDS and Triton X-100 [13,14]. These methods did not compromise the architecture of the airways or vasculature. DNA content was decreased to approximately 1-3% [13,14,31], while there was depletion of MHC-I and MHC-II markers [31]. Immunostaining demonstrated preservation of collagen, elastin and laminin in the matrices while a significant proportion of GAGs was lost [13,31]. Multiple other lung decellularization protocols have been published using different combinations of sodium deoxycholate, Triton X-100, NaCl and DNAse [18,32,33]. The delivery of decellularizing agents through the trachea apparently reduces decellularization times [34]. There are only few reports on the direct comparison of different decellularizing agents. For example, SDS-based protocols lead to a greater loss of collagen and decline in mechanical strength and elastic function when compared to a CHAPS-based protocol [35]. The comparison of three protocols (Triton-X100/sodium deoxycholate vs. SDS vs. CHAPS) showed significant differences in the retention of ECM components and intracellular proteins as well as mechanical properties [36]; however, these differences did not seem to have an impact on recellularization. Alternative methods for decellularization, such as freeze-thaw cycles [37] are currently not favored.
Decellularized lung scaffolds show reduced compliance and lower vital capacity [13,31], likely secondary to the lack of surfactant and edema after decellularization. Atomic force microscopy has shown micromechanical differences in decellularized scaffolds that may have consequences in the spatial distribution, differentiation and function of lung cells [38].
In terms of repopulation of lung scaffolds, vascular endothelial cells are delivered through the pulmonary artery or vein, while epithelial cells are delivered through the trachea. To achieve re-endothelialization of the pulmonary vasculature, HUVECs [13,14] and micro-vascular lung endothelial cells [31] have been used. For re-epithelialization of the airways, different cell lines have been used such as human lung cancer cells (e.g. A549) [13], rat fetal lung cells [13,14] and neonatal rat lung epithelial cells [31]. However, when using lung cancer cells as a proof-of-concept experiment, uncontrolled growth obliterated the airways after 6 days. In contrast, rat fetal or neonatal lung cells do not exhibit multilayer, tumor-like growth [13,14,31]. The presence of surfactant proteins A and C [13] and pro-surfactant proteins B and C [31] have been documented after a few days of in vitro culture. Negative pressure ventilation during biomimetic culture of repopulated lung scaffolds seems to be beneficial for the survival and differentiation of the epithelium and for the clearance of secretions [31].
Functionally, regenerated lungs have shown similar gas exchange, compliance and vital capacity when compared to cadaveric lungs [13,14], while others have found decreased compliance [31]. Regenerated rat lungs have been transplanted in an orthotopic position, showing gas exchange capacity [13,14,31], which was better when compared to pneumonectomized animals [13]. However, lung function was impaired secondary to pulmonary edema after 6 hours. On a follow up report [14], compliance and oxygenation in bioartificial lungs declined progressively, being no different than pneumonectomized rats 14 days after transplantation.
Recent years have also seen the application of this technology to large scaffolds relevant to human use. Successful decellularization of rabbit [39], sheep [13,34], porcine [13,40-42], non-human primate [13,43,44] and human [31,40-42,45] lungs has been achieved using similar methods.
Different cell types have been investigated for the repopulation of scaffolds to create functional bioartificial lungs such as are murine ESCs [32], bone marrow-derived stromal cells [33,36,46], mouse C10 lung epithelial cells [36, 46], bone marrow-derived MSCs [43], adipose-derived MSCs [43], human fetal lung cells [40] and primary human alveolar epithelial cells [40]. In general, the ideal candidate cells must be easily isolated from patients, expanded in culture and reseeded into decellularized lung scaffolds showing tissue-specific differentiation [47]; stem cells may be the ideal source. Recently, iPSC-derived type I and II lung epithelial cells were used to repopulate decellularized rat lung scaffolds and human lung slices [48]; functional outcomes of these constructs were not evaluated.
Finally, whether diseased organs not suitable for transplantation can be used in regenerative strategies remains a relevant question. In rodents, lung scaffolds obtained from older animals and those with induced emphysema or fibrosis can negatively impact the growth and differentiation of cells [46], which may limit the potential pool of donors.
Advances in kidney bioengineering
A very important milestone was achieved in 2013 when the first full report on the regeneration of a rat kidney was published [15]. Decellularized kidney scaffolds were obtained by perfusion-decellularization with a 1% SDS-based protocol, showing preservation of the microarchitecture, particularly the glomerular, Bowman's capsule, and tubular basement membranes. The total number of glomeruli, glomerular diameter, Bowman's space and glomerular capillary surface area were not different when compared to cadaveric kidneys using morphometric analysis [15]. DNA content was reduced to less than 10%, while concentrations of ECM components were similar. Others [49], have included enzymatic treatment with DNase during the decellularization process of kidneys.
Decellularized kidney scaffolds have been repopulated with HUVECs and rat neonatal kidney cells via the renal artery and ureter, respectively [15]. Cell seeding improved when applying a negative pressure gradient across the scaffolds, instead of positive pressure to the collecting system [15], achieving 70% of recellularized glomeruli. Mouse ESCs have also been used to repopulate whole-kidney scaffolds [49].
On functional testing, vascular resistance was higher in regenerated kidneys when compared to controls [15]. Decellularized kidneys produced nearly twice the volume of filtrate as cadaveric controls, while regenerated kidneys produced the least amount [15]. Creatinine clearance in regenerated kidneys was approximately 10% of that seen in cadaveric organs, which could be increased to 23% by increasing the arterial perfusion pressure [15]; albumin retention and glucose reabsorption were approximately 47% of that seen in controls, while electrolyte reabsorption was 50%.
Regenerated rat kidneys have been transplanted in an orthotopic position [15], showing production of urine shortly after unclamping of the vasculature. However, they produced a lesser amount of filtrate with higher concentrations of glucose and albumin, and lower concentrations of urea and creatinine when compared to controls [15].
Once again, the technology needs to be scaled up to produce human-sized scaffolds. Kidneys from pigs [15,22,50,51], non-human primates [44,52], and humans [15,53] have been decellularized using similar methods. As with other organs, there is interest in assessing whether human kidneys not suitable for transplantation can be used in regenerative applications [53].
Advances in liver bioengineering
Perfusion-decellularization to obtain decellularized liver scaffolds was first reported in 2010 [16,54]. Rat livers were perfused with SDS and Triton X-100 via the inferior vena cava or the portal vein achieving complete decellularization with preservation of the micro-architecture including the basement membranes and vascular network with retention of collagen type I and IV, fibronectin and laminin. Comparisons of different detergent solutions have been performed in an attempt to identify the best protocol [55]. Efforts to obtain human-sized liver scaffolds have been undertaken using sheep [56] and pigs [57-59].
Liver scaffolds have been repopulated with primary rat hepatocytes [16], human fetal hepatocytes cells [58,59] and human fetal stellate cells [59] via vascular perfusion. Delivered cells were observed to migrate out of the vessels and get distributed throughout the matrix. HUVECs and fetal endothelial cells have been used for re-endothelialization [16,58]. Additionally, hepatic stem cells have been seeded into decellularized liver matrix showing high engraftment rates and markers of hepatocytic and cholangiocytic differentiation [60].
Functionally, regenerated livers have shown urea and albumin synthesis capacity [16,59], however, albumin production was approximately 20% that of adult rats [16]. Gene expression of enzymes involved in drug metabolism was similar to parallel hepatocyte sandwich cultures. Regenerated grafts have been transplanted in a heterotopic position in rats demonstrating adequate perfusion and viability of nearly 80% of seeded hepatocytes after a short period of in vivo perfusion [16].
CONCLUSIONS
The use of decellularized scaffolds to bioengineer functional organs is an emerging field that has the potential to overcome the difficulties encountered in allogeneic organ transplantation: donor shortage and immunosuppression. Even though great advances have been made in small animal models, the field is still far from achieving the regeneration of a solid organ with a function similar to cadaveric organs. The same concepts used to bioengineer the organs mentioned above could be applied to produce other organs or tissues such as the esophagus [61-63], small bowel [63-65], pancreas [66,67], trachea [68], muscle or composite tissue grafts. Research efforts in the coming years should focus on specific goals (Table 1) to standardize the decellularization process, manipulate stem cells as desired, preserve scaffolds and organs, evaluate the immune response and reach the next mile-stone: the transplantation of bioengineered organs in a large animal model.
Table 1.
Future directions for the use of decellularized scaffolds to bioengineer functional organs
| Area | Specific questions | Impact |
|---|---|---|
| Scaffold decellularization methods | • Systematic direct comparisons between methods. • Identification of best method for each organ. • Standardization. |
Production of high-quality decellularized scaffolds in a reproducible way. |
| Stem cells | • Isolation from patients • Expansion in culture to large numbers. • Differentiation into specific phenotypes with high efficiency and purity. |
Repopulation of scaffolds to bioengineer functional organs in a bioreactor system. |
| Tissue preservation | • Long-term preservation of decellularized scaffolds (i.e. weeks to months). • Short-term ex vivo preservation of regenerated organs (i.e. days). |
Creation of a stock of decellularized scaffolds that can be used on demand. Preservation of bioengineered organs before transplantation. |
| Transplantation in large animal model | • Functional evaluation • Evaluation of mid- and long-term fate of scaffolds and regenerated organs. |
Assessment of outcomes of interest at a human-relevant scale. |
| Immunologic response | • Type of immunologic response to decellularized scaffolds and regenerated organs, if any. • Evaluation of the feasibility of using decellularized scaffolds from different species in organ bioengineering (“xenoscaffolds”). |
Determination of the need for any sort of immunosuppression after transplantation of bioengineered organs. Expansion of the tissue source pool to overcome donor shortage. |
KEY POINTS.
New technologies for organ replacement in patients with end-stage organ failure are needed to overcome the difficulties encountered with allogeneic organ transplantation, namely, donor shortage and life-long immunosuppression.
Decellularized whole-organ scaffolds can be obtained from virtually any organ in the body, while preserving the specific microarchitecture of the extracellular matrix. These scaffolds serve as a great platform for organ bioengineering as the preserve the organ's 3D blueprint.
The use of human pluripotent stem cells extracted from the patient needing organ replacement holds great promise to repopulate decellularized scaffolds in order to bioengineer functional organs.
Bioengineered organs have been successfully created in the laboratory and transplanted in small animal models; however, these regenerated organs have showed only rudimentary function.
The creation of a fully functional solid organ remains in the horizon of the tissue engineering and regenerative medicine fields.
ABBREVIATIONS
- CHAPS
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
- COPD
Chronic obstructive pulmonary disease
- DCA
Deoxycholic acid
- DNA
Deoxyribonucleic acid
- ECM
Extracellular matrix
- EDTA
Ethylenediaminetetraacetic acid
- ESRD
End-stage renal disease
- GAGs
Glycosaminoglycans
- hESCs
Human embryonic stem cells
- hMECs
Human mesendodermal cells
- HUVECs
human umbilical cord endothelial cells
- iPSCs
Induced-pluripotent stem cells
- PBS
Phosphate buffered saline
- SDS
Sodium dodecyl sulfate
- WHO
World Health Organization
Footnotes
4. CONFLICT OF INTEREST
HC Ott is founder and stockholder of IVIVA Medical Inc. This relationship did not affect the content or conclusions contained in this review.
REFERENCES AND RECOMMENDED READINGS
• of special interest
•• of outstanding interest
- 1.World Health Organization . World Health Statistics 2013. World Health Organization; Geneva, Switzerland: [Google Scholar]
- 2.United Nations . World Population to 2300. United Nations; New York: 2004. Department of Economic and Social Affairs, Population Division. [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute . Fact Book Fiscal Year 2012. US Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 2013. [Google Scholar]
- 5.Centers for Disease Control and Prevention [August 25, 2013];Viral hepatitis: statistics and surveillance. Available in: http://www.cdc.gov/hepatitis/Statistics.
- 6.US Renal Data System . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; Bethesda, MD: 2012. [Google Scholar]
- 7.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2012. [Google Scholar]
- 8.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D, OASIS Venus Ulcer Study Group Effectiveness of an extracellular graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41(5):837–43. doi: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 10.Boyd WD, Johnson WE, 3rd, Sultan PK, Deering TF, Matheny RG. Pericardial reconstruction using an extracellular matrix implant correlates with reduced risk of postoperative atrial fibrillation in coronary artery bypass surgery patients. Heart Surg Forum. 2010;13(5):E311–6. doi: 10.1532/HSF98.20091184. [DOI] [PubMed] [Google Scholar]
- 11.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancer. 2008;372(9655):2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 12.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 13.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 14.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti J, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92(3):998–1005. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 15•.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–51. doi: 10.1038/nm.3154. [This is the first report on full kidney bioengineering based on decellularized scaffolds. Bioengineered kidneys showed production of rudimentary filtrate after orthotopic transplantation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113(7):2217–22. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 18•.He M, Callanan A. Comparison of methods for whole-organ decellularization in tissue engineering of bioartificial organs. Tissue Eng Part B Rev. 2013;19(3):194–208. doi: 10.1089/ten.teb.2012.0340. [This review summarizes the decellularization and recellularization techniques used by different groups during attempts to bioengineer different organs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubin H, Kranz A, Hülsmann J, Lichtenberg A, Akhyari P. Decellularized whole heart for bioartificial heart. Methods Mol Biol. 2013;1036:163–78. doi: 10.1007/978-1-62703-511-8_14. [DOI] [PubMed] [Google Scholar]
- 20.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16(3):525–32. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remlinger NT, Wearden PD, Gilbert TW. Procedure for decellularization of porcine heart by retrograde coronary perfusion. J Vis Exp. 2012;(70):350059. doi: 10.3791/50059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KM, Woo HM. Porcine bioengineered scaffolds as new frontiers in regenerative medicine. Transplant Proc. 2012;44(4):1146–50. doi: 10.1016/j.transproceed.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, et al. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods. 2011;17(9):915–26. doi: 10.1089/ten.TEC.2011.0210. [DOI] [PubMed] [Google Scholar]
- 24.Witzenburg C, Raghupathy R, Kren SM, Taylor DA, Barocas VH. Mechanical changes in the rat right ventricle with decellularization. J Biomech. 2012;45(5):842–9. doi: 10.1016/j.jbiomech.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Merna N, Robertson C, La A, George SC. Optical imaging predicts mechanical properties during decellularization of cardiac tissue. Tissue Eng Part C Methods. 2013;19(10):802–9. doi: 10.1089/ten.tec.2012.0720. [This paper presents a novel imaging technique to assess the mechanical properties of decellularized heart without invading or destroying the tissue.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung JP, Squirrell JM, Lyons GE, Eliceiri KW, Ogle BM. Imaging cardiac extracellular matrices: a blueprint for regeneration. Trends Biotechnol. 2012;30(4):233–40. doi: 10.1016/j.tibtech.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32(30):7571–80. doi: 10.1016/j.biomaterials.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 28••.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013 Aug 14;4:2307. doi: 10.1038/ncomms3307. [This paper reports on the use of human iPS cells during heart bioengineering with a detailed functional analysis of the constructs.] [DOI] [PubMed] [Google Scholar]
- 29.Govoni M, Muscari C, Guarnieri C, Giordano E. Mechanostimulation protocols for cardiac tissue engineering. Biomed Res Int. 2013;2013:918640. doi: 10.1155/2013/918640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hülsmann J, Aubin H, Kranz A, Godehardt E, Munakata H, Kamiya H, et al. A novel customizable modular bioreactor system for whole-heart cultivation under controlled 3D biomechanical stimulation. J Artif Organs. 2013;16(3):294–304. doi: 10.1007/s10047-013-0705-5. [DOI] [PubMed] [Google Scholar]
- 31.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18(8):632–46. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18(1-2):1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maghsoudlou P, Georgiades F, Tyraskis A, Totonelli G, Loukogeorgakis SP, Orlando G, et al. Preservation of micro-architecture and angiogenic potential in a pulmonary acellular matrix obtained using intermittent intr-atracheal flow of detergent enzymatic treatment. Biomaterials. 2013;34(28):6638–48. doi: 10.1016/j.biomaterials.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195(3):222–31. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18(6):420–32. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonaka PN, Campillo N, Uriarte JJ, Garreta E, Melo E, de Oliveira LV, et al. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J Biomed Mater Res A. 2013 Mar 27; doi: 10.1002/jbm.a.34708. doi: 10.1002/jbm.a.34708 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Luque T, Melo E, Garreta E, Cortiella J, Nichols J, Farré R, et al. Local micromechanical properties of decellularized lung scaffolds measured with atomic force microscopy. Acta Biomater. 2013;9(6):6852–9. doi: 10.1016/j.actbio.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 39.Mahdavi Shahri N, Baharara J, Takbiri M, Khajeh Ahmadi S. In vitro decellularization of rabbit lung tissue. Cell J. 2013;15(1):83–8. [PMC free article] [PubMed] [Google Scholar]
- 40•.Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19(17-18):2045–62. doi: 10.1089/ten.tea.2012.0250. [One of the leading groups reports on the scaling up of this technology to obtain decellularized lung scaffolds from large animals and humans for regenerative purposes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, et al. Decellularization of human and porcine lung tissues for pulmonary tissue en gineering. Ann Thorac Surg. 2013;96(3):1046–56. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, et al. Decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J Heart Lung Transplant. 2013 doi: 10.1016/j.healun.2013.10.030. (In Press) [DOI] [PubMed] [Google Scholar]
- 43.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18(23-24):2437–52. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama KH, Lee CC, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8(5):e64134. doi: 10.1371/journal.pone.0064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186(9):866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Sokocevic D, Bonenfant NR, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013;34(13):3256–69. doi: 10.1016/j.biomaterials.2013.01.028. [This report describes the negative effects of advanced age and pre-existing pathologic conditions on lung scaffold repopulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, et al. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18(6):895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013 Oct 25; doi: 10.1172/JCI68793. Epub ahead of print. [This is a report from one of the leading groups in the field describing the techniques to differentiate iPSc into alveolar epithelium that can be used to repopulate decellularized matrices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross EA, Abrahamson DR, St John P, Clapp WL, Williams MJ, Terada N, et al. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis. 2012;8(2):49–55. doi: 10.4161/org.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33(31):7756–64. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 51.Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012;256(2):363–70. doi: 10.1097/SLA.0b013e31825a02ab. [DOI] [PubMed] [Google Scholar]
- 52.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16(7):2207–16. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Orlando G, Booth C, Wang Z, Totonelli G, Ross CL, Moran E, et al. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials. 2013;34(24):5915–25. doi: 10.1016/j.biomaterials.2013.04.033. [This paper shows that human kidneys not suitable for organ transplantation may be used to obtain decellularized scaffolds to be used in regenerative strategies.] [DOI] [PubMed] [Google Scholar]
- 54.Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis. 2010;6(2):134–6. doi: 10.4161/org.6.2.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, et al. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int. 2013;33(3):448–58. doi: 10.1111/liv.12088. [DOI] [PubMed] [Google Scholar]
- 56.Kajbafzadeh AM, Javan-Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for prepar ing a tissue scaffold of a human-sized liver tissue. Tissue Eng Part C Methods. 2013;19(8):642–51. doi: 10.1089/ten.TEC.2012.0334. [DOI] [PubMed] [Google Scholar]
- 57.Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant. 2013;22(2):231–42. doi: 10.3727/096368912X654939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–17. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 59.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, et al. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173(1):e11–25. doi: 10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53(1):293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 61.Totonelli G, Maghsoudlou P, Fishman JM, Orlando G, Ansari T, Sibbons P, et al. Esophageal tissue engineering: a new approach for esophageal replacement. World J Gastroenterol. 2012;18(47):6900–7. doi: 10.3748/wjg.v18.i47.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozeki M, Narita Y, Kagami H, Ohmiya N, Itoh A, Hirooka Y, et al. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A. 2006;79(4):771–8. doi: 10.1002/jbm.a.30885. [DOI] [PubMed] [Google Scholar]
- 63.Levin DE, Grikscheit TC. Tissue-engineering of the gastrointestinal tract. Curr Opin Pediatr. 2012;24(3):365–70. doi: 10.1097/MOP.0b013e328352ec19. [DOI] [PubMed] [Google Scholar]
- 64.Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33(12):3401–10. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patil PB, Chougule PB, Kumar VK, Almström S, Bäckdahl H, Banerjee D, et al. Recellularization of acellular human small intestine using bone marrow stem cells. Stem Cells Transl Med. 2013;2(4):307–15. doi: 10.5966/sctm.2012-0108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Conrad C, Schuetz C, Clippinger B, Vacanti JP, Markmann JF, Ott HC. Bio-engineered endocrine pancreas based on decellularized pancreatic matrix and mesenchymal stem cell/islet cell coculture. J Am Coll Surg. 2010;211(3):S62. [Google Scholar]
- 67.Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34(28):6760–72. doi: 10.1016/j.biomaterials.2013.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haag JC, Jungebluth P, Macchiarini P. Tracheal replacement for primary tracheal cancer. Curr Opin Otolaryngol Head Neck Surg. 2013;21(2):171–7. doi: 10.1097/MOO.0b013e32835e212b. [DOI] [PubMed] [Google Scholar]