Abstract
Decellularized organ scaffolds allow whole organ regeneration and study of cell behavior in three-dimensional culture conditions. Cell viability within the bio-engineered organ constructs is an essential parameter reflecting the performance of participating cells during long-term ex vivo culture, and is a prerequisite for further functional performance. Resazurin-based redox metabolic assays have been used to monitor cell viability in both two- and three-dimensional cell cultures. Here we developed a method for monitoring cell viability and proliferation in bio-engineered organ constructs using a resazurin perfusion assay. This method allows non-invasive, repetitive and rapid estimation of viable cell numbers during long-term ex vivo culture. As a proof-of-principle, we assessed the performance of two different endothelial sources and the impact of different perfusion programs on endothelial viability after re-endothelialization of decellularized lung scaffolds. The resazurin-based perfusion assay revealed changes in endothelial viability and proliferation during long-term ex vivo culture, which was consistent with histological assessment at different time points. Finally, we showed that this method could be used for assessment of proliferation and cytotoxicity after pharmacological treatment on a three-dimensional non-small cell lung cancer culture model.
1. Introduction
Whole-organ decellularization through detergent perfusion allows removal of cellular components while preserving fine structures of extracellular matrices [1–3]. Organ regeneration based on these decellularized scaffolds have been explored in heart [1], lung [4, 5], liver [6] and kidney [7], resulting in bio-engineered constructs partially recapitulating the natives organs’ functions in vitro and for short-term in vivo. Decellularized organ scaffolds also provide a novel platform to study cell behavior in three-dimensional culture conditions. Perfusable tumor nodules have been created by seeding human lung cancer cells into acellular rat lung scaffolds [8].
Both organ engineering and three-dimensional cell culture involve long-term in vitro culture. Moving cells from conventional two-dimensional culture vessels onto three-dimensional constructs represents a survival challenge. Proper viability of participating cells during the culture period is a prerequisite for in vitro tissue regeneration. The preservation of anatomical features and mechanical strength within decellularized whole organ scaffolds allows application of biomimetic culture conditions and various physiological stimulations to facilitate tissue maturation [1, 4]. The effect of these stimulations on cell viability needs to be evaluated in advance to ensure that they stay within a range without compromising cell viability. The development of a method allowing repetitive assessment of cell viability in three-dimensional, perfusable tissue-engineered constructs during the culture period will be helpful to facilitate the optimization of in vitro culture conditions.
Glucose consumption and lactate production rates are indicators of glycolytic metabolism of cells and therefore have been used to reflect cell viability within tissue-engineered constructs [9, 10]. These general metabolic assays compare medium composition before and after a culture period (generally a few days), and therefore only indicate trends in cell viability changes over a large time scale of days. The extended incubation time also makes these metabolic assays sensitive to experimental fluctuation during the entire culture period.
Several colorimetric and fluorometric assays have been developed and widely used to quantify viable cell numbers in conventional two-dimensional cell culture conditions. One of the most commonly used methods is based on tetrazolium salt MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide), which is reduced by live cells to purple formazan and thereby reflects the number of viable cells present [11]. MTT assay has been used to quantify cell numbers in small-size tissue-engineered constructs [12, 13]. However, MTT is generally regarded as an endpoint assay due to cytotoxicity and the requirement of final cell lysis before measurement [14]. More recently developed tetrazolium reagent XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) and resazurin-based reagents, such as AlamarBlue™ and CellTiter-Blue™, display improved cell permeability and lower cytotoxicity [15–18]. Besides cell proliferation, resazurin-based reagents can also be used to indicate cell apoptosis. Multiplexing assays combining resazurin and fluorometric caspase assays in drug-induced cell apoptosis models indicate well correlation between the increase in apoptotic caspase activation and decrease of resazurin metabolism [17, 19]. On three-dimensional cultures, resazurin-based AlamarBlue has been used to indicate cell viability and growth in hollow fiber bioreactors [20, 21] and in tissue-engineered bone constructs based on porous scaffolds [22, 23]. In these studies, perfusion through either the hollow fibers or porous scaffolds was performed to facilitate medium and resazurin distribution. In the culture of bio-engineered organ constructs based on native whole-organ scaffolds, perfusion was generally performed through the organ’s own vascular bed, which represents increased complexity and heterogeneity compared to synthetic scaffolds. Recently, resazurin was used to indicate metabolic activities in bio-engineered kidneys [24]. However, a systematic study establishing the feasibility and reliability of resazurin-based assays for long-term cell viability assessment in recellularized organ constructs is still missing.
A reliable colorimetric or fluorometric assay for repetitive quantification of viable cell numbers in large-size, three-dimensional bioengineered organ constructs needs to meet the following criteria. First, the diffusion of substrate dye and its metabolized product into and out of bioengineered constructs needs to be efficient, which ensures that the sampling from culture medium is representative of the composition of substrate dye and its metabolized product inside the constructs. Second, the reagents need to have minimal cytotoxicity during the assay period and need to be efficiently washed out after a measurement, thus enabling repetitive measurement during the entire culture period of bioengineered organ constructs. Here we demonstrate the feasibility of a resazurin-based PrestoBlue™ solution (Molecular Probes, Eugene, OR) as a potential candidate to meet these two criteria. PrestoBlue is metabolized by live cells and gives rise to a high fluorescent product that diffuses into surrounding medium without the need of cell lysis. When used in two-dimensional cell culture, PrestoBlue allows cell number quantification within 10 minutes comparing to 1 to 4 hours in MTT assay [25], demonstrating its fast metabolism and diffusion.
In this study, we demonstrated that a resazurin reduction assay is a reliable method to monitor changes of viable cell numbers in two-dimensional cell culture. We further developed a method to assess viable cell numbers in tissue-engineered whole-organ constructs within 1 hour by using a resazurin reduction perfusion assay. When used to compare the performance of two different endothelial cell lines in regenerated lung culture, whole-organ growth curves generated by resazurin reduction perfusion assay revealed cell viability changes consistent with histological assessment. Furthermore, we studied the effect of increasing perfusion rates during the culture of re-endothelialized lungs, and revealed no significant changes on endothelial cell viability. Finally, we applied the resazurin reduction perfusion assay to assess the proliferation and cytotoxic effect of a chemotherapy agent in a three-dimensional lung cancer model.
2. Methods
2.1 Isolation, culture and characterization of cord blood-derived endothelial progenitor cells (CB-EPCs)
Human umbilical cord blood was obtained from St. Louis Cord Blood Bank & Cellular Therapy Laboratory at SSM Cardinal Glennon Children’s Medical Center, St. Louis, MO, in accordance with an institutional review board–approved protocol. CB-EPCs were isolated from cord blood samples according to procedures used in previous studies [26–28] with minor modifications. Briefly, mononuclear cell (MNC) fractions was isolated by centrifugation using Vacutainer CPT Cell Preparation Tubes (BD Biosciences, San Jose, CA), and cultured on flasks coated with collagen I, rat tail (BD Biosciences, San Jose, CA). During the initial 2 days, MNCs were cultured in complete Endothelial Cell Growth Medium-2 (EGM-2, Lonza, Walkersville, MD) without hydrocortisone, supplemented with 20% defined fetal bovine serum (FBS) (Hyclone, Logan, UT), penicillin-streptomycin (P/S, Invitrogen, Camarillo, CA) and 15% autologous plasma. Unbound cells were removed after 2 days, and remaining cells were cultured in EGM-2 without hydrocortisone, supplemented with 20% defined FBS and P/S (this medium was referred to as CB-EPC medium). Once EPC colonies formed, cells were passaged and cultured on collagen I-coated flasks using CB-EPC medium.
CB-EPCs was characterized by immunofluorescence staining using anti-human CD31 antibody (DAKO, Carpinteria, CA). DiI-Ac-LDL uptake assay was performed by incubating CB-EPCs in medium containing DiI-Ac-LDL (10 μg/ml, Alfa Aesar, Ward Hill, MA) for 4 hours at 37 °C, followed by washing and nuclear staining using Hoechst 33342 (Invitrogen, Camarillo, CA). Matrigel tube formation assay was performed by seeding 50,000 CB-EPCs onto one well of a 24-well plate with 300 ul pre-solidified matrigel (BD Biosciences, San Jose, CA), and cultured in CB-EPC medium. The structures formed on top of matrigel were photographed using phase-contrast microscope (Nikon Instrument, Melville, NY) after 24 hours of incubation.
2.2 Culture of human non-small cell lung cancer cells
The human non-small cell lung cancer (NSCLC) cell line H358 (ATCC, Manassas, VA) was used for proliferation and cytotoxicity experiments. H358 is a bronchioloalveolar carcinoma cell line with epithelial features and was cultured in complete medium consisting of RPMI 1640 (Invitrogen, Camarillo, CA), supplemented with 10% FBS (Hyclone, Logan, UT) and P/S in an incubator at 37 °C supplemented with 5% CO2. Cells were passaged when 60–80% confluent at a 1:4 ratio.
2.3 Resazurin reduction assay on two-dimensional cell culture
Human umbilical vein endothelial cells (HUVECs, Lonza, Walkersville, MD) were maintained in complete EGM-2 medium. Resazurin reduction standard curves for HUVECs at different volume formats were generated by seeding serial dilutions of HUVECs with cell/medium ratios of 1, 0.5, 0.25, 0.1, 0.05, 0.025 and 0.01 million/ml onto 6-well plates in 2 ml volume and onto 96-well plates in 0.1 ml volume. Triplicate experiments were performed for each dilution of HUVECs and for each volume format. The resazurin-based PrestoBlue™ reagent (Molecular Probes, Eugene, OR) was added to each well 6 hours after HUVEC seeding at a resazurin/medium ratio of 1:20 (v/v). After 1 hour of incubation at 37 °C, fluorescence of resazurin-containing medium was read using SpectraMax Microplate Reader (Molecular Devices, Sunnyvale, CA) at 544 nm (ex)/590 nm (em).
HUVEC growth curves in two-dimensional culture were generated by seeding 0.5 or 1 million HUVECs onto T75 flasks in 10 ml EGM-2. Triplicate experiments were performed for each HUVEC seeding density. Resazurin reduction assays were performed as described above at 6 hour, 1 day, 2 days and 3 days after seeding. After each resazurin reduction assay, 10 ml fresh EGM-2 was used to replace the resazurin-containing medium. After the resazurin reduction assay on day 3, HUVECs were dissociated and cell numbers were counted with hemocytometer.
2.4 Perfusion decellularization of cadaveric rat lungs
All animal experiments were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and performed in compliance with the Animal Welfare Act. Cadaveric rat lungs were perfusion decellularized as previously described [4, 29]. In brief, cadaveric lungs were explanted from male Sprague-Dawley rats (250–300 g, Charles River Laboratories, Boston, MA) after systemic heparinization. The pulmonary artery (PA) was cannulated with an 18G cannula (McMaster-Carr, Santa Fe Springs, CA), and perfused (constant pressure, 40 mmHg) sequentially with heparinized (10 units/ml) phosphate-buffered saline (PBS, 10 min), 0.1% sodium dodecyl sulfate in deionized water (2 hours), deionized water (15 minutes) and 0.1% Triton X-100 in deionized water (10 minutes). Resulting scaffolds were washed with PBS containing antibiotics and antimytotics for 72 hours to remove residual detergent and cellular debris. All reagents are from Sigma Aldrich, St. Louis, MO.
2.5 Endothelial seeding into decellularized lung scaffolds and culture of re-endothelialized lungs
Medium used for re-endothelialized lung culture was the same as that for two-dimensional culture of corresponding endothelial cells. Decellularized rat lung scaffolds were placed inside a custom-made bioreactor allowing access to the PA. Before endothelial seeding, decellularized lung scaffolds were preconditioned with respective endothelial culture medium through PA perfusion at a rate of 2 ml/min for 1 hour. After preconditioning, 40 million HUVECs or CB-EPCs were resuspended in 100 ml of medium, and seeded into decellularized lung scaffolds through the PA under 30-mmHg pressure driven by the height of fluid column between the cell seeding chamber and lung bioreactor. Seeded lung scaffolds underwent 2-hour static culture to allow cell attachment before the initiation of PA perfusion at 2 ml/min. For culturing HUVEC and CB-EPC lungs with constant perfusion rate, PA perfusion rate was maintained at 2 ml/min throughout the entire 7 days of culture (n=4 for HUVEC lungs and n=3 for CB-EPC lungs). For culturing HUVEC lungs with increasing perfusion rate, PA perfusion rate was 2 ml/min during day 0 to day 1, 4 ml/min during day 1 to day 3 and 8 ml/min during day 3 to day 7 (n=3).
2.6 Seeding of human NSCLC cell line H358 into decellularized lung scaffolds and culture in bioreactor
Decellularized rat lung scaffolds (n=6) were placed inside a custom-made bioreactor allowing access to the PA and trachea. Before cell seeding, scaffolds were preconditioned with complete medium for H358 perfused via the PA at a rate of 10 ml/min for 1 hour. After preconditioning, perfusion was stopped and H358 cells were seeded into decellularized lung scaffolds by gravity-aided tracheal delivery of cell suspensions containing 20 million cells at a density of 0.8 million cells/ml. Static culture then took place for 1 hour to allow for cell attachment. After this, perfusion of 150 ml of complete medium via the PA was started at a constant rate of 10 ml/min throughout the entire culture period. Culture medium (150 ml) was changed on days 1, 3 and 5. H358-seeded decellularized lung scaffolds were cultured for 7 days inside an incubator at 37 °C supplemented with 5% CO2. A subset of H358-seeded lung scaffolds (n=3) received no further intervention, while the remaining tumor-seeded scaffolds (n=3) were used for cytotoxicity experiments. On day 5 of culture, these constructs were treated with a single dose of cisplatin (Teva Pharmaceutical Industries, Petach Tikva, Israel) in solution with H358 complete medium at a concentration of 110 μM and cultured for an extra 48 hours to complete 7 days of total culture time.
2.7 Resazurin reduction perfusion assay in re-endothelialized lungs
The resazurin reduction perfusion assay was performed in re-endothelialized lungs on day 1, 3, 5 and 7 of culture. The resazurin-based PrestoBlue reagent was diluted at 1:20 (v/v) in the medium used for re-endothelialized lung culture. Defined volumes of resazurin-medium mixture were used for each group of lung cultures (100 or 150 ml). 1 ml of resazurin-medium mixture was saved as non-metabolized control before adding the rest of mixture into lung culture. Re-endothelialized lungs were perfused with resazurin-medium mixture at 2 ml/min for 1 hour at 37 °C. The same perfusion rate (2 ml/min) was also used for resazurin reduction perfusion assays at each time point of HUVEC lungs cultured under increasing perfusion rates to ensure consistency of the assay. Fluorescence of resazurin-medium mixture after 1-hour perfusion was read using the SpectraMax Microplate Reader at 544 nm (ex)/590 nm (em), and was subtracted by that of non-metabolized control to yield net fluorescence increase corresponding to resazurin metabolism by re-endothelialized lungs. Cell/medium ratio of re-endothelialized lungs was calculated using the net fluorescence increase according to standard curves of HUVECs or CB-EPCs in two-dimensional cultures. After each resazurin reduction perfusion assay of re-endothelialized lungs, resazurin-medium mixture was replaced with 200 ml of fresh medium to continue the culture. To evaluate potential resazurin metabolism by decellularized scaffolds alone, resazurin reduction perfusion assays were also performed on decellularized lungs without endothelial seeding (n=3).
2.8 Time-lapse photography of resazurin reduction perfusion assay of lungs re-endothelialized with HUVECs
Resazurin perfusion was performed on decellularized lungs re-endothelialized with HUVECs on day 7 of culture as described above. For short-term 60-min perfusion, images of the re-endothelialized lung were taken right after the initiation of resazurin perfusion, and once every 10 min until the end of 60 min perfusion using a DSLR camera (Nikon, Melville, NY). Perfusion was then paused for 10 min, and another image of the lung was taken. A final image of the lung was taken after the re-endothelialized lung was re-perfused with fresh medium without resazurin for 10 min. 1 ml of medium was sampled at each 10 min interval during the resazurin perfusion, and their fluorescence intensities were measured using SpectraMax Microplate Reader at 544 nm (ex)/590 nm (em). For long-term 8-hour perfusion, images of the re-endothelialized lung were taken before and after the resazurin perfusion. 1 ml of medium was sampled at each 30-min interval during the resazurin perfusion, and their fluorescence intensities were measured using SpectraMax Microplate Reader at 544 nm (ex)/590 nm (em).
2.9 Resazurin reduction perfusion assay in NSCLC-seeded decellularized lung scaffolds
The resazurin reduction perfusion assay was performed in H358-seeded lung scaffolds on days 1, 3, 5 and 7 of culture. The resazurin-based PrestoBlue reagent was diluted at 1:20 (v/v) in the medium used for culture to make a total of 100ml resazurin-medium mixture. 1 ml of resazurin-medium mixture was saved as non-metabolized control before adding the rest of mixture into lung culture. H358-seeded lung scaffolds were perfused with resazurin-medium mixture at a rate of 10 ml/min for 1 hour at 37 °C. Fluorescence of resazurin-medium mixture afterwards was measured and calculated in a similar fashion as explained above in the resazurin assay in re-endothelialized lungs. Based on the net fluorescence increase, the total number of viable H358 cells within the decellularized lung scaffolds was estimated by extrapolation from a resazurin reduction standard curve of H358 cells in two-dimensional culture. The net fluorescence readings served to assess proliferation under three-dimensional culture conditions in H358-seeded scaffolds (days 1–5 of culture for all scaffolds and day 7 for those receiving no intervention on day 5), or to assess the cytotoxic effect of a single dose of cisplatin in H358-seeded scaffolds receiving the chemotherapeutic agent on day 5. After each resazurin reduction perfusion assay, resazurin-medium mixture was replaced with 150 ml of fresh culture medium to continue culture.
2.10 Glucose consumption measurement
Glucose consumption was measured during the culture of HUVEC and CB-EPC lungs under constant perfusion rate. Medium of re-endothelialized lung culture was sampled on day 3, 5 and 7 before resazurin reduction perfusion assays. Glucose concentrations in these medium samples were measured using Glucose (GO) Assay Kit (Sigma Aldrich, St. Louis, MO) according to manufacturer instructions. Glucose consumption was calculated based on the measured glucose concentration of fresh medium.
2.11 Histology
Re-endothelialized lungs were fixed with 4% paraformaldehyde overnight at 4 °C while H358-seeded lung scaffolds were fixed with 10% formalin for 48 hours, and processed for paraffin embedding and sectioning at 5 μm. Hematoxylin-Eosin (H&E, vector laboratories, Burlingame, CA) staining was performed according to manufacturer instructions. For immunohistochemistry staining, heat-induced antigen retrieval using sodium citrate buffer was performed after deparaffinization. Slides were blocked with 1% Bovine serum albumin (BSA, Sigma Aldrich, St. Louis, MO), and stained sequentially with mouse-anti-human CD31 antibody (1:200, M0823, sc-2005, DAKO, Carpinteria, CA) overnight at 4 °C and goat-anti-mouse-HRP antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) for 45 min at room temperature. Three washes with PBS were performed after each antibody incubation. After CD31 staining, slides were counterstained with Hematoxylin. For immunofluorescence staining, heat-induced antigen retrieval using sodium citrate buffer was also performed. Slides were blocked with 5% donkey serum (Millipore, Billerica, MA) and incubated sequentially with rabbit polyclonal anti-active and pro-Caspase 3 (1:100, ab13847, Abcam, Cambridge, MA) overnight at 4 °C and AlexaFluor® 647-conjugated donkey-anti-rabbit IgG H&L (1:400, ab150075, Abcam) for 1 hour at room temperature. A coverslip was mounted using ProLong® Gold Antifade Mountant with DAPI (Life Technologies, Grand Island, NY). TUNEL staining was performed using the DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI) according to manufacturer instructions.
2.12 Statistical analysis
Statistical analysis was performed by Student’s T-tests (two-tail comparisons) and statistically significant differences were defined as p < 0.05. Values in graphs were presented as means with standard deviations.
3. Results
3.1 Feasibility of a resazurin reduction assay for monitoring cell expansion in two-dimensional culture
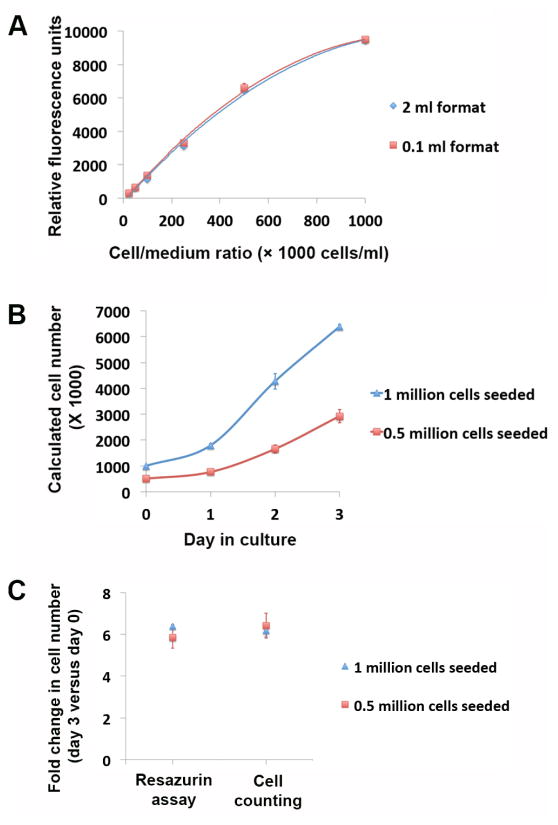
Colorimetric or fluorometric assays for cell number quantification depend on standard curves establishing the relation between colorimetric/fluorometric measurements and known numbers of cells. Tetrazolium or resazurin-based assays in two-dimensional cultures are usually performed in the same volume format for both the cell populations under quantification and those for generating standard curves. Ex vivo culture of bio-engineered organ constructs generally requires medium volumes of a few hundred milliliters or more. The generation of standard curve in such big volumes in two-dimensional cultures with matching cell densities is usually unpractical. To evaluate the feasibility of calculating cell numbers using resazurin reduction assays under different volume formats, a serial dilution of human umbilical vein endothelial cells (HUVECs) was seeded in two different volume formats (0.1 ml and 2 ml) with predefined cell number to medium volume ratio (cell/medium ratio). Resazurin reduction assays were performed 6 hours after HUVEC seeding. Standard curves generated from the two different volume formats showed close similarity with no significant difference detected for each cell/medium ratio point (Fig. 1A and Supplemental Table 1). This indicates resazurin reduction measurements correlate with cell/medium ratios instead of absolute cell numbers. Therefore, viable cell numbers in ex vivo cultured organ constructs can be calculated based on standard curves generated on regular multi-well plates.
Fig. 1.
Resazurin reduction assay on HUVECs in two-dimensional culture. (A) Fluorescence readings of resazurin reduction assay on serial dilutions of HUVECs (cell number to medium ratio of 1, 0.5, 0.25, 0.1, 0.05, 0.025 and 0.01 million/ml) in 2 ml and 0.1 ml volume formats. (B) Growth curve of HUVECs cultured in T75 flasks calculated from resazurin reduction assays according to the standard curve. Assays were performed on day 0 (6 hours after cell seeding), day 1, day 2 and day 3. Two groups of assays were performed with an initial seeding number of 0.5 and 1 million HUVECs per T75 flask. (C) Fold changes in HUVEC numbers over the 3 days of two-dimensional culture revealed by resazurin reduction assays and cell counting. Data were presented as means with standard deviations (n=3 for each experimental arm).
We further examined the feasibility of the resazurin reduction assay for monitoring viable cell number changes over long-term two-dimensional culture. Different numbers of HUVECs (1 and 0.5 million) were seeded onto T75 flasks. Resazurin reduction assays were performed on day 0 (6 hours after seeding), day 1, day 2, and day 3 of culture. HUVEC growth curves were generated from cell numbers calculated based on resazurin reduction measurements according to the standard curve (Fig. 1B). After the resazurin reduction assay on day 3, HUVECs were dissociated and their numbers were counted using a hemocytometer. Fold changes in HUVEC cell numbers over the 3 days of culture calculated from resazurin reduction assays were not significantly different from that revealed by cell counting (p=0.36 for 1 million cell seeded, and p=0.28 for 0.5 million cells seeded) (Fig. 1C).
3.2 Resazurin perfusion in ex vivo regenerated lung culture
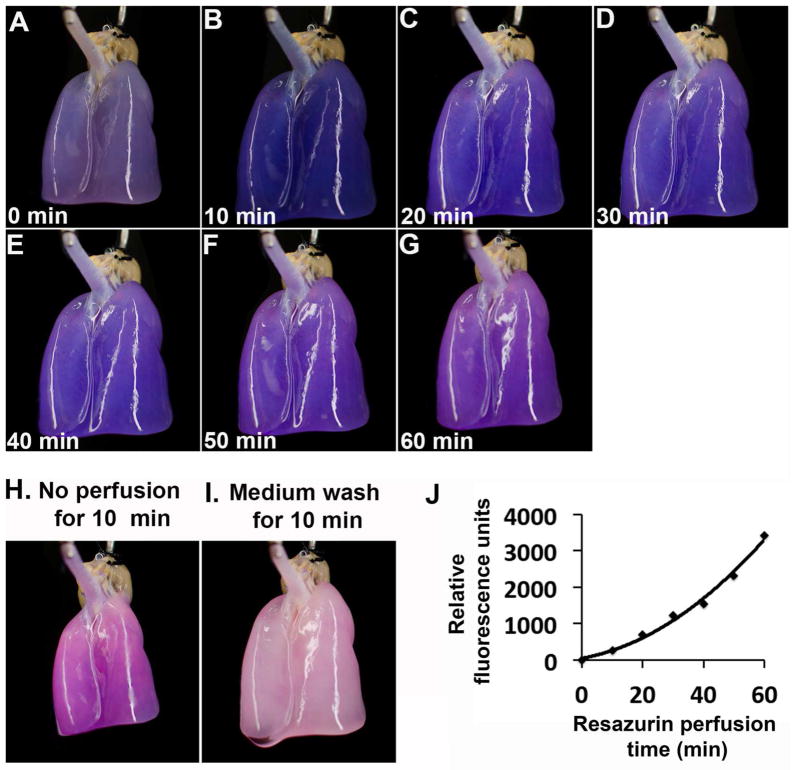
Due to limited diffusion, live cell number quantification using colorimetric assays such as MTT has been limited to small-size tissue-engineered constructs [12, 13]. To solve this issue, resazurin-based reagents have been delivered by perfusion in hollow fiber bioreactors [20, 21], in tissue-engineered bone constructs based on porous scaffolds [22, 23], and in regenerated kidneys based on native scaffolds [24]. The preservation of anatomical features in the decellularized whole organ construct allows medium to be perfused through the scaffold’s main artery and efficiently reach the entire organ’s vascular network. We attempted to perform resazurin reduction assay in regenerated rat lungs by perfusion. Resazurin-medium (1:20) mixture was perfused through the pulmonary artery (PA) of a decellularized rat lung re-endothelialized with HUVECs at a constant flow rate of 2 ml/min. Time-lapse photography of the regenerated lung during resazurin perfusion displayed gradual change of color from blue to red (Fig. 2A–G), indicating resazurin metabolism by viable cells within the regenerated organ. Medium sampled at the same time intervals indicated a steady increase in fluorescence intensity (Fig. 2J). We noticed an increased in the slope of resazurin metabolism over the 60-min perfusion. This was likely due to the time required for the initial perfusion of resazurin reagent into the bio-engineered construct from the medium reservoir, the time for initial resazurin metabolism, and the time for the release of the initial resazurin metabolite into surrounding medium. Similar increase in the slope of resazurin metabolism was also observed in the resazurin perfusion assay in HepG2 cells in three-dimensional bioreactor [20]. The color change within the regenerated lung paralleled well with that in its surrounding medium (Fig. S1), indicating efficient equilibration of resazurin and its metabolite composition inside and outside the organ construct. To confirm this idea, perfusion was paused for 10 min after the 60-min resazurin perfusion. The color of the lung further shifted towards red (Fig. 2H), indicating that perfusion is required for this efficient equilibration. After replacing the resazurin assay medium with fresh culture medium without resazurin and perfusing the lung for additional 10 min, the lung regained its original color similar to that before the assay (Fig. 2I). This makes repetitive measurement over long culture period possible. We also performed long-term perfusion of resazurin in a HUVEC-seeded lung for 8 hours, and observed the resazurin metabolism gradually reached a plateau (Fig. S2). Taken together, we demonstrated efficient delivery, metabolism, and equilibration with surrounding medium of a resazurin-based reagent during its perfusion of ex vivo cultured organ constructs.
Fig. 2.
Resazurin perfusion of a HUVEC-regenerated lung. (A–G) Time-lapse photography of the HUVEC-regenerated lung during resazurin perfusion. Images were taken at 0, 10, 20, 30, 40, 50 and 60 min after the start of resazurin perfusion. (H) An image was taken after the resazurin perfusion was paused for 10 min. (I) A final image was taken after the resazurin-containing medium was replaced with fresh medium without resazurin and perfused the lung for an additional 10 min. (J) Fluorescence intensities of medium sampled at each 10 min interval during the resazurin perfusion.
3.3 Growth curve of re-endothelialized lung scaffolds
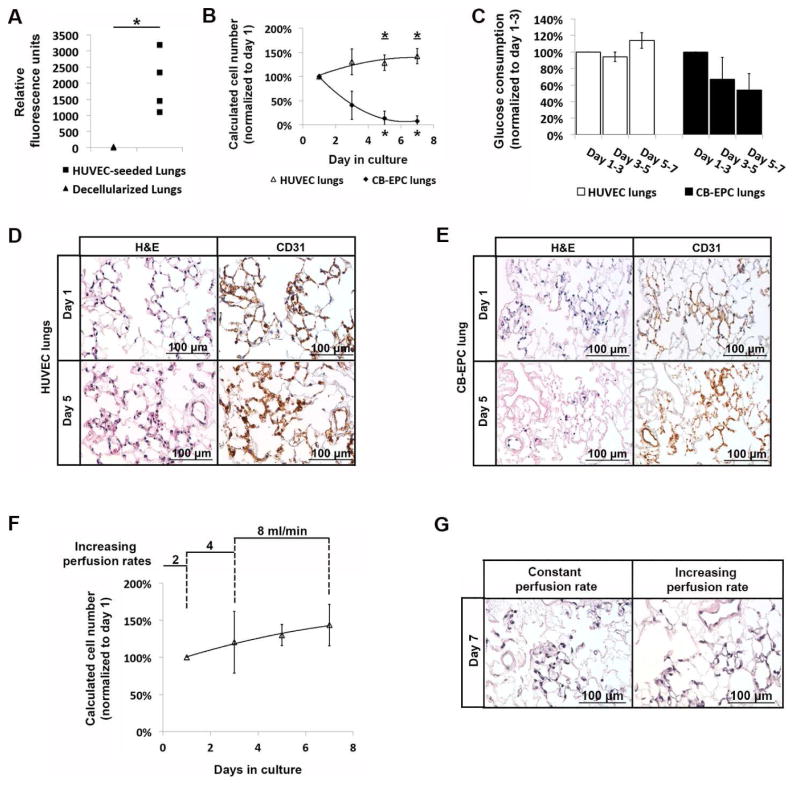
We performed resazurin reduction perfusion assays on decellularized rat lungs with and without endothelial seeding. 60-min perfusion of resazurin in decellularized lungs led to minimal increase in fluorescence intensity over the non-perfused resazurin-medium mixture control (22.2±13.8 rfu). Resazurin reduction perfusion assays in HUVEC-seeded lungs at 1 day after seeding generated pronounced increase in fluorescence intensity (2023.7±939.1 rfu), which was significantly higher than that generated by decellularized lungs alone (p=0.02) (Fig. 3A). The heterogeneity of resazurin reduction readings from the four HUVEC-seeded lungs at 1 day after seeding was likely due to experimental fluctuations in endothelial cell delivery and initial engraftment. This highlighted the necessity to monitor cell viability of individual regenerated lungs over the entire culture period to reveal its long-term change and response to specific experimental stimulations.
Fig. 3.
The resazurin reduction perfusion assay in re-endothelialized lung culture. (A) Fluorescence intensity of resazurin-medium mixture after perfusing decellularized rat lungs (n=3) or decellularized rat lungs seeded with 40 million HUVECs and after 1 day of culture (n=4). (B) Growth curve of HUVECs and CB-EPCs in regenerated lung culture with resazurin reduction perfusion assays performed on day 1, 3, 5 and 7 of culture. Viable cell numbers at each time point was calculated according to standard curves on two-dimensional culture, and normalized to that on day 1. The viable cell numbers in HUVEC lungs increased over the culture period with the calculated cell numbers on day 5 and on day 7 significantly different from that on day 1 (asterisk with underline indicated p<0.05). The viable cell numbers in CB-EPC lungs steadily decreased over the culture period with the calculated cell numbers on day 5 and on day 7 significantly different from that on day 1 (asterisk indicated p<0.05). Data were presented as means with standard deviations (n=4 for HUVEC lungs and n=3 for CB-EPC lungs). (C) Glucose consumption of HUVEC and CB-EPC lungs during day 1–3, 3–5 and 5–7. The level of glucose consumption during each culture period was normalized to that during day 1–3 for each lung culture. Glucose consumption in HUVEC lungs was 94%±5% during day 3–5 (p=0.13) and 114%±10% during day 5–7 (p=0.06) compared to that during day 1–3. Glucose consumption in CB-EPC lungs was 67%±27% during day 3–5 (p=0.16) and 54%±20% during day 5–7 compared to that during day 1–3 (p=0.06). Data were presented as means with standard deviations (n=4 for HUVEC lungs and n=3 for CB-EPC lungs). (D, E) H&E and CD31 immunohistochemical staining of HUVEC (D) and CB-EPC (E) lungs on day 1 and day 5 of culture. Matching of CD31 and hematoxylin signals was observed in HUVEC lungs on both day 1 and day 5, and was also observed in CB-EPC lungs on day 1. Widespread CD31 signals were observed in CB-EPC lungs on day 5, but the hematoxylin signal was sparse and showed signs of pyknosis. (F) Growth curve of HUVECs in regenerated lungs cultured at 2 ml/min during day 0–1, 4 ml/min during day 1–3 and 8 ml/min during day 3–7. Resazurin reduction perfusion assays performed on day 1, 3, 5 and 7 of culture. Viable cell numbers at each time point was calculated according the standard curve on two-dimensional culture, and normalized to that on day 1. (G) H&E staining of HUVEC regenerated lungs after 7 days of culture at constant perfusion rate of 2ml/min or at increasing perfusion rate as shown in (F).
We compared the viability of two different endothelial cell types (HUVEC and cord blood-derived endothelial progenitor cells) over 7 days of culture in decellularized lung scaffolds. Resazurin reduction perfusion assays were performed on day 1, 3, 5 and 7 of culture. For each regenerated lung, the cell number calculated at each time point was normalized to the cell number on day 1 of culture to minimize the effect of experimental fluctuations between biological replicates. Cord blood-derived endothelial progenitors (CB-EPCs) were characterized by colony formation, endothelial CD31 expression, DiI-Ac-LDL uptake and tube formation on matrigel (Fig. S3). Regenerated lungs seeded with HUVECs displayed an increase in viable cell numbers over the culture. When normalized to the calculated cell number on day 1, the viable cell numbers in HUVEC lungs reached 135.5%±26.7% on day 3 (p=0.11), 128.5%±16.0% on day 5 (p=0.04), and 142.3%±15.9% on day 7 (p=0.01). However, CB-EPCs did not survive well in three-dimensional lung culture under the same culture condition. When normalized to the calculated cell number on day 1, the viable cell numbers in CB-EPC lungs decreased to 40.5%±29.4% on day 3 (p=0.07), 13.8%±14.1% on day 5 (p<0.01), and 7.8%±10.9% on day 7 (p<0.01) (Fig. 3B).
In order to confirm the changes in endothelial viability in three-dimensional lung cultures revealed by the resazurin reduction perfusion assay, we measured glucose consumption between the two neighboring resazurin reduction measurements. Glucose consumption in HUVEC lungs was stable over the three time periods (day 1–3, day 3–5 and day 5–7), reaching 114%±10% during day 5–7 compared to that during day 1–3 (p=0.06). However, glucose consumption in CB-EPC lungs decreased over time, reaching 54%±20% during day 5–7 compared to that during day 1–3 (p=0.06). The changes in cell viability over the culture period were also confirmed by histological assessment of HUVEC and CB-EPC lungs on day 1 and day 5 of culture. In HUVEC lungs, CD31 immunohistochemical staining and nuclear hematoxylin staining matched well on both day 1 and day 5 (Fig. 3D), indicating maintenance of cell viability. Similar CD31 and hematoxylin matching was also observed in CB-EPC lungs on day 1 of culture, demonstrating cell delivery and initial attachment in CB-EPC lungs. However, on day 5 of culture, despite the widespread CD31 immunohistochemical signals in CB-EPC lungs, the nuclear staining was very sparse with signs of nuclear fragmentation indicating apoptosis and loss of cell viability (Fig. 3E). This was further confirmed by TUNEL staining showing the majority of the remaining cells in the CB-EPC lungs on day 5 of culture were undergoing apoptosis (Fig. S4).
The capability of monitoring cell viability in tissue-engineered constructs over long-term ex vivo culture allows study of the potential effects of different culture conditions on the viability of participating cells. Here we analyzed endothelial viability within regenerated lungs under different flow rate programs during culture. In the aforementioned group of HUVEC lung cultures, we used a constant flow rate of 2 ml/min from the PA throughout the entire 7 days of culture. As a comparison, we set up another group of HUVEC lung cultures, with increasing flow of 2 ml/min during day 0–1, 4 ml/min during day 1–3 and 8 ml/min during day 3–7 (Fig. 3F). In both groups of culture, resazurin reduction perfusion assays were performed using a flow rate of 2 ml/min to ensure consistency of the assay. No significant differences were observed between HUVEC lungs cultured under constant low flow rate (Fig. 3B) and those cultured under increasing flow rates (Fig. 3F), which was consistent with histological assessment on day 7 of culture (Fig. 3G).
3.4. Monitoring of proliferation and cytotoxicity of NSCLC cells in three-dimensional culture within decellularized lung scaffolds
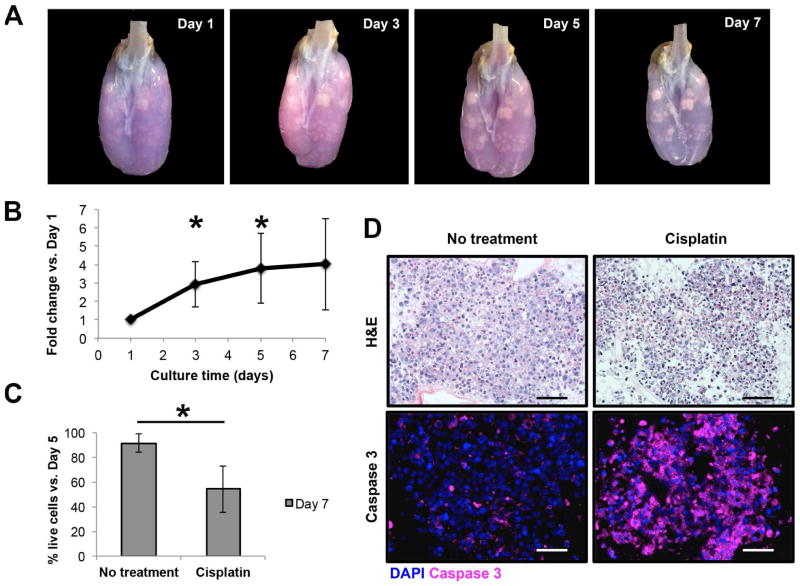
H358 cells seeded into decellularized lung scaffolds grew as three-dimensional tumor nodules that upon daily visual inspection increased progressively in size and density throughout the entire culture period (Fig. 4A). Serial resazurin reduction perfusion assays performed on H358-seeded decellularized lung scaffolds showed a progressively increasing net fluorescence signal suggesting increasing cell numbers, and therefore, proliferation of the lung cancer cells under three-dimensional culture conditions (Fig. 4B). These results correlated with the macroscopic visualization of tumor nodules within decellularized lung scaffolds, particularly in areas with relatively higher resazurin metabolism (Fig. 4A). Likewise, this assay was able to estimate the cytotoxic effect of a single dose of the chemotherapeutic agent cisplatin delivered on day 5 of culture. The resazurin reduction perfusion assay results on day 7 after 48 hours of cisplatin treatment showed a significant decrease in the number of viable cancer cells as compared to that on day 5 before cisplatin treatment (54.2%±18.8%). Viable cell numbers were generally maintained in H358-seeded decellularized lung scaffolds receiving no intervention (91.5%±7.6%) (p=0.03, compared to the viable cell number change in cisplatin treatment group) (Fig. 4C). The decrease in the number of viable cells, as suggested by the resazurin reduction perfusion assay, correlated with the observation of abundant apoptotic cells (i.e. ghost cells and cells with nuclear pyknosis or fragmentation) upon histologic examination and significantly higher number of cells with positive expression of activated caspase 3 (Fig. 4D).
Fig. 4.
The resazurin reduction perfusion assay in a three-dimensional culture model for non-small cell lung cancer within decellularized scaffolds. (A) Time-lapse photography of a decellularized scaffold seeded with H358 adenocarcinoma cells after the performance of the resazurin reduction perfusion assay. The resazurin reduction perfusion assay on day 1 facilitated the localization of engrafted cells within the decellularized lung scaffold by providing a blue background that contrasted with cells, but also by highlighting the specific sites with higher resazurin reduction activity in pink. The evolution of the tumor nodules in terms of size and density can be appreciated over the culture period. (B) Proliferation curve of H358 cells under three-dimensional culture conditions within decellularized lung scaffolds, based on the resazurin reduction perfusion assay, demonstrating increasing cancer cell numbers over the culture period. Cell number increase was compared to day 1 measurements to account for variations in cell engraftment between experimental replicates (n=6 for days 1, 3 and 5; n=3 for day 7). There was a significant increase in the number of live cells on day 3 (2.9±1.2-fold, p=0.012) and day 5 (3.8±1.9-fold, p=0.015) when compared to day 1. (C) Use of the resazurin reduction perfusion assay to assess the cytotoxic effect of a single dose of the chemotherapeutic agent cisplatin. Relative to pre-treatment results (day 5), the number of live cancer cells within the decellularized lung scaffolds decreased to 54.2%±18.8% after cisplatin treatment, as compared to a small decrease to 91.5%±7.6% in non-treated controls (p=0.03; n=3 for each experimental arm). (D) Histologic evaluation of H358 tumor nodules cultured within decellularized lung scaffolds. The H&E staining for control scaffolds showed tumor nodules with viable cancer cells, while for cisplatin-treated scaffolds there were several dead cells characterized by nuclear pyknosis and fragmentation. This observation correlated with activated caspase-3 staining thus confirming the cytotoxic effect of cisplatin. Data are presented as means with standard deviations. *p<0.05.
4. Discussion
The preservation of anatomical features and tissue compartmentalization in decellularized whole organ scaffolds allows for the experimental regeneration of complex tissues, ex vivo biomimetic culture and subsequent transplantation into animal models [2, 3]. Maturation of tissue-engineered constructs during long-term ex vivo culture is generally required before successful transplantation [2]. Maintenance of the viability of participating cells is a prerequisite for tissue maturation in vitro. Therefore, cell viability in tissue-engineered constructs is an essential parameter to be monitored during ex vivo culture in order to optimize culture conditions, including medium recipe, culture length and biomimetic stimulations. However, conventional methods for cell viability assessment, such as MTT, XTT and DNA quantification, are usually endpoint assays [14, 15, 17]. The application of colorimetric assays in large organ constructs is also challenging due to limited passive diffusion.
Perfusion of resazurin-based regents has been developed as an approach to indicate cell viability in three-dimensional cultures [20–24]. In the present study, we demonstrate the feasibility of a resazurin reduction perfusion assay for long-term cell viability monitoring in perfusable large tissue-engineered organs characterized by two features: first, the resazurin-based PrestoBlue reagent with low cytotoxicity, efficient metabolism and diffusion allows repetitive cell number quantification; second, resazurin delivery by means of perfusion through the organs’ own vascular bed greatly decreases diffusion distances allowing efficient and homogeneous distribution of the colorimetric and fluorometric reagent throughout tissue-engineered constructs. Moreover, we believe that the concept of performing a metabolic perfusion assay in ex vivo cultured three-dimensional tissue-engineered constructs could be extrapolated to other reagents besides resazurin, making this a versatile assay. On two-dimensional cultures, we demonstrated that resazurin reduction measurements were proportional to cell/medium ratio, and predicted cell number changes that were consistent with those revealed by direct cell counting after dissociation. This makes it possible to calculate viable cell numbers in tissue-engineered organs with large absolute cell numbers and culture volumes. We compared two different endothelial cell types (HUVECs and CB-EPCs) during the 7-day in vitro culture after seeding into decellularized lung scaffolds, and revealed their different trend of viability changes consistent with the trend of glucose consumption and histological assessment at different time points.
In the current study, we measured the fluorescence of resazurin/medium mixture in the medium reservoir to calculate the number of viable cells in the regenerated lungs. It is therefore an important consideration to make sure that the resazurin metabolism measured from the medium reservoir represents the overall metabolism in the regenerated lungs. As shown in Fig. 2 and Fig. S1, during resazurin perfusion, the color of the lung and that of the medium sampled from the reservoir were consistent at different time points. This indicated that constant perfusion allowed efficient equilibration of resazurin and its metabolite between the regenerated lung and its surrounding medium. As it is impractical to directly measure the fluorescence of the medium within the regenerated lungs, time-lapse photography can serve as a pilot experiment to ensure efficient equilibration. Perfusion rate is one of the main factors affecting the efficiency of equilibration between the bio-engineered construct and its medium reservoir. The perfusion rate may be increased to facilitate equilibration if desired.
We noticed that the standard curve of HUVECs in two-dimensional culture began to turn over above 500,000 cells/ml (Figure 1A), indicating reduced detection sensitivity at high cell/medium ratios. In the current study, most of the fluorescence readings after resazurin perfusion assays corresponded to cell/medium ratios below 500,000 cells/ml. To achieve high sensitivity of the assay, pilot experiments are desired to determine the optimal volume (and therefore the cell/medium ratio) of the resazurin perfusion assay to ensure the fluorescence readings fall into the high sensitivity area of the standard curve.
The resazurin reduction perfusion assay provided a general estimate of cell viability of the entire organ constructs, and revealed viability changes in these organ constructs consistent with histological assessment. Resazurin metabolism represents a total metabolic rate by all cells within the constructs. There is likely cellular heterogeneity within the constructs. As discussed above, the capability of cells metabolizing resazurin gradually decreased as they moving into the apoptotic pathway [17, 19]. Moreover, metabolic parameters, such as glucose and oxygen consumption per cell, vary depending on cell type, cell density, cell differentiation and oxygen availability [30–32]. For example, electrochemical monitoring of glucose consumption suggests that glucose consumption and cell population growth curve have a close correlation but different behavior, and glucose consumption per cell changes at different stages of cell population growth [33]. Similar phenomena may also apply to resazurin metabolism. In this study, the variability of resazurin metabolism by different cell types and different densities was taken into account by establishing a standard curve for each cell type at various cell densities (cell/medium ratios). In the culture of bio-engineered organs, oxygen availability depends on the perfusability of the decellularized scaffolds. To perform resazurin perfusion assays every other day for 7 days and normalize the calculated cell numbers to that on day 1 allowed assessment of resazurin metabolism in a stable context with similar scaffold perfusability and therefore oxygen availability.
Both resazurin reduction perfusion assay and glucose consumption measurement can be performed repetitively during ex vivo culture of tissue-engineered constructs to monitor cell viability. In this study, the resazurin reduction perfusion assay took 1 hour to finish, and therefore indicated cell viability at the specific time points. However, glucose consumption was calculated by measuring the glucose consumed over a few days of culture, and therefore indicated averaged cell viability over the culture period (between two resazurin reduction perfusion assay time points in this study). Consistent with this, the resazurin reduction perfusion assay revealed a sharper decrease in viable CB-EPC numbers than that indicated by glucose consumption assay. In experiments where fast changes in cell viability are expected, the resazurin reduction perfusion assay can be frequently performed, such as daily or even multiple times a day, to catch details in the fast changing kinetics. However, this is difficult to do with more general metabolic assays, such as glucose consumption, which require a few days of metabolism to accumulate changes in metabolite levels for reliable measurement.
Moving cells from conventional two-dimensional culture onto three-dimensional scaffolds represent a survival challenge. In the current study, we showed that the viability of HUVECs but not CB-EPCs could be maintained in decellularized lung scaffolds. Cell type-specific optimizations are required to improve their survival and engraftment in decellularized organ scaffolds. In the case of synthetic porous scaffolds, pore size has been shown to have an impact on cell viability by affecting oxygen and nutrient availability [34, 35]. Similar mechanism may also apply to cell seeding into native organ scaffolds, where cell type-specific optimization of perfusion parameters may be required. Moreover, immobilization of cell type-specific growth factors has also been shown to improve cell survival and proliferation in three-dimensional scaffolds [36, 37]. The resazurin reduction perfusion assay serves as a feasible method to monitor cell viability during the optimization of in vitro culture conditions.
Biomimetic culture conditions are usually introduced during ex vivo culture of tissue-engineered constructs to promote functional tissue formation. For example, both electrical and mechanical stimulations have been utilized to promote the assembly of contractile cardiac tissue formation [38, 39]. These stimulatory programs may potentially affect cell viability [40]. The ability to monitor cell viability within tissue-engineered constructs before and after stimulations will facilitate the optimization of stimulation programs and ensure they stay within a non-harmful range. Shear stress is one of the common stimulations for regenerating vasculature. As a proof-of-principle, we studied the effect of increasing perfusion rate on endothelial viability during the culture of re-endothelialized lungs. No significant difference was observed between HUVEC lungs cultured under constant flow rate (2 ml/min) and those cultured under flow rates that increased over time (up to 8 ml/min). This indicates that the flow rate for culturing re-endothelialized lungs can be gradually increased to get closer to the physiological value (50 ml/min for adult rats) [41], which will better prepare the bioengineered constructs for transplantation.
Initial cell delivery and retention within tissue-engineered constructs display variability between experimental replicates. When resazurin reduction perfusion assays were performed on the four replicates of HUVEC-seeded decellularized rat lung scaffolds on day 1 after seeding, up to a 3-fold difference in fluorescence readings can be observed between replicates, indicating heterogeneity between experimental replicates. Therefore, we normalized calculated cell numbers of each later time points to the value of day 1 after seeding for individual regenerated construct, and presented the organs’ growth curve as a fold change relative to day 1 of culture. In this way, the growth curve generated will mainly reflect the changes in cell viability over long-term culture with minimal carryover fluctuations from the initial cell retention. The heterogeneity of initial cell retention in native lung scaffolds was likely due to experimental fluctuations during the scaffold preparation and cell delivery. Further optimizations are required on both processes to improve the consistency of cell seeding into native organ scaffolds. On the other hand, the resazurin reduction perfusion assay performed right after cell delivery can serve as a quality control step to evaluate the consistency of initial cell retention. An inclusion/exclusion standard may be established based on this initial cell retention to improve the consistency before moving onto long-term ex vivo culture and functional examination of the bio-engineered grafts.
Here we demonstrate the feasibility and applicability of the resazurin reduction perfusion assay in a three-dimensional lung cancer culture model, which provides a different experimental setting for testing of this assay. First, the NSCLC cell line H358 was delivered via the trachea into the airway compartment of the decellularized lung scaffolds, as opposed to the vascular compartment in the case of endothelial cell experiments. Second, tumor cells tend to grow as nodules posing a challenge in terms of the diffusion capacity of the resazurin-based reagent. Our results show that relative cancer cell proliferation can be monitored, as well as their response to therapeutic agents in a non-invasive fashion by using this assay. The use of perfusion to deliver resazurin through the decellularized organ’s own vascular network greatly improves diffusion of the reagent, making it an useful tool when tissues grow thick, as is the case of lung cancer nodules presented here. The ability to monitor cell viability, proliferation and cytotoxicity in a perfusable, three-dimensional cancer model would allow a better understanding of cancer biology by taking cancer experimentation from the traditional two-dimensional culture systems, which are limited in their capacity to replicate in vivo conditions, to more complex models such as the one used here based on decellularized lung scaffolds.
Resazurin-based reagents, such as AlamarBlue, have been widely used to determine cytotoxicity, drug effects and cell proliferation during culture. AlamarBlue has been regarded as non-toxic to various cell lines, such as lymphocytes and corneal endothelial cells [21, 42, 43]. An AlamarBlue incubation time of 1 to 6 hours was recommended for various normal and cancer cell lines [44]. However, it has been noted that AlamarBlue will cause growth inhibition in some leukemic cell lines with an incubation time beyond 4 hours [21]. In the current study, we established a protocol allowing quantification of cell viability within 1-hour of resazurin perfusion. The short incubation time will minimize the potential adverse effect of the assay reagent on cell viability and proliferation. Consistent with our study, a stable increase of resazurin metabolism was observed in bio-engineered kidney constructs over 7 days of culture [24]. For analyzing drug cytotoxicity, proper control experiments without drug treatment and accompanied histological assessment will help to discriminate the drug effect versus the potential adverse effect of the resazurin reagent. For example, in the lung cancer model of this study, the loss of viable cells and increase of apoptotic cells was specific to the group of tumor-seeded scaffolds that received cisplatin and was not appreciated in the control group that received the same resazurin perfusion procedures.
4. Conclusion
The present work demonstrates the feasibility of using a resazurin reduction perfusion assay to quantify viable cell numbers in tissue-engineered organ constructs. This method allows efficient and repetitive measurement of cell viability and therefore monitoring the growth status of regenerated organ constructs during long-term ex vivo three-dimensional culture. This will facilitate the optimization of ex vivo organ culture conditions, including medium composition and various biomimetic stimuli. As decellularized organ scaffolds are also demonstrated as a model for three-dimensional cancer culture, resazurin perfusion will allow in vitro monitoring of tumor growth and the assessment of cytotoxic effects of potential therapeutic agents.
Supplementary Material
Fig. S1. Progressive blue-to-red color shift of resazurin-containing medium mixture during its perfusion of a HUVEC-regenerated lung. Photography of resazurin-containing medium sampled at 0, 10, 20, 30, 40, 50 and 60 min after the initiation of resazurin perfusion of the HUVEC regenerated lung.
Fig. S2. Long-term resazurin perfusion of a HUVEC-regenerated lung. (A,B) photography of the HUVEC lung before (A) and after 8-hour perfusion of resazurin (B). (C) Fluorescence intensities of medium sampled at each 30-min interval during the resazurin perfusion.
Fig. S3. Characterization of CB-EPCs. CB-EPCs were characterized by colony formation (A), CD31 expression (B), DiI-Ac-LDL uptake (C), and tube formation on matrigel (D).
Fig. S4. TUNEL staining of apoptotic cells in HUVEC (A) and CB-EPC (B) lungs on day 5 of culture.
Table S1. p-values of T-test comparing resazurin fluorescence intensities at each cell number/medium ratios (1, 0.5, 0.25, 0.1, 0.05, 0.025 and 0.01 million/ml) between two the volume formats (2 ml and 0.1 ml).
Acknowledgments
This work was supported by the United Therapeutics Corporation and the National Institutes of Health (NIH) Director’s New Innovator Award (DP2-OD008749-01). The authors thank St. Louis Cord Blood Bank & Cellular Therapy Laboratory at SSM Cardinal Glennon Children’s Medical Center, St. Louis, MO for providing the human umbilical cord blood samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 2.Badylak SF, Taylor D, Uygun K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren X, Ott HC. On the road to bioartificial organs. Pflugers Arch - Eur J Physiol. 2014:1–11. doi: 10.1007/s00424-014-1504-4. [DOI] [PubMed] [Google Scholar]
- 4.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 5.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-Engineered Lungs for in Vivo Implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–51. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra DK, Thrall MJ, Baird BN, Ott HC, Blackmon SH, Kurie JM, et al. Human Lung Cancer Cells Grown on Acellular Rat Lung Matrix Create Perfusable Tumor Nodules. The Annals of thoracic surgery. 2012;93:1075–81. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomaterialia. 2008;4:477–89. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnology and Bioengineering. 2003;82:403–14. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–51. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 13.Zund G, Ye Q, Hoerstrup SP, Schoeberlein A, Schmid AC, Grunenfelder J, et al. Tissue engineering in cardiovascular surgery: MTT, a rapid and reliable quantitative method to assess the optimal human cell seeding on polymeric meshes. European Journal of Cardio-Thoracic Surgery. 1999;15:519–24. doi: 10.1016/s1010-7940(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 14.Lü L, Zhang L, Wai MSM, Yew DTW, Xu J. Exocytosis of MTT formazan could exacerbate cell injury. Toxicology in Vitro. 2012;26:636–44. doi: 10.1016/j.tiv.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. Journal of Immunological Methods. 1991;142:257–65. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 16.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Research. 1988;48:4827–33. [PubMed] [Google Scholar]
- 17.Riss TL, Moravec RA, Niles AL, Benink HA, Worzella TJ, Minor L. Assay Guidance Manual: Cell Viability Assays. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004. [PubMed] [Google Scholar]
- 18.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 2000;267:5421–6. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 19.Węsierska-Gądek J, Gueorguieva M, Ranftler C, Zerza-Schnitzhofer G. A new multiplex assay allowing simultaneous detection of the inhibition of cell proliferation and induction of cell death. Journal of Cellular Biochemistry. 2005;96:1–7. doi: 10.1002/jcb.20531. [DOI] [PubMed] [Google Scholar]
- 20.Mueller D, Tascher G, Damm G, Nüssler A, Heinzle E, Noor F. Real-time in situ viability assessment in a 3D bioreactor with liver cells using resazurin assay. Cytotechnology. 2013;65:297–305. doi: 10.1007/s10616-012-9486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloeckner H, Jonuleit T, Lemke H-D. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue™. Journal of Immunological Methods. 2001;252:131–8. doi: 10.1016/s0022-1759(01)00347-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Holsbeeks I, Impens S, Sonnaert M, Bloemen V, Luyten F, et al. Noninvasive Real-Time Monitoring by AlamarBlue® During In Vitro Culture of Three-Dimensional Tissue-Engineered Bone Constructs. Tissue Engineering Part C: Methods. 2013;19:720–9. doi: 10.1089/ten.tec.2012.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gaalen SM, de Bruijn JD, Wilson CE, van Blitterswijk CA, Verbout AJ, Alblas J, et al. Relating cell proliferation to in vivo bone formation in porous Ca/P scaffolds. Journal of Biomedical Materials Research Part A. 2010;92A:303–10. doi: 10.1002/jbm.a.32380. [DOI] [PubMed] [Google Scholar]
- 24.Caralt M, Uzarski JS, Iacob S, Obergfell KP, Berg N, Bijonowski BM, et al. Optimization and Critical Evaluation of Decellularization Strategies to Develop Renal Extracellular Matrix Scaffolds as Biological Templates for Organ Engineering and Transplantation. American Journal of Transplantation. 2015;15:64–75. doi: 10.1111/ajt.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boncler M, Różalski M, Krajewska U, Podsędek A, Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. Journal of Pharmacological and Toxicological Methods. 2014;69:9–16. doi: 10.1016/j.vascn.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 27.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 28.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–5. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyette JP, Gilpin SE, Charest JM, Tapias LF, Ren X, Ott HC. Perfusion decellularization of whole organs. Nat Protocols. 2014;9:1451–68. doi: 10.1038/nprot.2014.097. [DOI] [PubMed] [Google Scholar]
- 30.Wagner BA, Venkataraman S, Buettner GR. The rate of oxygen utilization by cells. Free Radical Biology and Medicine. 2011;51:700–12. doi: 10.1016/j.freeradbiomed.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabater D, Arriarán S, Romero MdM, Agnelli S, Remesar X, Fernández-López JA, et al. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci Rep. 2014;4:3663. doi: 10.1038/srep03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bittner CX, Loaiza A, Ruminot I, Larenas V, Sotelo-Hitschfe T, Gutiérrez R, et al. High resolution measurement of the glycolytic rate. Frontiers in Neuroenergetics. 2010;2:26. doi: 10.3389/fnene.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F, Tian J, Wang L, He P, Chen Y. Correlation between cell growth rate and glucose consumption determined by electrochemical monitoring. Sensors and Actuators B: Chemical. 2011;156:416–22. [Google Scholar]
- 34.O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–41. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 35.Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Optimally Porous and Biomechanically Compatible Scaffolds for Large-Area Bone Regeneration. Tissue Engineering Part A. 2012;18:1376–88. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu LLY, Weisel RD, Li R-K, Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2011;5:69–84. doi: 10.1002/term.292. [DOI] [PubMed] [Google Scholar]
- 37.Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–41. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Govoni M, Muscari C, Guarnieri C, Giordano E. Mechanostimulation Protocols for Cardiac Tissue Engineering. BioMed Research International. 2013;2013:918640. doi: 10.1155/2013/918640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tandon N, Cannizzaro C, Chao P-HG, Maidhof R, Marsano A, Au HTH, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protocols. 2009;4:155–73. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuramochi Y, Guo X, Sawyer DB, Lim CC. Rapid electrical stimulation induces early activation of kinase signal transduction pathways and apoptosis in adult rat ventricular myocytes. Experimental Physiology. 2006;91:773–80. doi: 10.1113/expphysiol.2006.033894. [DOI] [PubMed] [Google Scholar]
- 41.Kohn DF, Clifford CB. Chapter 4 - Biology and Diseases of Rats. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine. 2. Burlington: Academic Press; 2002. pp. 121–65. [Google Scholar]
- 42.Larson EM, Doughman DJ, Gregerson DS, Obritsch WF. A new, simple, nonradioactive, nontoxic in vitro assay to monitor corneal endothelial cell viability. Investigative Ophthalmology & Visual Science. 1997;38:1929–33. [PubMed] [Google Scholar]
- 43.Ansar Ahmed S, Gogal RM, Jr, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. Journal of Immunological Methods. 1994;170:211–24. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. Journal of Immunological Methods. 1997;204:205–8. doi: 10.1016/s0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Progressive blue-to-red color shift of resazurin-containing medium mixture during its perfusion of a HUVEC-regenerated lung. Photography of resazurin-containing medium sampled at 0, 10, 20, 30, 40, 50 and 60 min after the initiation of resazurin perfusion of the HUVEC regenerated lung.
Fig. S2. Long-term resazurin perfusion of a HUVEC-regenerated lung. (A,B) photography of the HUVEC lung before (A) and after 8-hour perfusion of resazurin (B). (C) Fluorescence intensities of medium sampled at each 30-min interval during the resazurin perfusion.
Fig. S3. Characterization of CB-EPCs. CB-EPCs were characterized by colony formation (A), CD31 expression (B), DiI-Ac-LDL uptake (C), and tube formation on matrigel (D).
Fig. S4. TUNEL staining of apoptotic cells in HUVEC (A) and CB-EPC (B) lungs on day 5 of culture.
Table S1. p-values of T-test comparing resazurin fluorescence intensities at each cell number/medium ratios (1, 0.5, 0.25, 0.1, 0.05, 0.025 and 0.01 million/ml) between two the volume formats (2 ml and 0.1 ml).