Abstract
BACKGROUND
High expression of matrix metalloproteinase 9 (MMP9) during vascular injury and inflammation plays an important role in atherosclerotic plaque formation and rupture. In the process of atherosclerosis, oxidized low-density lipoprotein (oxLDL) upregulates MMP9 in human aortic vascular smooth muscle cells (HA/VSMCs). Adiponectin is an adipose tissue-derived hormone that has been shown to exert anti-atherogenic and anti-inflammatory effects. The aim of this study was to investigate the effect of adiponectin on MMP9 expression under pathogenic condition created by oxLDL in HA/VSMCs.
METHODS
In this experimental study, HA/VSMC were stimulated with oxLDL alone and in the presence of adiponectin for 24 and 48 h. The expression of MMP9 gene was determined by real-time polymerase chain reaction method. The protein level of this gene was investigated by western blotting technique.
RESULTS
An oxLDL increased MMP9 expression 2.16 ± 0.24- and 3.32 ± 0.25-fold after 24 and 48 h, respectively and adiponectin decreased oxLDL-induced MMP9 expression in a time-dependent manner.
CONCLUSION
These results show that adiponectin changes extracellular matrix by reducing MMP9 mRNA and protein, therefore, may stabilize lesions and reduce atheroma rupture.
Keywords: Matrix Metalloproteinase 9, Adiponectin, Oxidized Low Density Lipoprotein
Introduction
Atherosclerosis is a multifactorial disease that remains one of the leading causes of mortality worldwide.1 Obesity and its dependence on the pathogenesis of cardiovascular disease have evoked great interest in understanding the impact of adipokine secreted from adipose tissue on atherosclerosis.2 It has been well established that adipose tissue constitutes a versatile endocrine gland in the body and is actively involved in the regulation of many biological processes.3 Some adipose tissue-derived factors have proinflammatory activity and, in contrast, some factors like adiponectin inhibit inflammatory processes.4 Fluctuation in adipokines is a key mechanism that connects obesity to increased risk of vascular complications.5 Adiponectin has attracted special attention of investigators because of its ability to impact cardiovascular disease.6 According to clinical studies, high molecular weight form of adiponectin is more clinically relevant7 because it exerts the protective effects on vascular diseases.8
Matrix metalloproteinases (MMP) are a family of zinc-dependent proteolytic enzymes, which are collectively capable of degrading various components of the extracellular matrix.9
Human epidemiological and genetic studies show that (MMP9) is the strongest candidate for inducing plaque rupture.10,11 These studies confirmed that MMP9 played a basic role in progression of arterial lesions because it regulated vascular smooth muscle cells (VSMCs) proliferation and migration into the intima.12 Several studies have indicated that oxidized low-density lipoprotein (oxLDL) induced MMP9 gene expression in smooth muscle cells and macrophages.13-15 It is well known that oxLDL is involved in the induction and also in the progression of atherosclerosis.16 Furthermore, it is responsible for destabilizing plaques through increased expression of metalloproteinases.17 However, the functions of adiponectin in atherosclerosis have not yet been fully understood. Further research regarding the mechanism of adiponectin action will lead to better understanding of the pathogenesis of atherosclerosis. Due to importance role of oxLDL and MMP9 in atheroma formation and progression, it seems that atheroma progression can be reduced by using adiponectin. The purpose of this study was to investigate the effect of adiponectin on MMP9 expression under pathogenic condition created by oxLDL in human aortic VSMCs (HA/VSMCs).
Materials and Methods
In this experimental study, HA/VSMCs prepared from Pasteur Institute of Iran were maintained in F12K medium. F12 media contained 0.05 mg/ml ascorbic acid, 0.01 mg/ml insulin, 0.01 mg/ml transferrin, 10 ng/ml sodium selenite, 0.03 mg/ml endothelial cell growth supplement, 2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid (HEPES) to a final concentration of 10 mm, TES to a final concentration of 10 mm, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.01% amphotericin B. HA/VSMCs between passages 3 and 7 were used in this experiment. For treatment, we seed HA/VSMCs into 12-well plate at a density of 15 × 103 cells/well and incubated them at 37 °C in 5% CO2. Cells achieving 80% confluence were treated by oxLDL.18 We treated cells with oxLDL (100 µg/ml)17 alone and in combination with adiponectin (5 µg/ml)19 for 24 and 48 h. The cells without any treatment were used as the control.
The treated cells were washed with cold phosphate-buffered saline (PBS). Total RNA was extracted with Biozol reagent (BioFlux-China), according to the manufacturer’s instructions. Total RNA concentration and quality were evaluated by NanoDrop spectrophotometer (Thermo-USA). The single stranded cDNA was synthesized using cDNA Synthesis Kit (Thermo, Canada) with 1 µg total RNA. The cDNA was amplified by real time-polymerase chain reaction (PCR) using SYBR® Green PCR Master Mix (Qiagen, Germany). Gene expression was detected by Rotor-Gene 3000 (Corbett, Australia). Results were normalized against the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Primers for MMP9 were as follows: Forward: 5'-GCTCACCTTCACTCGCGTGTA-3', reverse: 5'-TCCGTGCTCCGCGACA-3', and primers for GAPDH; forward: 5'- ACACCCACTCCTCCACCTTTG -3', reverse: 5'- TCCACCACCCTGTTGCTGTAG -3'. The temperature profile for the reaction was an initial stage of 95 °C for 5 min then 40 cycles of 95 °C for 15 s, 59 °C for 20 s, and 72 °C for 30 s. Results were normalized against the housekeeping gene GAPDH mRNA. Data analysis was performed on the basis of the comparative delta CT method with the formula 2-ΔΔCT to perform relative quantification of target genes (gene expression).
To determine MMP9 expression at the protein level, cells were washed with PBS and lysed with radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail. The lysates were centrifuged at 10,000 g for 10 min at 4 °C. To equalize the concentrations, protein concentrations were determined by NanoDrop spectrophotometer. Protein lysates with an equal volume of Lameli buffer (0.125 M Tris-HCl 4%, sodium dodecyl sulfate (SDS) 20%, glycine 10%, and 2-mercaptoethanol) were mixed and boiled for 5 min. To separate based on the size equal amounts of protein (300 µg) per lane were loaded onto 10% SDS-polyacrylamide gel electrophoresis. The proteins were blotted onto polyvinylidene difluoride (PVDF) membranes (Roche-Germany) at 120 V for 2 h in transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol). PVDF membranes were blocked overnight at 4 °C with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST). After being washed 3 times with TBST buffer, the blots were incubated with antibodies against MMP9 (1:3000 dilution; Abcam, Cambridge, UK) and antie dies against MMP9 (1:300Abcam, Cambridge, UK) as an internal control in blocking buffer (skim milk and TBST) and were shaken for 2 h. We washed membranes again and incubated them with goat polyclonal secondary antibody to rabbit IgG (1:5000 dilution; Abcam, Cambridge, UK) for 90 min. Finally, bands were visualized using BM blue-POD substrate (Roche-Germany).
All experiments were done in triplicate. Statistical analysis was done using by nonparametric Kruskal-Wallis test and pairwise comparisons among groups were performed by Mann-Whitney test. All statistical analyses were performed with Graph Pad Prism for Windows (version 5, Graph Pad, Software Inc., San Diego, CA, 2005). Graphs were represented as a mean ± standard error of mean, and P < 0.05 was considered as the level of significance.
Results
To determine the role of adiponectin protein in MMP9 expression in HA/VSMCs in the presence of oxLDL, the cells were stimulated with oxLDL alone and in the presence of adiponectin for 24 and 48 h.
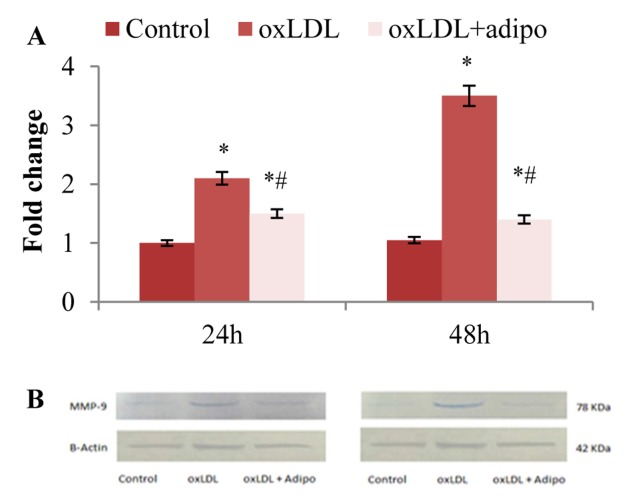
Our results show that oxLDL increased MMP9 expression 2.16 ± 0.24- and 3.32 ± 0.25-fold after 24 and 48 h, respectively (P < 0.05). An oxLDL-induced MMP9 expression was markedly inhibited by adiponectin. Adiponectin decreased oxLDL-induced MMP9 expression 34 and 61% after 24 and 48 h, respectively (P < 0.05) (Figure 1a).
Figure 1.
The effect of adiponectin on oxLDL-induced MMP9 expression after 24 and 48 h; (A) Real time PCR analysis of MMP9 mRNA expression levels in HA/VSMCs in the presence of oxLDL with or without adiponectin after 24 and 48 h. Values are expressed as mean ± SE from two independent experiments performed in triplicate. Differences between groups were determined as significant at P < 0.05; (B) MMP9 protein expression levels by western blotting for 24 and 48 h. β-actin was used as internal control * Means P< 0.05 compared to the control VSMCs; # Means P < 0.05 compared to the oxLDL-exposed VSMCs; oxLDL: Oxidized low-density lipoprotein; VSMCs: human aortic vascular smooth muscle cells; MMP9: matrix metalloproteinase 9; PCR: Polymerase chain reaction
Melting curves confirmed that the desired fragments were specifically amplified. In a parallel experiment, western blot analysis confirmed the changes observed at mRNA level (Figure 1b).
Discussion
Our findings indicate that adiponectin reduces gene expression and protein mass of MMP9 in a time-dependent manner. According to the anti-inflammatory properties of adiponectin and inflammatory role of MMP9, reduction of MMP9 mRNA observed by the adiponectin in this study was not unexpected. Adiponectin exerts protective effects on vascular diseases through its direct actions on vascular component cells including endothelial cells, macrophages, and smooth muscle cells.8,20,21 In vitro studies on VSMCs have shown that adiponectin can inhibit proliferation and migration of VSMCs induced by several atherogenic growth factors through inhibition of extracellular signal-regulated kinase activation.22 The results of the present study are consistent with previous results, and indicate that adiponectin through reducing MMP9 can be effective in reducing the risk of cardiovascular disease.22 Animal studies results have indicated a key role for adiponectin in the progression of atherosclerosis.22 Considering the fact that, the progression and stability of atherosclerotic lesions are associated with concentrations of metalloproteinases and their inhibitors,23 the effect of adiponectin on atheroma lesions in these experiments may be due to changes occurring in the extracellular matrix. The present study has shown that changes in the extracellular matrix occurred as the changes in MMP9 concentration happened. Our study has shown that reduction in MMP9 protein concentration is due to decrease in gene expression following adiponectin treatment. Experiment on macrophage shows that adiponectin augmented tissue inhibitor of metalloproteinases-1 (TIMP-1) expression without affecting the mRNA, protein levels, and activities of MMP-9.24 These results together with the fact that adiponectin with MMP9/TIMP-1 ratio has an inverse relationship in patients with acute coronary syndrome, suggest that adiponectin through the balancing of this ratio modulates plaque stability.25 The general conclusion from these studies that indicate adiponectin has protective properties is consistent with our findings. Studies performed on macrophages have shown that adiponectin has no effect on MMP9 expression,24 whereas our study shows that adiponectin has a direct effect on the mRNA and protein levels of MMP9. It could be concluded that the beneficial effect of adiponectin is more intense under pathogenic conditions that we have established in the presence of oxLDL. This demonstrates the value of clinical use of adiponectin. In general, adiponectin through reducing gene expression of MMP9 might contribute to change in the extracellular matrix, hence leading to its vasculoprotective activity through stabilization of atheroma lesions and reducing rupture of atheroma. It is hoped that these findings will lead to better understanding of the pathology of atherosclerosis and its treatment.
Conclusion
In summary, our findings in this experimental study indicate that adiponectin decreased oxLDL-induced MMP9 expression. These results suggest that adiponectin protects smooth muscle in the vessel wall from the damaging effect of oxLDL in pathologic condition.
Acknowledgments
This work has been obtained from the MSc thesis of Maryam Saneipour. We would like to thank the Research and Technology Deputy of Shahrekord University of Medical Science, Iran.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Tunstall-Pedoe H. Preventing Chronic Diseases. A Vital Investment: WHO Global Report. Geneva: World Health Organization, 2005. pp 200. CHF 30.00. ISBN 92 4 1563001. Int J Epidemiol. 2006;35(4):1107. [Google Scholar]
- 2.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ17229978. Mol Cell Endocrinol. 2010;316(2):129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94(10):2143–9. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Guzik TJ, Mangalat D, Korbut R. Adipocytokines - novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57(4):505–28. [PubMed] [Google Scholar]
- 6.Toth PP. Adiponectin and high-density lipoprotein: a metabolic association through thick and thin. Eur Heart J. 2005;26(16):1579–81. doi: 10.1093/eurheartj/ehi374. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29(6):1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114(5):361–74. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 9.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–85. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 11.Peeters W, Moll FL, Vink A, van der Spek PJ, de Kleijn DP, de Vries, et al. Collagenase matrix metalloproteinase-8 expressed in atherosclerotic carotid plaques is associated with systemic cardiovascular outcome. Eur Heart J. 2011;32(18):2314–25. doi: 10.1093/eurheartj/ehq517. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Ning W, Tan Z, Gong Z, Li X. Mechanism of matrix metalloproteinase axis-induced neointimal growth. J Mol Cell Cardiol. 2014;66:116–25. doi: 10.1016/j.yjmcc.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Wilson D, Massaeli H, Pierce GN, Zahradka P. Native and minimally oxidized low density lipoprotein depress smooth muscle matrix metalloproteinase levels. Mol Cell Biochem. 2003;249(1-2):141–9. [PubMed] [Google Scholar]
- 14.Ardans JA, Economou AP, Martinson JM, Zhou M, Wahl LM. Oxidized low-density and high-density lipoproteins regulate the production of matrix metalloproteinase-1 and -9 by activated monocytes. J Leukoc Biol. 2002;71(6):1012–8. [PubMed] [Google Scholar]
- 15.Xu XP, Meisel SR, Ong JM, Kaul S, Cercek B, Rajavashisth TB, et al. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation. 1999;99(8):993–8. doi: 10.1161/01.cir.99.8.993. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D. Hypercholesterolemia and inflammation in atherogenesis: two sides of the same coin. Mol Nutr Food Res. 2005;49(11):995–8. doi: 10.1002/mnfr.200500081. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Song L, Wu S, Fan F, Lopes-Virella MF. Oxidized LDL differentially regulates MMP-1 and TIMP-1 expression in vascular endothelial cells. Atherosclerosis. 2001;156(1):119–25. doi: 10.1016/s0021-9150(00)00638-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang YS, Wang HY, Liao YC, Tsai PC, Chen KC, Cheng HY, et al. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc Res. 2012;95(4):517–26. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 19.Ding M, Xie Y, Wagner RJ, Jin Y, Carrao AC, Liu LS, et al. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arterioscler Thromb Vasc Biol. 2011;31(6):1403–10. doi: 10.1161/ATVBAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, et al. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29(13):3487–99. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285(9):6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94(10):2137–42. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 24.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109(17):2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 25.Cheng M, Hashmi S, Mao X, Zeng QT. Relationships of adiponectin and matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio with coronary plaque morphology in patients with acute coronary syndrome. Can J Cardiol. 2008;24(5):385–90. doi: 10.1016/s0828-282x(08)70602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]