Abstract
BACKGROUND
Transcatheter aortic valve implantation (TAVI) was known as an alternative technique for treatment of severe aortic stenosis (AS). This technique is controversial in bicuspid aortic valve (BAV). Here, we report TAVI for severe AS in a BAV setting in a patient with serious lung disease.
CASE REPORT
A 68-year-old woman with a history of coronary artery bypass graft, BAV and severe AS, asthma, who had repeatedly denied any suggestion for open heart surgery, was our volunteer candidate for TAVI. The peak and mean pressure gradient decreased from 53 and 43 mm Hg to 13and 6 mm Hg respectively.
CONCLUSION
TAVI could be a viable option for highly selected patients with AS and BAV who have a prohibitive risk for open heart surgery.
Keywords: Transcatheter Aortic Valve Implantation, Aortic Stenosis, Bicuspid Aortic Valve
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease1 with a potential to progress finally to aortic stenosis (AS). In patients over 80 years of age, BAV is the cause of 30-50% of the cases with severe AS, necessitating invasive treatment.1,2
There is a limited experience with transcatheter aortic valve implantation (TAVI) in the setting of a bicuspid valve. Incomplete valve expansion or malposition are the most frequent problems in this setting. There is, therefore, a concern over scheduling these patients for TAVI due to the bulky and asymmetric leaflets in a BAV, which may result in hemodynamic disturbances, reduced durability, paravalvular leaks, or embolization. Another complication that may occur in these patients is the occlusion of the left main artery by the bulky valve.
Many authors believe that congenital BAV is a contraindication for TAVI1,3-5 however, successful cases of TAVI in patients with severe symptomatic AS who were very high risk for open heart surgery have recently been published.1,3,4
Here we introduce a patient with severe AS due to BAV who was not a candidate for open heart surgery and was finally treated with TAVI.
Case Report
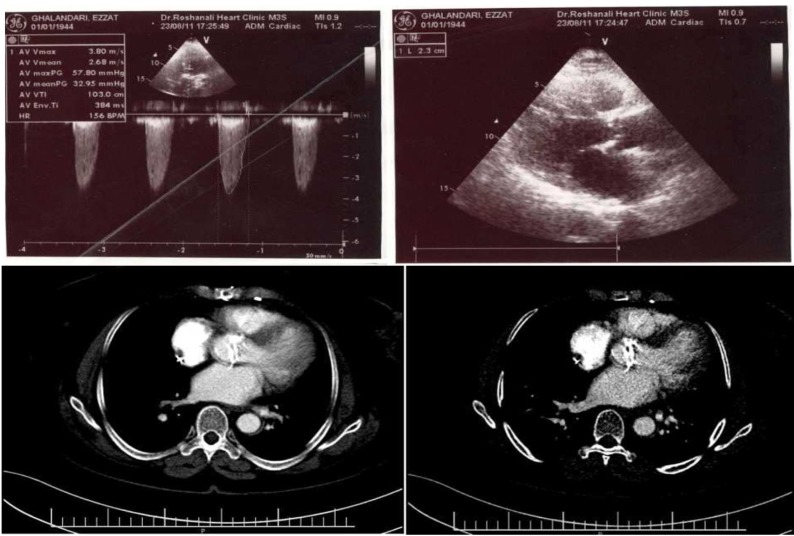
A 68-year-old woman with a history of coronary artery disease and coronary artery bypass graft surgery in 2001, presented with dyspnea (The Canadian Cardiovascular Society Functional Class IV) and chest pain. The echocardiography (ECG) findings included normal sinus rhythm, normal axis, heart rate of 72 beats/min, ST depression, and T inversion in leads I, AVL, and V1-V6. Echocardiography revealed BAV with severe AS and moderate thickening and calcification and trivial aortic insufficiency. In addition, the ascending aorta was mildly dilated to 3.9 cm with mild left ventricular (LV) hypertrophy, diastolic LV dysfunction, good LV size and systolic function, and good right ventricular size and function. The aortic annular diameter and aortic valve area were about 2.3 cm and 0.6 cm respectively (Figure 1).
Figure 1.
Echocardiography and computed tomography angiography of the patient showing a bicuspid aortic valve with a peak pressure gradient of 57.8 mm Hg
On her coronary angiography in December 2011, the left anterior descending artery had significant stenosis in the proximal portion with a good competitive flow from the left internal mammary artery. The left circumflex artery was cut-off at its distal portion, and the right coronary artery was diffusely diseased. The saphenous vein graft on the posterior descending artery was patent, and so was the left internal mammary artery on the left anterior descending artery. The saphenous vein graft on the obtuse marginal was occluded based on its stump on aortography. Multi-slice computed tomography angiography showed the left internal mammary artery graft was exactly behind the sternum suggesting a possible risk of compromising the left internal mammary artery during surgery.
The patient had moderate asthma, for which she had been on corticosteroids for quite a long period of time. She had repeatedly denied any suggestion for open heart surgery during the previous years. Considering the patient’s past medical history and her clinical conditions, our cardiac surgeons refused to perform open-heart surgery, and she was referred for TAVI.
The details of the TAVI procedure, especially drawbacks of this technique for patients with BAV were described for the patient and her family, followed by written consents from them. General anesthesia, transesophageal echocardiography, pre-implantation balloon aortic valvuloplasty, and rapid ventricular pacing were carried out as a routine standard protocol. Arteriotomy was performed to obtain an appropriate femoral access. An 18 French 26 mm Nova flex Edwards-Sapien XT valve (Edwards Lifesciences, Inc., Irvine, California) was selected and all the stages of implantation were evaluated via intra-procedural transesophageal echocardiography (Figures 2 and 3).
Figure 2.
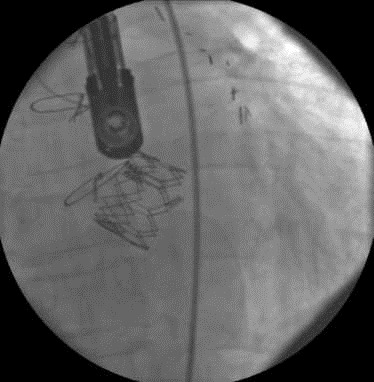
The implanted prosthetic aortic valve
Figure 3.
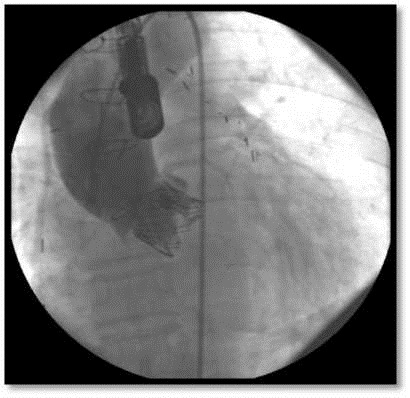
Aortography after transcatheter aortic valve implantation, showing no obvious aortic insufficiency
Pre-implantation balloon valvuloplasty was done with a 20-40 balloon, and full balloon expansion could be achieved. The above-mentioned valve was thereafter successfully implanted. The peak pressure gradients (PPG) and mean pressure gradients (MPG) by echocardiography decreased from 57 mm Hg to 13 mm Hg and from 43 mm Hg to 6 mm Hg, respectively. No obvious aortic insufficiency was detected. A 30 day follow-up showed significant improvement in clinical and hemodynamic findings in that the patient had milder dyspnea (The Canadian Cardiovascular Society functional Class II), prosthetic aortic valve PPG of 13.1 mm Hg, MPG of 6.5 mm Hg, mild tricuspid regurgitation, and systolic pulmonary artery pressure of 35 mm Hg (Figure 4).
Figure 4.
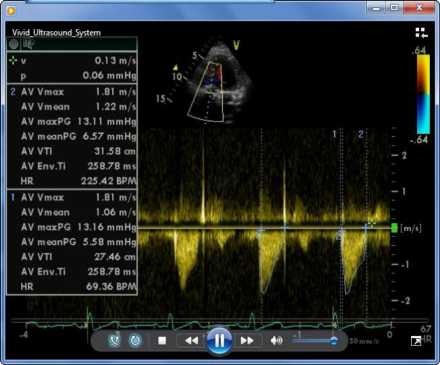
Doppler echocardiography after transcatheter aortic valve implantation showed peak pressure gradient: 13.1 mean pressure gradients: 6.5 mmHg
Discussion
Up to one-third of patients who require life-saving surgical aortic valve replacement are denied surgery due to high co-morbidities resulting in a higher operative mortality rate.6 In the past, such patients could only be treated with medical therapy or percutaneous aortic valvuloplasty. Neither of those approaches was demonstrated to improve the survival. With advances in interventional cardiology, transcatheter methods have been developed for aortic valve replacement with an aim of offering a therapeutic solution for patients unfit for surgical therapy.6
Transcatheter stent-valve implantation in stenotic congenital BAV is under debate. Heavily calcified elliptic bicuspid valves represent a contraindication to catheter-based valve therapies because of concerns over the risks for stent-valve displacement, distortion, or malfunctioning after implantation.5 Some previous studies have advised against TAVI in heavily calcified aortic valves because of the very high possibility of congenital malformation (BAV or unicuspid aortic valve) in those valves.7 Given that most of those concerns about poor results of TAVI in BAV originated from the experimental studies done by self-expandable stents,1 the recommendations against TAVI in patients with BAV are not based on well-designed clinical studies. Indeed, there have recently been a few reports of successful TAVI in patients with BAV,1,3,5,8 consequently, TAVI seems promising in the treatment of the severe stenosis of BAV, especially in high-risk patients.
In our case, significant clinical improvement (The Canadian Cardiovascular Society functional Class IV to II) was achieved, and PPG and MPG decreased from 57 mm Hg to 43 mm Hg to 13 mm Hg to 6 mm Hg, respectively.
Wijesinghe et al.1 published their experience on 11 patients and reported significant symptomatic and hemodynamic improvement in 91% of their patients. In their study, MPG decreased from 41 ± 22.4 mm Hg to 13.4 ± 5.7 mm Hg. Baralis et al.3 and Ferrari et al.5 reported successful TAVI in a BAV setting in two separate cases with significant improvement in clinical situation. Himbert et al. reported the largest series (15 cases) of TAVI in BAV:9 the MPG decreased from 60 ± 19 mm Hg to 11 ± 4 mm HG and significant clinical improvement was achieved in 80% of the patients and after 7-8 months follow-up, one patient had died due to aortic dissection and two patients had hospitalization because of dyspnea (functional class higher than II).
In our patient, there was no obvious paravalvular leakage on intraoperative transesophageal echocardiography and on transthoracic echocardiography in 1-month follow-up. In contrast, in the Himbert et al.’s study, periprosthetic leaks (< 1+) were observed in 13 of 15 patients.9 Wijesinghe et al. reported 2 out of 11 patients with moderate paravalvular leaks.1
Conclusion
Contrary to concerns raised in recent studies, our experience of TAVI in a patient with BAV shows that this modality could be a viable option for patients with prohibitive risk for open heart surgery. This technique should, however, be performed cautiously, paying sufficient heed to preoperative and intraoperative valve imaging, in meticulously selected patients.
Acknowledgments
We would like to thank the patient for her participation in the present study.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Wijesinghe N, Ye J, Rodes-Cabau J, Cheung A, Velianou JL, Natarajan MK, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2010;3(11):1122–5. doi: 10.1016/j.jcin.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Roberts WC, Janning KG, Ko JM, Filardo G, Matter GJ. Frequency of congenitally bicuspid aortic valves in patients >/=80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol. 2012;109(11):1632–6. doi: 10.1016/j.amjcard.2012.01.390. [DOI] [PubMed] [Google Scholar]
- 3.Baralis G, Di Gregorio O, Riva L, Steffenino G, Grossi C, Locatelli A. Transcatheter aortic valve implantation in bicuspid aortic valve: never say never. G Ital Cardiol (Rome) 2012;13(1):67–70. doi: 10.1714/1015.11058. [DOI] [PubMed] [Google Scholar]
- 4.Zegdi R, Lecuyer L, Achouh P, Didier B, Lafont A, Latremouille C, et al. Increased radial force improves stent deployment in tricuspid but not in bicuspid stenotic native aortic valves. Ann Thorac Surg. 2010;89(3):768–72. doi: 10.1016/j.athoracsur.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari E, Locca D, Sulzer C, Marcucci C, Rizzo E, Tozzi P, et al. Successful transapical aortic valve implantation in a congenital bicuspid aortic valve. Ann Thorac Surg. 2010;90(2):630–2. doi: 10.1016/j.athoracsur.2009.12.080. [DOI] [PubMed] [Google Scholar]
- 6.Akin I, Kische S, Rehders TC, Nienaber CA, Rauchhaus M, Ince H, et al. Indication for percutaneous aortic valve implantation. Arch Med Sci. 2010;6(3):296–302. doi: 10.5114/aoms.2010.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts WC, Ko JM, Filardo G. Comparison of heavier versus lighter operatively excised stenotic aortic valves in adults with aortic stenosis and implications for percutaneous aortic valve implantation without replacement. Am J Cardiol. 2009;104(3):393–405. doi: 10.1016/j.amjcard.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Jilaihawi H, Asgar A, Bonan R. Good outcome and valve function despite Medtronic-corevalve underexpansion. Catheter Cardiovasc Interv. 2010;76(7):1022–5. doi: 10.1002/ccd.22648. [DOI] [PubMed] [Google Scholar]
- 9.Himbert D, Pontnau F, Messika-Zeitoun D, Descoutures F, Detaint D, Cueff C, et al. Feasibility and outcomes of transcatheter aortic valve implantation in high-risk patients with stenotic bicuspid aortic valves. Am J Cardiol. 2012;110(6):877–83. doi: 10.1016/j.amjcard.2012.04.064. [DOI] [PubMed] [Google Scholar]