Abstract
BACKGROUND
The effect of legume-based hypocaloric diet on cardiovascular disease (CVD) risk factors in women is unclear. This study provides an opportunity to find effects of high-legume diet on CVD risk factors in women who consumed high legumes at baseline.
METHODS
This randomized controlled trial was undertaken in 34 premenopausal women with central obesity. After 2 weeks of a run-in period on an isocaloric diet, subjects were randomly assigned into two groups: (1) hypocaloric diet enriched with legumes (HDEL) (n = 17) (two servings per day) and (2) hypocaloric diet without legumes (HDWL) (n = 17) for 6 weeks. The following variables were assessed before intervention, 3, and 6 weeks after it: Waist to hip ratio (WHR), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high-sensitive-C-reactive protein (hs-CRP), total antioxidant capacity (TAC), nitric oxides (NOx), and Malondialdehyde (MDA).
RESULTS
Both hypocaloric diets reduced hs-CRP in 3 weeks and returned it to basal values after 6 weeks (P = 0.004). HDWL significantly reduced WHR [P = 0.010 (3.2%)] and increased TC [P < 0.001 (6.3%)]. Despite the significant effect of HDEL on increasing TAC in 3 weeks [P = 0.050 (4%)], the level of TAC remained the same in 6 weeks. None of the diets had any significant effects on NOx and MDA.
CONCLUSION
The study indicated that beneficial effects of legumes on TC, LDL-C, and hs-CRP were achieved by three servings per week, and consuming more amounts of these products had no more advantages.
Keywords: Legume, Cardiovascular Disease, Caloric Restriction, Central Obesity, Premenopause
Introduction
Cardiovascular disease (CVD) is number one killer in the world.1 Although many factors can increase the risk of CVDs, central obesity is usually considered underlying risk factor for CVD, which can disrupt lipid profile and endothelial function and enhance inflammatory and oxidative markers.2 Central obesity is more prevalent among men around the globe; however, some studies shows that it is more common across women in Middle East countries.3
Although CVDs are one of the major causes of morbidity and mortality, some aspects of the relationship between nutrition and CVD are still unknown. Legumes are one of the main important fractions of healthy dietary patterns and they are also relatively inexpensive sources of protein, fiber, phytochemicals, vitamins and minerals.4 That is the reason why studies related to legumes and their beneficial effects on cardiovascular risk factors is the focus of a body of recent nutrition research.5-7 The beneficial effects of soybeans on lipids profile are well known.8 Soy beans can protect the body from oxidative stress because it has isoflavones, saponins, and other components with anti-oxidant capacity.8 Low amounts of soy beans are consumed in Iran, while non-soy legumes such as red, white and wax beans, cowpea, chickpeas, split peas, and lentils are conventional foods, and thinking to cook without non-soy legumes is a hard task. Non-soy legumes and soy beans have a similar composition. Nutritionists are increasingly concerned about the beneficial effects of non-soy legumes on CVD risks.5,9-11 Several studies showed different aspects of the beneficial effects of legumes on CVD. Kabagambe et al. concluded the risk of myocardial infarction was reduced by 38% in the subjects who consumed one serve of beans per day in a case-control study9 and similar results were reported in a cohort study conducted by Bazzano et al.10 Two RCTs showed that an isocaloric diet with high legumes could reduce total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL-C) more than an isocaloric diet containing no legumes in men. Other RCTs which prescribed hypocaloric diets with high legumes showed more reduction in weight, TC, LDL, systolic blood pressure, Malondialdehyde (MDA), CRP, complement C3 and urinary 8-Isoprostane F2a in both genders compared to a legume-less hypocaloric diet.6,11,12 Our present study takes advantages of higher consumption of legumes among subjects at baseline in comparison with other similar studies.13-15 The baseline amount of legume consumption in participants is effective in outputs of studies prescribing legume enriched diets. In Middle East countries, the consumption of legumes is more common than western countries. The mean intake of legumes among Iranians is almost three servings per week compared to two servings per week in US and Spain.13-15 To our knowledge, this is the first study that investigates the role of legume-based hypocaloric diet with the exclusion of soy bean on CVD risk factors among women with abdominal obesity.
Materials and Methods
The study was a voluntary based randomized control trial with an 8 weeks follow-up period. To select the participants, we published advertisements in local newspapers. A total of 257 pre-menopausal women were found eligible to enter the study.
Inclusion criteria were age (pre-menopausal women aged 20-50 years), waist circumference (WC) >88 cm, no participation in weight-reduction programs, and maintenance of a stable weight (± 2 kg) during the previous 6 months.
Exclusion criteria were: any secondary cause of hyperglycemia (as trauma) or hypertension (as renal disease); treatment with oral hypoglycemic agents or insulin, consumption of anti-lipemic drugs or anti-hypertensive drugs; consumption of vitamin or mineral supplements or antacids containing magnesium or calcium; psychiatric disorders; untreated hypothyroidism; cancer; hepatic, systemic, pulmonary, renal or CVDs; inflammatory or infectious diseases; smoking; alcoholism; and legume intolerance.
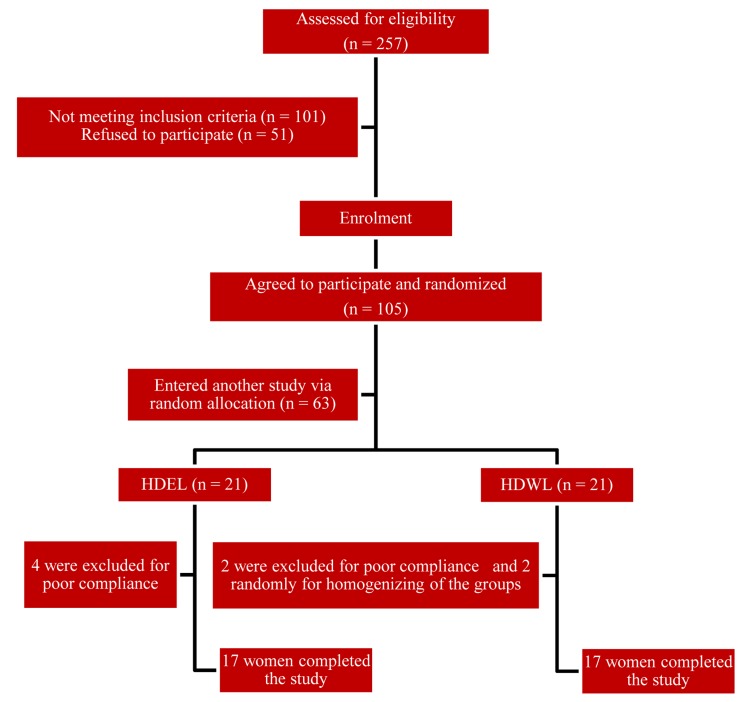
Finally, 42 pre-menopausal women were enrolled. Of these, 8 subjects did not complete the study: 4 patients in hypocaloric diet enriched with legumes (HDEL) group, and 2 patients in hypocaloric diet without legumes (HDWL) group because of poor compliance, and 2 patients in the HDWL group to homogenize the groups. Thirty-four women remained for the study analysis. Figure 1 shows the flowchart of the participants of the study.
Figure 1.
Flowchart for enrolment of participants HDEL: Hypocaloric diet enriched in legumes; HDWL: Hypocaloric diet without legumes
The study was registered at www.irct.ir (irct ID: irct138712101720N1) and approved by the Ethics Committee of Tabriz University of Medical Sciences, Iran. Written informed consent was obtained from all selected subjects.
The caloric needs for each subject were determined separately using the equation from the Food and Nutrition Board of the Institute of Medicine.16 In the run-in period, the participants consumed an isocaloric diet for 2 weeks. In the intervention period, members of first group ate HDEL (which comprised two servings or 1 cup per day of cooked non-soy legumes including red, white and wax beans; cowpea, chickpeas, split peas; and lentil instead of meat), and second group ate HDWL. The composition of each diet was 55% carbohydrate, 30% fat, and 15% protein. All participants in two groups were given a diet of 500-kcal less than their caloric needs in the intervention period. Participants were being visited every week; each session for each participant lasted 20-30 min. Behavioral counseling was instigated for all participants. The nutritionist described the advantages of diets for the participants, explaining that the central obesity could be controlled by continuing the diets. Diets were prescribed individually using a calorie count system. An “exchange list” was also given to each participant for calorie counting and exchanging food items. A nutritionist taught participants how to write “food diaries”. Each participant had to write her 3 days diet and physical-activity records before the run-in period as well as before, in the middle, and at the end of the intervention. The diaries were evaluated by the investigators. A validated menu of 7 days with 42 meals and snacks at 17 calorie levels (1,200-2800) was developed for each diet. Participant compliance was evaluated by reviewing the 3 days food records and weekly visits. Each week, the number of reported food-group exchanges from 3 days food records was compared with prescribed exchanges. Participants who completed ≥ 80% of the planned diets were encouraged to follow it diligently for the subsequent weeks. Volunteers who did not complete ≥ 80% of the prescribed diets for 2 successive weeks were excluded from the study (n = 6).
We made weekly contacts with the subjects during the 8 weeks period of the study. The true isocaloric needs of some of the subjects were different from the amount determined in the equation from the Food and Nutrition Board at the Institute of Medicine. In such cases, an isocaloric diet would cause the reduction or gain of weight rather than weight maintenance. Such individuals could cause biases in the study. Hence, among individuals eligible to enter the study, only those who maintained their weight at the end of the run-in period using an isocaloric diet calculated according to the equation were selected (n = 42). Moreover, the dietary pattern of each participant was different and could affect the results of interventions. Therefore, a run-in period was planned to standardize macronutrient consumption and to get detailed information about the study population. After 2 weeks of the run-in period on an isocaloric diet, subjects were randomly assigned into two groups: (1) HDEL and (2) HDWL for 6 weeks. To get randomized and matched groups, all participants were divided into four groups using factor analysis method. Participants in each group were similar in general characteristics and CVD risk factors. Participants in each group were then randomly allocated to two study groups. We repeated random allocation several times and selected the most homogenous groups. For the allocation of the participants, a computer-generated list of random numbers was used.
The measurements were obtained before, in the middle, and at the end of the intervention. Participants were told not to vary their usual physical activity during the study.
All measurements were conducted by the same researcher using the same instrument at the baseline and follow-up assessments. Laboratory staff was blinded about grouping of the participants. WC was measured (to the nearest 0.1 cm) at the narrowest point and the hip circumference at the largest circumference without pressure to the body surface by the light clothing using a standard tape measure.
After 12 h fasting, blood samples were taken, centrifuged (500 g, 10 min, 4 °C), and serums were separated. All parameters except MDA, total antioxidant capacity (TAC) and nitric oxides (NOx) were measured on the day of blood collection. Serums were frozen at -80 °C until they were analyzed for the assessment of other parameters.
Levels of TC were measured by enzymatic means (ParsAzmoun, Tehran, Iran). Levels of LDL-C were calculated using the Friedewald formula. Plasma concentrations of high-sensitive-C-reactive protein (hs-CRP) were measured using an immunoturbidimetric assay with an enzymatic kit (ParsAzmoun).17
Levels of nitrites/nitrates were measured concurrently using the Griess reaction.18 Briefly, nitrates were reduced to nitrites by vanadium (III), and then the level of total nitrites measured. MDA was measured using a modified version of the Yagi et al. protocol based on the thiobarbituric acid reaction.19 We used the ferric reducing ability of plasma assay for measuring TAC based on the protocol devised by Benzie and Starin.20
Inter- and intra-assay coefficients of variation were 1.22 and 0.61% for TC and 1.7 and 1% for hs-CRP, respectively.
Additional covariate information was obtained by the validated questionnaires. “Chronic dieters” were distributed into the groups as they were likely to lose less weight with hypocaloric diet. Subjects were stratified into three levels considering the following parameters: (a) education level (not completed high school, diploma, and university graduates); (b) subject income level (no income (housewife), < $350 (USA) per month, and > $350 (USA) per month); (c) family income level [< $350 (USA) per month, ≥ $350 (USA) < $700 (USA), ≥ $700 (USA)]; (d) overweight subjects and the metabolic syndrome in the family (any relative, first-degree relative, and second-degree relative diagnosed overweight or with metabolic syndrome). A participant was characterized as overweight if the body mass index was > 25 kg/m2. Definition of the metabolic syndrome was based on the criteria set by the Adult Treatment Panel III.21
The sample size for each group was estimated based on the studies conducted on obese women.22,23 With a 1-β = 95% and 1-α = 95%, the maximum sample size was achieved from WC indicators and were evaluated to be 16 persons. Values are means ± standard deviation at each time interval.
We used nested M-ANOVA repeated measurements of a multifactor model by a Minitab (version 13, Minitab, State College, PA, USA). to recognize the effect of interventions on variables. In this method, we also used another model for controlling the effect of the reduction in WC. Mauchly’s sphericity test was used to validate repeated measure analysis. If the condition of sphericity was met, univariate model of repeated measurement was used. Multivariate test statistics (MANOVA) was used in instances that sphericity was violated. ANOVA repeated measurements with Bonferroni post hoc analysis was used for evaluating the within group effect of interventions in each group.
We used independent t test and χ2 test to assess significant differences in baseline values among two groups. For appropriate variables, we merged subclasses of variables and then used the χ2 test. P < 0.050 (two-tailed) was considered significant.
Results
The general characteristics of the subjects are shown in table 1. The mean age of obesity onset in all participants was 16.4 years. Thirty percent of participants did not complete high school, and 65% of participants were housewives. Family income per month for 50% of the participants was ≥ $350 (USA) < $700 (USA). Only 6% of the participants did not have overweight member, and 47% did not have a history of the metabolic syndrome among first- and second-degree relatives. Mean values for the number of diets completed and weight losses were 1 and 6 kg, respectively, but only 3% of participants maintained their weight loss. There were no significant differences in the general characteristics of the two groups.
Table 1.
Baseline characteristics of the groups
| Baseline characteristics | Treatments |
Total | P | |
|---|---|---|---|---|
| HDEL | HDWL | |||
| n | 17 | 17 | 34 | - |
| Age (year) (mean ± SE) | 35.5 ± 8.6 | 36.8 ± 7.8 | 36.1 ± 8.2 | 0.340* |
| Height (cm) (mean ± SE) | 158.6 ± 7.0 | 157.0 ± 5.5 | 157.8 ± 6.0 | 0.510* |
| Age of obesity onset (year) (mean ± SE) | 17.2 ± 8.7 | 15.6 ± 11.3 | 16.4 ± 9.8 | 0.330* |
| Education [n (%)] | ||||
| Not obtained a high-school diploma | 8 (47) | 2 (12) | 10 (30) | 0.160** |
| High school diploma | 4 (23) | 8 (47.1) | 12 (35) | |
| University graduates | 5 (29) | 7 (41) | 12 (35) | |
| Income status [n (%)] | ||||
| Without income (housewife) | 12 (70) | 10 (59) | 22 (65) | 0.250** |
| < $350 (USA) per month | 2 (12) | 1 (6) | 3 (9) | |
| ≥ $350 (USA) per month | 3 (18) | 6 (35) | 9 (26) | |
| Overweight subjects in family [n (%)] | ||||
| Any relative | 1 (6) | 1 (6) | 2 (6) | 0.120** |
| First-degree relatives | 13 (76) | 15 (88) | 28 (82) | |
| Second-degree relatives | 3 (18) | 1 (6) | 4 (12) | |
| The metabolic syndrome in family [n (%)] | ||||
| Any relative | 9 (53) | 7 (41) | 16 (47) | 0.190** |
| First-degree relatives | 7 (41) | 9 (53) | 16 (47) | |
| Second-degree relatives | 1 (6) | 1 (6) | 2 (6) | |
| Family economic status [n, (%)] | ||||
| < $350 (USA) per month | 4 (23) | 3 (17) | 7 (20) | 0.150** |
| ≥ $350 (USA)> $700 (USA) per month | 7 (41) | 10 (59) | 17 (50) | |
| ≥ $700 (USA) per month | 6 (35) | 4 (24) | 10 (30) | |
| Dieting history [n (%)] | ||||
| Yes | 9 (53) | 14 (82) | 23 (68) | 0.270** |
| No | 8 (47) | 3 (18) | 11 (32) | |
| Number of diets completed (n) (mean ± SE) | 1.1 ± 1.6 | 0.9 ± 0.5 | 1.0 ± 1.2 | 0.910* |
| Dieting duration (day) (mean ± SE) | 253.0 ± 877.0 | 81.0 ± 97.0 | 167.0 ± 620.0 | 0.400* |
| Weight loss in dieting periods (kg) (mean ± SE) | 4.2 ± 8.3 | 7.8 ± 9.2 | 6.0 ± 7.8 | 0.180* |
| Time of dieting [n (%)] | ||||
| Any time | 8 (46) | 3 (18) | 11 (32) | 0.220** |
| 6 months until 1 year ago | 3 (18) | 5 (29) | 8 (23) | |
| 1-5 years ago | 3 (18) | 7 (41) | 10 (30) | |
| 5 years ago | 3 (18) | 2 (12) | 5 (15) | |
| Weight maintenance in past diets [n (%)] | ||||
| No dieting | 9 (53) | 3 (17) | 12 (35) | 0.120** |
| Maintenance of redaction | 0 (0) | 1 (6) | 1 (3) | |
| Some maintenance | 0 (0) | 2 (12) | 2 (6) | |
| No maintenance | 8 (47) | 11 (65) | 19 (56) | |
HDEL: Hypocaloric diet enriched in legumes; HDWL: Hypocaloric diet without legumes; SE: Standard error
Independent t test was used;
χ2 test was used
Table 2 represents food intake of the groups, calorie intake, and calories expended in activities before the run-in period. The mean intake of milk and fruit in both groups was low. There were no differences before the run-in period with respect to food intake between the groups.
Table 2.
Intake of food, calorie intake and calories expended in activity before the run-in period
| Variables | HDEL | HDWL | P |
|---|---|---|---|
| Milk (serving/day) | 0.6 ± 0.6 | 0.6 ± 0.5 | 0.940 |
| Vegetable (serving/day) | 2.6 ± 0.2 | 2.0 ± 1.1 | 0.500 |
| Fruit (serving/day) | 1.6 ± 1.2 | 1.9 ± 1.3 | 0.450 |
| Meat (serving/day) | 2.9 ± 1.6 | 3.4 ± 1.0 | 0.330 |
| Cereal (serving/day) | 9.0 ± 3.8 | 8.5 ± 3.8 | 0.780 |
| Legumes (serving/day) | 0.5 ± 0.5 | 0.4 ± 0.3 | 0.620 |
| Sugar (serving/day) | 2.4 ± 1.1 | 2.4 ± 1.2 | 0.900 |
| Fat (serving/day) | 11.1 ± 7.6 | 12.5 ± 5.5 | 0.770 |
| Activity calories (kcal/day) | 324.0 ± 186.0 | 295.0 ± 205.0 | 0.120 |
| Calories intake (kcal/day) | 1883.0 ± 725.0 | 1929.0 ± 520.0 | 0.240 |
Values are means ± SE; HDEL: Hypocaloric diet enriched in legumes; HDWL: Hypocaloric diet without legumes; SE: Standard error
The effect of interventions on CVD risk factors are outlined in table 3. No significant difference was found in basal (before intervention) measurements between two groups (not shown in table 3).
Table 3.
Effect of interventions on cardiovascular risk factors by nested M-ANOVA for repeated measurements of a multi-factor model
| Variables | Treatment |
P(hypocaloric diet) | P(legumes) | P(hypocaloric diet and legume) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HDEL |
HDWL |
||||||||
| T1 (mean ± SE) | T2 (mean ± SE) | T3 (mean ± SE) | T1 (mean ± SE) | T2 (mean ± SE) | T3 (mean ± SE) | ||||
| WHR | 0.8 ± 0.05 | 0.8 ± 0.05 | 0.8 ± 0.05 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.23 | 0.97 | 0.95 |
| TC (mg/dl) | 188.4 ± 31.5 | 197.9 ± 34.8 | 191.0 ± 42.2 | 188.0 ± 19.9 | 193.8 ± 28.3 | 199.6 ± 25.2 | 0.41 | 0.78 | 0.12 |
| LDL-C (mg/dl) | 111.6 ± 30.0 | 120.9 ± 32.0 | 117.5 ± 40.0 | 110.4 ± 17.8 | 124.1 ± 20.2 | 123.4 ± 15.6 | 0.19 | 0.69 | 0.35 |
| hs-CRP (mg/l) | 2.5 ± 1.7 | 1.2 ± 1.1 | 2.2 ± 1.6 | 2.4 ± 1.9 | 1.3 ± 1.5 | 1.8 ± 1.5 | 0.004 | 0.87 | 0.28 |
| TAC (μmol/l) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.88 | 0.88 | 0.14 |
| NOx (μmol/l) | 29.3 ± 33.9 | 37.2 ± 38.8 | 33.0 ± 30.4 | 29.0 ± 62.2 | 31.0 ± 65.9 | 49.8 ± 76.8 | 0.53 | 0.74 | 0.43 |
| MDA (nmol/ml) | 2.2 ± 0.7 | 2.4 ± 1.0 | 2.3 ± 0.9 | 2.2 ± 0.2 | 2.3 ± 1.0 | 2.4 ± 1.0 | 0.74 | 0.92 | 0.73 |
Values are means ± SE; HDEL: Hypocaloric diet enriched in legumes; HDWL: Hypocaloric diet without legumes; T1: Before intervention; T2: Three weeks after intervention; T3: Six weeks after intervention; WHR: Waist to hip ratio; TC: Total cholesterol; LDL-C: Low-density lipoprotein-cholesterol; hs-CRP: High-sensitivity C-reactive protein; TAC: Total antioxidant capacity; NOx: Nitrite/nitrate; MDA: Malondialdehyde; SE: Standard error
After 6 weeks HDEL and HDWL administration, the following results were obtained (Table 3): (1) Time effect: Both HDEL and HDWL significantly reduced the hs-CRP level in the first 3 weeks and returned it to basal levels in the subsequent 3 weeks (P = 0.004) but this significant effect disappeared after controlling of WC and/or weight reduction. (2) Treatment effect: No significant effect of legumes on parameters was found. (3) Interaction of time and treatment: No significant effect because of the interaction of time and treatment was found.
By within group analysis, the following results were obtained (Table 4): (1) HDWL significantly increased TC in 6 weeks as much as 6.3% (P = 0.000); (2) HDWL marginally increased LDL-C in the first 3 weeks (8.4%, P = 0.060); (3) both HDEL and HDWL significantly reduced the hs-CRP level in the first 3 weeks and HDEL returned it to basal levels in the subsequent 3 weeks; (4) and HDEL increased TAC in 3 weeks (4%, P = 0.050).
Table 4.
Within group effect of interventions on risk factors for cardiovascular disease (CVD) by ANOVA repeated measurements with Bonferroni post-hoc analysis
| Variables | Intervention |
|||||
|---|---|---|---|---|---|---|
| HDEL |
HDWL |
|||||
| PT2,T1 | PT3,T2 | PT3,T1 | PT2,T1 | PT3,T2 | PT3,T1 | |
| (change percent) | (change percent) | (change percent) | (change percent) | (change percent) | (change percent) | |
| WHR | 0.07↓ (2 ± 3.3) | 0.09↓ (2.1 ± 3.8) | 0.01↓ (1.1 ± 1.4) | 0.01↓ (3.2 ± 4.2) | ||
| TC (mg/dl) | 0.00↑ (6.3 ± 6.6) | |||||
| LDL-C (mg/dl) | 0.06↑ (8.4 ± 11.9) | |||||
| hs-CRP (mg/l) | 0.04↓ (19.2 ± 73.3) | 0.00↑ (59.1 ± 104.7) | 0.03↓ (49.8 ± 40.7) | |||
| TAC (μmol/l) | 0.05↑ (4 ± 5.8) | |||||
Values are means ± SE; HDEL: Hypocaloric diet enriched in legumes; HDWL: Hypocaloric diet without legumes; T1: Before intervention; T2: Three weeks after intervention; T3: Six weeks after intervention; WHR: Waist to hip ratio; TC: Total cholesterol; LDL-C: Low density lipoprotein-cholesterol; hs-CRP: High sensitivity C-reactive protein; TAC: Total antioxidant capacity; SE: Standard error
Discussion
This clinical trial explored the effects of high-legume hypocaloric diet on cardiovascular risk factors among women with central obesity. We observed that HDEL did not affect fasting concentrations of TC, while HDWL increased it. In the previous studies both high-legume hypocaloric diets and high-legume isocaloric diets led to greater declines in both TC and LDL-C compared to diets without legumes.5,12 In this study, the mean legume intake at baseline diet was 2.94 servings per week (increased to 2 servings per day in intervention period) compared to 1serving per week in Hermsdorff et al. study (increased to 4 servings per week in intervention period),6 and 1.3 servings per week in Zhang et al.5 and Hartman et al.7 study (increased to 3 servings per day in isocaloric diet and 3.8 servings per day in hypocaloric diet in intervention period). In fact, the baseline legume intake in our study was almost 3 times more than baseline legume intake in previous RCTs. The inconsistency of the results of the current study can be attributed to the participants’ higher legumes intake at baseline in comparison to other studies. In the two recent RCTs, the intake of legumes even after intervention reached to the baseline level of the our study.6,12 In HDWL group which participants consumed legume-less diet in intervention period, fasting concentration of TC increased because of replacing legumes with animal foods. In HDWL group adverse effects of legumes-less diet on TC were not compensated by beneficial effects of low-calorie diet.
We observed that both hypocaloric diets (HDEL and HDWL) reduced hs-CRP in 3 weeks and returned it to basal values after 6 weeks. In previous isocaloric and hypocaloric diets legumes favorably improved CRP concentrations compared with legume-less diets after 4 and 8 weeks.6,7 If the fasting concentration of hs-CRP was being measured after 3 and 6 weeks in previous studies, probably we would not be observing any contradiction among studies. In general, our study did not show any advantage to legumes in reducing hs-CRP. With precise scrutiny of previous studies we can see that there was a direct correlation between hs-CRP and TC.6 We did not observe any reduction in TC in HDEL group because of high consumption of legumes at baseline, therefore, any expectation for reduction of hs-CRP would not be reasonable. On the other hand, it seems that lowering the calorie intake and weight is more effective in reducing hs-CRP than diet composition.24 Since the results showed the significant effect of both diets on hs-CRP was disappeared after adjustment for WC and/or weight. Consistent with this study, Belza et al. did not find any reduction in hs-CRP after 8 weeks via weight reducing hypocaloric diet and only hs-CRP reduction was observed after 16 weeks.25 In our study, it seems that in the first half of the study hypocaloric diets did not increase plasma levels of free fatty acids but in the second half of the study enhanced it. High plasma levels of free fatty acids in the second half of the study probably produced acute-phase reactants and cytokines in the liver and masked the beneficial effects of the weight reducing hypocaloric diets on hs-CRP.
It seems that there is no linear correlation between higher intake of legumes and low fasting concentration of TC, LDL-C and hs-CRP meaning that its beneficial effects reaches to a plateau. This conclusion has been confirmed by Kabagambe et al. study in which the consumption of 1/3 cup (86 gr) cooked beans decreased myocardial infarction risk by 38% and more amounts showed no beneficial effects.9 The baseline level of legume intake in the our study is almost equal to what Kabagambe et al. defined as beneficial level. Although no positive effect was found by increasing legume intake, omitting usual consumption of legumes raised TC.9
Two meta-analyses have confirmed the beneficial effects of legumes on TC and LDL-C. Based on these studies, the beneficial effects of legumes can be mediated via soluble fiber, plant protein, oligosaccharides, isoflavones, phospholipids, fatty acids, phytosterols, saponins and other components. They also concluded that legumes can probably decrease the risk of CVD.26,27
In this study, none of the two interventions had any effect on NOx and MDA however HDEL increased TAC only in the first-half of the study. In Crujeiras et al. study, high-legume hypocaloric diet reduced MDA compared to basal values and had no effect on MDA and TAC compared to legume-less hypocaloric diet.12 Inconsistent results of the two studies can be related to high basal consumption of legumes in our study and/or MDA measurement methods. We measured MDA using a modified version of the Yagi et al.19 protocol based on the thiobarbituric acid reaction, while Crujeiras et al. used Kits for its measurement.12
Consistent with many hypocaloric diets both of interventional diets reduced waist to hip ratio (WHR) and, the study showed privilege to legume less diet in reduction of WHR. Probably bloating due to legumes caused this result.
To our knowledge, this is the first research investigated a legume based hypocaloric diet exclusively in women. The advantage of our study was the special population of the study which their mean usual intake of legumes was almost threefold of the usual intakes of the previous RCTs.6,7 This study provides an opportunity to find the effects of legume enriched diet on CVD risk factors in participants who consumed high legumes at baseline. Our study had two limitations. First, the participants’ reasons for leaving the study were not evaluated. Second, we could not provide food to the participants. Third, we did not blind participants and researchers due to the nature of the study.
Conclusion
We concluded that both hypocaloric diets reduced hs-CRP in 3 weeks and returned it to basal values after 6 weeks (P = 0.004). HDWL significantly reduced WHR (3.2%, P = 0.01) and increased TC (6.3%, P < 0.01). Despite the significant effect of HDEL on increasing TAC in 3 weeks (4%, P = 0.05), the level of TAC remained the same in 6 weeks. None of the diets have any significant effects on NOx and MDA. It seems that there is not reverse linear correlation between intake of legumes and fasting concentration of TC, LDL-C and hs-CRP meaning that its beneficial effects reaches to a plateau. Planning long-term studies with different servings of legumes, study on diverse population with different usual intakes of legumes, and study of these aspects in high-legumes isocaloric diets are necessary for validating the results of the present study.
Acknowledgments
This work was supported by Tabriz University of Medical Sciences (Grant no. 5.4.8491), Nutrition Research Center (Grant no. 5.71.2419) and Liver and Gastrointestinal Disease Research Center (Grant no. GT-660). We thank the participants of this study for their enthusiastic support.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.World Health Organization. Fact sheets [Online]. 2012. Available from: URL: http://www.who.int/mediacentre/factsheets/fs317/en/index.html.
- 2.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595–600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 3.Azizi F, Azadbakht L, Mirmiran P. Trends in overweight, obesity and central fat accumulation among Tehranian adults between 1998-1999 and 2001-2002: Tehran lipid and glucose study. Ann Nutr Metab. 2005;49(1):3–8. doi: 10.1159/000084171. [DOI] [PubMed] [Google Scholar]
- 4.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3 Suppl):439S–50S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Lanza E, Kris-Etherton PM, Colburn NH, Bagshaw D, Rovine MJ, et al. A high legume low glycemic index diet improves serum lipid profiles in men. Lipids. 2010;45(9):765–75. doi: 10.1007/s11745-010-3463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermsdorff HH, Zulet MA, Abete I, Martinez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50(1):61–9. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 7.Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, et al. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140(1):60–7. doi: 10.3945/jn.109.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiseman H, O'Reilly JD, Adlercreutz H, Mallet AI, Bowey EA, Rowland IR, et al. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 2000;72(2):395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 9.Kabagambe EK, Baylin A, Ruiz-Narvarez E, Siles X, Campos H. Decreased consumption of dried mature beans is positively associated with urbanization and nonfatal acute myocardial infarction. J Nutr. 2005;135(7):1770–5. doi: 10.1093/jn/135.7.1770. [DOI] [PubMed] [Google Scholar]
- 10.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, et al. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med. 2001;161(21):2573–8. doi: 10.1001/archinte.161.21.2573. [DOI] [PubMed] [Google Scholar]
- 11.Duane WC. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res. 1997;38(6):1120–8. [PubMed] [Google Scholar]
- 12.Crujeiras AB, Parra D, Abete I, Martinez JA. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res. 2007;41(4):498–506. doi: 10.1080/10715760601131935. [DOI] [PubMed] [Google Scholar]
- 13.Aranceta J. Spanish food patterns. Public Health Nutr. 2001;4(6A):1399–402. doi: 10.1079/phn2001227. [DOI] [PubMed] [Google Scholar]
- 14.Ayatollahi SM. Nutritional assessment of lactating women in Shiraz in relation to recommended dietary allowances. East Mediterr Health J. 2004;10(6):822–7. [PubMed] [Google Scholar]
- 15.McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. Pulse consumption, satiety, and weight management. Adv Nutr. 2010;1(1):17–30. doi: 10.3945/an.110.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington, DC: National Academies Press; 2005. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SP, Barlow IM. Immunoturbidimetric C-reactive protein kit adapted to the Technicon RA-1000. Clin Chem. 1988;34(1):172. [PubMed] [Google Scholar]
- 18.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 19.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15(2):212–6. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 20.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239(1):70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183(2):308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137(4):992–8. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 24.Clifton PM. Diet and C-reactive protein. Curr Atheroscler Rep. 2003;5(6):431–6. doi: 10.1007/s11883-003-0032-z. [DOI] [PubMed] [Google Scholar]
- 25.Belza A, Toubro S, Stender S, Astrup A. Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory markers in obese subjects. Int J Obes (Lond) 2009;33(4):456–64. doi: 10.1038/ijo.2009.27. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: potential in the prevention of cardiovascular disease. Br J Nutr. 2002;88(Suppl 3):S263–S271. doi: 10.1079/BJN2002716. [DOI] [PubMed] [Google Scholar]
- 27.Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(2):94–103. doi: 10.1016/j.numecd.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]