Abstract
BACKGROUND
Today, the fractional flow reserve (FFR) guides the physician to select suitable patients with intermediate severity coronary lesions in angiography that should be treated or not with stent. The aim of this study was to evaluate the impact of using FFR in the selection of appropriate treatment strategy in angiographic intermediate coronary lesions and their short-term outcome in a sample of Iranian population.
METHODS
In a prospective cohort, 34 patients who had intermediate coronary artery lesion(s), defined as having a 40-70% diameter stenosis, as determined by visual estimation or quantitative coronary angiography were enrolled through a convenience sampling method. All patients underwent FFR measurement to decide whether percutaneous coronary intervention should be performed. The results of visual assessment, quantitative coronary angiography, and functional assessment of the severity of coronary stenosis were compared. Significant stenosis was defined as FFR < 0.80. All patients were followed for 6 months for the incidence of major advanced cardiac events.
RESULTS
In this study, 34 patients (22 male and 12 female) with mean age of 57 ± 8 (range 45-70) were included. In 26.47% (9/34) of patients, FFR was < 0.80, they underwent coronary angioplasty. The correlation between visual estimation and quantitative assessment of lesion diameter was 0.804 (P < 0.001). During the follow-up period, no major advanced cardiac events were reported. In addition, 5.88 (2/34) of patients had a left main (LM) lesion with FFR > 0.80 and stenting was done to the other vessels with significant coronary lesions.
CONCLUSION
Measurement of FFR is a useful approach in making clinical decisions about revascularization procedures in patients with moderate coronary artery lesion severity, especially in LM and multivessel disease. This study showed that not only FFR can change treatment plan of the patients, but also it would improve clinical outcomes.
Keywords: Fractional Flow Reserve Myocardial, Coronary Stenosis, Coronary Angiography
Introduction
Intermediate coronary artery lesions are defined as a diameter narrowing of ≥ 40% to ≤ 70%1 and is reported in about one third to half of coronary angiograms.2 The clinical significance and management strategy of these lesions are important.3 Visual estimation of coronary lesion severity in routine coronary angiography may be inaccurate because of two-dimensional views and inter/interobservervariability.3,4 Quantitative coronary angiograms are better suited, but they also have the same limitations in assessing coronary artery stenosis.1 In patients with angina pectoris and moderate coronary artery stenosis severity, as assessed by visual estimation in a coronary angiogram, decision making for the selection of treatment strategies, stenting, or medical follow-up is a dilemma, and evaluation and treatment of lesions are challenging.3 In a considerable number of patients, coronary artery revascularization is performed without definite evidence that coronary stenosis is causing symptoms.5,6 In recent years, technical advances have provided new diagnostic devices to catheterization laboratories to evaluate the severity of coronary artery lesions. One of the diagnostic modalities for assessment of the functional importance of intermediate coronary lesion is fractional flow reserve (FFR), which is carried out by intracoronary pressure guide wires.7,8 Based on the pressure-flow analysis of coronary stenosis during maximal flow reserve,9 the concept of a myocardial FFR has been developed as an invasively determined index of the functional severity of coronary stenosis in intermediate lesion.10-13 FFR is defined as the ratio of maximum coronary blood flow in a stenotic area to maximum blood flow in the same vessel that is completely normal.10,14 In other words, FFR can be derived from the ratio of the mean distal coronary artery pressure (post stenotic-Pd) to the aortic pressure (Pa) during maximal hyperemia (FFR = Pd/Pa).10,15 This index is independent of changes in systemic blood pressure, heart rate, and left ventricular function, and it is unaffected by conditions known to increase the baseline myocardial flow.2,12 The normal value of the index is 1.0, regardless of the patient or the specific vessel studied.13 The FFR has been shown to correlate well with other noninvasive tests for the detection of ischemia.16-18 In contrast to most other invasive indexes, FFR has a direct clinical relevance.10,13,14,19 For these reasons, FFR may be regarded as a gold standard for the evaluation of the physiological significance of intermediate coronary stenosis in catheterization laboratories, with extensive validation in randomized controlled trials.6
We believe using FFR have been shown to improve patient outcomes in the short- and long-term2,20,21 reduce the number of stents implanted by approximately 30% and are cost effective.21 Therefore, without using these devices, stents (usually drug eluting stents) may be inserted in non-significant lesions wrongly.3
Our center (Chamran Heart Hospital, Isfahan, Iran) has been using intracoronary pressure guide wires since January 2013. Therefore, the purpose of this study was to describe our experiences with the use of this device.
This study aimed to evaluate the impact of FFR in decision making for revascularization in patients with intermediate coronary stenosis and relevant angina in an Iranian population. It aimed to determine the complications associated with the use of FFR devices also.
Materials and Methods
The patient population consisted of 34 stable angina patients who consecutively underwent FFR assessment to decide whether to perform percutaneous coronary intervention (PCI) for de novo intermediate coronary lesions between January and December 2013. All cases were selected from patients who came to our center for coronary angiography as outpatients. An intermediate coronary artery lesion was defined as a 40-70% diameter1 stenosis through the visual estimation of two cardiologists separately. If their estimation was different, opinion of third cardiologist was considered as a final decision. A single operator blinded to clinical and FFR data performed an off-line quantitative coronary angiogram on moderate coronary lesions to determine lesion length. For this study, the target vessel was a lesion in the proximal or mid part of a major epicardial coronary artery with a reference vessel diameter larger than 2.5 mm. Patients were excluded if they were in the setting of ST elevation of myocardial infarction for primary PCI; in the setting of acute coronary syndrome; had a major life-threatening illness; experienced contraindication to adenosine and anticoagulant or antiplatelet, or had prior coronary artery bypass surgery. Patients were eligible for enrollment if they had at least one intermediate lesion in their coronary artery tree. The cutoff value of FFR in the FFR-guided PCI group was 0.80.2,14,22 PCI was done if the FFR was > 80% and all implanted stents were commercially available third-generation drug-eluting stents. The patients gave us informed consent.
Major advanced cardiac events defined as death, myocardial infarction, and ischemic driven target vessel revascularization (TVR) at 6 months follow-up of all the patients were evaluated. During the follow-up period, all patients in the vascularized group received appropriate doses of Aspirin, metoprolol, an angiotensin-converting enzyme inhibitor, nitrate, and clopidogrel.
Immediately after coronary angiography with standard technique,2 the coronary artery was selectively engaged with a 6F guiding catheter without side holes, and 200 µg nitroglycerin was administered intracoronary. A 0.014ʺ pressure guide wire was calibrated at zero, advanced into the coronary artery, and positioned distal to the stenosis to be measured. FFR was determined during maximum hyperemia using the ratio Pd/Pa; Pd represents mean coronary pressure distal to the stenosis segment measured via the pressure wire, and Pa represents mean Pa measured via the guiding catheter. Maximum hyperemia was induced by intracoronary adenosine (≥ 30 µg in the right and ≥ 40 µg in the left coronary artery).23
All selected patients underwent coronary angiography by standard techniques via femoral approach. Coronary angiography was performed in multiple orthogonal views. Patients requiring FFR performance were chosen based on a visual estimation of coronary lesion severity by at least two cardiologists in each coronary artery. If patients were eligible for the study, informed consent was obtained. A single operator blinded to clinical and FFR data performed an off-line quantitative coronary angiogram on coronary moderate lesions. The most severe narrowing in no foreshortening view was used for quantitative coronary measurements (Siemens software, Siemens Healthcare GmbH, Germany); lesion length was obtained and recorded.
All patients were followed for about 6 months, and the primary outcome was defined as a composite of major adverse cardiac event (MACE), defined as death, myocardial infarction, and ischemia-driven TVR at 12 months after the index procedure. Death was defined as all-cause mortality. The diagnosis of myocardial infarction was based on either the development of new pathological Q-waves in two contiguous electrocardiogram leads and/or cardiac enzyme level elevation 3 times the upper limit of normal value. TVR included target lesion PCI and bypass surgery of the target lesion. TVR was performed only in the presence of symptoms and/or signs of ischemia3, so MACEs were recorded, if present.
All values are expressed as mean ± standard deviation for continuous variables or as counts and percentages for categorical variables. All variables were compared using an appropriate statistical test. Kolmogorov-Smirnov test was used to evaluate if the distribution of data was normal. Pearson correlation coefficient tests were used for the correlation between quantitative variables and linear regression curves were drawn using the least square method. Independent t-test was used to compare means of vessel diameter and a minimal luminal diameter between patients with FFR ≥ 0.80 and those with lower values. Statistical significance was assessed as P < 0.050 using a two-tailed probability analysis. All data were analyzed SPSS software for Windows (version 20, SPSS Inc., Chicago, IL, USA).
Results
From the beginning of the January to 31 December 2013, 10,000 angiography and interventional procedures were carried out in our center. Of these, 34 patients (22 male and 12 female) with mean age 57 ± 8 (range 45-70) who were undergoing clinically indicated coronary angiography and met inclusion criteria were enrolled in the study and underwent FFR assessment, pending informed consent from the patient and their physician. Clinical characteristic of all patients, including age, sex, ordinary atherosclerotic risk factors, and so on, were summarized in table 1.
Table 1.
Baseline characteristics of the patients (n = 34)
| Variables | n (%) | Mean ± SD |
|---|---|---|
| Sex | ||
| Female | 12 (35.3) | - |
| Male | 22 (64.7) | - |
| Age | ||
| Female | - | 59.50 ± 10.30 |
| Male | - | 56.20 ± 8.40 |
| Total | - | 57.50 ± 9.20 |
| Risk factors | ||
| DM | 10 (29.4) | - |
| HTN | 19 (55.9) | - |
| DLP | 15 (44.1) | - |
| Smoking | 14 (41.2) | - |
| FH | 10 (29.4) | - |
| LVEF | - | 51.76 ± 4.58 |
| Angina class | ||
| I | 10 (29.4) | - |
| II | 18 (53.0) | - |
| III | 5 (14.7) | - |
| IV | 1 (2.9) | - |
| Angiography results | ||
| Visual estimation | - | - |
| Diameter stenosis | - | 59.60 ± 8.00 |
| QCA | ||
| Reference diameter | - | 31.50 ± 0.49 |
| Diameter stenosis | - | 56.60 ± 7.80 |
| Lesion length | - | 15.00 ± 4.80 |
| FFR | - | 0.86 ± 0.09 |
| Treatment strategy | ||
| PCI on target vessel | 9 (26.5) | - |
| Medical | 23 (67.6) | - |
| PCI on non-target vessel | 2 (5.9) | - |
| Number of diseased vessels | ||
| Single vessel | 25 (83.5) | - |
| Two vessels | 8 (23.5) | - |
| Three vessel | 1 (2.9) | - |
| Type of diseased vessel | ||
| LM | 3 (8.8) | - |
| LAD | 29 (25.3) | - |
| LCX | 4 (11.8) | - |
| RCA | 9 (26.5) | - |
SD: Standard deviation; DM: Diabetes mellitus; HTN: Hypertension; DLP: Dyslipidemia; FH: Familial hypercholesterolemia; LVEF: Left ventricular ejection fraction; QCA: Quantitative coronary angiography; FFR: Fractional flow reserve; PCI: Percutaneous coronary intervention; LM: Left main; LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery
FFR measurements were done in 34 patients. There were no significant differences in reference vessel diameter and a minimal luminal diameter between patients with FFR ≥ 0.80 and those with lower values (P = 0.332 and P = 0.724, respectively). Angiography data, numbers of diseased vessels, type of involved vessel, lesion length, extent of stenosis, and FFR values are shown in table 1, as well.
In 26.47% (9/34) of the patients, FFR was lower than 0.8 and they underwent coronary angioplasty and stenting with a drug-eluting stent. Two patients (5.88 %) had FFR above 0.80 in the intermediate lesion and stenting of the other vessel with significant lesions (stenosis > 70%) was done. For the other patients, the FFR was more than 80% and revascularization was not performed. In this study, three cases (8.82%) of left main (LM) with intermediate lesions were enrolled. If the FFR measurements showed no significant LM disease, revascularization protocol would change from urgent coronary bypass graft surgery to stenting of the other vessel with significant lesions.
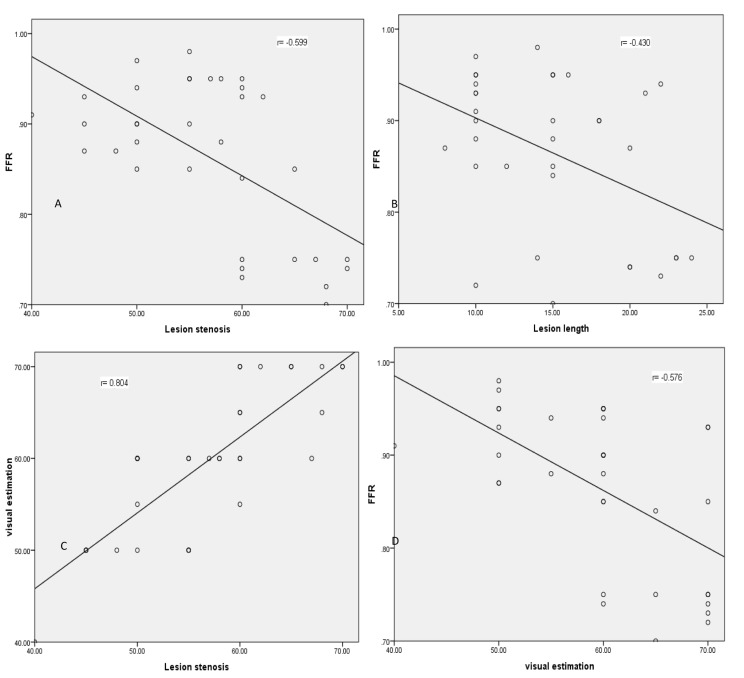
The correlation coefficient between FFR and lesion length and FFR and luminal stenosis was -0.599 (P < 0.001) and -0.430 (P = 0.011), respectively. The correlation between visual estimation of lesion diameter and quantitative measurement of lesion diameter was 0.804 (P < 0.001). Visual estimation of lesion diameter and FFR showed a correlation of -0.576 (P < 0.001), as shown in figure 1.
Figure 1.
Scatter plots and linear regression curves of fractional flow reserve versus visual and quantitative indices of coronary lesions FFR: Fractional flow reserve
During 6 months follow-up period no any MACEs were reported in both groups.
Significant complications did not occur during coronary adenosine administration, except for a transient severe bradycardia in nine patients and a transient complete heart blockage in one case. One lesion was not studied with pressure guide wires in the first attempt due to guide wire cross failure; the failed guide wire was then substituted with the next wire. Successful FFR measurements increased progressively after the early months of using pressure guide wires and increasing operator experience. The mean procedure time for the FFR measurement of a single lesion was 25 minutes (15-45), a period that included calibration, equalization, and hyperemia induction. This timeframe was not significantly different versus single vessel angioplasty, but in the cases of multivessel disease or vessels with multiple lesions, this was important. In the current study, misclassification of lesions with angiographic assessment alone amounted to more than 25%.
Discussion
This study demonstrated a new experience of an Iranian center in the use of pressure guide wires as a new technology for the assessment of intermediate coronary stenosis.
Routine coronary angiography is not accurate in assessing the functional significance of coronary stenosis when compared with FFR, not only in the 50-70% category, but also in the 70-90% category of angiographic severity.4 This is because of inter-/intra-observer variability that is about 27% and 15%, respectively, in this study. In our study all PCI was done by new third generation drug eluted stents, therefore, good outcomes and low MACEs in follow-up period may be for this reason, however, more participants are needed to evaluate this subject. A prominent physician from our center refused to use FFR measurements in the assessment of coronary lesion severity because of cost, time constraints, radiation exposure, and a higher likelihood of volume of contrast. This study, along with Leesar et al., showed that FFR procedures for measurement are safe, resulting in decreased radiation exposure, and no change in the amount of contrast in comparison to conditions with inappropriate stenting.24
Despite the fact that myocardial single photon emission tomography (SPECT) has shown a high sensitivity of 90% or greater in the detection of multivessel coronary artery disease, accuracy is limited in the identification of each individual stenosis. Detection of reversible perfusion abnormalities, especially of the culprit lesion, might fail in cases of a missing reference area, and the allocation of perfusion defects to target vessels is a well-known problem in these patients.25 In our study, some of the cases involved multivessel disease, so myocardial perfusion SPECT may not be useful for the functional assessment of intermediate lesions. Leesar et al. showed that the FFR significantly shortens the duration of hospitalization compared with stress perfusion scintigraphy.24
Technical developments have made newer and better-designed tools available for coronary interventionist procedures. Sometimes, the complexity of devices, their cost, their limited field of application, or the scant yield of relevant information in an interventionist procedure mean that new devices are used only for research in few hemodynamic laboratories, or in sporadic cases with unusual presentation or evolution.26 In the present study, the FFR was used in < 0.5% of the interventionist procedures in our center, but it had a great negative impact on the need to do revascularization in the patient with intermediate lesions. It has an indication in about 30% of cases. Previous studies have shown that FFR above 0.75-0.80 was a strong predictor of favorable clinical outcomes in patients with intermediate LM disease.27-31
One of the major findings of the present study is the effect of FFR measurement in intermediate lesions of LM or proximal left anterior descending (LAD) or left circumflex (LCX). If FFR showed insignificant lesions in LM, proximal lesions of LAD or LCX can change the strategy for revascularization, [coronary artery bypass grafting (CABG) or stenting] medical treatment. This study showed that lesion length with a severity of lesion stenosis predicted lower FFR. Thus, FFR measurement is appropriate to identify patients with intermediate LM stenosis in whom deferral of revascularization may be associated with excellent outcomes.32
In this study, from 35 patients coronary stenting was done in only 9 (26.5%) lesions that FFR showed significant stenosis, so this procedure prevented from inappropriate stenting of others (73.5%). The therapeutical decisions were changed from PCI or CABG in 23 patients (67.5%) to medical treatment. The treatment approach was changed from urgent CABG to Medical treatment or PCI on other lesions in three cases that had LM coronary artery lesions but without significant FFR findings. The current study with a low sample size showed that not only FFR can change treatment plan of the patients, but also it would improve clinical and economical outcomes by lowering inappropriate stenting.4
Limitations
The presence of small vessel disease, diffuse coronary artery disease, and left ventricular hypertrophy restrict the hyperemia induced by pharmacologic vasodilatation, so decreasing distal coronary pressure and the calculation of FFR measurements is limited.33 This study included a relatively small number of patients and a short course follow-up for event recording.
Conclusion
Measurement of FFR during coronary angiography is a useful method of assessing whether an intermediate coronary lesion based on a routine angiography is functionally significant and may be responsible for future cardiac events. Although this procedure is underused in catheterization laboratories, it is believed that FFR is a useful approach in clinical decision making about revascularization procedures in patients with moderate coronary artery lesion severity, especially in cases of LM and multivessel disease. This procedure had a significant negative effect in coronary revascularization in these patients. Therefore, this study is a good foundation for the increased use of functional assessments in our and other catheterization laboratories, and it should be a basis for new randomized trials.
Acknowledgments
We would like to thank the personnel of the Interventional and Catheterization Angiography Laboratory of Isfahan Chamran Heart Hospital. This study was approved and supported by Isfahan Medical School, Isfahan University of Medical Sciences. This paper is a part of fellowship thesis in School of Medicine Isfahan University of Medical Sciences.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Iguchi T, Hasegawa T, Nishimura S, Nakata S, Kataoka T, Ehara S, et al. Impact of lesion length on functional significance in intermediate coronary lesions. Clin Cardiol. 2013;36(3):172–7. doi: 10.1002/clc.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 3.Nam CW, Yoon HJ, Cho YK, Park HS, Kim H, Hur SH, et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease: fractional flow reserve-guided versus intravascular ultrasound-guided. JACC Cardiovasc Interv. 2010;3(8):812–7. doi: 10.1016/j.jcin.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55(25):2816–21. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 5.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 6.Kern MJ, Donohue TJ, Aguirre FV, Bach RG, Caracciolo EA, Wolford T, et al. Clinical outcome of deferring angioplasty in patients with normal translesional pressure-flow velocity measurements. J Am Coll Cardiol. 1995;25(1):178–87. doi: 10.1016/0735-1097(94)00328-n. [DOI] [PubMed] [Google Scholar]
- 7.Kern MJ, Puri S, Craig WR, Bach RG, Donohue TJ. Hemodynamic rounds series II: Coronary hemodynamics for angioplasty and stenting after myocardial infarction: use of absolute, relative coronary velocity and fractional flow reserve. Cathet Cardiovasc Diagn. 1998;45(2):174–82. doi: 10.1002/(sici)1097-0304(199810)45:2<174::aid-ccd16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49(1):1–16. doi: 10.1002/(sici)1522-726x(200001)49:1<1::aid-ccd1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15(2):459–74. doi: 10.1016/s0735-1097(10)80078-6. [DOI] [PubMed] [Google Scholar]
- 10.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–67. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 11.De Bruyne B, Paulus WJ, Pijls NH. Rationale and application of coronary transstenotic pressure gradient measurements. Cathet Cardiovasc Diagn. 1994;33(3):250–61. doi: 10.1002/ccd.1810330312. [DOI] [PubMed] [Google Scholar]
- 12.Pijls NH, Bech GJ, el Gamal MI, Bonnier HJ, De Bruyne B, Van Gelder B, et al. Quantification of recruitable coronary collateral blood flow in conscious humans and its potential to predict future ischemic events. J Am Coll Cardiol. 1995;25(7):1522–8. doi: 10.1016/0735-1097(95)00111-g. [DOI] [PubMed] [Google Scholar]
- 13.Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183–93. doi: 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–8. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 15.De Bruyne B, Baudhuin T, Melin JA, Pijls NH, Sys SU, Bol A, et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation. 1994;89(3):1013–22. doi: 10.1161/01.cir.89.3.1013. [DOI] [PubMed] [Google Scholar]
- 16.Bartunek J, Marwick TH, Rodrigues AC, Vincent M, Van Schuerbeeck E, Sys SU, et al. Dobutamine-induced wall motion abnormalities: correlations with myocardial fractional flow reserve and quantitative coronary angiography. J Am Coll Cardiol. 1996;27(6):1429–36. doi: 10.1016/0735-1097(96)00022-8. [DOI] [PubMed] [Google Scholar]
- 17.Bartunek J, Van Schuerbeeck E, De Bruyne B. Comparison of exercise electrocardiography and dobutamine echocardiography with invasively assessed myocardial fractional flow reserve in evaluation of severity of coronary arterial narrowing. Am J Cardiol. 1997;79(4):478–81. doi: 10.1016/s0002-9149(96)00788-6. [DOI] [PubMed] [Google Scholar]
- 18.Caymaz O, Fak AS, Tezcan H, Inanir SS, Toprak A, Tokay S, et al. Correlation of myocardial fractional flow reserve with thallium-201 SPECT imaging in intermediate-severity coronary artery lesions. J Invasive Cardiol. 2000;12(7):345–50. [PubMed] [Google Scholar]
- 19.Lederman SJ. Brief review: Fractional flow reserve. ACC Curr J Rev. 1997:34–5. [Google Scholar]
- 20.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 21.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, Van't Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–11. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 22.De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 2001;104(2):157–62. doi: 10.1161/01.cir.104.2.157. [DOI] [PubMed] [Google Scholar]
- 23.McGeoch RJ, Oldroyd KG. Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheter Cardiovasc Interv. 2008;71(2):198–204. doi: 10.1002/ccd.21307. [DOI] [PubMed] [Google Scholar]
- 24.Leesar MA, Abdul-Baki T, Akkus NI, Sharma A, Kannan T, Bolli R. Use of fractional flow reserve versus stress perfusion scintigraphy after unstable angina. Effect on duration of hospitalization, cost, procedural characteristics, and clinical outcome. J Am Coll Cardiol. 2003;41(7):1115–21. doi: 10.1016/s0735-1097(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 25.Hacker M, Rieber J, Schmid R, Lafougere C, Tausig A, Theisen K, et al. Comparison of Tc-99m sestamibi SPECT with fractional flow reserve in patients with intermediate coronary artery stenoses. J Nucl Cardiol. 2005;12(6):645–54. doi: 10.1016/j.nuclcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Palop R, Pinar E, Lozano I, Carrillo P, Cortes R, Pico F, et al. Clinical utilization of the coronary pressure wire. Rev Esp Cardiol. 2002;55(3):251–7. doi: 10.1016/s0300-8932(02)76593-7. [DOI] [PubMed] [Google Scholar]
- 27.Bech GJ, Droste H, Pijls NH, De Bruyne B, Bonnier JJ, Michels HR, et al. Value of fractional flow reserve in making decisions about bypass surgery for equivocal left main coronary artery disease. Heart. 2001;86(5):547–52. doi: 10.1136/heart.86.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindstaedt M, Yazar A, Germing A, Fritz MK, Holland-Letz T, Mugge A, et al. Clinical outcome in patients with intermediate or equivocal left main coronary artery disease after deferral of surgical revascularization on the basis of fractional flow reserve measurements. Am Heart J. 2006;152(1):156–9. doi: 10.1016/j.ahj.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez-Navarro M, Hernandez-Garcia JM, Alonso-Briales JH, Kuhlmorgen B, Gomez-Doblas JJ, Garcia-Pinilla JM, et al. Should we treat patients with moderately severe stenosis of the left main coronary artery and negative FFR results? J Invasive Cardiol. 2004;16(8):398–400. [PubMed] [Google Scholar]
- 30.Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110(18):2831–6. doi: 10.1161/01.CIR.0000146338.62813.E7. [DOI] [PubMed] [Google Scholar]
- 31.Courtis J, Rodes-Cabau J, Larose E, Potvin JM, Dery JP, Larochelliere RD, et al. Usefulness of coronary fractional flow reserve measurements in guiding clinical decisions in intermediate orequivocal left main coronary stenoses. Am J Cardiol. 2009;103(7):943–9. doi: 10.1016/j.amjcard.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 32.Kang SJ, Lee JY, Ahn JM, Song HG, Kim WJ, Park DW, et al. Intravascular ultrasound-derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv. 2011;4(11):1168–74. doi: 10.1016/j.jcin.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 33.de Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–17. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]