Significance
Two-component systems represent the most common bacterial sensors for perceiving environmental stimuli. Extracytoplasmic sensing kinases of this type consist of an extracytoplasmic sensor domain, transmembrane helices (TMs), and a cytoplasmic His-kinase. Conformational changes in sensor domains upon ligand binding suggest transmembrane signal transduction by a piston-type displacement of TMs. Here, transmembrane signaling was probed for the C4-dicarboxylate sensor kinase (DcuS). Cysteine accessibility studies of TM2 showed fumarate-driven displacement of TM2 that is directly related to the activation of DcuS. TM2 repositioning is paralleled by corresponding energy optima for membrane insertion and effector-induced compaction of the corresponding binding domain. These experiments show signal transduction by a piston-type movement, which could be generally representative for bacterial sensor kinases.
Keywords: DcuS sensor kinase, transmembrane signaling, bacteria, piston-type, SCAM
Abstract
The C4-dicarboxylate sensor kinase DcuS is membrane integral because of the transmembrane (TM) helices TM1 and TM2. Fumarate-induced movement of the helices was probed in vivo by Cys accessibility scanning at the membrane–water interfaces after activation of DcuS by fumarate at the periplasmic binding site. TM1 was inserted with amino acid residues 21–41 in the membrane in both the fumarate-activated (ON) and inactive (OFF) states. In contrast, TM2 was inserted with residues 181–201 in the OFF state and residues 185–205 in the ON state. Replacement of Trp 185 by an Arg residue caused displacement of TM2 toward the outside of the membrane and a concomitant induction of the ON state. Results from Cys cross-linking of TM2/TM2′ in the DcuS homodimer excluded rotation; thus, data from accessibility changes of TM2 upon activation, either by ligand binding or by mutation of TM2, and cross-linking of TM2 and the connected region in the periplasm suggest a piston-type shift of TM2 by four residues to the periplasm upon activation (or fumarate binding). This mode of function is supported by the suggestion from energetic calculations of two preferred positions for TM2 insertion in the membrane. The shift of TM2 by four residues (or 4–6 Å) toward the periplasm upon activation is complementary to the periplasmic displacement of 3–4 Å of the C-terminal part of the periplasmic ligand-binding domain upon ligand occupancy in the citrate-binding domain in the homologous CitA sensor kinase.
Two-component systems consisting of a sensor kinase and a response regulator are the most versatile sensors for the bacterial response to environmental stimuli (1). The minimal version of sensor kinases consists of a sensory and a transmitter domain, including the kinase domain (2). Membrane-embedded sensor kinases require additional domains for the transfer of the signal across the membrane (transmembrane signaling). Although sensing and kinase reactions and domains are known in some detail, transmembrane signaling is mostly unknown, and mechanisms have been inferred primarily from structural analysis of the extracytoplasmic-binding domains (3–5).
The C4-dicarboxylate sensor (DcuS) kinase belongs to the two-component DcuS–C4-dicarboxylate response (DcuR) system that responds to C4-dicarboxylates and related di-anions (6–8). DcuS consists of an extracytoplasmic (or periplasmic) sensory Per-ARNT-Sim (PASP) or PDC (PhoQ-DcuS-CitA) domain that is anchored to the membrane by transmembrane (TM) helices TM1 and TM2, a cytoplasmic PAS (PASC) domain, and the C-terminal kinase (3, 8–10). Effector binding by PASP has been characterized for DcuS and the closely related citrate sensor kinase (CitA) (3, 8, 10, 11), but transmembrane signaling has not yet been studied. However, structural changes in the periplasmic binding sites of DcuS and CitA suggest the mode of transmembrane signal transduction by this type of sensor. The effector domain PASP of CitA is compacted upon citrate binding (3). The compaction results in an uplift of the C-terminal end of the domain and of the attached helix α6 that is continued in TM2. This compaction is thought to pull TM2 out of the membrane toward the periplasmic side along the helical axis, with some contribution from rotational movement. A similar movement was postulated for DcuS, based on the structural similarity of CitA and DcuS (7).
In the current work, to obtain information about the movement of the TM helices of DcuS during signal transduction, the water accessibility of amino acid residues of TM2 and TM1 at the water–membrane interface was characterized. The changes in the accessibility of TM1 and TM2 were tested after activation of DcuS by fumarate binding or other parameters. TM2 showed an accessibility shift to the periplasmic side that was related to the induction of the DcuS-dependent dcuB–lacZ reporter gene fusion. These data directly support a piston-type movement of TM2 as the basis for transmembrane signaling by DcuS.
Results
Cys Accessibility as a High-Resolution Topology Scan for TM1 and TM2 of DcuS.
The topology of TM1 and TM2 was studied by a Cys accessibility approach in vivo to retain the full structural and functional integrity of the system. For the study, we used the substituted Cys accessibility method (SCAM) (12), combined with Cys scanning (Scan-SCAM). A Cys-less version of DcuS was applied in which Cys residues were introduced by mutational replacement at suitable positions. DcuS comprises two intrinsic Cys residues, C199 and C471, that can be replaced by Ser residues (DcuS-C199S-C471S, hereafter referred to as “DcuS-Cys−”) without loss of functionality (13). Cys residues often are tolerated in proteins and allow specific labeling of the thiol group (14, 15). The repositioning of a TM helix is predicted to alter the localization of its residues in relation to the water–membrane boundaries. For differentiation of water-accessible and -inaccessible positions, Cys residues were blocked by N-ethylmaleimide (NEM) in a water-dependent reaction. Labeling of unblocked (water-inaccessible) Cys residues was performed after solubilization of the membrane with low amounts of SDS with the thiol reagent methoxypolyethylene glycol maleimide (PEG-mal) (12). As a result of the polyethylene glycol chain (>10 kDa) of PEG-mal, pegylated proteins could be recognized by an additional band of higher molar mass after SDS/PAGE and Western blotting with DcuS-specific antisera (Fig. 1B). Thus, wild-type DcuS containing Cys199 that is inaccessible from the water space and Cys471 that is inaccessible to NEM because of intermolecular disulfide bond formation (13) showed two additional bands (lanes 1 and 2) of single- and double-pegylated DcuS. DcuS-Cys− was not labeled by PEG-mal (lanes 4–6). A Cys residue in the periplasmic region (S80C) revealed no labeling by PEG-mal (lane 9), which was the result of blocking by NEM (lane 7). In contrast, a Cys residue in TM2 (C199) was inaccessible to NEM and therefore was labeled by PEG-mal (lanes 10 and 11). Labeling was always dependent on the specific reaction of PEG-mal with unblocked Cys residues because solubilization before NEM treatment blocked all Cys residues (lanes 3, 6, 9, and 12). Thus, Cys accessibility can be used to distinguish residues within or outside the cytoplasmic membrane.
Fig. 1.
Proof of principle for differentiation between water-accessible and membrane-hidden Cys residues by Scan-SCAM. Covalently bound PEG-mal (A) induces a mass shift to DcuS during SDS/PAGE that is visualized by Western blotting using antiserum against DcuS-PASP (B). NSP (sequential treatment by NEM, SDS; and PEG-mal): Blocking of water-accessible Cys residues by NEM is followed by cell solubilization (SDS) and labeling of unblocked Cys residues by PEG-mal. SP (sequential treatment by SDS and PEG-mal): There is no masking with NEM to PEGylate all Cys residues of the protein. SNP (sequential treatment by SDS, NEM, and PEG-mal): Cells are permeabilized before NEM masking, and no Cys residue is PEGylated.
Fixed Position of TM1 and Fumarate-Induced Repositioning of TM2 in the Membrane.
A plasmid harboring DcuS-Cys− (pMW336) was used to generate a library of 60 single-Cys variants of DcuS covering all residues around the water–membrane interfaces of TM1 and TM2. Compared with wild-type DcuS, none of the variants was inhibited by more than 13% in the fumarate-stimulated induction of the reporter gene dcuB–lacZ (Fig. 2A) that is mediated by DcuS. The variants were used for Cys accessibility testing by the Scan-SCAM method (Fig. 2B). An intramembrane location is predicted for residues 21–41 and 182–202 of TM1 and TM2, respectively. The water-protected regions span positions 20–40 and 182–201, respectively (Fig. 2B, Left), which correspond to the regions predicted for TM1 and TM2. Deviating from this pattern are accessible residues 24, 25, 37, and 199 within the otherwise inaccessible range and inaccessible residues 17 and 209 outside the inaccessible area. Residues 17 and 209 may be inaccessible because they are protected for steric reasons within the protein or by other components.
Fig. 2.
Accessibility of Cys residues around the first (TM1) and second (TM2) transmembrane helix with and without fumarate (A and B) and in the genetic ON-variants DcuS-W181R and W185R (C and D). (A) DcuS-dependent stimulation of dcuB-lacZ by fumarate. Expression was measured in strain IMW260 complemented with plasmid-encoded DcuSWT (pMW181) after anaerobic growth in eM9 medium with glycerol plus DMSO, without (light gray) or with (dark gray) 50 mM sodium fumarate. (B) Schematic representation of TM1 and TM2, their accessibility pattern, and the accessibility shift induced by fumarate. Accessible (unPEGylated) Cys residues are shown in green, and inaccessible (PEGylated) Cys residues are shown in red. The plus and minus signs indicate the periplasmic and cytoplasmic sides of the membrane, respectively. (C) Fumarate-independent stimulation of dcuB-lacZ by the weak ON-variant DcuS-W181R and the strong ON-variant DcuS-W185R. Expression was measured with plasmid-encoded DcuS-W181R (pMW1545) and DcuS-W185R (pMW1546), respectively. (D) Schematic representation of TM2, its accessibility pattern in the variants DcuS-W181R and DcuS-W185R, and the accessibility shift induced by fumarate in both variants. The pattern is shown for only a selection of residues. The plus and minus signs indicate the periplasmic and cytoplasmic sides of the membrane, respectively. Scan-SCAM Western blots of all Cys variants of DcuS or selected Cys variants of DcuS-W181R and DcuS-W185R, with or without fumarate, are shown in Figs. S1 and S2, respectively.
Fig. S1.
Accessibility of Cys residues around TM1 and TM2 in the absence (−F) and presence (+F) of fumarate. SCAM Western blots of 60 single Cys variants of DcuS with substituted Cys residues around TM1 and TM2, once in the absence (−F) and once in the presence (+F) of 50 mM sodium fumarate during labeling. Accessible (unPEGylated) Cys residues are shown in green, and inaccessible (PEGylated) Cys residues are shown in red. Arrows indicate Cys residues with a shifted accessibility between both activation states.
Fig. S2.
Accessibility of selected Cys residues around TM2 in the ON variants DcuS-W181R and W185R, in the absence (−F) and presence (+F) of fumarate. SCAM Western blots of single Cys variants of the DcuS ON-variants DcuS-W181R and DcuS-W185R with substituted Cys residues TM2, once in the absence and once in the presence of 50 mM sodium fumarate during labeling. Accessible (unPEGylated) Cys residues are shown in green, and inaccessible (PEGylated) Cys residues are shown in red. Arrows indicate Cys residues with a shifted accessibility between both activation states.
The presence of fumarate as the ligand (Fig. 2B, Right) affected the accessibility of a significant number of residues compared with the fumarate-deficient state. The changes were minor for TM1, where the accessibility of only two positions was affected. In contrast, within and around TM2, seven positions altered their accessibility. Therefore it can be concluded that TM1 shows a mostly fixed position within the membrane, and TM2 is more mobile.
Movement of TM2 Toward the Periplasm upon Activation.
In the periplasmic half of TM2, Cys residues at position 173 lost accessibility, and those at positions 179, 182, and 185 gained accessibility upon binding of fumarate, whereas in the cytoplasmic half residues 199 and 205 became inaccessible, and residue 209 became accessible in the presence of fumarate. When residues 173 and 209 were disregarded because of their remote position from the water–membrane interfaces, there was an opposite change in accessibility on the two ends of TM2. In the periplasmic half of TM2, more residues became water-accessible and reactive, whereas the cytoplasmic part entered a water-hidden environment. These changes are in line with a shifting of TM2 toward the periplasm during fumarate activation.
In the reverse approach, we tested whether artificial displacement of TM2 by Arg substitutions could activate DcuS-dependent gene expression (Fig. 2 C and D). Positively charged Arg residues in a TM helix tend to move to a hydrophilic region to interact by H-bonds and electrostatically (16, 17). To reach this environment, Arg residues must move vertically toward the nearest water–membrane interface with a corresponding shift of the TM helix. Arg residues were introduced into TM2 by substitution at the last water-accessible positions of the OFF state and the fumarate-activated ON state (positions 181 and 185) in the periplasmic half of TM2. The DcuS variants were tested for function (dcuB–lacZ expression) and for Cys accessibility. In terms of Cys accessibility, DcuS-W181R resembled the noninduced wild type (labeling at positions 182, 185, 199, and 209) but was labeled at two positions (179 and 205) at which the fumarate-activated wild type was labeled (Fig. 2D). In contrast, DcuS-W185R was more similar to the fumarate-stimulated wild type (labeling at positions 179, 181, 182, and 209), and the labeling of only two residues (199 and 205) resembled that of the noninduced state (Fig. 2D). Coincidently, in the absence of fumarate DcuS-W185R had a stronger effect than DcuS-W181R on dcuB–lacZ stimulation (7.7-fold vs. 2.3-fold) (Fig. 2C). Thus, artificial displacement of TM2 by Arg residues activates DcuS, and the magnitude of displacement is related to the level of activation.
DcuS Activity Is Fine-Tuned by a Variable TM2 Repositioning.
The sensor kinase DcuS responds to a wide range of C4-dicarboxylates (8, 18, 19), but the level of reporter gene expression varies for the effectors (Fig. 3). Fumarate and l-malate are the strongest effectors, followed by maleate, l-tartrate, and the non–C4-dicarboxylate citrate and 3-nitropropionate. Cys accessibility in TM2 was tested in the presence of the same effectors. As did fumarate (Fig. 2A), l-malate changed the Cys accessibility at six positions (residues 179, 182, 185, 199, 205, and 209), and maleate and l-tartrate did so at three (residues 179, 182, and 205) and two (residues 179 and 205) positions, respectively. Finally, citrate and 3-nitropropionate shifted only one Cys residue (residue 205). In any example, the changes at the given residues were the same as those for fumarate induction. Plotting the number of positions with changed accessibility versus the level of reporter gene expression demonstrated a correlation of both parameters with the characteristics of a saturation curve (Fig. 3). This correlation indicated that the total number of positions with changed accessibility represented a measure for the displacement of TM2 in the membrane and the extent of DcuS activation.
Fig. 3.
Cys accessibility of TM2 in the presence of various effectors and correlation to the level of reporter gene expression: Correlation between the total number of positions with a changed accessibility between both signaling states and the level of dcuB-lacZ expression. Expression was measured in strain IMW237 after anaerobic growth in eM9 medium with glycerol plus DMSO with 50 mM of the respective effector (3-Nitropropionate: 5 mM). The dotted line represents a polynomial regression of measured values. SCAM Western blots of 22 single-Cys variants of DcuS in the presence of l-malate, maleate, l-tartrate, citrate, or 3-nitropropionate are shown in Fig. S3.
Fig. S3.
Accessibility of Cys residues around TM2 in the presence of different effectors. SCAM Western blots of 22 single-Cys variants of DcuS with substituted Cys residues around TM2 in the presence of 50 mM l-malate, 50 mM maleate, 50 mM l-tartrate, 50 mM citrate, or 5 mM 3-nitropropionate during labeling. Accessible (unPEGylated) Cys residues are shown in green, and inaccessible (PEGylated) Cys residues are shown in red.
A Symmetric Repositioning of the TM2 Helices in the DcuS Dimer.
DcuS is a functional homodimer or higher oligomer, irrespective of its signaling state (13), and interaction of the TM2 helices is thought to contribute to DcuS dimerization (20). To study quaternary structural changes in the DcuS homodimer during transmembrane signaling, we tested oxidative Cys cross-linking between protomers of the homodimer. This method has been used to determine oligomerization (21), dimerization interface (22), structural dynamics, flexibility (23), and the position of two TM helices relative to each other (24). In this assay, two Cys residues that are close together in the initial position form a disulfide bond that could be affected by reorganization of TM2/TM2′ interaction upon activation. Parallel (piston-type) displacement of both TM2 helices is supposed to have no effect on cross-linking, whereas rotation or a scissors-like reorganization of the TM2/TM2′ interfaces is thought to affect cross-linking.
For TM2 cross-linking, the same single-Cys variants were used as in the accessibility approach, namely those at the periplasm–membrane transition zone (172–186). The Cys residues for disulfide bond formation within one dimer (provided steric compatibility) were oxidized by copper (II)-(1,10-phenanthroline)3 (25). The CuII complex is used for the oxidation of neighboring Cys thiolates in water or at the water–membrane interface of membrane proteins (26). The extent of Cys cross-linking was estimated from the band intensities of cross-linked and nonlinked DcuS as obtained after SDS/PAGE and immunostaining (Fig. 4). The efficiency of cross-linking ranged from 0–45% with an insignificant mean difference of less than 1% between the absence and presence of fumarate. Thus, cross-linking patterns were almost identical in the induced and noninduced states, indicating a symmetrical piston-type movement of both TM2 helices within one dimer. The local maxima of cross-linking efficiency showed a specific periodicity (Fig. 4) with a 4-3–4-3 distance pattern corresponding to the periodicity of a continuous α-helix. The cross-linked region ranged from residues 172–186 comprising most of the C-terminal α6 helix of PASP (PASP-α6) and the periplasmic part of TM2. The experimental results suggest that PASP-α6 and TM2 form a continuous helix in the ON and OFF states of DcuS and move in parallel in the DcuS dimer upon fumarate activation, without changes in the helical interface. A cross-linking pattern of this type is in agreement with a joint piston-type shift of TM2/TM2′.
Fig. 4.
Intermolecular oxidative disulfide cross-linking in DcuS. Single-Cys variants of DcuS around TM2 were cross-linked oxidatively in vivo using copper phenanthroline as the catalyst, once in the absence (gray) and once in the presence (blue) of fumarate. The amount of cross-linking product among total DcuS was calculated after SDS/PAGE in the Western blot by measuring the band intensities with the software GelPro Analyzer. For each Cys residue the cross-linking efficiency was determined in three independent experiments, and the arithmetic average is plotted as percentage against the respective position. Arrows indicate local maxima of the cross-linking efficiency. Western blots of the cross-linking experiments are shown in Fig. S4.
Fig. S4.
Intermolecular oxidative disulfide cross-linking in DcuS. Single-Cys variants of DcuS around TM2 were cross-linked oxidatively in vivo using copper phenanthroline as the catalyst, once in the absence (−F) and once in the presence (+F) of fumarate. Each cross-linking experiment was done in triplicate.
Membrane Insertion of TM2 and Energetics of Relocation.
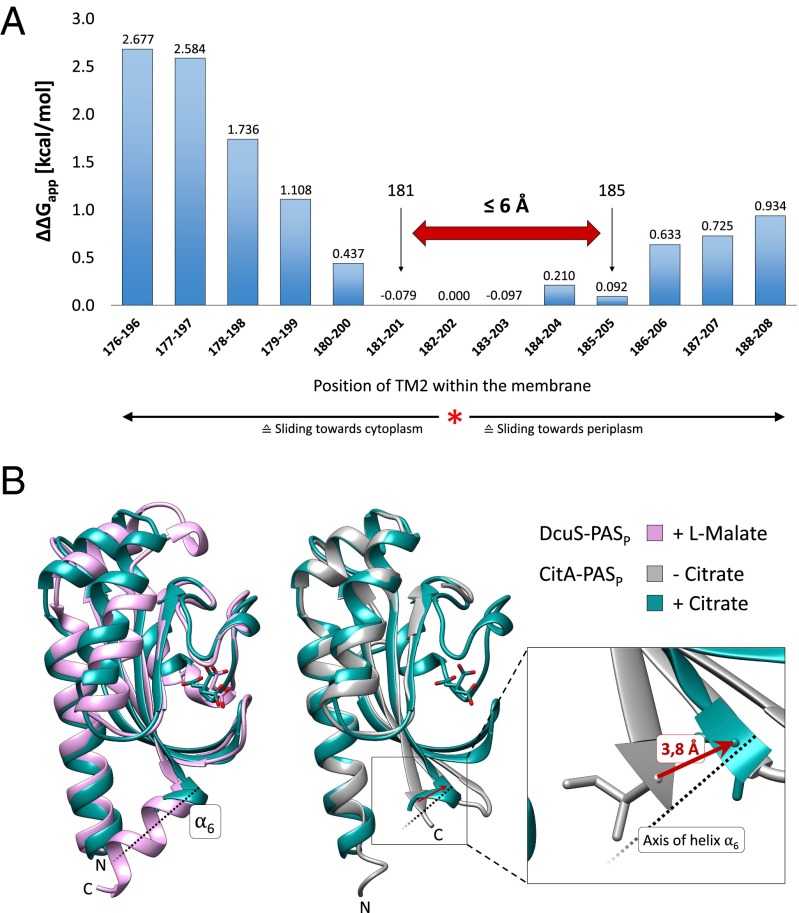
The likelihood of membrane insertion of a given polypeptide and the energy required for insertion can be estimated by means of the ∆G prediction server, which calculates the apparent free energy of insertion (∆Gapp) as a function of the amino acid sequence (25). The ∆Gapp was calculated for different supposed positions of TM2 for the ranges 176–196 to 188–208 (Fig. 5A). The free energies were compared with the position of TM2 (182–202) that is predicted by Uniprot (nar.oxfordjournals.org/content/43/D1/D204) and showed a low amount of free energy for membrane insertion (red asterisk). These ∆∆Gapp values for the hypothetical other TM positions relative to the 182–202 position (Fig. 5A) served as a measure for the energy difference of both positions and of the energy demand for repositioning TM2 from position 182–202 to the other positions. There was a continuum with low energy spanning TM helices 181–202 to 185–205. This region was framed by the two signaling states, as obtained by the Cys accessibility experiment (Fig. 2B). Therefore, the shift from position 181–185 for the last accessible residue (periplasmic side) is reflected by the low energy barrier and increasing energy levels on both sides.
Fig. 5.
Estimation of the amplitude of piston-type sliding by structure comparison, Cys accessibility, and energetic considerations. (A) Change in the apparent free energy (∆∆Gapp) for the insertion of TM2 at various positions compared with the predicted default state (182–202; red asterisk). The ∆Gapp for the different positions was calculated by the ∆G prediction server v 1.0 (27). Calculations are based on the probability that TM helices will be inserted into the membrane and use parameters such as the identity and position of a particular amino acid and the overall length of the sequence. The black arrows indicate the C-terminal ends of TM2 in the ON and OFF signaling states as derived from Scan-SCAM. The red arrow reflects a potential longitudinal displacement of TM2 between the two signaling states. (B) Comparison of the structure of the periplasmic PASP domains of DcuS (pink; PDB ID #3BY8) (10) and CitAKp with citrate (green; PDB ID #2J80) and without citrate (gray, PDB ID #2V9A) (3). Structures were superimposed using Chimera software (42). The C terminus of CitAKp is shown in an enlarged view; its uplift in parallel with the helix axis (derived from DcuS-PASP helix α6; dotted line) is shown by a red arrow.
The ∆∆Gapp profile of TM1 showed a different pattern (Fig. S5). There was one position with a low ∆∆Gapp value for TM position 21–41, which was very close to the region determined by the accessibility studies (region 20–40). The ∆∆Gapp values increased rapidly from this position when the helix moved in either direction. The prediction of one energetically preferred position is paralleled by the experimental data for one invariant position of TM1 in the membrane.
Fig. S5.
Change in the apparent free energy (∆∆Gapp) for shifting TM1 from the preferred position to other positions. Change in the apparent free energy (∆∆Gapp) for the insertion of TM1 in various positions compared with the predicted default state (21–41; red asterisk). The ∆Gapp for the different positions was calculated by the ∆G prediction server v. 1.0 (27), based on the respective amino acid sequences.
Discussion
A Long-Range Piston-Type Displacement of TM2 upon Activation.
The Scan-SCAM method was used to determine the positions of amino acid residues of TM1 and TM2 of DcuS that are accessible to the water space during the OFF/ON state transition of DcuS. SCAM has been used for studies of the topology of TM regions (12) and changes in topology organization (28). Combining SCAM with Cys scanning according to a previous suggestion (12) provided a tool for testing changes in the accessibility of DcuS TM2 during the OFF/ON activity shift. The validity of the method was supported by the reverse response on both sides of the membrane and by demonstrating TM2 shifts after charged amino acid residues were introduced into the TM helix. In addition, the data on the changes in Cys accessibility could be correlated with the functional changes of the DcuS sensor kinase and with the energetically preferred positions of the TM helices. The data coincidently are compatible with a piston-type shift of TM2 for transmission of the signal across the membrane upon fumarate binding at the periplasmic PASP domain. The periodicity in the Cys cross-linking pattern for the PASP-TM2 region (residues 172–186 of DcuS) indicates a continuous α-helix for α6 of PASP and TM2 and an important role for this region in DcuS homodimerization. The region is obviously displaced in parallel upon activation, and the parallel displacement of a long continuous helix is compatible with a piston-type shift of TM2 in response to a ligand-induced compaction at PASP. Persistence of the interaction for a long helical region at the TM2–TM2′ interface of DcuS is not compatible with a rotational mode of signal transduction by TM2 and is not easily compatible with a scissors-like movement.
Structural data for PASP of the closely related citrate sensor CitAKp of Klebsiella pneumoniae support a shift of TM2 toward the periplasmic side of the membrane after ligand binding. Citrate binding induces a compaction of the central β-sheet around citrate that pulls the C-terminal end of the domain to the binding site in the periplasm, away from the membrane surface (3, 11). The uplift includes the PASP C-terminal helix α6 and comprises ∼3.8 Å (Fig. 5B). For DcuS, a high-resolution structure is available only for the ligand-bound form. The effector-bound structures of PASP from CitAKp and DcuS are almost identical, with an overall rmsd of 1.6 Å (Fig. 5B). Given the similar structures of the effector-bound forms, a similar compaction of DcuS-PASP is predicted upon fumarate or l-malate binding. In the Cys accessibility experiments, the last water-accessible Cys residue on the periplasmic side of TM2 moved from position 181 to 185. A shift of four residues corresponds to 1.1 turns or to 4–6 Å at the Cα-atoms of an α-helix (Fig. 5B), consistent with an uplift of the region by the extent shown for PASP of CitA. Remarkably, the physiologically (fumarate) induced shift by four residues can be replaced in part by shifting of TM2 induced by a Trp/Arg substitution and is paralleled by the energetically preferred continuum for the TM dislocation over the same range (Fig. 5A).
Whether TM2 shows a perpendicular or tilted arrangement relative to the membrane surface is not known. In the structure of the isolated PASP, α6 represents the loose end of the domain and provides no reliable information about the arrangement of α6 and TM2 in full-length DcuS. Because of the lack of information, a perpendicular arrangement of TM2 is assumed in the scheme in Fig. 2, but a slightly tilted arrangement of TM2 also is compatible with the accessibility pattern. In the SCAM experiments, the periplasmic and cytoplasmic sides of TM2 did not show the same increase and decrease, respectively, in accessibility upon transfer to the ON state, possibly indicating a tilted arrangement of TM2 in the membrane or some contribution of a scissors-type movement. More likely, however, the uneven labeling might result from the protection of some sites from reaction by protein–protein interaction within the DcuS dimer (13) or by DctA or DcuB forming a complex with DcuS (29, 30).
At the cytoplasmic side, the transmembrane signal from TM2 is perceived by a cytoplasmic PASC domain and transmitted to the kinase. By the presence of the PASC domain, DcuS is different from the nitrate (NarX), trimethylamine N-oxide (TorS), and cationic antimicrobial peptide (PhoQ) sensor kinases where the TM region is followed by the histidine kinases, adenylate cyclases, methyl-accepting chemotaxis protein, and phosphatases (HAMP) domains. In DcuS, TM2 is connected by a short linker to the N-terminal region of PASC, which has a high plasticity that is central to its function in signal perception and transmission (20). As suggested in the scheme of Fig. S6, activation of the kinase domain is based on the destabilization of PASC dimerization (20, 31). The destabilization is the supposed response of PASC to the transmembrane signaling by a piston-type movement of TM2 and the pulling of TM2 at the N terminus of PASC. Destabilization of the PASC dimer and activation of the kinase also can be achieved by mutations in the PASC dimerization sites (20, 31).
Fig. S6.
Scheme for the conversion of DcuS from the OFF to the ON state. Binding of fumarate or l-malate at the DcuS dimer causes closing of the binding site and compaction of PASP with an uplift of α6 from PASP (red arrow) (3, 4, 7) and of TM2 (red arrow) by one helical turn in TM2 [shifting the periplasmic membrane–water interface from Trp181 to Trp185 (upper and lower balls)]. Pulling of TM2 at the N-terminal region of PASC results in the relief of PASC dimerization (20, 31) and kinase activation (31).
Transmembrane Signaling in Sensor Kinases.
For the nitrate and trimethylamine-N-oxide sensor kinases NarX and TorS, ligand-induced conformational changes at the binding domain have been thought to cause piston-type movements in signal transduction (4, 5, 32), similar to the piston-type mode of signal transduction by the bacterial chemoreceptor Tar (33–36). In Tar, a piston displacement toward the cytoplasm upon attractant binding triggers the cellular chemotactic response (34, 35). The vertical displacement for Tar (1.6 Å) and the supposed displacements for NarX and TorS (<2 Å) are distinctly smaller than that described for DcuS (4–6 Å). The question has arisen of whether TM shifts of <2 Å are sufficient for transmembrane signaling (37) by sensors that are not organized in large chemotactic protein complexes such as Tar. The large shift suggested for DcuS (4–6 Å) is compatible with the supposed need for large transmembrane displacement. It must be kept in mind, however, that the sensor kinases with small transmembrane displacement (e.g., NarX and TorS) contain HAMP domains for signal transmission from the membrane to the kinase, whereas the PASC domain serves that function in DcuS.
In the PhoQ/PhoR two-component system of Escherichia coli or Salmonella typhimurium that responds to antimicrobial peptides and cations, transmembrane signaling by the PhoQ sensor kinase involves a complex repacking of the transmembrane helices in a scissors-like mode (37, 38). Remarkably, PhoQ contains a sensory PAS domain with a fold similar to DcuS. The domain belongs to the PDC family (10, 39) (an alternative designation for PASP). Despite its structural similarity, the sensory PAS domain of PhoQ contains no defined binding pocket for the ligands, and perception of the signal is created by binding/repulsion of a surface region of the PAS domain that is transduced to and across the membrane in a scissors-like mode (37, 38). PhoQ contains a HAMP domain on the cytoplasmic side for signal transmission to the kinase. Thus, despite their structural similarity, the periplasmic PAS domains of DcuS and PhoQ apparently differ in their mode of stimulus perception and in the way they transmit the stimulus to the membrane.
Materials and Methods
Bacteria and Molecular Genetics Methods.
Derivatives of E. coli K12 and plasmids used are listed in Table S1. All molecular methods were performed according to standard procedures (40). Plasmids were isolated using the GenElute HP Plasmid Miniprep Kit, and PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma-Aldrich). Oligonucleotides were synthesized by Sigma-Aldrich. Transformation of E. coli was accomplished through electroporation or heat shock. Point mutations were created with Phusion HF DNA Polymerase in combination with DpnI endonuclease and heat shock-competent XL1-Blue (Agilent Technologies). Antibiotics were used at the following concentrations: 20 µg/mL chloramphenicol, 50 µg/mL kanamycin, 50 µg/mL spectinomycin, and 15 µg/mL tetracycline. The concentration was halved if two or more antibiotics were used simultaneously.
Table S1.
Strains of E. coli and plasmids used in this study
| Strain or plasmid | Genotype | Reference or source |
| Escherichia coli K12 | ||
| C43(DE3) | Strain for overexpression of membrane proteins carrying a chromosomal T7 polymerase | (40) |
| MC4100 | F− araD139 Δ(argF-lac)U169 rpsL150 ΔlacZ relA1 flbB530 deoC1 ptsF25 rbsR | (6, 31) |
| IMW237 | MC4100 λ[Φ(dcuB’-‘lacZ)hyb, bla+] | (6) |
| IMW260 | MC4100 λ[Φ(dcuB’-‘lacZ)hyb, bla+] dcuS::Camr | (6) |
| Plasmids | ||
| pET28a | Expression vector, pBR ori, T7 Promoter, His6-tag (Kanr) | Novagen |
| Plasmids for reporter gene measurements | ||
| pMW181 | pET28a with dcuS (2.2 kb XbaI/HindIII fragment) (Kanr) | (8) |
| pMW1545 | pMW181 but DcuS-W181R (Kanr) | This study |
| pMW1546 | pMW181 but DcuS-W185R (Kanr) | This study |
| Plasmids for SCAM and in vivo cross-linking | ||
| pMW151 | His6-DcuS expression plasmid, pET28a derivative (Kanr) | (18) |
| pMW324 | pMW151 but DcuS-C199S (Kanr) | (13) |
| pMW325 | pMW151 but DcuS-C471S (Kanr) | (13) |
| pMW336 | pMW151 but DcuS-C199S-C471S (= DcuS-Cys−) (Kanr) | (13) |
| pMW1588 | pMW336 but DcuS-D178C (Kanr) | This study |
| pMW1589 | pMW336 but DcuS-S179C (Kanr) | This study |
| pMW1590 | pMW336 but DcuS-R180C (Kanr) | This study |
| pMW1591 | pMW336 but DcuS-W181C (Kanr) | This study |
| pMW1592 | pMW336 but DcuS-S182C (Kanr) | This study |
| pMW1593 | pMW336 but DcuS-I183C (Kanr) | This study |
| pMW1594 | pMW336 but DcuS-I184C (Kanr) | This study |
| pMW1595 | pMW336 but DcuS-W185C (Kanr) | This study |
| pMW1596 | pMW336 but DcuS-S186C (Kanr) | This study |
| pMW1597 | pMW336 but DcuS-V187C (Kanr) | This study |
| pMW1598 | pMW336 but DcuS-L188C (Kanr) | This study |
| pMW1840 | pMW336 but DcuS-I196C (Kanr) | This study |
| pMW1841 | pMW336 but DcuS-G197C (Kanr) | This study |
| pMW1842 | pMW336 but DcuS-T198C (Kanr) | This study |
| pMW1843 | pMW336 but DcuS-I200C (Kanr) | This study |
| pMW1844 | pMW336 but DcuS-L201C (Kanr) | This study |
| pMW1845 | pMW336 but DcuS-V202C (Kanr) | This study |
| pMW1846 | pMW336 but DcuS-K203C (Kanr) | This study |
| pMW1847 | pMW336 but DcuS-V204C (Kanr) | This study |
| pMW1848 | pMW336 but DcuS-L205C (Kanr) | This study |
| pMW1849 | pMW336 but DcuS-K206C (Kanr) | This study |
| pMW1850 | pMW336 but DcuS-K207C (Kanr) | This study |
| pMW1851 | pMW336 but DcuS-I208C (Kanr) | This study |
| pMW1852 | pMW336 but DcuS-L209C (Kanr) | This study |
| pMW1853 | pMW336 but DcuS-F210C (Kanr) | This study |
| pMW1854 | pMW336 but DcuS-G211C (Kanr) | This study |
| pMW1855 | pMW336 but DcuS-L212C (Kanr) | This study |
| pMW1856 | pMW336 but DcuS-E213C (Kanr) | This study |
| pMW1930 | pMW336 but DcuS-K16C (Kanr) | This study |
| pMW1931 | pMW336 but DcuS-L17C (Kanr) | This study |
| pMW1932 | pMW336 but DcuS-S18C (Kanr) | This study |
| pMW1933 | pMW336 but DcuS-T19C (Kanr) | This study |
| pMW1934 | pMW336 but DcuS-T20C (Kanr) | This study |
| pMW1935 | pMW336 but DcuS-V21C (Kanr) | This study |
| pMW1936 | pMW336 but DcuS-I22C (Kanr) | This study |
| pMW1937 | pMW336 but DcuS-L23C (Kanr) | This study |
| pMW1938 | pMW336 but DcuS-M24C (Kanr) | This study |
| pMW1939 | pMW336 but DcuS-V25C (Kanr) | This study |
| pMW1977 | pMW336 but DcuS-V35C (Kanr) | This study |
| pMW1978 | pMW336 but DcuS-V36C (Kanr) | This study |
| pMW1979 | pMW336 but DcuS-H37C (Kanr) | This study |
| pMW1980 | pMW336 but DcuS-L38C (Kanr) | This study |
| pMW1981 | pMW336 but DcuS-I39C (Kanr) | This study |
| pMW1982 | pMW336 but DcuS-Y40C (Kanr) | This study |
| pMW1983 | pMW336 but DcuS-F41C (Kanr) | This study |
| pMW1984 | pMW336 but DcuS-S42C (Kanr) | This study |
| pMW1985 | pMW336 but DcuS-Q43C (Kanr) | This study |
| pMW1986 | pMW336 but DcuS-I44C (Kanr) | This study |
| pMW1987 | pMW336 but DcuS-S45C (Kanr) | This study |
| pMW1988 | pMW336 but DcuS-D46C (Kanr) | This study |
| pMW2032 | pMW336 but DcuS-S179C-W181R (Kanr) | This study |
| pMW2033 | pMW336 but DcuS-W181R-S182C (Kanr) | This study |
| pMW2034 | pMW336 but DcuS-W181R-W185C (Kanr) | This study |
| pMW2035 | pMW325 but DcuS-W181R (Kanr) | This study |
| pMW2036 | pMW336 but DcuS-W181R-L205C (Kanr) | This study |
| pMW2037 | pMW336 but DcuS-W181R-L209C (Kanr) | This study |
| pMW2038 | pMW336 but DcuS-S179C-W185R (Kanr) | This study |
| pMW2039 | pMW336 but DcuS-W181C-W185R (Kanr) | This study |
| pMW2040 | pMW336 but DcuS-S182C-W185R (Kanr) | This study |
| pMW2041 | pMW325 but DcuS-W185R (Kanr) | This study |
| pMW2042 | pMW336 but DcuS-W185R-L205C (Kanr) | This study |
| pMW2043 | pMW336 but DcuS-W185R-L209C (Kanr) | This study |
| pMW2129 | pMW336 but DcuS-L169C (Kanr) | This study |
| pMW2130 | pMW336 but DcuS-S170C (Kanr) | This study |
| pMW2131 | pMW336 but DcuS-R171C (Kanr) | This study |
| pMW2132 | pMW336 but DcuS-V172C (Kanr) | This study |
| pMW2133 | pMW336 but DcuS-T173C (Kanr) | This study |
| pMW2134 | pMW336 but DcuS-Q174C (Kanr) | This study |
| pMW2135 | pMW336 but DcuS-Q175C (Kanr) | This study |
| pMW2136 | pMW336 but DcuS-I176C (Kanr) | This study |
| pMW2137 | pMW336 but DcuS-N177C (Kanr) | This study |
Scan-SCAM.
Water accessibility of Cys residues was analyzed by a two-step masking and labeling procedure (12). For this purpose, derivatives of pMW336 encoding single-Cys variants of an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible and otherwise Cys-less form of DcuS (DcuS-Cys−) were transformed into E. coli strain C43(DE3). Cells were grown in 500 µL LB medium per well with antibiotics in 48-well plates at 1,000 rpm aerobically for 6 h; DcuS expression was induced after 2 h with 1 mM IPTG. Where indicated, the LB medium contained 50 mM of the respective effector (e.g., sodium fumarate). Cells were harvested, washed in high-salt/50 mM Hepes buffer (pH 6.8), and resuspended in 50 mM Hepes buffer (pH 6.8). Masking of water-exposed Cys residues was started with 100 µg of total cell protein and 2 mM NEM in a 50-µL reaction. After 1.5 h at 25 °C and occasional vortexing, the reaction was stopped by two washes in Hepes, a final centrifugation step, and removal of the supernatant. Cells were permeabilized, and proteins were denatured by resuspension of the resulting cell pellet in 40 µL Hepes + 1.3% (wt/vol) SDS. Labeling of former unblocked Cys residues was done with 5 mM PEG-mal (Sigma-Aldrich) in a total volume of 45 µL, again for 1.5 h at 25 °C and with occasional vortexing. The reaction was stopped with 55 µL 2× SDS sample buffer containing 250 mM DTT and boiling at 95 °C for 5 min.
Oxidative Disulfide in Vivo Cross-Linking.
In vivo cross-linking was done as described previously (41). Strains, plasmids, growth conditions, and effector use were the same as in the SCAM experiments. At the end of cultivation, 100 µL of cell suspension was mixed with 100 µL of a copper (II) phenanthroline solution (4 mM CuSO4, 13 mM 1,10-phenanthroline, 3.33 mM NaPi, pH 7.4) in a cavity of a 48-well plate and was incubated for 10 min at 25 °C with vigorous shaking. The reaction was terminated with 50 µL of a combined stop solution/nonreducing sample buffer [20 mM Tris, 8 mM NaH2PO4 (pH 7.8), 12.5 mM EDTA, 12.5 mM NEM, 1.25% (wt/vol) SDS, 12.5% (wt/vol) sucrose, 2.5 mg/mL bromophenol blue] and boiling at 95 °C for 5 min.
SDS/PAGE, Western Blot, and Immunostaining.
Samples from SCAM or oxidative disulfide cross-linking were subjected to SDS/PAGE (stacking gel: 4%; resolving gel: 10%). Gels were run at constant voltage (250 V) for 40 min and subsequently were transferred onto a nitrocellulose membrane. Semidry Western blotting was carried out at a constant current (1.6 mA/cm2) for 45 min. For immunostaining, rabbit polyclonal antiserum raised against the periplasmic domain of DcuS (Eurogentec) was combined with peroxidase-coupled goat anti–rabbit-IgG secondary antibody (Sigma-Aldrich). A chemiluminescent substrate (HRP; Merck Millipore) and X-ray films (Advansta) were used to visualize bands. Quantification of the cross-linking product among total DcuS was achieved by measuring the band intensities with GelPro Analyzer software.
β-Galactosidase Assay.
The dcuB–lacZ expression was measured as described elsewhere (31).
Acknowledgments
We thank R. Krämer and D. Schneider for helpful and stimulating discussions and C. Griesinger for carefully reading the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grant UN49/17-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507217112/-/DCSupplemental.
References
- 1.Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev. 2006;70(4):910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grebe TW, Stock JB. The histidine protein kinase superfamily. Adv Microb Physiol. 1999;41:139–227. doi: 10.1016/s0065-2911(08)60167-8. [DOI] [PubMed] [Google Scholar]
- 3.Sevvana M, et al. A ligand-induced switch in the periplasmic domain of sensor histidine kinase CitA. J Mol Biol. 2008;377(2):512–523. doi: 10.1016/j.jmb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Cheung J, Hendrickson WA. Structural analysis of ligand stimulation of the histidine kinase NarX. Structure. 2009;17(2):190–201. doi: 10.1016/j.str.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore JO, Hendrickson WA. Structural analysis of sensor domains from the TMAO-responsive histidine kinase receptor TorS. Structure. 2009;17(9):1195–1204. doi: 10.1016/j.str.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Zientz E, Bongaerts J, Unden G. Fumarate regulation of gene expression in Escherichia coli by the DcuSR (dcuSR genes) two-component regulatory system. J Bacteriol. 1998;180(20):5421–5425. doi: 10.1128/jb.180.20.5421-5425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheu PD, Kim OB, Griesinger C, Unden G. Sensing by the membrane-bound sensor kinase DcuS: Exogenous versus endogenous sensing of C(4)-dicarboxylates in bacteria. Future Microbiol. 2010;5(9):1383–1402. doi: 10.2217/fmb.10.103. [DOI] [PubMed] [Google Scholar]
- 8.Kneuper H, et al. The nature of the stimulus and of the fumarate binding site of the fumarate sensor DcuS of Escherichia coli. J Biol Chem. 2005;280(21):20596–20603. doi: 10.1074/jbc.M502015200. [DOI] [PubMed] [Google Scholar]
- 9.Pappalardo L, et al. The NMR structure of the sensory domain of the membranous two-component fumarate sensor (histidine protein kinase) DcuS of Escherichia coli. J Biol Chem. 2003;278(40):39185–39188. doi: 10.1074/jbc.C300344200. [DOI] [PubMed] [Google Scholar]
- 10.Cheung J, Hendrickson WA. Crystal structures of C4-dicarboxylate ligand complexes with sensor domains of histidine kinases DcuS and DctB. J Biol Chem. 2008;283(44):30256–30265. doi: 10.1074/jbc.M805253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinelt S, Hofmann E, Gerharz T, Bott M, Madden DR. The structure of the periplasmic ligand-binding domain of the sensor kinase CitA reveals the first extracellular PAS domain. J Biol Chem. 2003;278(40):39189–39196. doi: 10.1074/jbc.M305864200. [DOI] [PubMed] [Google Scholar]
- 12.Bogdanov M, Zhang W, Xie J, Dowhan W. Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAM(TM)): Application to lipid-specific membrane protein topogenesis. Methods. 2005;36(2):148–171. doi: 10.1016/j.ymeth.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheu PD, et al. Oligomeric sensor kinase DcuS in the membrane of Escherichia coli and in proteoliposomes: Chemical cross-linking and FRET spectroscopy. J Bacteriol. 2010;192(13):3474–3483. doi: 10.1128/JB.00082-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popot JL, Saraste M. Engineering membrane proteins. Curr Opin Biotechnol. 1995;6(4):394–402. doi: 10.1016/0958-1669(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 15.MacCallum JL, Bennett WFD, Tieleman DP. Distribution of amino acids in a lipid bilayer from computer simulations. Biophys J. 2008;94(9):3393–3404. doi: 10.1529/biophysj.107.112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Vorobyov I, Allen TW. The different interactions of lysine and arginine side chains with lipid membranes. J Phys Chem B. 2013;117(40):11906–11920. doi: 10.1021/jp405418y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draheim RR, Bormans AF, Lai RZ, Manson MD. Tryptophan residues flanking the second transmembrane helix (TM2) set the signaling state of the Tar chemoreceptor. Biochemistry. 2005;44(4):1268–1277. doi: 10.1021/bi048969d. [DOI] [PubMed] [Google Scholar]
- 18.Janausch IG, Garcia-Moreno I, Unden G. Function of DcuS from Escherichia coli as a fumarate-stimulated histidine protein kinase in vitro. J Biol Chem. 2002;277(42):39809–39814. doi: 10.1074/jbc.M204482200. [DOI] [PubMed] [Google Scholar]
- 19.Krämer J, et al. Citrate sensing by the C4-dicarboxylate/citrate sensor kinase DcuS of Escherichia coli: Binding site and conversion of DcuS to a C4-dicarboxylate- or citrate-specific sensor. J Bacteriol. 2007;189(11):4290–4298. doi: 10.1128/JB.00168-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etzkorn M, et al. Plasticity of the PAS domain and a potential role for signal transduction in the histidine kinase DcuS. Nat Struct Mol Biol. 2008;15(10):1031–1039. doi: 10.1038/nsmb.1493. [DOI] [PubMed] [Google Scholar]
- 21.Milligan DL, Koshland DE., Jr Site-directed cross-linking. Establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J Biol Chem. 1988;263(13):6268–6275. [PubMed] [Google Scholar]
- 22.Pakula AA, Simon MI. Determination of transmembrane protein structure by disulfide cross-linking: The Escherichia coli Tar receptor. Proc Natl Acad Sci USA. 1992;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falke JJ, Koshland DE., Jr Global flexibility in a sensory receptor: A site-directed cross-linking approach. Science. 1987;237(4822):1596–1600. doi: 10.1126/science.2820061. [DOI] [PubMed] [Google Scholar]
- 24.Hughson AG, Hazelbauer GL. Detecting the conformational change of transmembrane signaling in a bacterial chemoreceptor by measuring effects on disulfide cross-linking in vivo. Proc Natl Acad Sci USA. 1996;93(21):11546–11551. doi: 10.1073/pnas.93.21.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bass RB, Butler SL, Chervitz SA, Gloor SL, Falke JJ. Use of site-directed cysteine and disulfide chemistry to probe protein structure and dynamics: Applications to soluble and transmembrane receptors of bacterial chemotaxis. Methods Enzymol. 2007;423:25–51. doi: 10.1016/S0076-6879(07)23002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughson AG, Lee GF, Hazelbauer GL. Analysis of protein structure in intact cells: Crosslinking in vivo between introduced cysteines in the transmembrane domain of a bacterial chemoreceptor. Protein Sci. 1997;6(2):315–322. doi: 10.1002/pro.5560060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hessa T, et al. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 2007;450(7172):1026–1030. doi: 10.1038/nature06387. [DOI] [PubMed] [Google Scholar]
- 28.Long JC, DeLeon-Rangel J, Vik SB. Characterization of the first cytoplasmic loop of subunit a of the Escherichia coli ATP synthase by surface labeling, cross-linking, and mutagenesis. J Biol Chem. 2002;277(30):27288–27293. doi: 10.1074/jbc.M202118200. [DOI] [PubMed] [Google Scholar]
- 29.Witan J, et al. Interaction of the Escherichia coli transporter DctA with the sensor kinase DcuS: Presence of functional DctA/DcuS sensor units. Mol Microbiol. 2012;85(5):846–861. doi: 10.1111/j.1365-2958.2012.08143.x. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz PA, Wörner S, Unden G. Differentiation of DctA and DcuS function in the DctA/DcuS sensor complex of Escherichia coli: Function of DctA as an activity switch and of DcuS as the C4-dicarboxylate sensor. Mol Microbiol. 2014;94(1):218–229. doi: 10.1111/mmi.12759. [DOI] [PubMed] [Google Scholar]
- 31.Monzel C, Degreif-Dünnwald P, Gröpper C, Griesinger C, Unden G. The cytoplasmic PASC domain of the sensor kinase DcuS of Escherichia coli: Role in signal transduction, dimer formation, and DctA interaction. MicrobiologyOpen. 2013;2(6):912–927. doi: 10.1002/mbo3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falke JJ, Erbse AH. The piston rises again. Structure. 2009;17(9):1149–1151. doi: 10.1016/j.str.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milburn MV, et al. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991;254(5036):1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- 34.Chervitz SA, Falke JJ. Molecular mechanism of transmembrane signaling by the aspartate receptor: A model. Proc Natl Acad Sci USA. 1996;93(6):2545–2550. doi: 10.1073/pnas.93.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falke JJ, Hazelbauer GL. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem Sci. 2001;26(4):257–265. doi: 10.1016/s0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szurmant H, White RA, Hoch JA. Sensor complexes regulating two-component signal transduction. Curr Opin Struct Biol. 2007;17(6):706–715. doi: 10.1016/j.sbi.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falke JJ. Piston versus scissors: Chemotaxis receptors versus sensor His-kinase receptors in two-component signaling pathways. Structure. 2014;22(9):1219–1220. doi: 10.1016/j.str.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molnar KS, et al. Cys-scanning disulfide crosslinking and bayesian modeling probe the transmembrane signaling mechanism of the histidine kinase, PhoQ. Structure. 2014;22(9):1239–1251. doi: 10.1016/j.str.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung J, Hendrickson WA. Sensor domains of two-component regulatory systems. Curr Opin Microbiol. 2010;13(2):116–123. doi: 10.1016/j.mib.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. 3rd Ed. Vol 3 Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 41.Lee GF, Lebert MR, Lilly AA, Hazelbauer GL. Transmembrane signaling characterized in bacterial chemoreceptors by using sulfhydryl cross-linking in vivo. Proc Natl Acad Sci USA. 1995;92(8):3391–3395. doi: 10.1073/pnas.92.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]