Significance
Small ubiquitin-like modifier (SUMO) is thought to function by acting as a protein recruitment platform. To date, studies have focused on the role of mono-SUMO and poly-SUMO in the form of linear chains. However, recent findings suggested a role for multi-SUMOylation where several SUMO moieties are spread across numerous proteins found at sites of DNA damage. Here, we used a novel screen to identify dozens of multi-SUMO–binding proteins. We investigated one of these in detail and demonstrate that a multi-SIM–containing SUMO-binding module is required for recruitment of the transcriptional regulator ZMYM2 to chromatin. Because little is known about the function of multi-SUMOylation and multi-SIM–binding proteins, this represents an important conceptual advance in our thinking about how protein SUMOylation might exert its molecular effects.
Keywords: SUMO, ZMYM2, chromatin, SIM, ZNF198
Abstract
Protein SUMOylation has emerged as an important regulatory event, particularly in nuclear processes such as transcriptional control and DNA repair. In this context, small ubiquitin-like modifier (SUMO) often provides a binding platform for the recruitment of proteins via their SUMO-interacting motifs (SIMs). Recent discoveries point to an important role for multivalent SUMO binding through multiple SIMs in the binding partner as exemplified by poly-SUMOylation acting as a binding platform for ubiquitin E3 ligases such as ring finger protein 4. Here, we have investigated whether other types of protein are recruited through multivalent SUMO interactions. We have identified dozens of proteins that bind to multi-SUMO platforms, thereby uncovering a complex potential regulatory network. Multi-SUMO binding is mediated through multi-SIM modules, and the functional importance of these interactions is demonstrated for the transcriptional corepressor ZMYM2/ZNF198 where its multi-SUMO–binding activity is required for its recruitment to chromatin.
Protein modification with small ubiquitin-like modifier (SUMO) has emerged as a major regulatory event that impacts on the activities of hundreds of proteins associated with a diverse array of biological activities (reviewed in refs. 1–3). In particular, a number of roles have been ascribed to nuclear functions, predominantly in the area of chromatin function, DNA repair, and transcriptional regulation (reviewed in ref. 4). SUMO can act at the molecular level in many different ways but a major mode of action is through providing an additional binding surface for attracting coregulatory proteins to a SUMOylated protein (reviewed in refs. 2 and 5). These interactions are driven by short hydrophobic regions on binding partners known as SUMO-interacting motifs (SIMs) (6, 7). However, their binding affinity for SUMO is generally in the micromolar range (e.g., ref. 6), meaning that additional contacts between the SUMOylated protein and its binding partner are needed to drive interactions. Alternatively, by incorporating multiple SIMs, this can be sufficient to recruit a protein to a SUMOylated binding partner, but multiple SUMO moieties must be present in the form of poly-SUMO chains (reviewed in ref. 8). This is exemplified by PML, which becomes poly-SUMOylated upon treatment of cells with arsenic trioxide, and results in the recruitment of the ubiquitin ligase ring finger protein 4 (RNF4), which contains four closely juxtaposed SIMs (9, 10). More recently, mass spectrometry studies have led to the identification of hundreds of proteins that become poly-SUMOylated upon heat shock, and can then bind to RNF4 (11). The functional importance of these chains has been elucidated in yeast, where numerous roles have been attributed to poly-SUMOylation, including a major defect in higher-order chromatin structure maintenance (12). Thus, the promotion of poly-SUMOylation represents a potential regulatory route for specifically recruiting binding partners that contain multiple SIM motifs.
Many proteins contain multiple sites for modification by SUMO. For example, a bioinformatics approach identified 448 human proteins containing two or more motifs corresponding to the extended negatively charged amino acid-dependent SUMOylation motif (NDSM) (13) Thus, there is a huge potential for widespread multi-SUMOylation of proteins to occur. Indeed, several of these proteins have been shown to be SUMOylated on multiple sites, including megakaryoblastic leukemia (translocation) 1 (MKL1) (14), CREB-binding protein (CBP) (15), and PEA3/ETV4 (16). Furthermore, two recent proteomic studies emphasize the potential for more widespread multi-SUMOylation as they found that a large proportion of all SUMO2-modified proteins contain two or more modified lysine residues, with one study reporting that over one-half of the proteins fell into this category, with proteins like ZNF451 having 40 such sites (17, 18). In principle, multi-SUMOylation could provide a platform for recruiting proteins containing multiple SIMs as has already been observed for poly-SUMOylation in the form of linear chains. Moreover, the aggregation of several SUMOylated proteins into protein complexes, as seen in many transcriptional regulatory complexes, provides yet more potential for presenting a multi-SUMO–binding surface for recruiting coregulatory partners. Indeed, a recent study demonstrated that SUMOylation of many different proteins involved in homologous recombination acts synergistically to promote efficient DNA repair (19).
In this study, we therefore investigated whether multi-SUMOylation could provide a means for driving protein recruitment through multi-SIM–containing protein partners. We used a novel multi-SUMO scaffold to identify over 100 potential multi-SUMO–binding proteins. We verified several of these interactions and demonstrated the importance of a multi-SUMO–binding multi-SIM module for anchoring the transcriptional corepressor protein zinc finger, MYM-type 2 (ZMYM2)/zinc finger protein 198 (ZNF198) to chromatin.
Materials and Methods
Information regarding the plasmids, cell lines, transfections, and reporter gene assays used in this study can be found in SI Materials and Methods.
Microarray Analysis, RT-PCR, and siRNAs.
The microarray expression data have been submitted to ArrayExpress (accession no. E-MEXP-3880). Methods of the microarray and quantitative RT-PCR can be found in SI Materials and Methods. SMARTpool siRNA duplexes against ZMYM2, UBE2I/UBC9, and nontargeting control (NTC) were purchased from Dharmacon.
ChIP-qPCR and ChIP-seq Analysis.
ChIP-qPCR was performed at least in duplicate, from at least two independent experiments, and analyzed as described previously (20).
ChIP-seq accession numbers are E-MTAB-2701 (ZMYM2 and FOXO3) and E-MTAB-3084 (SUMO3). Methodology can be found in SI Materials and Methods.
Protein Analysis.
All of the proteomic methodology used in this paper, including protein purification, pulldown, SIM peptide competition, nuclear extract preparation, mass spectrometry, Western blotting, immunofluorescence, and surface plasmon resonance can be found in SI Materials and Methods.
SIM Motif Search.
A motif string search script was written in Python 3. SIM motifs were searched in either the proteins identified by mass spectrometry or in 1,000 different sets of random proteins with similar length distributions to those identified in this study. Random protein sequences were retrieved from UniProt. To determine the significance of enrichment of this motif in the identified proteins, a one-sample t test was carried out. For annotating our list of proteins identified in the mass spectrometry assay, the presence of SIM motifs was determined using the program GPS-SUMO (21) set to high threshold.
SI Materials and Methods
Plasmid Constructs.
The following plasmids were used in mammalian cell transfections. pAS1143 (encoding FLAG-SENP3 C532S) (42), pAS4162 (encoding FLAG-PIAS1; kindly provided by Zongling Ji, University of Manchester), pRC/CMV-flagmPTRF [kindly provided by Ingrid Grummt, German Cancer Research Center (DKZF), Heidelberg], pCS2-myc-ZNF198 full length (kindly provided by Hongtao Yu, University of Texas Southwestern Medical Centre, Dallas; ref. 35), pEF6/Myc-HisC-BLM (kindly provided by Ian Hickson, University of Copenhagen, Copenhagen), pcDNA3.1-myc-SRBC (kindly provided by Richard Anderson, University of Texas Southwestern Medical Center, Dallas; ref. 43), pBicep-3×-FLAG-Kaiso and pBicep-3×-FLAG-ZBTB4 (kindly provided by Pierre-Antoine Defossez, University Paris Diderot, Paris), L8G5E1a-Luc and LexA-VP16 constructs (kindly provided by C. Lemercier, Institut Albert Bonniot, Grenoble, France; ref. 44) have been described elsewhere. pCS2-myc-ZNF198(SIM1mut) (pAS4079), pCS2-myc-ZNF198(SIM2mut) (pAS4152), pCS2-myc-ZNF198(SIM3mut) (pAS4153), pCS2-myc-ZNF198(SIM1,2mut) (pAS4080), pCS2-myc-ZNF198(SIM1,3mut) (pAS4154), and pCS2-myc-ZNF198(SIM1,2,3mut) (pAS4151) were constructed by QuikChange mutagenesis (Stratagene), using the following templates and pair of primers, pAS2784, ADS3284/ADS3285; pAS2784, ADS3304/ADS3305; pAS2784, ADS3288/ADS3289; pAS4079, ADS3286/3287; pAS4079, ADS3288/3289; pAS4081, ADS3505/3506, respectively. The following plasmids were used to create stable cell lines that inducibly express FOXO3 or ZNF198/ZMYM2; pcDNA5/FRT/TO-3×-FLAG-FOXO3 (pAS3012), pcDNA5/FRT/TO-3×-FLAG-ZNF198(WT) (pAS4155), pcDNA5/FRT/TO-3×-FLAG-ZNF198(SIM2mut) (pAS4156), and pcDNA5/FRT/TO-3×-FLAG-ZNF198(SIM1,2,3mut) (pAS4157), and they were constructed by ligating either a BamHI/NotI fragment from pAS3010 (FOXO3) or BamHI/NotI-cleaved PCR products (ZMYM2; primers ADS2717/ADS2722 and the templates pAS2784, pAS4152, or pAS4151, respectively) into the same sites in pcDNA5/FRT/TO (Invitrogen). pcDNA5-FRT-TO-Gal4DBD (pAS4014) was constructed by subcloning the Gal4DBD fragment from pAS2440 in the HindIII/BamHI sites of pcDNA5-FRT-TO. Gal4-ZMYM2-WT (pAS4163) and Gal4-ZMYM2-SIM1,2,3 (pAS4165) were created by subcloning the ZMYM2 fragment from pAS4155 or pAS4157 into the BamHI/NotI sites of the pcDNA5-FRT-TO-Gal4DBD (pAS4014) plasmid.
The following plasmids were used for bacterial expression: MBP-RNF4(WT) and MBP-RNF4(SIM2mut), both in pLou3 (9), pAS2974 (encoding GST-SUMO1), pAS2976 (encoding GST-SUMO3) (45), and pHis-COMP-chMp7IVP (kindly provided by Richard Kammerer, Paul Scherrer Institut, Villigen, Switzerland) have been described elsewhere. Plasmids expressing His-tagged SUMO2 chains with two or four SUMOs have been described previously (9). pAS4179 (encoding GST-SUMO3×4 chain) was cloned by inserting the BamHI/NotI fragment from pHis-4×-DN-SUMO3 into the same sites in pGEX-6P1. pCDNA5/FRT-TO/3×FLAG-SUMO3(K11R/Q90P) (pAS4172) was created by first inserting the HindIII/NotI fragment, containing HA-SUMO3(K11R), from pAS2987 into the same sites in pcDNA5/FRT/TO (creating pAS4170). Amino acid 90 was mutated to proline by site-directed mutagenesis using the pair of primers ADS3778/ADS3779 (creating pAS4171). The 3×-FLAG tag was PCR amplified using primers ADS3784/ADS3785. The PCR fragment was then cloned into the HindIII/BamHI site of pAS4171 replacing the HA tag with the 3×-FLAG tag (creating pAS4172).
pAS4012 (encoding His-COMP-SUMO1) was constructed by inserting the MunI-cleaved PCR fragment (generated using the primer pair ADS2569/ADS2567 and template pAS2974) into the EcoRI site in pHis-COMP-chMp7IV, and pAS4013 (encoding His-COMP-SUMO3) was constructed by inserting an EcoRI-cleaved PCR fragment (generated using the primer pair ADS2567/ADS2581 and the template pAS976) into the same sites in pHis-COMP-chMp7IV. pAS4017 (encoding GST-COMP) was constructed by inserting a BamHI-EcoRI fragment from pHis-COMP-chMp7IV into pGEX-6P-1. pAS4018 (encoding GST-COMP-SUMO1) was constructed by inserting the MunI-cleaved PCR fragment (generated using the primer pair ADS2569/ADS2567 and templates pAS2974) into the EcoRI site in GST-COMP (pAS4017), and pAS4020 (GST-COMP-SUMO3) was constructed by inserting an EcoRI-cleaved PCR fragment (generated using the primer pair ADS2567/ADS2581 and template pAS4013) into the same site in GST-COMP (pAS4017). pAS4097 [encoding GST-COMP-SUMO3(3A)] was constructed by QuikChange mutagenesis (Stratagene), using the template pAS4020 and the primer pair ADS3459/ADS3460. pAS4158 (encoding GST-ZMYM2 amino acids 1–200) was constructed by inserting a BamHI/NotI-cleaved PCR fragment (primer pair ADS2717/ADS3584 and template pAS4081) into the same sites in pGEX-6P1. For in vitro transcription/translation, pAS4044 (encoding full-length Kaiso/ZBTB33) was constructed by inserting a BamHI/XhoI-cleaved PCR fragment [primer pair ADS3143/ADS3144 and template pAS4029 (kindly provided by Egor Prokhortchouk, Center Bioengineering Russian Academy of Sciences, Moscow)] into the same sites in pcDNA3C-FLAG.
Tissue Culture, Cell Transfections, and Reporter Gene Assays.
HEK293T, HeLa S3, U2OS, and U2OS-Flp-In cells were grown in DMEM supplemented with 10% (vol/vol) FBS. Growth media for U2OS-Flp-In cells also contained blasticidin (10 μg/mL). HeLa S3 cells were first grown as monolayer before transferring them to a spin-flask culture, where they were grown with constant agitation. Plasmid transfection of HEK293T cells was performed using Polyfect (Qiagen); U2OS-Flp-In cells were transfected using X-Treme HP DNA (Roche) for plasmids, and Lipofectamine RNAi-Max (Invitrogen) for siRNA transfection according to the manufacturers’ instructions. U2OS-Flp-In cells were used to create the stable cell lines expressing the following FLAG-tagged proteins under a doxycycline-inducible promoter; WT ZMYM2 (WT), ZMYM2(SIM2mut), ZMYM2(SIM1,2,3mut), FOXO3, and SUMO3(K11R/Q90P). pAS4155, pAS4156, pAS4157, pAS3012, or pAS4173 was cotransfected with the Flp recombinase encoding plasmid pOG44 (Invitrogen) into U2OS cells containing a FRT recombination site and the T-REx tetracycline controlled repressor (kindly provided by Catherine Millar, University of Manchester). Hygromycin-resistant cells were selected, expanded 24 h after transfection, and maintained as polyclonal lines. Expression of the transgenes was induced by treating the cells with 1 ng/mL (for FOXO3), 100 ng/mL [for ZMYM2(WT)], or 50 ng/mL [for ZMYM2(SIM2mut) and SUMO3(K11R/Q90P)] doxycycline (Sigma) for 48 h. For luciferase assays, 293T cells were transfected with 250 ng of reporter plasmid, 50 ng of pCH110, 150 ng of Lex-VP16, and increasing amounts of Gal-fused protein. Transfections were carried out using Polyfect (Qiagen) according to the manufacturer’s instructions. Luciferase and β-galactosidase activities were measured 24 h posttransfection using Dual-Light luciferase reporter assay system (Applied Biosystems). The relative luciferase activity was measured as a ratio of the β-galactosidase control.
RNA Isolation and RT-PCR.
Total RNA was isolated using the RNAeasy plus Kit (Qiagen) following the manufacturer’s instructions. Quantitative RT-PCR was performed using 30 ng of total RNA and QuantiTect SYBR Green RT-PCR kit (Qiagen) according to the supplier’s protocol. Data were analyzed by Qiagen Rotor-Gene Q Series software and presented after normalization against the control gene GAPDH. The following primer pairs were used for RT-PCR experiments: ARMC7, ADS3619/ADS3620 (5′-TCGCCAACTTCGCTTATGAC/GCCTCCAATAGCAAACTCCAC-3′), CHGB, ADS3611/ADS3612 (5′-GAAGGAATGGTGACTCGCTG-3′/5′-TCTACTCGTCTTCAGGACTTGG-3′), CCNF, ADS2896/ADS2897 (5′-TAAGAAGTGCTTCCATGATGAC-3′/5′-TCTTGTGTCACTCCTAATGC-3′), EPHX2, ADS3615/3616 (5′-GGAAGTGTTATGTAAGGAGATGGT-3′/5′-TGGGATTTGCTGGTATGAAGG-3′), RNF7, ADS4091/ADS4092 (5′-CCAGGTGATGGATGCCTGTCT/GCTCTGAACAACTGCGCTAAG), TMEM154, ADS3587/3588 (5′-GAACTAAACAAGAACCTTCTAGCC-3′/5′-GGTAGGTAAACATTCAAAGTCGG-3′), GAPDH, ADS2184/ADS2185 (5′-ACAGTCAGCCGCATCTTCTT-3′/5′-TTGATTTTGGAGGGATCTCG-3′).
ChIP-qPCR and ChIP-seq Analysis.
ChIP assays were carried out using 5 × 106 U2OS-ZMYM2(WT) or ZMYM(SIM2mut) cells treated for 48 h with 100 ng/mL [for ZMYM2(WT)] or 50 ng/mL [for ZMYM2(SIM2mut)] doxycycline (Sigma). Where indicated, cells were heat shocked at 43 °C for 30 min before harvesting. Assays were performed as described previously (46) using control IgG (Millipore), anti-FLAG (Sigma), anti-ZMYM2 (Bethyl Laboratories), or SUMO2/3 (Biomol) antibodies. Bound regions were detected by quantitative PCR using the following combinations of primers to detect the loci associated with the indicated genes: EMX2, ADS3764 (CTGATCTTGGCTGGGCTTAC) and ADS3765 (CTGGGCTAGCATTTTGGAAG); FAM109A, ADS4348 (AACACCGGTCATAGCAGCTT) and ADS4349 (CTGGTGATGACTGCCTGTTG); GCNT7, ADS3770 (ACTTCTCCTGCAACCTGGAA) and ADS3771 (AACAGGCCCAGACATACTGC); MCC, ADS3774 (CCAAGACCTGCTCAAGGTTC) and ADS3775 (TTCACGAAACCACAAATTGC); NEAT1, ADS3724 (CAAAAGTTGTGGCAAGTCCA) and ADS3725 (GCCCCCTCGTCTCATCTAAC); PADI4, ADS3772 (AGGAATGCAGGGAGGCTAGT) and ADS3773 (CCATCTCCTTCCATCCAGAA); SSBP3, ADS3776 (CCAGGTCAGGCACAGTTCTT) and ADS3777 (GGCCTTGGATTGTCAGATGT); TXNL4, ADS3760 (TGCCCTTCTCCTCTTTCCTT) and ADS3761 (GGGTGGAAAGCTAGGGTCTT); and ZC3H3, ADS3768 (TTTTACCGTGTTAGCCAGGA) and ADS3769 (CGGTGCAAGATTTCATCACA).
For ChIP-seq, ChIP was carried out using 60 × 106 U2OS-FOXO3-FLAG, U2OS-ZMYM2(WT), or U2OS-ZMYM2(SIM2mut) cells treated for 48 h with 1 ng/mL (for FOXO3), 100 ng/mL [for ZMYM2(WT)], or 50 ng/mL [for ZMYM2(SIM2mut)] doxycycline (Sigma); 6 μg of anti-FLAG (Sigma) antibody. The control experiment (Control-IP) was performed in the U2OS parental cell line that does not have any FLAG-tagged proteins using the anti-FLAG antibody. For SUMO2/3, ChIP-seq was performed using 3 × 106 U2OS cells grown under standard culture conditions using 3 μg of anti-SUMO2 (Life Technologies) antibody. The ChIPed DNA and input DNA were amplified as previously described (47). Libraries were generated, and sequencing was performed on an Illumina HiSeq according to the manufacturer’s protocols. Single ChIP-seq libraries from each cell line were analyzed by trimming reads to 50 bp and mapping these to the human genome (hg19) using Bowtie1.0 (settings: v = 0, m = 1) (48). Peaks were called using HOMER4.2 with default settings (49) using Control-IP as a background. ZMYM2 binding regions were linked to their nearest gene transcription start sites from Ensembl, version 72 (50), using an in-house script. Accession numbers are E-MTAB-2701 (ZMYM2 and FOXO3) and E-MTAB-3084 (SUMO3). Reads were mapped to the human genome (hg19) and peaks called relative to input control using the same methods as for FOXO3/ZMYM2 datasets. Tag density distributions around TF peak regions were calculated using annotatePeaks.pl functionality in the HOMER suite and visualized using JavaTreeView (51) and plotted as mean numbers of tags per 10 bp per peak per million reads. Preferential binding of WT ZMYM2 to chromatin was determined using the getDifferentialPeaks functionality of HOMER, version 4.7.2, with “-size fixed” option to compare the number of tags within each of the union peak set. For correlating with SUMO occupancy, the top 1,000 ZMYM2(WT) and FOXO3 binding regions were selected, ranked by HOMER4.2 normalized tag score. We then calculated the density of SUMO tags across a 1-kbp interval around the centers of these regions. To correlate ZMYM2 occupancy with the requirement of its SIM motifs, the total number of tags from either the ZMYM2(WT) or ZMYM2(SIM2) ChIP-seq experiments that fall within 1 kb of the centers of 3,984 ZMYM2 bound regions were counted. To compare SUMO occupancy at ZMYM2 bound regions, the peaks were remapped using 101-bp reads and recalled using Homer, version 4.7.2. From the union of peaks of the ZMYM2(WT) or ZMYM2(SIM2) ChIP-seq experiments, peaks were removed that had fewer than five tags resulting in 1,805 peaks. These peaks were then ranked by the excess of WT over SIM2IP tags within the entire peaks according to the ratio of WT to SIM2mutant ZMYM2 and divided into 10 cohorts, from the top 10% to the bottom 10% most WT tag-enriched. Finally, the mean number of SUMO tags across the regions within each cohort was calculated.
Microarray Analysis.
After 48 h of doxycycline treatment, total RNA from the U2OS stable cell lines expressing either ZMYM2(WT) or ZMYM2(SIM2mut) was isolated using the RNeasy plus kit (Qiagen). The quality and size distribution of the RNA were assessed with the RNA Nano Lab on a Chip kit (Agilent Technologies). Sample labeling and hybridization to Affymetrix GeneChip Human Genome U133 Plus 2.0 arrays were performed according to the manufacturer’s instructions. A single microarray experiment was performed for RNA samples from each cell line. Upon collection of signal, technical quality control was performed with dChip (V2005) using default settings. Background correction, quantile normalization, and gene expression analysis were performed using Propagating Uncertainty in Microarray Analysis software (PUMA) (52). Principal component analysis (PCA) was performed with Partek Genomics Solution (version 6.5; copyright 2010; Partek, Inc.).
Protein Purification, GST Pulldown Assay, SIM Peptide Competition Assays, Nuclear Extract Preparation, and Mass Spectrometry.
Recombinant proteins were expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL (Stratagene). Recombinant GST-fusion and His-tagged proteins were purified using glutathione (Sigma) or Ni-NTA agarose beads (Qiagen), respectively.
GST pulldown assays were carried out using purified recombinant GST proteins (generally 1–2 μg) and either cell lysates from HEK293T cells transfected with the desired expression construct, in vitro translated proteins, total cell lysates from E. coli transformed with the desired expression construct, or His-tagged purified proteins as described previously (9). For pulldowns involving ZMYM2, the binding buffer was modified by removing DTT and adding ZnCl2 to a final concentration of 100 μM. SIM peptide competition assays were carried out as described for the GST pulldown assay, but increasing amounts of a SIM peptide from PIAS2 (VDVIDLTIEEDE) (11) was added to the samples during the binding step.
To isolate proteins binding to the GST-COMP-SUMO3 multi-SUMO scaffold, nuclear extracts were prepared from 3 L of HeLa S3 cells grown in suspension as described previously (53). Final extracts were dialyzed against pulldown buffer (9). Next, the nuclear extract was mixed with 1 μg of glutathione Sepharose-bound GST-COMP and incubated at room temperature for 1 h. The supernatant, containing proteins that did not interact with GST or COMP, was added to 1 μg of glutathione Sepharose-bound GST-COMP-SUMO3 and incubated for 1 h at room temperature. After extensive washes with SUMO wash buffer [50 mM Tris, pH 7.5, 0.1% Triton X-100, 50 mM NaCl, 2 mM DTT, and 5% (vol/vol) glycerol], the bound proteins were released from the Sepharose by overnight treatment with PreScission protease (8 units; GE Healthcare). Proteins were resolved by SDS/PAGE and visualized by colloidal Coomassie blue stain. Bands corresponding to proteins that interacted with multi-SUMO3 were excised from the gel and subjected to mass spectrometry. First, the gel slices were dehydrated using acetonitrile followed by vacuum centrifugation. Proteins in the dried gel pieces were reduced with 10 mM DTT and alkylated with 55 mM iodoacetamide. Gel pieces were then washed alternately with 25 mM ammonium bicarbonate followed by acetonitrile. This was repeated, and the gel pieces were dried by vacuum centrifugation. Samples were digested with trypsin overnight at 37 °C. Digested samples were analyzed by liquid chromatography (LC)-MS/MS using a NanoAcquity LC (Waters) coupled to a LTQ Velos (Thermo Fisher Scientific) mass spectrometer. Peptides were concentrated on a precolumn (20 mm × 180 μm i.d.; Waters). The peptides were then separated using a gradient from 99% A (0.1% FA in water) and 1% B (0.1% FA in acetonitrile) to 25% B, in 45 min at 200 nL⋅min−1, using a 75 mm × 250 μm i.d., 1.7 μM BEH C18, analytical column (Waters). Peptides were selected for fragmentation automatically by data-dependent analysis. Data produced were searched using Mascot (Matrix Science), against the (contam.ipi_human.v3_63_4_0) database. Data were validated using Scaffold (Proteome Software).
Surface Plasmon Resonance Assay.
For surface plasmon resonance assays, GST-fused proteins were purified and GST-ZMYM2 was eluted off the beads by incubating with 10 mM reduced glutathione for 45 min. GST-COMP, GST-COMP-SUMO3, and GST-SUMO3×4 were released from the GST moiety while still bound to the beads by overnight treatment with PreScission protease (8 units; GE Healthcare). The buffer was then exchanged using ZebaTM spin desalting columns following the manufacturer’s instructions (Life Technologies).
Experiments were performed using the ProteOn XPR36 instrument (Bio-Rad Laboratories). Running buffer was 100 mM NaCl, 10 mM Hepes, 0.1% (vol/vol) Igepal, pH 7.0. GST-ZMYM2 or the control protein GST-kistrin (54) was immobilized on a GLC chip (Bio-Rad Laboratories) in the vertical orientation. Two channels were activated with a mixture of 25 mM N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC) with 15 mM sulfo-N-hydroxysuccinimide (sulfo-NHS) at a flow rate of 30 μL/min for 5 min. GST proteins were immobilized in acetate buffer, pH 4.5, at a flow rate of 30 μL/min. The immobilization level of GST proteins was ∼1,600 resonance units (RU). Remaining cross-linking sites were blocked by an injection of 150 μL of 1 M ethanolamine–HCl (pH 8.5). SUMO fusion proteins or the control COMP protein at a concentration of 5 μg/mL, were used in three parallel horizontal channels at a flow rate of 100 μL/min. Binding experiments were performed using an injection time of 60 s and a dissociation time of 300 s. Regeneration of the surface was accomplished with a 30-s pulse of 50 mM NaOH at 100 μL/min. All experiments were performed at 25 °C. All binding sensorgrams were collected, processed, and analyzed using the integrated ProteOn Manager software (Bio-Rad Laboratories). The complex nature of the binding kinetics precluded fitting to standard kinetic models; however, dissociation rates could be calculated using off-rate analysis with the following equation:
Western Blotting and Immunofluorescence Analysis.
Western blotting was carried out with the primary antibodies; anti-FLAG (Sigma), anti-MBP (Cell Signaling), anti-myc (Santa Cruz), anti-His (GE Healthcare), anti-ZMYM2 (Bethyl laboratories), anti-Ubc9 (BD Transduction Laboratories), anti-Lamin B (Santa Cruz), anti-Gal4 (Santa Cruz), and anti-Tubulin (Sigma). The proteins were detected using SuperSignal Dura (Pierce) chemiluminescent substrates or infrared dye-conjugated secondary antibodies (LI-COR Bioscience; IRDye 800CW and IRDye 680LT). The signal was collected with either a Chemidoc imaging system (Bio-Rad) or with a LI-COR Odyssey Infrared Imager. Data were quantified using Quantity One software (Bio-Rad) or Odyssey software (LI-COR Bioscience; Odyssey Infrared Imaging system application software, version 3.0.25).
For immunofluorescence, transfected U2OS-Flp-In cells were fixed and permeabilized with 3.7% (vol/vol) paraformaldehyde–0.18% Triton X-100. Anti-myc was used as primary antibody and anti-mouse Alexa Fluor 594 as secondary antibody. DNA was stained with Hoechst. Images were acquired on a Delta Vision RT (Applied Precision) restoration microscope using a 100×/1.40 Plan Apo objective. The images were collected using a Coolsnap HQ (Photometrics) camera with a Z optical spacing of 0.2 μm. Raw images were then deconvolved using the Softworx software, and maximum-intensity projections of these deconvolved images are shown in Results.
Results
Identification of Multi-SUMO–Binding Proteins.
Previous studies established that RNF4 binds to proteins containing multiple SUMO moieties in the form of linear poly-SUMO chains, through its multiple SIM motifs (9). However, it is equally plausible that multi-SIM–containing proteins might be able to recognize multiple single SUMO moieties conjugated to different sites in a protein or protein complex (Fig. 1A). To date, RNF4 and the closely related protein Arkadia are the only known multi-SIM–containing proteins with the potential to bind poly- or multi-SUMOylated proteins (9, 22). We therefore sought to investigate how widespread this phenomenon is likely to be, by searching for proteins that could bind to a multi-SUMO platform. To identify multi-SUMO–binding proteins, we created a fusion protein, containing SUMO fused to GST through a linker region corresponding to the coiled-coil pentamerization domain of cartilage oligomeric matrix protein (COMP) (23). This allowed us to create a scaffold which presents five copies of SUMO in a form that resembles multi-SUMOylation rather than the single SUMO molecules presented on a standard GST-fusion protein (Fig. 1B). As expected, in comparison with GST-SUMO fusion proteins, GST-COMP-SUMO fusion proteins migrated as higher molecular-weight species in native polyacrylamide gels (Fig. 1C, Bottom; compare lanes 2 and 3 with lanes 5 and 6). To test whether GST-COMP-SUMO acted in the predicted manner, we examined interactions with RNF4. Little binding of RNF4 to GST-COMP or GST-SUMO3 was observed, but binding was readily observable to GST-COMP-SUMO3 (Fig. 1D). Binding appears to be SUMO paralog specific as comparatively little RNF4 binding was observed to GST-COMP-SUMO1 (Fig. S1). Importantly, upon mutation of the SIM2 motif in RNF4, this mutant protein showed reduced binding to GST-COMP-SUMO3 (Fig. 1E), which resembled the reduced binding seen with poly-SUMO chains (9). These findings therefore establish GST-COMP-SUMO3 as a suitable platform for use in the identification of multi-SUMO–binding proteins.
Fig. 1.
Identification of multi-SUMO–binding proteins. (A) Schematic representation of potential mechanisms through which a multi-SIM–containing protein can interact with a SUMOylated substrate (X) through binding to poly-SUMO chains (Left) or a multiple SUMO molecules (S) present on a platform of several mono-SUMOylated lysine (K) residues (Right). (B) Schematic representation of GST-SUMO and “pentameric” GST-COMP-SUMO. (C) The indicated purified GST-fused proteins were resolved by denaturing (Top) or native (Bottom) polyacrylamide gel electrophoresis. Proteins were visualized with Coomassie blue stain. (D and E) GST pulldown assays of WT or mutant MBP-RNF4 with GST or the indicated GST-fusion proteins. (D) RNF4 binding is shown at the Top using an anti-MBP antibody for immunoblot (IB), and a Ponceau-stained membrane at the Bottom shows the GST bait input proteins. The 5% input protein is shown (lane 1). (E) Coomassie-stained gel showing the GST-COMP-SUMO bait proteins and pulled down (PD) WT and SIM2 mutant versions of MBP-RNF4. (F) Strategy for identifying multi-SUMO interacting proteins, from nuclear extracts by binding to GST-COMP-SUMO3. (G) Coomassie-stained gel showing bait proteins (lanes 3 and 4) and proteins pulled down by GST-COMP and GST-COMP-SUMO3. Molecular-weight markers (M1 and M2) are indicated. (H) Pie chart showing the cellular function of the GST-COMP-SUMO3 binding proteins found by mass spectrometry.
Fig. S1.
Paralog specificity of RNF4 binding to multi-SUMO scaffolds. GST-pulldown assay of FLAG-tagged RNF4 (expressed in HEK293T cell lysates) with the indicated GST-tagged COMP fusion proteins. Proteins pulled down (PD) (lanes 5–7) or the unbound protein in the flow through (FT) (lanes 2–4) were detected by IB with an anti-FLAG antibody (Top). Proteins used as baits are shown by Coomassie stain at the Bottom. The 5% input protein is shown (lane 1). RNF4 shows specificity toward binding to SUMO3 in the context of the GST-COMP-SUMO3 multi-SUMO scaffold.
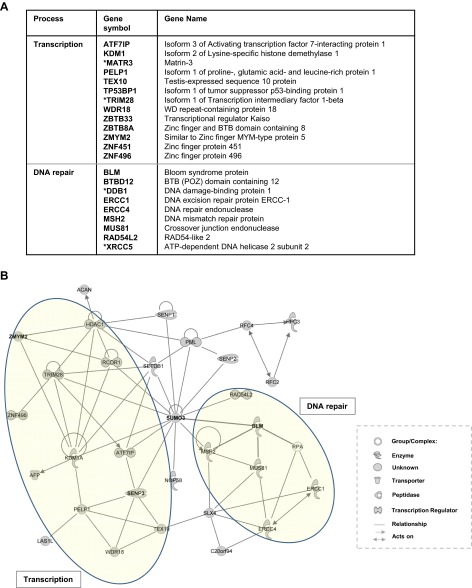
To identify novel multi-SUMO–binding proteins, a pulldown assay was carried out using GST-COMP-SUMO3 as bait, and HeLa nuclear extracts as the source of potential interacting proteins. We first removed nonspecific binding proteins by incubating the nuclear extract with glutathione-agarose bead-immobilized GST-COMP (Fig. 1F). Proteins remaining in the supernatant were subsequently incubated with glutathione-agarose bead-immobilized GST-COMP-SUMO3. Following extensive washing, the remaining bound proteins were eluted from the beads by cleaving the GST tag and subjected to SDS/PAGE (Fig. 1G, lane 8). Bound proteins were then eluted from the gel, and their identities were determined by mass spectrometry. We identified a total of 163 proteins, over one-half of which can be considered higher confidence due to not being commonly identified components of coimmunoprecipitation experiments (Dataset S1). This list includes several involved in protein SUMOylation (PIAS1, SENP1, SENP2, and SENP3) and also many associated with transcriptional regulation and DNA repair, two processes that are often associated with SUMOylation (Fig. 1H and Fig. S2A; reviewed in ref. 4). Many of these proteins have previously been shown to interact either physically or functionally, thereby suggesting the existence of important underlying SUMO-dependent networks (Fig. S2B). Several of the proteins had previously been shown to bind to monomeric SUMO including BLM1 (24) and RCOR1 (25).
Fig. S2.
Interaction network of proteins found to interact with multi-SUMO by mass spectrometry. (A) Proteins involved in transcription or DNA repair that were identified by mass spectrometry after binding to the GST-COMP-SUMO3 multi-SUMO scaffold. Proteins denoted with an asterisk were from the lower confidence dataset. (B) A network of known interactions was constructed using Ingenuity Systems software based on using SUMO as the starting protein and all of the proteins identified as binding to the multi-SUMO scaffold. Parts of the network associated with transcriptional control and DNA repair are highlighted.
To verify the mass spectrometry results, we transfected HEK293T cells with expression constructs for epitope-tagged versions of PTRF, BLM, and ZMYM2/ZNF198, and determined whether GST-COMP-SUMO3 could bind to any of these in the context of cell lysates. All three of these proteins bound strongly to GST-COMP-SUMO3 (Fig. 2A, lane 7). In contrast, little binding was seen to GST alone or GST-COMP (Fig. 2A, lanes 2 and 5), and, importantly, little binding was seen to GST-SUMO3 (Fig. 2A, lane 4), demonstrating the importance of presenting SUMO3 in a multimeric form on a pentameric scaffold. Interestingly, there also appears to be SUMO paralog specificity, as comparatively little binding to GST-COMP-SUMO1 was observed (Fig. 2A, lane 6). To further demonstrate the specificity of these interactions, we examined ZBTB33/Kaiso and demonstrated that, although it preferentially binds to GST-COMP-SUMO3 over GST-COMP, the closely related zinc finger and ZBT domain-containing protein ZBTB4 is unable to bind to GST-COMP-SUMO3 (Fig. 2B).
Fig. 2.
Validation of multi-SUMO–binding interactions. (A) GST pulldown analysis of PTRF, BLM, and ZMYM2 binding to the indicated GST-fusion proteins. Epitope-tagged PTRF, BLM, or ZMYM2 were overexpressed in HEK293T cells, and total cell lysates were used for the pulldown. Proteins were detected by IB using anti-FLAG (PTRF) or anti-Myc (BLM and ZMYM2) antibodies. Arrows indicate the positions of bands corresponding to the full-length proteins. Bait proteins were stained with Ponceau (Bottom). (B) Interaction of ZBTB33 or ZBTB4 with COMP-SUMO3 was analyzed by GST-pulldown. Total cell extracts of transfected HEK293T cells were used for the pulldown. Input and precipitated ZBTB33 and ZBTB4 were detected using an anti-FLAG antibody. Proteins used as bait were stained with Ponceau (Bottom).
We also identified proteins like SRBC that could not bind specifically to GST-COMP-SUMO3, unless coexpressed with its binding partner PTRF (26) (Fig. S3 A and B). This raised the possibility that some of the interactions we have observed might be indirect and mediated by other partners in multiprotein complexes. We therefore further investigated whether ZBTB33 binding to GST-COMP-SUMO3 could be observed when it was expressed in vitro. Again, strong binding was only observed with GST-COMP-SUMO3 among the GST-fusion proteins tested (Fig. S3C). Similarly, we demonstrated direct interactions with multi-SUMO by demonstrating that recombinant GST-tagged ZMYM2(1–200) could bind specifically to recombinant His-tagged COMP-SUMO3 (Fig. S3D).
Fig. S3.
Multi-SUMO interactions with SRBC and ZMYM2. (A) The interaction of SRBC with the indicated GST-fusion proteins was analyzed by GST pulldown using total cell extracts of HEK293T cells transfected with a myc-tagged SRBC expression construct. SRBC was detected by IB using an anti-myc antibody. A long exposure of the blot is presented here, showing low-level nonspecific binding of SRBC to the GST-COMP fusion proteins. The input sample lane (5% input) shows saturation (lane 1; gray line). (B) GST pulldown analysis of the interaction of myc-tagged SRBC with the indicated GST-fusion proteins when coexpressed with PTRF in HEK293T cell lysates. The binding of PTRF when expressed alone to the different GST-fusion constructs is shown in Fig. 2A. Specific binding of SRBC to GST-COMP-SUMO3 is revealed in the presence of coexpressed PTRF. (C) In vitro-translated ZBTB33 was used in a GST pulldown assay with the indicated GST-fusion proteins. Bait proteins were stained with Coomassie (Bottom), and ZBTB33 was detected by autoradiography (Top). In all cases, 10% input protein is shown. (D, Left) Coomassie-stained SDS/PAGE of the purified GST and GST-ZMYM2 input proteins. Arrow indicates the position of full-length GST-ZMYM2(1–200). (Right) IB of recombinant purified His-tagged COMP (Top) or COMP-SUMO3 (Bottom) following pulldown with GST or GST-ZMYM2(1–200). Proteins were detected using an anti-His antibody. The 5% input is shown (lane 1). Direct specific interactions are observed between ZMYM2 and the multimeric SUMO3 scaffold. (E) GST pulldown analysis of the interaction of purified recombinant GST-ZMYM2(1–200) with multi or poly forms of His-tagged SUMO. Poly-SUMO is presented in the form of linear fusion proteins. (Top) Schematic illustration of the constructs used for the analysis. (Bottom Left) Coomassie-stained SDS/PAGE of the His-tagged purified proteins. (Bottom Right) Proteins binding to ZMYM2 are shown by immunoblot (IB) with anti-His antibody (Top), and the GST bait proteins are shown on a Ponceau-stained membrane (Bottom). (F) Binding of SUMO domain proteins to immobilized control protein GST-Kistrin by SPR. At t = 0, either COMP (gray sensorgram), COMP-SUMO3 (blue sensorgram), or SUMO3×4 (orange sensorgram) was injected. (G) Immunoprecipitation (IP) analysis of ZMYM2 from U2OS-Flp-In cells containing doxycycline (Dox)-inducible FLAG-tagged SUMO3(K11R/Q90P) in the absence and presence of Dox. Coprecipitated SUMO3 was detected by IB with anti-FLAG antibody.
In summary, we have identified a large number of novel multi-SUMO–binding proteins, and at least some of these are likely to directly interact with SUMO.
Characterization of the SIM–Multi-SUMO Binding Interactions.
SUMO2 and 3 have been shown to contain a conserved binding surface that is used for binding to SIMs, which conform to the typical consensus of a short hydrophobic core surrounded by acidic residues such as found in PIAS2 (6, 7). This surface on SUMO2/3 contains a mixture of hydrophobic and basic residues that acts as a reciprocal interface for SIMs (27) (Fig. 3A). We therefore asked whether the proteins that we identified bind through this same surface of SUMO by using a mutant SUMO3 derivative, SUMO3(3A), in which three residues in the SIM binding surface have been replaced with alanines (Fig. 3A). As expected, RNF4 binding to GST-COMP-SUMO3 was reduced upon mutation of the SIM-interaction surface on SUMO3 (Fig. 3B). Similarly, binding of ZMYM2 to SUMO3 was also severely compromised upon introduction of these mutations (Fig. 3B), and three more of the proteins tested, ZBTB33, PTRF, and PIAS1, showed the same binding profile (Fig. 3C). As predicted, SENP3 showed identical binding to wild-type (WT) and mutant versions of SUMO3, in agreement with the observation that SUMO proteases use additional contact regions in their interaction with SUMO (28, 29). Thus, SENP3 uses a different mode of interaction to the other proteins identified in our screen.
Fig. 3.
Characterization of SIM–multi-SUMO binding interactions. (A) Structure of SUMO3 indicating the surface involved in the interaction with SIMs (41). Hydrophobic amino acids are shown in black; basic amino acids are colored blue. The locations of three residues in the surface that we mutated to alanine to disrupt SIM interactions are shown in yellow (Right). The sequences surrounding these amino acids in wild-type (WT) SUMO3 or the triple-alanine mutant SUMO3(3A) are shown below. Mutated amino acids are in bold. (B–E) GST pulldown (PD) assays with the indicated GST-fusion proteins. After the PD, bait proteins were stained with Ponceau (Bottom). (B) Binding of RNF4 or ZMYM2 (from lysates of HEK293T cells overexpressing the proteins) to WT GST-COMP-SUMO3 or GST-COMP-SUMO3(3A). Interacting proteins were detected by IB with anti-FLAG (RNF4) or anti-myc (ZMYM2) antibodies (Top). (C) Interaction of FLAG-tagged ZBTB33, PTRF, PIAS1, or SENP3cs from lysates of HEK293T cells overexpressing these proteins with WT SUMO3 or SUMO3(3A). Proteins were detected by immunoblot using anti-FLAG antibodies (Top). (D and E) Peptide competition assays. Bait proteins were stained with Ponceau (Bottom). (D, Top) Sequence of the SIM peptide used for the competition assays. Acidic amino acids are underlined, and the hydrophobic core boxed. (Bottom) IB using anti-FLAG antibody showing RNF4 binding to GST-COMP-SUMO3 after incubation with increasing concentrations of SIM peptide (0, 0.5, 5, and 50 ng). RNF4 was from cell lysates of transfected HEK293T cells. (E) Assay was carried out as described in D, but only 0, 25, and 50 ng of SIM peptide were used and the cell lysates were from HEK293T cells expressing catalytically inactive SENP3cs or ZMYM2. Interacting proteins were detected by IB using an anti-FLAG (for SENP3CS) or anti-myc (for ZMYM2) antibody.
Given the observed binding to the SIM interaction surface on SUMO, this indicated that many of the proteins that we identified as binding to multi-SUMO might do so through canonical SIM-like motifs. We therefore searched the sequences of the proteins we identified for the well-characterized motifs conforming to SIM2 in RNF4 [ED][ED][IVL][IVL][DE][IVL]xx[ED] and the SIM in PIAS2 [DE][IVL][IVL][DE][VIL]xx[ED][ED]. These SIM motifs were enriched in the mass spectrometry-identified proteins compared with random background data sets (values of P < 0.0013 and 0.00018, respectively). Moreover, 14 of the proteins contained two or more canonical SIMs, consistent with a potential role in multi-SUMO binding (Dataset S1). To investigate this phenomenon further, we used an alternative approach, involving a peptide competition with a peptide corresponding to the SIM in PIAS2, and incorporated this into binding assays with WT GST-COMP-SUMO3. As expected, increasing amounts of this peptide disrupted interactions between RNF4 and GST-COMP-SUMO3 (Fig. 3D). Similarly, the SIM peptide competed with ZMYM2 for binding to GST-COMP-SUMO3 but was unable to compete with binding of SENP3 (Fig. 3E, top two panels). This is consistent with ZMYM2 and SENP3 each using different interaction surfaces on SUMO3.
Together, these results demonstrate that many of the factors that we have identified as multi-SUMO–binding proteins, share similar binding properties to those previously defined for canonical SIM–SUMO interactions. However, there are likely other proteins typified by SENP3 that use alternative binding modes.
ZMYM2 Contains Multiple Functional SIMs.
RNF4 contains several SIMs, which enables it to make simultaneous contact with multiple SUMO moieties (9). Thus, it appears likely that, by analogy with RNF4, many of the numerous multi-SUMO–binding proteins we have identified will operate a similar interaction mechanism. One such protein is ZMYM2, which contains two motifs (SIM1 and 2), that closely resemble the canonical SIMs found in RNF4 and PIAS2 with a hydrophobic core and multiple flanking acidic residues and a third motif with only one flanking acidic residue (SIM3) (Fig. 4A). To establish the importance of these motifs in ZMYM2 for multi-SUMO binding, we mutated them by substituting three or four residues of the core hydrophobic motifs for alanines and tested the resulting proteins for binding to GST-COMP-SUMO3. The mutation of individual motifs had differing effects on binding, with SIM1 and SIM3 mutations having moderate and little effect, whereas mutating SIM2 virtually eliminated multi-SUMO binding (Fig. 4B). Such pronounced effects of a single dominant SIM have been observed for RNF4 and Arkadia (9, 22). However, combinatorial mutation of SIM1 and SIM3 caused a loss of binding to GST-COMP-SUMO3 (Fig. 4B), demonstrating their functional importance in multi-SUMO binding.
Fig. 4.
Mapping the SIMs in ZMYM2. (A) Schematic illustration of ZMYM2 showing the location and sequence of three putative SIMs. Acidic amino acids are colored in red. Regions containing hydrophobic amino acids mutated to alanine are boxed. (B) GST pulldown analysis of the indicated GST-fusion proteins to WT or mutant forms of ZMYM2. Total cell lysates of HEK293T cells overexpressing the ZMYM2 proteins were used for the PD. ZMYM2 was detected by IB using anti-myc antibody. The 5% input is shown. Quantification of ZMYM2 binding to GST-COMP-SUMO3 is shown on the Right as a percentage relative to the input lane and is the average of two experiments. Error bars represent SE. (C) GST pulldown analysis of the interaction of the purified recombinant GST-fusion proteins, COMP, COMP-SUMO3 (multi-SUMO), and SUMO3×4 (poly-SUMO is presented in the form of a linear fusion protein) with the indicated proteins from transiently transfected HEK293T cell lysates. (Top) Schematic illustration of the constructs used for the analysis. (Bottom) Proteins binding to the indicated GST-fusion proteins are shown by immunoblot (IB) with antibodies to the specific tags (Fig. 2) (top panels) and the GST bait proteins are shown on a Ponceau-stained membrane (bottom panel). Quantification of binding of each protein to each of the GST-fusion proteins is shown on the Right as a percentage relative to binding to GST-SUMO3×4 (taken as 100%). Data are the average of three experiments, and error bars represent SD. (D) GST pulldown analysis of GST-COMP-SUMO3 or GST-SUMO3×4 to WT or mutant forms of ZMYM2. Total cell lysates of HEK293T cells overexpressing the ZMYM2 proteins were used for the PD. ZMYM2 was detected by IB using anti-myc antibody. The 5% input is shown. (E) Binding of the indicated SUMO fusion proteins to immobilized GST-ZMYM2(1–200) by SPR. At t = 0, either COMP (gray sensorgram), COMP-SUMO3 (blue sensorgram), or SUMO×4 (orange sensorgram) was injected. Dissociation rates (kd) were 6.05 ± 1.11 × 10−4 s−1 (measured between 200 and 300 s) for COMP-SUMO3 and 7.51 ± 1.64 × 10−2 s−1 (measured between 62 and 100 s) for SUMO3×4. The kd values are mean ± SD from three separate experiments.
Next, we asked whether ZMYM2 and several other multi-SUMO proteins we had identified preferred the presentation of multiple SUMO moieties on either a linear poly-SUMO chain or on a multi-SIM scaffold. Although RNF4 preferentially binds multi-SUMO rather than mono-SUMO (Fig. 1), it showed a clear preference for poly-SUMO chains found in GST-SUMO3 × 4 over the multi-SUMO GST-COMP-SUMO3 scaffold (Fig. 4C). In contrast, ZMYM2 bound strongly to both forms of multimerized SUMO, and ZBTB33 and PTRF showed intermediate relative levels of binding to the GST-COMP-SUMO3 scaffold (Fig. 4C). Given the strong multi-SIM–mediated binding characteristics of ZMYM2, we focused on this further. A previous study identified the zinc finger motifs of ZMYM2 as necessary and sufficient for binding to poly-SUMO chains (30). Importantly, full-length ZMYM2 lacking SIM2, which is critically important for binding the multi-SUMO scaffold, still retained poly-SUMO chain binding activity (Fig. 4D). We therefore focused on the N-terminal region of ZMYM2, which contains two functional canonical SIMs, and asked whether this was sufficient for multi-SUMO binding. This region has previously been shown to be required for colocalization with PML and copurification of SUMOylated proteins (30). Although ZMYM2(1–200) was able to efficiently bind directly to His-tagged COMP-SUMO3 in pulldown assays, it was unable to bind to either dimeric or tetrameric SUMO chains (Fig. S3E). Furthermore, surface plasmon resonance (SPR) studies demonstrated that ZMYM2(1–200) showed specific binding to both multi-SUMO in COMP-SUMO3 and poly-SUMO in SUMO×4 (Fig. 4E and Fig. S3F). However, only COMP-SUMO3 showed stable binding to ZMYM2 with the majority of COMP-SUMO3 binding having a slow dissociation rate (kd = 6.05 ± 1.11 × 10−4 s−1), whereas the poly-SUMO chain had a very rapid dissociation rate (kd = 7.51 ± 1.64 × 10−2 s−1).
Finally, we asked whether we could find evidence for interactions between ZMYM2 and any multi-SUMO–containing proteins in vivo. To do this, we created a doxycycline-inducible stable cell line expressing a FLAG-tagged version of SUMO3 lacking a key lysine residue needed for polychain formation (K11R). Furthermore, to enhance our ability to detect multi-SUMOylated species, we also introduced the Q90P mutation into SUMO3, which renders it noncleavable from substrates by SENPs (31, 32). As predicted, SUMO3 was detected in cells expressing SUMO3(K11R/Q90P) as a series of high–molecular-weight bands (Fig. S3G, lane 2). This is suggestive of the generation of multi-SUMOylated proteins, although due to the presence of endogenous SUMO these SUMO conjugates may contain a mixture of multi and poly-SUMO species. Importantly, immunoprecipitation of ZMYM2 from these cells yielded coprecipitation of a number of these high–molecular-weight SUMOylated species (Fig. S3G, lane 4). Thus, multi-SUMO–binding proteins show a range of binding propensities for different multi-SUMO scaffolds, with ZMYM2 containing an N-terminal multi-SUMO–binding module and exhibiting the greatest ability to bind to a multi-SUMO scaffold.
ZMYM2 has been shown to form part of the CoREST transcriptional corepressor complex (33, 34), and hence likely plays a role in transcriptional repression. To establish the functional importance of the SIMs, we first analyzed the subcellular distribution of WT ZMYM2, the SIM2 mutant and a form with all three SIMs mutated. However, all of the forms of ZMYM2 remained nuclear (Fig. S4A). Next, we asked whether the nuclear function of ZMYM2 as a corepressor protein was perturbed upon mutation of its SIMs. Stable U2OS cell lines were created containing an inducible form of FLAG-tagged WT ZMYM2 or mutant forms with either SIM2 or all three SIMs mutated. Equal expression of all three forms of ZMYM2 was observed, and this was similar to the endogenous protein (Fig. S4 B and C). We then took an unbiased approach to identify the effects of disrupting the multi-SUMO–binding activity of ZMYM2, by using microarray analysis to assess the differences in gene expression in cells ectopically expressing WT or SIM2 mutant version of ZMYM2. We found that 168 genes were up-regulated by more than fivefold and 626 genes were down-regulated by more than fivefold in cells expressing ZMYM2(SIM2mut) (Dataset S2), indicating a pleiotropic change in the gene expression profiles in these cells. As ZMYM2 is thought to be involved in transcriptional repression (35), we focused on genes that were up-regulated in cells expressing ZMYM2(SIM2mut) to identify effects that were potentially caused as a direct effect of disrupting ZMYM2 function. Three genes in this category, CHGB, EPHX2, and TMEM154, were selected, and their increased expression in cells expressing ZMYM2(SIM2mut) was verified by RT-qPCR analysis (Fig. S4D). In contrast, the control genes ARMC7, CCNF, and RNF7 showed little evidence of up-regulation. Importantly, the same genes were also up-regulated in a second cell line expressing ZMYM2(SIM1,2,3mut) (Fig. S4E). The likely explanation for the effect of SIM mutations is a loss of transcriptional repressive activity of ZMYM2. To confirm that ZMYM2 acted as a repressor of the same target genes, we depleted ZMYM2 and assessed their expression levels. In all cases, increased expression was observed upon ZMYM2 depletion, and comparatively little effect was seen on control genes (Fig. S5 A and B). These results suggest that ZMYM2 acts to repress gene expression through its SIM motifs, presumably by interacting with multi-SUMOylated coregulators. If this is the case, reductions in SUMOylation should lead to derepression of the same target genes. We therefore depleted the SUMO E2 conjugating enzyme UBC9/UBE2I (Fig. S5C) and assessed the expression of the same set of genes. In all cases, increased expression was observed upon UBC9/UBE2I depletion (Fig. S5D), demonstrating the importance of SUMOylation in their mediating repression.
Fig. S4.
The SIMs of ZMYM2 are important for transcriptional regulation. (A) Immunofluorescence of transiently transfected U2OS cells expressing WT, SIM2mut, or SIM1,2,3mut myc-tagged ZMYM2. Proteins were detected using an anti-myc primary and anti-mouse Alexa Fluor 594 secondary antibodies, and DNA was detected by Hoechst staining. Projections of deconvolved images and a merge of the two signals (DNA, blue, and ZMYM2, red) are shown. (B) Expression of wild-type (WT) or the SIM2 mutant form of ZMYM2 was detected by IB with anti-FLAG antibodies. Stably transfected U2OS cells were treated with doxycycline (Dox) for 48 h, where indicated. Lamin B was detected by IB as loading control. (C) Immunoblot of ZMYM2 expression in the parental U2OS Flp-In cell line or U2OS cells stably transfected with constructs expressing WT, SIM2mut, or SIM1,2,3mut and treated for 48 h with doxycycline. Proteins were detected using anti-ZMYM2 (Top) or anti-FLAG (Bottom) antibodies. Gray arrows indicate the endogenous protein, black arrows indicate the exogenous FLAG-tagged ZMYM2, and the asterisk indicates an unspecific band. (D) RT-PCR analysis of the expression of the indicated genes in U2OS cells stably expressing the SIM2 mutant form of ZMYM2. Data are the average of two (for TMEM154) or three independent experiments and are presented as log10 of the fold change in ZMYM2(SIM2mut) cells relative to the transcript levels in WT ZMYM2-expressing cells (taken as 1). Asterisks (*) denote P < 0.05 in Student’s t test and a fold change >5. (E) RT-PCR analysis of the expression of the indicated genes in U2OS cells stably expressing the SIM1,2,3 mutant form of ZMYM2. Data are the average of three independent experiments and are presented as log10 of the fold change relative to the transcript levels in WT ZMYM2-expressing cells (taken as 1). Asterisks (*) denote P < 0.05 in Student’s t test and a fold change >1.6.
Fig. S5.
Effect of ZMYM2 and UBE2I depletion on ZMYM2 target gene expression. (A) IB analysis of ZMYM2 expression in U2OS Flp-In cells following depletion with siZMYM2 and a nontargeting (siNT) control. Lamin B was used as loading control. (B) RT-PCR analysis of the expression of the indicated genes in U2OS cells transfected with control siNT (dark bars) or a siRNA duplex directed against ZMYM2 (lighter bars). Data are the average of three independent experiments and are presented as fold change relative to the transcript levels in control siNT-transfected cells (taken as 1). Asterisks (*) denote P < 0.05 in Student’s t test and a fold change >1.5. (C) IB analysis of UBC9/UBE2I expression following depletion with siUBC9/UBE2I or a nontargeting (siNT) control. Tubulin was used as loading control. (D) RT-PCR analysis of the expression of the indicated genes in U2OS Flp-In cells transfected with control siNT (dark bars) or a siRNA duplex directed against UBC9/UBE2I (lighter bars). Data are the average of two (for TMEM154) or three independent experiments and are presented as fold change relative to the transcript levels in control siNT-transfected cells (taken as 1). Asterisks (*) denotes P < 0.05 in Student’s t test and a fold change >1.5.
Together, these results therefore demonstrate the functional importance of the SIMs found in ZMYM2 and indicate that a multi-SUMO–binding mode is used in mediating its transcriptional regulatory activities.
Multi-SUMO–Binding Activity Promotes ZMYM2 Recruitment to Chromatin.
Having demonstrated that the SIM motifs of ZMYM2 play an important role in its gene-regulatory activities, we next investigated the molecular basis to this phenomenon. First, we tested whether mutation of the SIMs in ZMYM2 affected its transcriptional repression activity. We used a reporter assay using a luciferase reporter, driven by a LexA-VP16 fusion protein, and asked whether a Gal4-ZMYM2 fusion protein could repress transcription in this context (Fig. 5A). Here, we fused full-length ZMYM2 to the Gal4 DNA binding. Gal4-ZMYM2 was a highly active transcriptional repressor (Fig. 5A). However, this transcriptional repressive activity was retained in the Gal4-ZMYM2(SIM1,2,3mut) protein, which lacked all three SIMs we have identified (Fig. 5A). Thus, the SIM motifs are not required for the intrinsic transcriptional repressive properties of ZMYM2.
Fig. 5.
ZMYM2 SIMs are required for its recruitment to chromatin. (A) Reporter gene assay with constant LexA-VP16 but increasing amounts of the indicated Gal4-ZMYM2 derivatives. A schematic of the reporter gene is shown above, and the expression of the different Gal4 fusion proteins is shown in the Western blot as an Inset. Lamin B is a loading control, and the vertical line indicates that an intervening irrelevant lane has been removed. Data are shown relative to the internal β-galactosidase control (normalized to Gal4 DBD alone, taken as 1) and are the mean of two independent experiments, each performed in triplicate. (B) University of California, Santa Cruz genome browser view of three example loci and the binding peaks of the indicated ZMYM2 derivatives. (C) ChIP analysis of ZMYM2(WT) or ZMYM2(SIM2mut) binding to the indicated loci in the respective stable U2OS cell lines. Data are presented relative to input for each locus (n = 3), and statistically significant differences are indicated (P value <0.005). Control ChIPs with IgG are shown at each locus for each cell line.
A second possibility is that the SIMs could be required for recruitment of ZMYM2 to chromatin. To address this possibility, we used ChIP-seq to analyze the binding of exogenous ZMYM2 to chromatin in our U2OS cell lines containing inducible forms of FLAG-tagged WT ZMYM2 or ZMYM2 with the critical SIM for multi-SUMO binding (SIM2) mutated. We initially identified 4,361 binding regions in the ZMYM2(WT) cell line but only 3,168 binding regions in the ZMYM2(SIM2mut) cell line. Taking the union of ZMYM2 binding sites for the WT and SIM2mut proteins gave 6,693 distinct regions, of which 3,069 loci were identified where significantly stronger binding (more than fourfold greater tag density; Poisson P value < 1 × 10−4) of WT ZMYM2 was observed. The remainder showed similar or lower levels of WT ZMYM2 compared with the ZMYM2(SIM2mut) mutant version (see examples in Fig. 5B). We validated three sites that showed stronger binding by the WT protein and five sites that showed equivalent levels of binding by ChIP-qPCR (Fig. 5C). Thus, the SIMs are required for recruitment of ZMYM2 to a subset of genomic loci. One prediction of our results is that the genomic loci that show differential requirement for the ZMYM2 SIMs for its recruitment to chromatin, should harbor either a multi-SUMOylated protein or a cluster of SUMOylated proteins. To address this issue, we first compared our ZMYM2 ChIP-seq data with ChIP-seq data for SUMO3 from the same cell line. SUMO binding occurs around many sites where we observed binding of WT ZMYM2 (Fig. 6A). To establish whether this association is more relevant to ZMYM2 or is typical of other transcriptional regulators, we compared SUMO occupancy around the summits of the binding regions for the transcriptional activator FOXO3 and found that SUMO3 is substantially more enriched around ZMYM2 binding summits (Fig. 6B). Given this association between ZMYM2 and SUMO, we next examined whether SUMO occupancy was higher around sites where ZMYM2 binding was affected by mutation of its SIMs as might be expected if SUMO is involved in ZMYM2 recruitment. ZMYM2-binding regions were partitioned into deciles, depending on the enrichment of ChIP-seq signal for WT versus SIM mutant form of ZMYM2. Importantly, the top decile, where binding of WT ZMYM2 was favored, showed the largest SUMO tag density, and this enrichment for SUMO co-occupancy decreased in the bottom decile, where the dependency on SIMs for ZMYM2 binding was lowest (Fig. 6C). Consistent with these results, stronger binding of SUMO was demonstrated at several of the sites that we identified as preferentially bound by WT ZMYM2 (e.g., TNXL4 and EMX2; Figs. 5C and 6D). Finally, we asked whether the coassociation with SUMO on chromatin is functionally linked to ZMYM2 recruitment. To do this, we took advantage of a ChIP-seq dataset for SUMO done under heat shock conditions (36). Although heat shock is generally thought to promote SUMOylation, decreases in SUMOylation occurred at many loci. Importantly, this occurred at all three loci where we saw differential binding between WT and SIM mutant forms of ZMYM2 and was accompanied by reduced binding of endogenous ZMYM2 following heat shock (e.g., EMX2; Fig. 6D). In contrast, heat shock did not affect ZMYM2 binding at loci such as PADI4 and ZC3H3 where basal levels of SUMO are low and there is little difference in binding of WT and SIM2 mutant forms of ZMYM2 (Fig. 6D).
Fig. 6.
Multi-SUMO binding promotes ZMYM2 recruitment to chromatin. (A) Heat map of tag densities from ZMYM2, SUMO, or control IgG ChIP-seq experiments plotted around 5 kb either side of the ZMYM2 binding peak summits. Binding regions are ranked according to ZMYM2 tag density. (B) Average tag density profiles of SUMO binding plotted onto 1-kb regions surrounding the summits of the top 1,000 most enriched peaks for either ZMYM2 or FOXO3. (C) Enrichment of SUMO associated with WT ZMYM2 binding relative to ZMYM2 SIM2mut binding. Total SUMO tags are plotted for the top 10% most ZMYM2(WT) tag enriched and the bottom 10% binding regions relative to ZMYM2mut binding. (D) ChIP analysis of SUMO2/3 (Left) or ZMYM2 (Right) binding to the indicated loci in U2OS cells at either 37 °C (black bars) or 43 °C (gray bars). Data are presented relative to input for each locus (n = 3) and statistically significant differences are indicated (value of P < 0.005). (E) Model showing different possible modes of SUMO (S) interactions with the multi-SIM module in ZMYM2 on chromatin. ZMYM2 could interact with multi-SUMOylated repressor (Rep) or corepressor (CoRep) proteins, multiple SUMO motifs found in just one protein, or multiple SUMO motifs found in different SUMOylated proteins including core histones.
In summary, these results are consistent with a model whereby the multiple SIM motifs in ZMYM2 are required for recruitment of ZMYM2 to a subset of chromatin regions in a SUMO-dependent manner (Fig. 6E).
Discussion
Protein SUMOylation has been implicated in numerous nuclear processes, and in particular chromatin regulation, transcriptional control, and DNA repair have emerged as major areas of influence (reviewed in ref. 4). Although it is now appreciated that a major role for SUMO is to provide a binding platform for other proteins, we still know relatively little about how specificity is generated or how these interactions are controlled. Recently, poly-SUMOylation has been shown to act as a binding platform for SUMO-targeted ubiquitin ligases (STUbLs), thereby coupling protein SUMOylation and ubiquitination (9, 22). We have extended these findings and demonstrate that STUbLs might also be targeted to substrates by interacting with alternative multi-SUMO interfaces, although poly-SUMO chain binding was clearly preferred. Nevertheless, these findings on STUbLs raised the possibility that multi-SUMO might act as a binding platform for other multi-SIM–containing proteins. Here, we identified dozens of potential multi-SUMO–binding proteins, validated our findings on several of these proteins, and, through detailed analysis of ZMYM2, identified a multi-SIM module and demonstrated its functional importance.
Both RNF4 and ZMYM2 can bind to multi-SUMO either as a multimerized SUMO array or as a poly-SUMO chain, and in keeping with this observation, each protein contains multiple SIMs, presumably so that each SIM can bind simultaneously to a different SUMO moiety (Fig. 1A). In principle, the binding modes for poly-SUMO chains and multi-SUMO platforms might be expected to be similar, but although poly-SUMO chains are likely to be uniform linear arrays, the structure of multi-SUMO platforms is dependent on the relative stereospecific presentation of the SUMO conjugates on the protein surface. Although ZMYM2 has the capacity to recognize both forms of multi-SUMO scaffold, RNF4 clearly has a preference for multi-SUMO in the context of poly-SUMO chains. Other proteins show intermediate preferences for the two forms of multimerized SUMO, presumably due to differences in how their SIMs are presented. Specific multi-SUMO platforms could be assembled either in cis on a single protein, or in trans on two or more proteins in a complex (Fig. 6E). Hints at both types of mechanisms are provided by the recent demonstration that SUMOylation of many different proteins involved in homologous recombination acts synergistically to promote efficient DNA repair (19). Conversely, juxtaposed SIMs can be presented in the form of a flexible chain as observed in RNF4 or be presented according to their locations on the surface of a folded protein, and such a scenario appears likely for ZMYM2. It is also possible that individual SIMs could be contributed by several different members of a protein complex. Thus, there are a large number of possible assembly mechanisms involving multiple SIMs and multi-SUMO–containing surfaces.
One emerging concept is that one or a subset of SIMs is “dominant” as defined by the large effects on binding seen upon their mutation (22). In the case of ZMYM2, one of the SIMs (SIM2) is “dominant,” whereas the roles of the other SIMs are only revealed through combinatorial mutation. It is not clear what causes this dominant effect, but a likely cause is that these SIMs might orchestrate the clustering of the other SIMs into a suitable conformation that enables multi- or poly-SUMO binding. In this context, it is notable that, unlike RNF4, ZMYM2 can bind equally well to a multi-SUMO platform and poly-SUMO chains, thereby further suggesting that the overall conformation of the SIM clusters and the SUMO binding platform is important for directing binding events.
It is also important to consider the functional significance of multi-SUMO binding and why this might be important. One attractive hypothesis is that a multi-SUMO–binding surface will only be created when either several SUMOylated proteins aggregate together or high levels of SUMOylation are initiated on one or more proteins. This would then provide a molecular rheostat whereby recruitment of multi-SIM–containing partner proteins would only be triggered once high-level SUMOylation is achieved. Similar mechanisms involving a different posttranslational modification operate in the context of cell cycle control. Here, multisite phosphorylation, as exemplified by CDK-mediated Sic1 phosphorylation, triggers “switch-like” behavior through promoting SCF recruitment and Sic1 destruction (37).
Another interesting finding of our results was that, when tested for SUMO paralog binding, all of the multi-SUMO proteins that we identified showed a strong preference for SUMO2/3 binding over SUMO1 (Fig. 2). This might be expected given the fact that we used a GST-COMP-SUMO3 scaffold to isolate these proteins in the first place, but this does emphasize that SUMO1 and SUMO3 binding through SIMs is likely to be a highly selective process. Indeed, others have documented selective binding to SUMO3 over SUMO1 such as was observed for USP25 binding to SUMO-thioester charged UBC9/UBE2I (38). Furthermore, we also find that RNF4 preferentially binds multi-SUMO3 over multi-SUMO1. Conversely, several proteins have been shown to be able to bind both SUMO1 and SUMO3 (7), with acidic residues around SIMs apparently favoring SUMO1 binding. However, given that the SIMs in ZMYM2 resemble canonical SIMs with surrounding acidic residues and that ZMYM2 preferentially binds to SUMO3, this does not fit with this simplified view and suggests that there is much to learn about the specificity mechanisms used in SUMO paralog recognition. Moreover, such specificity mechanisms would open up additional regulatory possibilities. For example, it is possible that, in some cases, a mixture of SUMO1 and SUMO3 could constitute a multi-SUMO–binding surface, and this would result in the recruitment of proteins with a distinct mixture of SIMs recognizing these two different paralogs.
ZMYM2 has previously been identified in a proteomic screen for GST-SUMO3–binding proteins (39), and in a yeast two-hybrid screen using GAL-SUMO1 and GAL-SUMO3 as baits (7), although in the latter case, it was one of the weaker interactors. Thus, ZMYM2 has the potential to bind to nonmultimerized SUMO. However, we clearly demonstrate that ZMYM2 binds more strongly to multi-SUMO than to GST-SUMO alone. It should be noted in this context that both GAL4 and GST-fusion proteins are dimeric in nature, so are not strictly “mono-SUMO” scaffolds. It does, however, remain a possibility that ZMYM2 might function through both mono- and multi-SUMO binding in different contexts. Recently, ZMYM2 was shown to bind specifically to the SUMO-modified form of HDAC1, but this interaction was driven through its zinc finger region rather than through the functionally important SIMs we have identified here (35). Moreover, a recent paper demonstrated mono-SUMO and poly-SUMO chain binding activity for ZMYM2, and again this was attributed to sequences contained in the zinc finger region of the protein (30). These findings support the idea that this protein might have different ways of interaction with SUMOylated partner proteins, but future studies are required to determine the precise nature of the multi-SUMOylated species that ZMYM2 binds to in the cell. Interestingly, the N-terminal region of ZMYM2 has been shown to be fused to FGFR1 in human leukemic cells (40). One major function of ZMYM2 in this context is to promote the constitutive dimerization, and hence activation, of the FGFR1 kinase activity (40). However, our results indicate that this fusion protein will also have the possibility of binding to multi-SUMOylated proteins through its N-terminal multiple SIM motifs, and that this could contribute to its activity as an oncogenic protein.
In summary, we have demonstrated the importance of multivalent SUMO–SIM interactions for ZMYM2 recruitment to chromatin. More generally, we have demonstrated that multi-SUMOylation has the potential to have an equally widespread impact on SUMO-mediated control mechanisms in the cell as poly-SUMO chain formation. Our identification of a large number of multi-SUMO–binding proteins such as ZMYM2 provides an opportunity for the community to further investigate this phenomenon.
Supplementary Material
Acknowledgments
We thank Karren Palmer for excellent technical assistance; Catherine Millar, Alan Whitmarsh, Shen-Hsi Yang, and other members of our laboratory for comments on the manuscript and stimulating discussions; Richard Kammerer, Frances Fuller-Pace, Hongtao Yu, Ian Hickson, Pierre-Antoine Defossez, Egor Prokhortchouk, Richard Anderson, and Ingrid Grummt for reagents; Martin Lowe for help with the tissue culture; Stacey Warwood in the Core Biological Mass Spectrometry Facility; Peter March in the Bioimaging Facility; Michael Smiga in the Genomics Technology Facility; and Ian Donaldson and Leo Zeef in the Bioinformatics Facility. This work was supported by the Wellcome Trust and a Royal Society–Wolfson award (to A.D.S.) and the Wellcome Trust (098391/Z/12/Z) (to R.T.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the ArrayExpress database [accession nos. E-MEXP-3880 (microarray data), E-MTAB-2701 (ChIP-seq data), and E-MTAB-3084 (ChIP-seq data)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509716112/-/DCSupplemental.
References
- 1.Heun P. SUMOrganization of the nucleus. Curr Opin Cell Biol. 2007;19(3):350–355. doi: 10.1016/j.ceb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Finkbeiner E, Haindl M, Raman N, Muller S. SUMO routes ribosome maturation. Nucleus. 2011;2(6):527–532. doi: 10.4161/nucl.2.6.17604. [DOI] [PubMed] [Google Scholar]
- 3.Barry J, Lock RB. Small ubiquitin-related modifier-1: Wrestling with protein regulation. Int J Biochem Cell Biol. 2011;43(1):37–40. doi: 10.1016/j.biocel.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Cubeñas-Potts C, Matunis MJ. SUMO: A multifaceted modifier of chromatin structure and function. Dev Cell. 2013;24(1):1–12. doi: 10.1016/j.devcel.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Dominguez M, Reyes JC. SUMO association with repressor complexes, emerging routes for transcriptional control. Biochim Biophys Acta. 2009;1789(6-8):451–459. doi: 10.1016/j.bbagrm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci USA. 2004;101(40):14373–14378. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I. Specification of SUMO1- and SUMO2-interacting motifs. J Biol Chem. 2006;281(23):16117–16127. doi: 10.1074/jbc.M512757200. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich HD. The fast-growing business of SUMO chains. Mol Cell. 2008;32(3):301–305. doi: 10.1016/j.molcel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Tatham MH, et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10(5):538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 10.Lallemand-Breitenbach V, et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10(5):547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 11.Bruderer R, et al. Purification and identification of endogenous polySUMO conjugates. EMBO Rep. 2011;12(2):142–148. doi: 10.1038/embor.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srikumar T, et al. Global analysis of SUMO chain function reveals multiple roles in chromatin regulation. J Cell Biol. 2013;201(1):145–163. doi: 10.1083/jcb.201210019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SH, Galanis A, Witty J, Sharrocks AD. An extended consensus motif enhances the specificity of substrate modification by SUMO. EMBO J. 2006;25(21):5083–5093. doi: 10.1038/sj.emboj.7601383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa K, Kuzumaki N. Transcriptional activity of megakaryoblastic leukemia 1 (MKL1) is repressed by SUMO modification. Genes Cells. 2005;10(8):835–850. doi: 10.1111/j.1365-2443.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuo HY, et al. SUMO modification negatively modulates the transcriptional activity of CREB-binding protein via the recruitment of Daxx. Proc Natl Acad Sci USA. 2005;102(47):16973–16978. doi: 10.1073/pnas.0504460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo B, Sharrocks AD. Extracellular signal-regulated kinase mitogen-activated protein kinase signaling initiates a dynamic interplay between sumoylation and ubiquitination to regulate the activity of the transcriptional activator PEA3. Mol Cell Biol. 2009;29(11):3204–3218. doi: 10.1128/MCB.01128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tammsalu T, et al. Proteome-wide identification of SUMO2 modification sites. Sci Signal. 2014;7(323):rs2. doi: 10.1126/scisignal.2005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks IA, et al. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol. 2014;21(10):927–936. doi: 10.1038/nsmb.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell. 2012;151(4):807–820. doi: 10.1016/j.cell.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, et al. The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Mol Cell Biol. 2013;33(2):227–236. doi: 10.1128/MCB.00881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Q, et al. GPS-SUMO: A tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res. 2014;42(Web Server issue):W325–W330. doi: 10.1093/nar/gku383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Hunter T. Poly-small ubiquitin-like modifier (PolySUMO)-binding proteins identified through a string search. J Biol Chem. 2012;287(50):42071–42083. doi: 10.1074/jbc.M112.410985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP: A prototype ion channel? Science. 1996;274(5288):761–765. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, et al. Small ubiquitin-related modifier (SUMO) binding determines substrate recognition and paralog-selective SUMO modification. J Biol Chem. 2008;283(43):29405–29415. doi: 10.1074/jbc.M803632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang J, Gill G. SUMO engages multiple corepressors to regulate chromatin structure and transcription. Epigenetics. 2009;4(7):440–444. doi: 10.4161/epi.4.7.9807. [DOI] [PubMed] [Google Scholar]
- 26.Bastiani M, et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185(7):1259–1273. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekiyama N, et al. Structure of the small ubiquitin-like modifier (SUMO)-interacting motif of MBD1-containing chromatin-associated factor 1 bound to SUMO-3. J Biol Chem. 2008;283(51):35966–35975. doi: 10.1074/jbc.M802528200. [DOI] [PubMed] [Google Scholar]
- 28.Shen LN, Dong C, Liu H, Naismith JH, Hay RT. The structure of SENP1-SUMO-2 complex suggests a structural basis for discrimination between SUMO paralogues during processing. Biochem J. 2006;397(2):279–288. doi: 10.1042/BJ20052030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reverter D, Lima CD. Structural basis for SENP2 protease interactions with SUMO precursors and conjugated substrates. Nat Struct Mol Biol. 2006;13(12):1060–1068. doi: 10.1038/nsmb1168. [DOI] [PubMed] [Google Scholar]
- 30.Guzzo CM, et al. Characterization of the SUMO-binding activity of the myeloproliferative and mental retardation (MYM)-type zinc fingers in ZNF261 and ZNF198. PLoS One. 2014;9(8):e105271. doi: 10.1371/journal.pone.0105271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malakhov MP, et al. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5(1-2):75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- 32.Owerbach D, McKay EM, Yeh ET, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun. 2005;337(2):517–520. doi: 10.1016/j.bbrc.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 33.Hakimi MA, Dong Y, Lane WS, Speicher DW, Shiekhattar R. A candidate X-linked mental retardation gene is a component of a new family of histone deacetylase-containing complexes. J Biol Chem. 2003;278(9):7234–7239. doi: 10.1074/jbc.M208992200. [DOI] [PubMed] [Google Scholar]
- 34.Shi YJ, et al. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Gocke CB, Yu H. ZNF198 stabilizes the LSD1-CoREST-HDAC1 complex on chromatin through its MYM-type zinc fingers. PLoS One. 2008;3(9):e3255. doi: 10.1371/journal.pone.0003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifert A, Schoefield P, Barton GJ, Hay RT. Changes in the chromatin landscape of SUMO modification in response to proteotoxic stress. Sci Signal. 2015;8(384):rs7. doi: 10.1126/scisignal.aaa2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kõivomägi M, et al. Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature. 2011;480(7375):128–131. doi: 10.1038/nature10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meulmeester E, Kunze M, Hsiao HH, Urlaub H, Melchior F. Mechanism and consequences for paralog-specific sumoylation of ubiquitin-specific protease 25. Mol Cell. 2008;30(5):610–619. doi: 10.1016/j.molcel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Rosendorff A, et al. NXP-2 association with SUMO-2 depends on lysines required for transcriptional repression. Proc Natl Acad Sci USA. 2006;103(14):5308–5313. doi: 10.1073/pnas.0601066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao S, McCarthy JG, Aster JC, Fletcher JA. ZNF198-FGFR1 transforming activity depends on a novel proline-rich ZNF198 oligomerization domain. Blood. 2000;96(2):699–704. [PubMed] [Google Scholar]
- 41.Ding H, et al. Solution structure of human SUMO-3 C47S and its binding surface for Ubc9. Biochemistry. 2005;44(8):2790–2799. doi: 10.1021/bi0477586. [DOI] [PubMed] [Google Scholar]
- 42.Witty J, Aguilar-Martinez E, Sharrocks AD. SENP1 participates in the dynamic regulation of Elk-1 SUMOylation. Biochem J. 2010;428(2):247–254. doi: 10.1042/BJ20091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon KA, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28(8):1001–1015. doi: 10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemercier C, et al. mHDA1/HDAC5 histone deacetylase interacts with and represses MEF2A transcriptional activity. J Biol Chem. 2000;275(20):15594–15599. doi: 10.1074/jbc.M908437199. [DOI] [PubMed] [Google Scholar]
- 45.Yang SH, Sharrocks AD. The SUMO E3 ligase activity of Pc2 is coordinated through a SUMO interaction motif. Mol Cell Biol. 2010;30(9):2193–2205. doi: 10.1128/MCB.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1(2):729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt D, et al. ChIP-seq: Using high-throughput sequencing to discover protein-DNA interactions. Methods. 2009;48(3):240–248. doi: 10.1016/j.ymeth.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinsella RJ, et al. Ensembl BioMarts: A hub for data retrieval across taxonomic space. Database (Oxford) 2011;2011:bar030. doi: 10.1093/database/bar030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 52.Pearson RD, et al. puma: A Bioconductor package for propagating uncertainty in microarray analysis. BMC Bioinformatics. 2009;10:211. doi: 10.1186/1471-2105-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higgins SJ, Hames BD. RNA Processing: A Practical Approach. Vol I IRL; Oxford: 1994. [Google Scholar]
- 54.Tselepis VH, Green LJ, Humphries MJ. An RGD to LDV motif conversion within the disintegrin kistrin generates an integrin antagonist that retains potency but exhibits altered receptor specificity. Evidence for a functional equivalence of acidic integrin-binding motifs. J Biol Chem. 1997;272(34):21341–21348. doi: 10.1074/jbc.272.34.21341. [DOI] [PubMed] [Google Scholar]
- 55.Mellacheruvu D, et al. The CRAPome: A contaminant repository for affinity purification-mass spectrometry data. Nat Methods. 2013;10(8):730–736. doi: 10.1038/nmeth.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.