Significance
Ozonolysis of alkenes produces highly reactive Criegee intermediates. Whereas water dimer efficiently scavenges the simplest Criegee intermediate CH2OO in the troposphere, this study clear demonstrates that water vapor does not react with dimethyl substituted Criegee intermediate (CH3)2COO, at least not fast enough to significantly consume (CH3)2COO in the troposphere. On the other hand, (CH3)2COO reacts with SO2 three times faster than CH2OO does, indicating Criegee intermediates of a structure similar to (CH3)2COO are potential candidates for an efficient oxidant in the atmospheric SO2 oxidation.
Keywords: atmospheric chemistry, Criegee intermediate, SO2 oxidation, chemical kinetics
Abstract
Criegee intermediates are thought to play a role in atmospheric chemistry, in particular, the oxidation of SO2, which produces SO3 and subsequently H2SO4, an important constituent of aerosols and acid rain. However, the impact of such oxidation reactions is affected by the reactions of Criegee intermediates with water vapor, because of high water concentrations in the troposphere. In this work, the kinetics of the reactions of dimethyl substituted Criegee intermediate (CH3)2COO with water vapor and with SO2 were directly measured via UV absorption of (CH3)2COO under near-atmospheric conditions. The results indicate that (i) the water reaction with (CH3)2COO is not fast enough (kH2O < 1.5 × 10−16 cm3s−1) to consume atmospheric (CH3)2COO significantly and (ii) (CH3)2COO reacts with SO2 at a near–gas-kinetic-limit rate (kSO2 = 1.3 × 10−10 cm3s−1). These observations imply a significant fraction of atmospheric (CH3)2COO may survive under humid conditions and react with SO2, very different from the case of the simplest Criegee intermediate CH2OO, in which the reaction with water dimer predominates in the CH2OO decay under typical tropospheric conditions. In addition, a significant pressure dependence was observed for the reaction of (CH3)2COO with SO2, suggesting the use of low pressure rate may underestimate the impact of this reaction. This work demonstrates that the reactivity of a Criegee intermediate toward water vapor strongly depends on its structure, which will influence the main decay pathways and steady-state concentrations for various Criegee intermediates in the atmosphere.
Unsaturated hydrocarbons are emitted into the atmosphere in large quantities from either human or natural sources. Ozonolysis of unsaturated hydrocarbons produces highly reactive Criegee intermediates (CIs) (1), which may (i) decompose to radical species like OH radicals or (ii) react with a number of atmospheric species, for example, with SO2 to form SO3 and with NO2 to form NO3 (2, 3). The SO2 oxidation by CIs has gained special attentions because the SO3 product would be converted into H2SO4, an important constituent of aerosols and acid rain (4–8). For example, Mauldin et al. (4) have speculated that Criegee intermediate reactions with SO2 may account for the discrepancy between the observed and modeled concentrations of H2SO4 in a boreal forest region, where various alkenes are emitted by trees.
Recently, Welz et al. (2) demonstrated an efficient method to prepare a CI in a laboratory by the reaction of iodoalkyl radical with O2 (for example, CH2I + O2 → CH2OO + I). This method can produce a CI of high enough concentration that allows direct detection. With photoionization mass spectrometry (PIMS) detection, Welz et al. (2) measured the rate coefficients of the simplest CI (CH2OO) reactions with SO2 and NO2. Notably, these new rate coefficients, confirmed by a few later investigations (9–11), are orders of magnitude larger than those previously used (12, 13) in atmospheric models (e.g., MCM v3.3, available at mcm.leeds.ac.uk/MCM/browse.htt?species=CH2OO), suggesting a greater role of CIs in atmospheric chemistry. This result also indicates previous ozonolysis analyses may be affected by complicated and partly unknown side reactions and may contain errors in some of the reported rate coefficients.
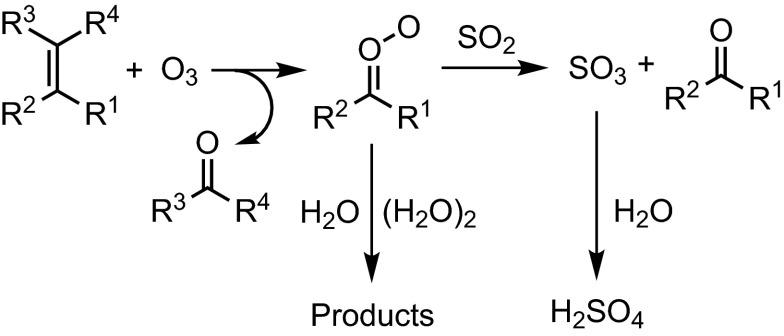
Typical water concentration in the troposphere (1.3 × 1017 to 8.3 × 1017 cm−3 at the dew point of 0–27 °C) is orders of magnitude higher than those of atmospheric trace gases like SO2, NO2, and volatile organic compounds (VOC) (on the order of 1012 cm−3 or less). Although it has been shown that CIs may react very fast with SO2, NO2, and organic acids (2, 3, 14), the reactions of CIs with atmospheric water vapor would still strongly influence the fates and concentrations of atmospheric CIs (see Fig. 1 for a simplified schematic). As expected, the reactivity of CIs toward water vapor would govern the modeling results of atmospheric H2SO4 formation from CIs (8, 15, 16).
Fig. 1.
Reaction scheme showing competitions for CIs between reactions with water (monomer and dimer) and with SO2.
However, there had been discrepancies about the reactivity of CIs toward water. Whereas studies (17–20) using C2H4 ozonolysis as a CH2OO source show substantial reactivity of CH2OO toward water vapor, despite a large scatter (10−17 to 10−12 cm3s−1) in the reported rate coefficient, other studies (2, 10, 21) using the CH2I+O2 reaction as a CH2OO source reported negative observation for the CH2OO reaction with water vapor.
More recently, Chao et al. (22) and Berndt et al. (23) investigated the reaction of CH2OO with water vapor using the CH2I+O2 reaction and the C2H4 ozonolysis as their CH2OO sources, respectively. Both groups observed clear second-order kinetics with respect to the concentration of water and concluded that reaction with water dimer predominates in the decay of CH2OO under atmospheric conditions and that previous studies may require some reinterpretations. The reported rate coefficient of the CH2OO reaction with water dimer is large, about 7 × 10−12 cm3s−1 (22), leading to extremely fast decay rate of CH2OO under typical tropospheric conditions (Table 1).
Table 1.
Reported bimolecular rate coefficients k and effective first-order rate coefficients (keff = k[Coreactant]) for simple CI reactions with H2O, (H2O)2, and SO2 at a given atmospheric condition
| CI | Coreactant | [Coreactant]/cm−3 | k/cm3s−1 | keff/s−1 | Reference |
| CH2OO | H2O | 5.4 × 1017 | <1.5 × 10−15 | <810 | (22) |
| (H2O)2 | 6.0 × 1014 | 6.5 × 10−12 | 3,900 | (22) | |
| SO2 | 1.2 × 1012 | 3.9 × 10−11 | 47 | (2) | |
| anti-CH3CHOO | H2O | 5.4 × 1017 | 1.0 × 10−14 | 5,400 | (3) |
| 2.4 × 10−14 | 13,000 | (24) | |||
| SO2 | 1.2 × 1012 | 6.7 × 10−11 | 80 | (3) | |
| 2.2 × 10−10 | 260 | (24) | |||
| syn-CH3CHOO | H2O | 5.4 × 1017 | <4 × 10−15 | <2,200 | (3) |
| <2 × 10−16 | <110 | (24) | |||
| SO2 | 1.2 × 1012 | 2.4 × 10−11 | 29 | (3) | |
| 2.9 × 10−11 | 35 | (24) | |||
| (CH3)2COO | H2O | 5.4 × 1017 | <1.5 × 10−16* | <81* | This work |
| (H2O)2 | 6.0 × 1014 | <1.3 × 10−13* | <78* | This work | |
| SO2 | 1.2 × 1012 | 1.3 × 10−10 | 160 | This work |
The assumed concentrations of H2O and SO2 correspond to a relative humidity RH = 70% and a SO2 mixing ratio of 50 ppb at 298 K and 1 atm. Only data from direct kinetic measurements are selected.
The rate constant of the H2O reaction with (CH3)2COO is obtained by assuming the rate constant of the (H2O)2 reaction with (CH3)2COO is zero and vice versa (SI Appendix, Table S2); thus, these two effective decay rates should not be added together.
Taatjes et al. (3) and Sheps et al. (24) have reported that the anti- form of methyl-substituted CI (CH3CHOO, R1 = H in Fig. 1) reacts with water vapor much faster than the syn- form (R1 = CH3 in Fig. 1) does. Quantum-chemical investigations (25–27) as well as studies of alkene ozonolysis (20, 28) also indicate that the structure of a CI strongly influences its reactivity toward water vapor. If one type of CI reacts slowly with water vapor but reacts quickly with SO2, these CIs may accumulate to higher concentrations and have higher probability to oxidize atmospheric SO2. Table 1 shows selected rate coefficients for relevant CI reactions and the effective first-order decay rate coefficients (keff) of small CIs under an atmospheric condition.
As will be discussed in detail in Discussion and Conclusions, the steady-state concentration of a particular CI would depend on its formation rate and effective decay rate coefficient; its impact on the SO2 oxidation would further depend on its concentration and reaction rate coefficient with SO2. Experimental results (Table 1) show that CH2OO and anti-CH3CHOO react with water vapor very quickly (3, 22–24). Thus, their steady-state concentrations would be too low to have a significant impact in SO2 oxidation under typical atmospheric conditions, as shown in modeling results (15, 16). On the other hand, previous experimental data for syn-CH3CHOO (3, 24) are not precise enough to determine its main decay pathways in the atmosphere.
Quantum-chemistry (25–27) calculations predicted that the anti- form of CIs (CIs with R1 = H in Fig. 1, including CH2OO) react with water vapor very quickly and that the syn- form of CIs (CIs with R1 ≠ H in Fig. 1, including dialkyl-substituted CIs) react slowly with water vapor. Here, steric hindrance of the alkyl group may account for the structure dependence in the reactivity. However, due to uncertainty in the calculated rate coefficients, it is unclear about the main decay channels of the syn-CIs in the atmosphere. For example, some theoretical investigation (29) shows that the reactions of water vapor with syn-CIs may still be fast enough (with a large uncertainty) to efficiently scavenge atmospheric syn-CIs, whereas some other calculations (25) suggest that these reactions are too slow to consume syn-CIs significantly. If the latter is the case, syn-CIs may have higher steady-state concentrations in the troposphere and may still play an important role in the SO2 oxidation; otherwise the steady-state concentrations of syn-CIs would still be low due to their fast consumption by reactions with water vapor (unless their sources are significantly larger than current estimation) and we might need to find another candidate for the unknown oxidant [oxidant X in the work by Mauldin et al. (4)] in the SO2 atmospheric chemistry.
To shed some light on this important issue, we performed direct kinetic measurements of the reactions of dimethyl-substituted CI, (CH3)2COO, with water vapor and with SO2 under near-atmospheric conditions. By introducing the water reactant at high concentrations, the rate coefficient of (CH3)2COO reaction with water can be better constrained. In contrast with the fast reaction of CH2OO with water dimer, this result shows that the relative probabilities of (CH3)2COO reactions with SO2 and with water vapor are comparable in the troposphere, so water alone would not completely scavenge (CH3)2COO, suggesting CIs of similar structures may play a more important role in the atmospheric oxidation of SO2.
Results
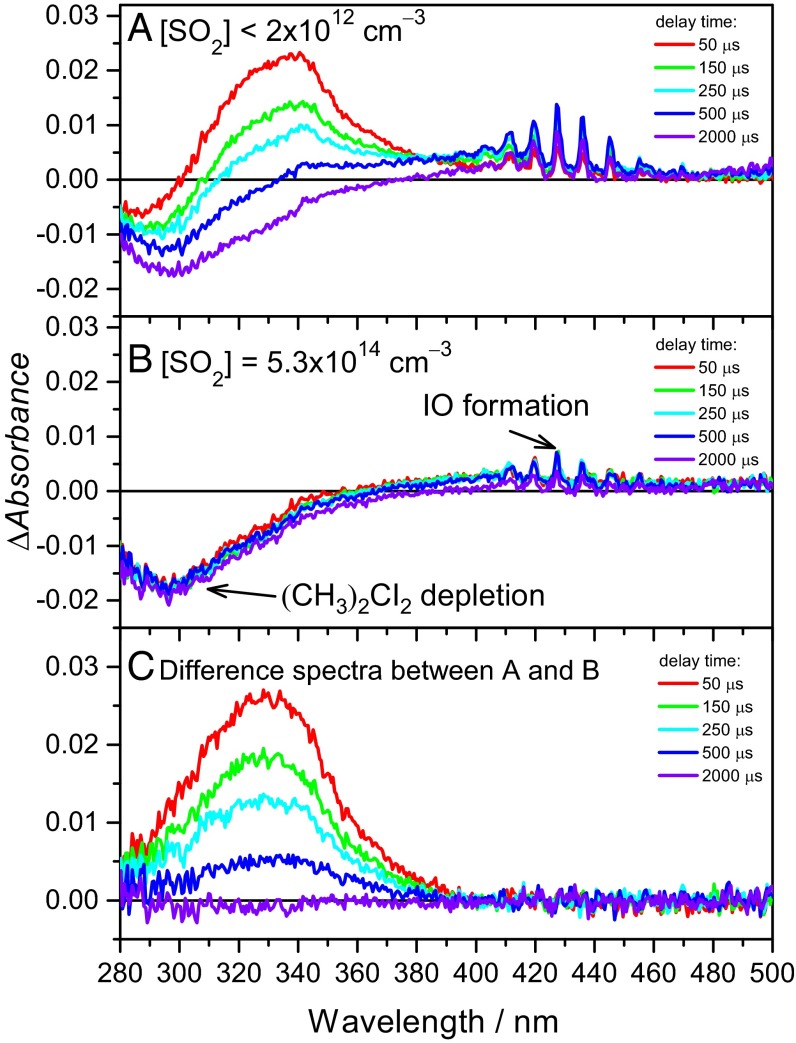
Fig. 2A shows time-resolved difference absorption spectra recorded in the (CH3)2CI2/O2 pulsed photolysis system, in which the laser pulse defines delay time t = 0. The photodissociation of the (CH3)2CI2 precursor produces (CH3)2CI radicals; (CH3)2COO was formed through the reaction of (CH3)2CI + O2 → (CH3)2COO + I (30). At t = 50 μs, a strong absorption band peaked at 330 nm was observed, which decays with the delay time. At very long delay time (e.g., 2,000 μs), a negative difference absorption signal peaked at 296 nm was observed, which corresponds to the depletion of the (CH3)2CI2 precursor. Fig. 2B shows the spectra recorded at a very similar condition but adding SO2 gas to scavenge CIs. The absorption signal in Fig. 2B consists mainly of the depletion of (CH3)2CI2 and a small amount of IO [a byproduct, likely from I + (CH3)2COO → IO + (CH3)2CO]. Other possible species in the absorption cell are SO3 and acetone, which absorb rather weakly in the studied wavelength region [the absorption cross-sections σ for the relevant species are σ(SO3) < 1 × 10−21 cm2, σ(acetone) < 5 × 10−20 cm2, and σ ∼ 10−17 cm2 for a CI] (30, 31). Following the established method of spectral analysis in similar systems (22, 31–33), the absorption band of (CH3)2COO can be obtained from the difference between the spectra in Fig. 2 A and B and is shown in Fig. 2C. The resultant spectrum of (CH3)2COO is slightly broader than but still consistent with the jet-cooled spectrum reported by Liu et al. (30). See SI Appendix, Fig. S2.
Fig. 2.
Typical transient absorption spectra recorded in the (CH3)2CI2/O2 photolysis system at selected delay times after the photolysis laser pulse (t = 0). Total pressure was 100.1 torr (PN2 = 89.9 torr, PO2 = 10.2 torr, [(CH3)2CI2]0 = 1.1 × 1014 cm−3). (A) Spectra recorded without adding SO2. (B) Spectra recorded at [SO2] = 5.3 × 1014 cm−3; (CH3)2COO was consumed by its reaction with SO2. (C) Difference spectra between the two sets of spectra in A and B. The main spectral carrier is assigned to (CH3)2COO because the reaction of (CH3)2COO with SO2 is fast. The residue contributions of the IO byproduct and the (CH3)2CI2 precursor are minor and have been removed in C.
In the kinetic measurements, we chose a detection window of 335–345 nm to monitor the change in (CH3)2COO concentrations. In this detection window, the absorption signal of IO is negligible [σ(IO) = 2.7 × 10−19 cm2 << σ((CH3)2COO) ∼ 10−17 cm2] (22); the absorption of the precursor (CH3)2CI2 is small but not negligible. Fortunately, Fig. 2B shows that the depletion of the precursor is a constant after the photolysis laser pulse, which would not affect our kinetic analysis for (CH3)2COO.
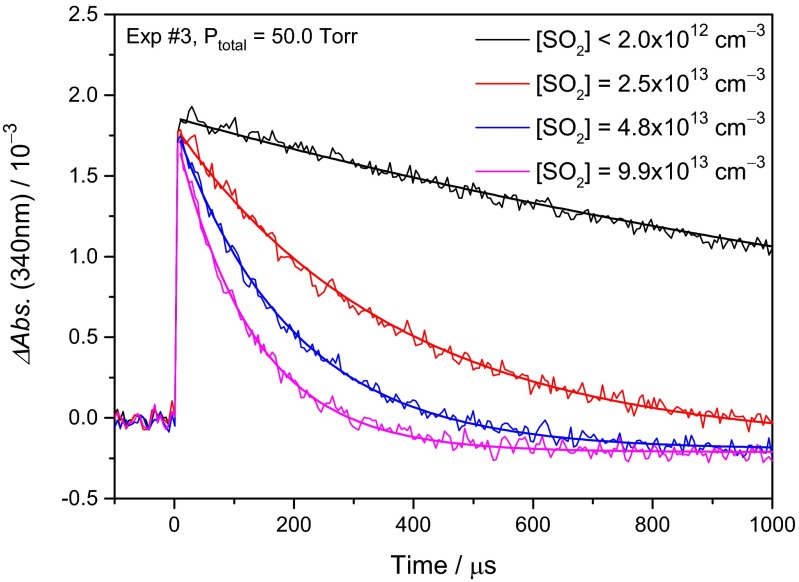
Representative difference transient absorption traces recorded at various SO2 concentrations are shown in Fig. 3; a more complete set of the experimental data can be found in SI Appendix, Figs. S3–S6 and Table S1. As mentioned above, (CH3)2COO is the main spectral carrier for the absorption at 340 nm. The rapid rise of the signal after the photolysis laser pulse was due to (CH3)2COO formation. When SO2 is added, the reaction of (CH3)2COO with SO2 dominates in the observed decay of (CH3)2COO. When no SO2 is added, the (CH3)2COO decay is due mainly to reactions of (CH3)2COO with radical species, including I atoms, OH radicals [possibly from decomposition of (CH3)2COO (30)], and (CH3)2COO itself, similar to the case of CH2OO (33). Because the concentration of (CH3)2COO in our experiment was low (on the order of 1011 cm−3), the self-reaction between two (CH3)2COO molecules did not dominate in the decay of (CH3)2COO, leading to the observed first-order–like decay.
Fig. 3.
Typical temporal profiles of the absorbance change at 340 nm. The change in absorbance was mainly due to (CH3)2COO and was monitored in real time with a balanced photodiode detector at various SO2 concentrations. The smooth lines are single exponential fit to the data.
The decay of the (CH3)2COO signals can be well described by Eqs. 1 and 2. Under the high O2 pressure (∼10 torr) used in this study, the formation time of (CH3)2COO is within 1 μs. This formation time is relatively short in comparison with the decay time of (CH3)2COO. Thus, the fitting for the decay typically started at t = 10 μs to decouple the formation kinetics.
| [1] |
| [2] |
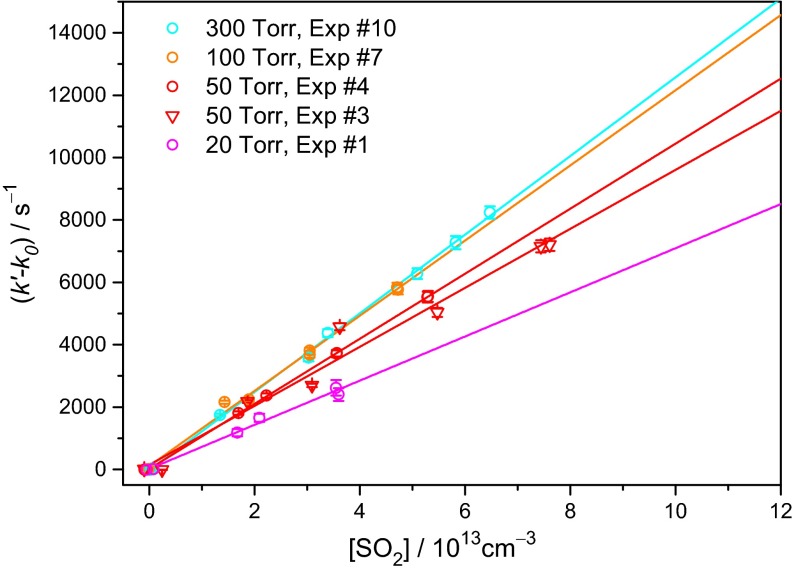
Fig. 4 shows the observed pseudo–first-order rate coefficient (k′− k0) as a function of SO2 concentration, where the k0 term (Eq. 2) accounts for the (CH3)2COO decay when no SO2 was added (see SI Appendix, Table S1 for the values of k0). A linear relationship between k′ and [SO2] is found with the slope corresponding to the rate coefficient kSO2 for the (CH3)2COO reaction with SO2. In Fig. 4, it is obvious that the slope is different at different total pressure.
Fig. 4.
Pseudo–first-order rate coefficient of (CH3)2COO reaction with SO2 plotted as a function of [SO2]. k0 is the rate coefficient at zero [SO2]. The measurements were performed at 298 K with N2 as the buffer gas at the indicated total pressure. The error bars indicate the SDs; the number of independent data can be found in SI Appendix, Table S1 for each experiment. Solid lines are linear fit to the data at the corresponding pressure. Data at higher and lower pressure ranges are shown in SI Appendix, Figs. S7 and S8.
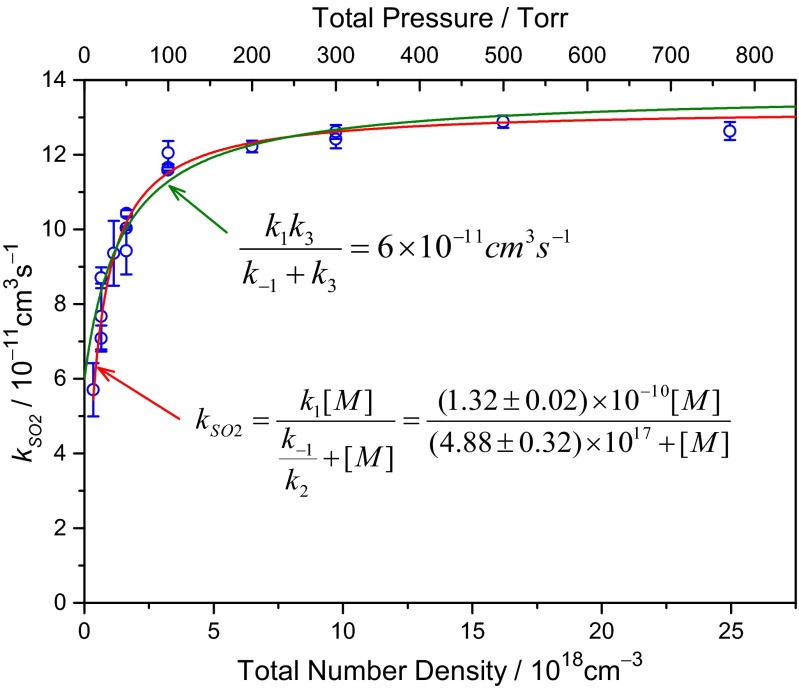
Fig. 5 plots the reaction rate coefficient kSO2 as a function of the total pressure with N2 as the buffer gas. At pressure higher than 100 torr ([M] = 3.2 × 1018 cm−3), the rate coefficient is leveled. This high-pressure-limit rate coefficient is 1.3 × 10−10 cm3s−1, about 3 times the reported rate coefficient of CH2OO reaction with SO2 [kCH2OO+SO2 = (3.9 ± 0.7) × 10−11 cm3s−1] (2). At low pressure, the rate coefficient drops. A simple model in Fig. 6 is used to analyze the experimental data.
Fig. 5.
Observed second-order rate coefficient of (CH3)2COO reaction with SO2 plotted as a function of the total pressure and number density. The fitting is plotted as solid lines. The error bars of the data indicate the SDs. The errors in the fitted equation are the SDs resulting from the fitting only, not including any possible systematic uncertainty yet. There are 18 independent measurements.
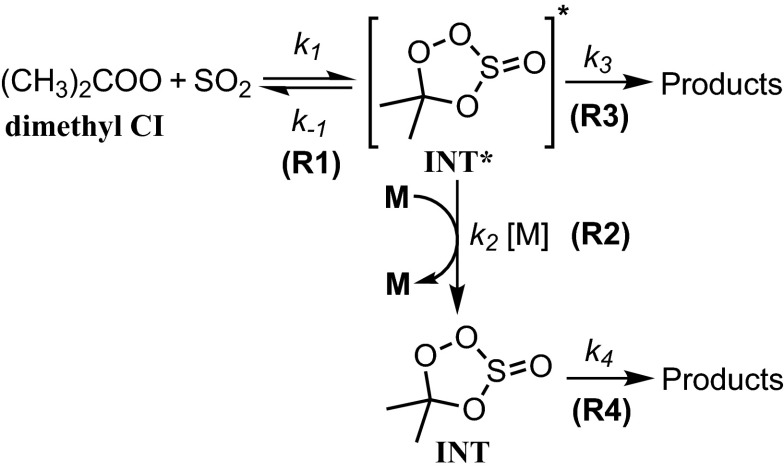
Fig. 6.
Proposed mechanism for CI reaction with SO2.
Quantum-chemistry studies (29) suggested that CI reaction with SO2 would first go through a barrierless formation of a cyclic intermediate, INT* (R1). In addition, because the formation of the cyclic intermediate is quite exothermic, its stabilization requires collision with a third body (R2). Furthermore, the energized intermediate INT* may dissociate back to the reactants or form products directly (likely SO3 + carbonyl); (R4) connects the stabilized intermediate INT to the final products.
Applying steady-state approximation to INT* leads to the following:
| [3] |
| [4] |
Theoretical analysis (29) suggests that (R2) is the main pathway (97%) for the (CH3)2COO reaction with SO2 under atmospheric pressure. Thus, we may simplify the mechanism by assuming k2[M] >> k3, unless the pressure is low. (R4) may be a slow reaction, but if there are no other competing processes, the yield of R4 may still be high, as indicated by previous ozonolysis studies (20, 28, 34).
The best fit of Eq. 4 to the data of Fig. 5 is shown as the red line (the best fit gives k3 =0). At the high-pressure limit, kSO2 = k1 = 1.32 × 10−10 cm3s−1. Nonzero k3 leads to a nonzero low-pressure-limit rate constant k1k3/(k-1+k3) but a value larger than 6 × 10−11 cm3s−1 for this term cannot fit the data satisfactorily (green line in Fig. 5). The error in k1 mostly comes from the uncertainty in the absolute concentrations of SO2, which is less than 10%. Thus, we report k1 = (1.32 ± 0.13)×10−10 cm3s−1.
To further elucidate the role of the buffer gas, we changed the buffer gas from N2 to CO2 or Ne and performed similar experiments at 50 torr. SI Appendix, Fig. S9 shows that CO2 is a more efficient collider than N2 and Ne is a less efficient collider, as one may expect. Furthermore, we also investigated the pressure effect in the reaction of CH2OO with SO2. However, the reaction rate coefficient of CH2OO with SO2 does not exhibit significant pressure dependence (see SI Appendix, Figs. S13–S15 and Table S3 and ref. 10).
The pressure dependence of the reaction of (CH3)2COO with SO2 has implications in atmospheric chemistry. Applying low-pressure rates in a model may underestimate the impact of the (CH3)2COO reaction with SO2. Similarly, the pressure dependence of the reactions of SO2 with other CIs needs to be investigated because quite a number of rate coefficients of CI reactions are measured under low-pressure conditions [∼4 torr for PIMS experiments (2, 3, 14)].
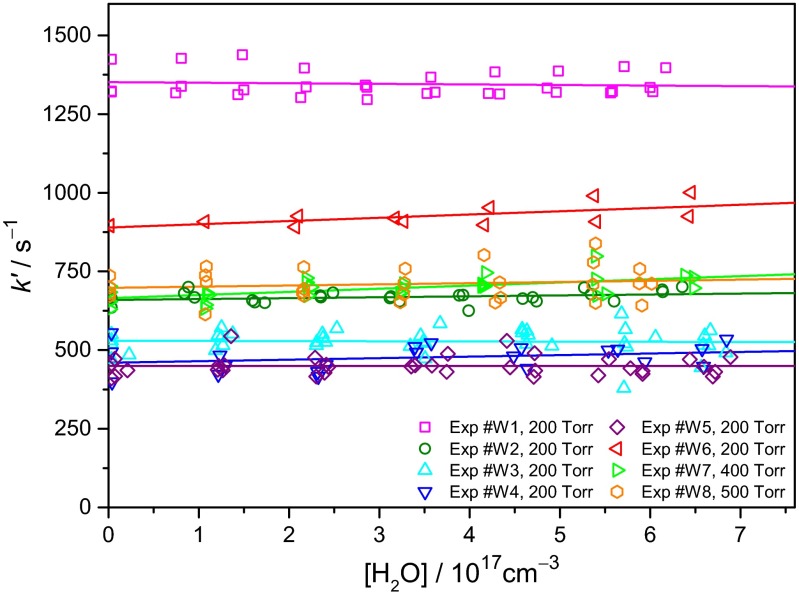
The kinetics of the (CH3)2COO reaction with water vapor were also measured. In sharp contrast with the fast reaction of CH2OO with water vapor, the decay of (CH3)2COO barely depends on [H2O], even for high water concentration up to 7 × 1017 cm−3 (RH = 90% at 298 K). Fig. 7 shows the first-order rate coefficients k′ of (CH3)2COO decay as a function of [H2O]. We have varied the experimental conditions including the total pressure, precursor concentration, photolysis laser power, etc. (SI Appendix, Table S2) and the results show that the effect of water in the decay of (CH3)2COO is extremely weak. More detailed analysis indicates the decay rate coefficient of (CH3)2COO depends mainly on the radical concentration (SI Appendix, Fig. S12). We estimate an upper limit of 1.5 × 10−16 cm3s−1 for the rate coefficient of the (CH3)2COO reaction with H2O, based on the fluctuation of the experimental data (SI Appendix, Table S2).
Fig. 7.
Pseudo–first-order rate coefficient of (CH3)2COO as a function of [H2O]. Solid lines are linear fit to the data of each experiment. There are 223 data points (see SI Appendix, Table S2 for the experimental conditions).
Discussion and Conclusions
So far, there is no method available to detect the concentration of any CI in the atmosphere. The estimation of the CI concentration can only be done by knowing its formation rate and consumption rate. The impact of a given CI (e.g., to the SO2 oxidation) depends on its concentration and the rate coefficient of its reaction with SO2, as shown in the following (Eqs. 5–9):
| [5] |
| [6] |
| [7] |
| [8] |
| [9] |
where [CI]ss is the steady-state concentration of the CI; kform is the formation rate constant of the CI from its precursors (e.g., ozone and an alkene); kdecay would be the summation of the effective decay rate constants keff of all possible decay pathways; ktherm is the thermal decomposition rate coefficient; kH2O, kw2, kSO2, and kNO2 are the bimolecular rate coefficients of CI reactions with H2O, (H2O)2, SO2, and NO2, respectively. The ktherm for (CH3)2COO has been reported recently to be 3 ± 0.4 s−1 at 293 K (34) with a significant temperature dependence (28).
Considering the possible concentrations of H2O and SO2 in the troposphere, we can evaluate the effective decay rate constants of simple CIs by water reaction and by SO2 reaction. As shown in Table 1, the consumptions of CH2OO and anti-CH3CHOO by water (including monomer and dimer) are extremely fast, >103 s−1, predominating in their kdecay. The very large kdecay would result in very low steady-state concentrations for these CIs, limiting their roles in oxidizing other atmospheric species like SO2 and NO2 (see Eqs. 7–9).
On the other hand, the reaction of (CH3)2COO with H2O is much slower, such that the water reaction would not limit the (CH3)2COO concentration in the troposphere anymore. In addition, the rate coefficient of (CH3)2COO reaction with SO2 is larger than those of other known CI reactions with SO2, indicating a greater role of (CH3)2COO in the atmospheric SO2 oxidation. In other words, there is a strong structure dependence in the CI oxidation of SO2. For (CH3)2COO, its kdecay is smaller and its kSO2 is larger, such that the SO2 oxidation rate by (CH3)2COO is faster in comparison with those for CH2OO and anti-CH3CHOO, assuming similar formation rates (see Eq. 9). In addition, for a CI with small kH2O and kw2, its ktherm would be another important factor that also influences its [CI]ss and oxidation capacity. A related issue is that the thermal decomposition of syn-CIs [including (CH3)2COO] may form OH radicals through a 1,4 H-migration process (30), which may be an important nonphotolytic OH source in the troposphere. Again, structure dependence of ktherm for various CIs would need further investigation.
Previous studies of ozonolysis of 2,3-dimethyl-2-butene (tetramethyl ethylene, R1 = R2 = R3 = R4 = CH3) (20, 28, 34) have provided some information about the (CH3)2COO reactions with H2O and with SO2. But, the results are far from consistency. First, the reported kSO2 are on the order of 10−13 cm3s−1 (34), much smaller than the value determined from the direct kinetic measurement (Table 1). A similar situation also happens for smaller CIs like CH2OO and CH3CHOO (12, 13, 35), suggesting there might be some systematic issues in the ozonolysis experiments. Very recently, Newland et al. (20) measured the removal of SO2 in the presence of alkene–ozone systems and concluded that the SO2 removal displays a clear dependence on relative humidity for all four alkene ozonolysis systems [ethene (to form CH2OO), cis-2-butene (to form CH3CHOO), trans-2-butene (to form CH3CHOO), and 2,3-dimethyl-2-butene (to form (CH3)2COO)], confirming a significant reaction for stabilized CIs with H2O. However, Berndt et al. (28) investigated the H2SO4 formation as a function of [H2O] in similar ozonolysis reactions and reported quite different values. The results for (CH3)2COO are summarized in Table 2. The result by Newland et al. is not consistent with the results of Berndt et al. and this work. The reason for this discrepancy may originate from the complexity of the ozonolysis experiments, in which it is difficult to fully quantify the side reactions during the long reaction time.
Table 2.
Ratio of the rate coefficients of (CH3)2COO reactions with H2O and with SO2, kH2O/kSO2
It is important to note that the reactivity of a particular CI toward water vapor strongly depends on its structure. Table 1 indicates that a methyl group substitution for R1 may alter the rate coefficient by orders of magnitude. Although ozonolysis experiments (20, 28, 34) have also shown a trend for the structure dependence, the magnitude of the reactivity difference is much smaller than what is observed in the direct kinetic measurements (Table 1). In addition, the ozonolysis experiments cannot distinguish the anti- and syn- conformers of CIs, which have quite different reactivity.
The results summarized in Table 1 give direct evidence that the reaction of water with (CH3)2COO is greatly hindered by the methyl group at the R1 position. Interestingly, methyl substitution seems to enhance the reactivity of CIs toward SO2. One can imagine that CIs with more complicated substitution groups may also react with water slowly but react with SO2 quickly, similar to (CH3)2COO. Such CIs may be the candidate for the oxidant X in the SO2 oxidation, an important issue raised recently (4).
Considering that much of the VOC emissions consist of a large variety of alkenes, ranging from simple alkenes like C2H4 and C3H6 to bigger alkenes like isoprene, monoterpenes, sesquiterpenes, etc., various CIs are expected to form in the atmospheric ozonolysis reactions. To assess the impact of the CI+SO2 reaction class on the atmosphere, it is critical to know the atmospheric concentration of CIs. As mentioned above, it requires the relevant rate coefficients to estimate the atmospheric concentration of a CI. Water is the third most abundant molecule in the air. The reactions of CIs with water are crucial in determining the concentrations and fate of the CIs. The slow rates of water reactions with (CH3)2COO or with similar CIs are very difficult to measure, but very important in estimating the concentrations of those CIs and thus the oxidizing capacity of the atmosphere.
Materials and Methods
The experimental setup has been described in detail elsewhere (22, 32). In brief, (CH3)2COO was generated from photolysis of a gaseous mixture consisting of (CH3)2CI2, O2, and buffer gas (N2) at 248 nm (KrF excimer laser) via the established preparation method: (CH3)2CI2 + hν → (CH3)2CI + I; (CH3)2CI +O2 → (CH3)2COO + I (2, 30). (CH3)2COO was monitored via its strong UV absorption (30). Continuous probe light went through the photolysis reactor (25 or 20 mm inner diameter, 76 cm long) six or eight times to enhance the absorption signal.
Spectral Measurements.
Transient absorption spectra of the reaction system were measured with a continuous broadband light source (Energetiq, EQ-99) and a time-gated iCCD spectrometer (Andor, SR303i and DH320T-18F-03). The light source was projected to the entrance of the absorption cell by an achromatic lens (Thorlabs ACA254-100-UV). To enhance the absorption signal, the probe light was reflected eight times through the photolysis reactor by a spherical mirror (R = 1 m, Thorlabs, CM750-500-F01) and a SiO2 prism. The probe beam and the photolysis beam were overlapped collinearly in the photolysis reactor. For the iCCD measurement, the reference spectrum was recorded 200 μs before the photolysis pulse. Transient spectra at delay times 50, 100, 150, 200, 250, 300, 500, 1,000, and 2,000 μs were recorded.
Kinetics Measurements.
Absorption signal at 340 nm was measured in real time by using a balanced photodiode detector (Thorlabs, PDB450A) and a bandpass filter (Edmund Optics, 65129, 10-nm OD4 band pass filter) and the same light source. A time-dependent transmittance change (<1%) was observed after the photolysis pulse even without adding any sample. This background did not depend on [H2O], or on [SO2]. It can be subtracted by performing a background run under the same experimental condition except adding (CH3)2CI2. The presented data are after the background subtraction.
[SO2] Measurements.
The SO2 concentration was adjusted by controlling the amount of SO2 in buffer gas with mass flow controllers (Brooks, 5850E or 5800E) and monitored by its UV absorption (190–330 nm) with a D2 lamp (Ocean Optics, D-2000) and a spectrometer (Ocean Optics, Maya2000 Pro).
Relative Humidity Measurements.
The relative humidity was adjusted by controlling the mixing ratio of dry and moisturized buffer gases with mass flow controllers (Brooks, 5850E or 5800E) and monitored with a humidity sensor (Rotronic, HC2-S). The temperature for measuring the water reaction was controlled at 298.2 ± 0.5 K.
Precursor Preparation.
The precursor, (CH3)2CI2, was synthesized following a reported method (36). In brief, acetone was added to hydrazine monohydrate (80 °C, >1 h) to obtain acetone hydrazone (CH3)2C=NNH2. Saturated solution of iodine in ethyl ether was added to acetone hydrazone (at room temperature), which is mixed with ethyl ether and triethylamine, to obtain the final product. The structure of the synthesized (CH3)2CI2 was checked with H-NMR spectroscopy [(CH3)2CI2: 3.00 ppm (6H, s, Me) in CDCl3] (37).
Supplementary Material
Acknowledgments
The authors thank Prof. Jim-Min Fang, Ms. Ling-Wei Li, and Ms. Che-Hsuan Chang for help in organic synthesis and NMR measurement; Ms. Liang-Chun Lin and Mr. Chun-Hung Chang for help in experiments; and Prof. Yuan-Tseh Lee for discussion. This work was supported by Academia Sinica and Ministry of Science and Technology, MOST 103-2113-M-001-019-MY3, Taiwan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513149112/-/DCSupplemental.
References
- 1.Criegee R. Mechanism of ozonolysis. Angew Chem Int Ed Engl. 1975;14(11):745–752. [Google Scholar]
- 2.Welz O, et al. Direct kinetic measurements of Criegee intermediate (CH₂OO) formed by reaction of CH₂I with O₂. Science. 2012;335(6065):204–207. doi: 10.1126/science.1213229. [DOI] [PubMed] [Google Scholar]
- 3.Taatjes CA, et al. Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science. 2013;340(6129):177–180. doi: 10.1126/science.1234689. [DOI] [PubMed] [Google Scholar]
- 4.Mauldin RL, 3rd, et al. A new atmospherically relevant oxidant of sulphur dioxide. Nature. 2012;488(7410):193–196. doi: 10.1038/nature11278. [DOI] [PubMed] [Google Scholar]
- 5.Boy M, et al. Oxidation of SO2 by stabilized Criegee intermediate (sCI) radicals as a crucial source for atmospheric sulfuric acid concentrations. Atmos Chem Phys. 2013;13(7):3865–3879. [Google Scholar]
- 6.Ehn M, et al. A large source of low-volatility secondary organic aerosol. Nature. 2014;506(7489):476–479. doi: 10.1038/nature13032. [DOI] [PubMed] [Google Scholar]
- 7.Berresheim H, et al. Missing SO2 oxidant in the coastal atmosphere?–observations from high-resolution measurements of OH and atmospheric sulfur compounds. Atmos Chem Phys. 2014;14(22):12209–12223. [Google Scholar]
- 8.Sarwar G, et al. Impact of sulfur dioxide oxidation by stabilized Criegee intermediate on sulfate. Atmos Environ. 2014;85(3):204–214. [Google Scholar]
- 9.Sheps L. Absolute ultraviolet absorption spectrum of a Criegee intermediate CH2OO. J Phys Chem Lett. 2013;4(24):4201–4205. doi: 10.1021/jz402191w. [DOI] [PubMed] [Google Scholar]
- 10.Stone D, Blitz M, Daubney L, Howes NU, Seakins P. Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure. Phys Chem Chem Phys. 2014;16(3):1139–1149. doi: 10.1039/c3cp54391a. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Bayes KD, Sander SP. Measuring rate constants for reactions of the simplest Criegee intermediate (CH2OO) by monitoring the OH radical. J Phys Chem A. 2014;118(4):741–747. doi: 10.1021/jp407058b. [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama S, Akimoto H. Reactions of Criegee intermediates in the gas phase. Res Chem Intermed. 1994;20(3-5):503–524. [Google Scholar]
- 13.Johnson D, Marston G. The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem Soc Rev. 2008;37(4):699–716. doi: 10.1039/b704260b. [DOI] [PubMed] [Google Scholar]
- 14.Welz O, et al. Rate coefficients of C1 and C2 Criegee intermediate reactions with formic and acetic acid near the collision limit: Direct kinetics measurements and atmospheric implications. Angew Chem Int Ed Engl. 2014;53(18):4547–4550. doi: 10.1002/anie.201400964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarwar G, et al. Potential impacts of two SO2 oxidation pathways on regional sulfate concentrations: Aqueous-phase oxidation by NO2 and gas-phase oxidation by stabilized Criegee intermediates. Atmos Environ. 2013;68:186–197. [Google Scholar]
- 16.Li J, Ying Q, Yi B, Yang P. Role of stabilized Criegee intermediates in the formation of atmospheric sulfate in eastern United States. Atmos Environ. 2013;79:442–447. [Google Scholar]
- 17.Suto M, Manzanares ER, Lee LC. Detection of sulfuric acid aerosols by ultraviolet scattering. Environ Sci Technol. 1985;19(9):815–820. doi: 10.1021/es00139a008. [DOI] [PubMed] [Google Scholar]
- 18.Becker K, Bechara J, Brockmann K. Studies on the formation of H2O2 in the ozonolysis of alkenes. Atmos Environ. 1993;27(1):57–61. [Google Scholar]
- 19.Leather K, et al. Acid-yield measurements of the gas-phase ozonolysis of ethene as a function of humidity using chemical ionisation mass spectrometry (CIMS) Atmos Chem Phys. 2012;12(1):469–479. [Google Scholar]
- 20.Newland MJ, et al. Kinetics of stabilised Criegee intermediates derived from alkene ozonolysis: Reactions with SO2, H2O and decomposition under boundary layer conditions. Phys Chem Chem Phys. 2015;17(6):4076–4088. doi: 10.1039/c4cp04186k. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang B, McLeod MW, Jones RL, Bloss WJ. NO3 radical production from the reaction between the Criegee intermediate CH2OO and NO2. Phys Chem Chem Phys. 2013;15(40):17070–17075. doi: 10.1039/c3cp53024h. [DOI] [PubMed] [Google Scholar]
- 22.Chao W, Hsieh J-T, Chang C-H, Lin JJ-M. Atmospheric chemistry. Direct kinetic measurement of the reaction of the simplest Criegee intermediate with water vapor. Science. 2015;347(6223):751–754. doi: 10.1126/science.1261549. [DOI] [PubMed] [Google Scholar]
- 23.Berndt T, et al. Competing atmospheric reactions of CH2OO with SO2 and water vapour. Phys Chem Chem Phys. 2014;16(36):19130–19136. doi: 10.1039/c4cp02345e. [DOI] [PubMed] [Google Scholar]
- 24.Sheps L, Scully AM, Au K. UV absorption probing of the conformer-dependent reactivity of a Criegee intermediate CH3CHOO. Phys Chem Chem Phys. 2014;16(48):26701–26706. doi: 10.1039/c4cp04408h. [DOI] [PubMed] [Google Scholar]
- 25.Ryzhkov AB, Ariya PA. A theoretical study of the reactions of parent and substituted Criegee intermediates with water and the water dimer. Phys Chem Chem Phys. 2004;6(21):5042–5050. [Google Scholar]
- 26.Kuwata KT, Hermes MR, Carlson MJ, Zogg CK. Computational studies of the isomerization and hydration reactions of acetaldehyde oxide and methyl vinyl carbonyl oxide. J Phys Chem A. 2010;114(34):9192–9204. doi: 10.1021/jp105358v. [DOI] [PubMed] [Google Scholar]
- 27.Anglada JM, González J, Torrent-Sucarrat M. Effects of the substituents on the reactivity of carbonyl oxides. A theoretical study on the reaction of substituted carbonyl oxides with water. Phys Chem Chem Phys. 2011;13(28):13034–13045. doi: 10.1039/c1cp20872a. [DOI] [PubMed] [Google Scholar]
- 28.Berndt T, et al. H2SO4 formation from the gas-phase reaction of stabilized Criegee intermediates with SO2: Influence of water vapour content and temperature. Atmos Environ. 2014;89:603–612. [Google Scholar]
- 29.Vereecken L, Harder H, Novelli A. The reaction of Criegee intermediates with NO, RO2, and SO2, and their fate in the atmosphere. Phys Chem Chem Phys. 2012;14(42):14682–14695. doi: 10.1039/c2cp42300f. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Beames JM, Green AM, Lester MI. UV spectroscopic characterization of dimethyl- and ethyl-substituted carbonyl oxides. J Phys Chem A. 2014;118(12):2298–2306. doi: 10.1021/jp412726z. [DOI] [PubMed] [Google Scholar]
- 31.Ting W-L, Chen Y-H, Chao W, Smith MC, Lin JJ. The UV absorption spectrum of the simplest Criegee intermediate CH2OO. Phys Chem Chem Phys. 2014;16(22):10438–10443. doi: 10.1039/c4cp00877d. [DOI] [PubMed] [Google Scholar]
- 32.Smith MC, et al. UV absorption spectrum of the C2 Criegee intermediate CH3CHOO. J Chem Phys. 2014;141(7):074302. doi: 10.1063/1.4892582. [DOI] [PubMed] [Google Scholar]
- 33.Ting W-L, et al. Detailed mechanism of the CH₂I + O₂ reaction: Yield and self-reaction of the simplest Criegee intermediate CH₂OO. J Chem Phys. 2014;141(10):104308. doi: 10.1063/1.4894405. [DOI] [PubMed] [Google Scholar]
- 34.Berndt T, et al. Gas-Phase ozonolysis of selected olefins: The yield of stabilized Criegee intermediate and the reactivity toward SO2. J Phys Chem Lett. 2012;3(19):2892–2896. [Google Scholar]
- 35.Jenkin ME, et al. The MCM v3.3 degradation scheme for isoprene. Atmos Chem Phys Discuss. 2015;15:9709–9766. [Google Scholar]
- 36.Pross A, Sternhell S. Oxidation of hydrazones with iodine in the presence of base. Aust J Chem. 1970;23(5):989–1003. [Google Scholar]
- 37.Karabatsos G, Osborne C. Structural studies by nuclear magnetic resonance—XVI: Conformations and configurations of hydrazones. Tetrahedron. 1968;24(8):3361–3368. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.