Significance
Emerging influenza subtypes, such as the recently identified H7N9 strains, are of considerable public health concern. Although vaccines are an important countermeasure, influenza vaccines tend to be subtype- and strain-specific, such that they may not be widely available in the event of the human adaptation and spread of an unanticipated strain or subtype. Additionally, influenza strains have demonstrated the ability to develop resistance to existing antivirals, including oseltamivir. As such, there is a need for novel interventions that can treat and/or prevent serious influenza infection. We demonstrate that VIS410, a human mAb, neutralizes a wide range of influenza A viruses and effectively manages H7N9 infection.
Keywords: antibody, influenza, virus, H7N9
Abstract
Emerging strains of influenza represent a significant public health threat with potential pandemic consequences. Of particular concern are the recently emerged H7N9 strains which cause pneumonia with acute respiratory distress syndrome. Estimates are that nearly 80% of hospitalized patients with H7N9 have received intensive care unit support. VIS410, a human antibody, targets a unique conserved epitope on influenza A. We evaluated the efficacy of VIS410 for neutralization of group 2 influenza strains, including H3N2 and H7N9 strains in vitro and in vivo. VIS410, administered at 50 mg/kg, protected DBA mice infected with A/Anhui/2013 (H7N9), resulting in significant survival benefit upon single-dose (−24 h) or double-dose (−12 h, +48 h) administration (P < 0.001). A single dose of VIS410 at 50 mg/kg (−12 h) combined with oseltamivir at 50 mg/kg (−12 h, twice daily for 7 d) in C57BL/6 mice infected with A/Shanghai 2/2013 (H7N9) resulted in significant decreased lung viral load (P = 0.002) and decreased lung cytokine responses for nine of the 11 cytokines measured. Based on these results, we find that VIS410 may be effective either as monotherapy or combined with antivirals in treating H7N9 disease, as well as disease from other influenza strains.
Influenza, a zoonotic viral disease, is responsible for substantial human morbidity and mortality yearly, with periodic elevations due to emergence of novel viral strains, either through mutation or genetic reassortment in a variety of animal reservoirs, including pigs, birds, and seals. Antigenic naivety within the population, coupled with the advent of a virus strain that can effectively transmit via respiratory droplets, can lead to epidemic or pandemic outbreaks. In addition, viruses with increased virulence, such as H5N1 and H7N9, are associated with enhanced morbidity and case fatality, estimated at 30–60% despite the availability of current antiviral therapy.
Patients hospitalized with H7N9 infection typically manifest a high fever and cough, hypoxemia, and opacities and/or consolidations on chest radiology, with associated findings including shock, acute kidney injury, and the development of acute respiratory distress syndrome (ARDS). The high mortality associated with H7N9 infection and development of ARDS is similar to what has been reported for H5N1. An associated cytokine storm has been described in both of these patient groups, with proinflammatory cytokines/chemokines documented in plasma and pulmonary lavage samples (1–3). The increased cytokine responses have recently been correlated with increased severity and mortality observed in patients (2–4). Elevated levels of interleukin (IL)-10, IL-6, IL-8, and macrophage inflammatory protein-1β (MIP-1β) in plasma were found to be predictive of a less favorable or fatal outcome. Furthermore, IL-1β, interferon (IFN)-γ, MIP-1α, and MIP-1β were all significantly elevated in the bronchial lavage samples at a 100- to 1,000-fold increase compared with plasma concentrations, and tumor necrosis factor (TNF)-α was only detected in the lavage samples. Mouse models for H5N1 and H7N9 infection mimic this cytokine response and the lung pathology of ARDS (2). We therefore sought to examine the role of a broadly neutralizing antibody, VIS410, in mitigating this “cytokine storm” in infected mice and lowering lung viral concentrations in this sublethal H7N9 model. Since this agent would likely be used in combination with a neuraminidase inhibitor, we investigated the effect of VIS410 compared to, and in combination with, oseltamivir.
Additionally, the DBA mouse has been found to have much higher susceptibility to influenza infection than either C56BL/6 or BALB/c mice (5, 6). A variety of influenza viruses, including H5N1 and influenza B viruses, have been shown to be lethal to DBA mice without prior adaptation (7, 8). We reasoned that to complement the cytokine measurements in BALB/c mice, a lethal DBA mouse model could be used to examine the effect of VIS410 on mortality, thereby providing an appropriate model of the significant morbidity and mortality associated with H7N9 infection in humans. We therefore additionally evaluated VIS410 in a lethal model of H7N9 disease.
Results
Identification, Recombinant Expression, and Characterization of VIS410.
Our goal was to achieve broad-spectrum coverage with an antibody-based approach that could overcome some of the historical challenges associated with deployment of such approaches, such as limited spectrum of coverage and/or potency.
To target influenza hemagglutinin (HA), we attempted to engineer an antibody using available structural information on antibody–antigen interfaces. For this effort, we sought to identify an antibody targeting amino acids within the trimeric interface within the stem region of HA, which are highly networked, and therefore potentially limited in their ability to mutate (9). Using a database of nonredundant combinations of complementary determining region (CDR) canonical structures (antibody scaffolds), we selected multiple antibody templates satisfying shape complementarity criteria and systematically engineered energetically favorable, hotspot-like interactions between CDR residues and these anchor residues on HA. The engineering constructs from the multiple templates were evaluated first through in silico methods and subsequently through experimentation to test their ability to bind to one or more HAs from group 1 and group 2 (SI Text, Table S1, and Fig. S1). The data from each engineering iteration were used for the subsequent design cycle. VIS410 was selected after multiple rounds of this effort based on its breadth of binding and physicochemical properties, including stability and expression levels. VIS410 is biophysically stable, with a thermal melting transition temperature of ∼70 °C, consistent with other commercial antibody products. Analytical analysis of the product indicates that it exists as >98% monomer and is stable to aggregation under accelerated conditions (37 °C). In addition to inhibiting HA-mediated membrane fusion (Fig. S2), VIS410 exhibits antibody-dependent, cell-mediated cytotoxicity in in vitro assays (Fig. S3), suggesting infected cells can also be targeted for elimination by VIS410.
Table S1.
Top ranking VH and VL templates to target the trimeric interface of HA
| GenBank ID | Protein Data Bank ID | Description | Germ line | CDR canonical | GenBank ID | Protein Data Bank ID | Description | Germ line | CDR canonical |
| 218681906 | 3EYQ_D | Chain D, crystal structure of Mj5 Fab, a germ line antibody variant of antihuman CMV antibody 8f9 | IGHV3-33*05 | 1/10A, 3/10B | 213424087 | 3BDY_L | Dual specific bH1 Fab in complex with VEGF | IGKV1-NL1*01 | ?/15A, 1/7A, 1/9A |
| 33318928 | N/A | Ig heavy chain variable region of EBV | IGHV3-7*03 | 1/10A, 2/10A | 33356966 | 1H0D_A | NCBI:B3 | ?/15A, 1/7A, 1/9A | |
| 185486 | N/A | Ig heavy chain | IGHV3-33*05 | 1/10A, 3/10B | 157831893 | 1LVE_A | Structure of the variable domain of human Ig K-4 light chain Len | NCBI:B3 | 3/17A, 1/7A, 1/9A |
N/A, not available.
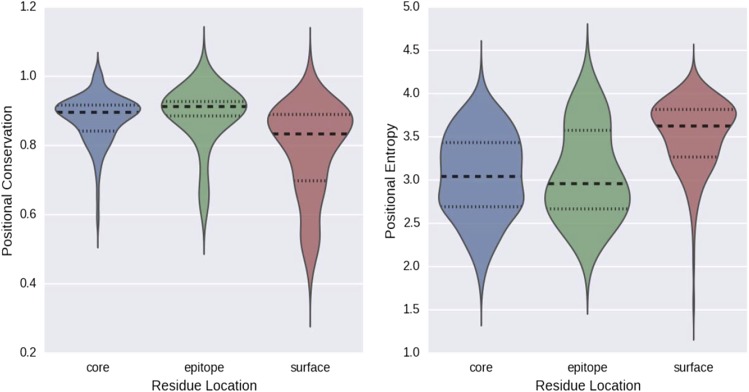
Fig. S1.
(Left) Distribution of positional conservation among available H3 HA sequences. In this analysis, a higher value indicates a greater degree of conservation. (Right) Distribution of observed site entropy for the same positions within H1N1. In this case, lower values indicate less tolerance to mutation.
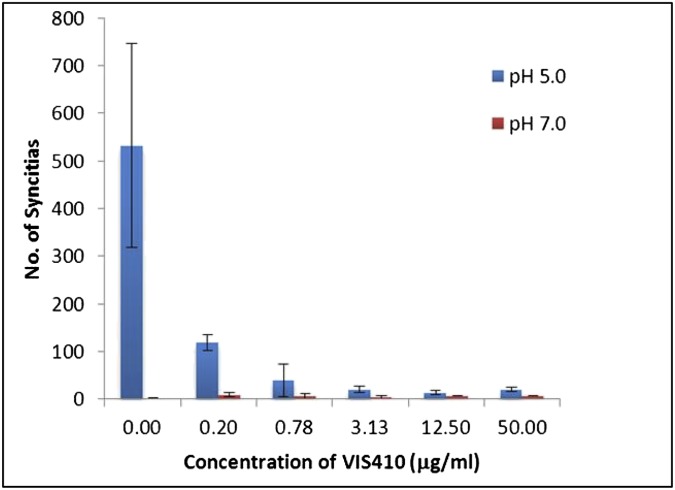
Fig. S2.
Inhibition of syncytia formation by VIS410.
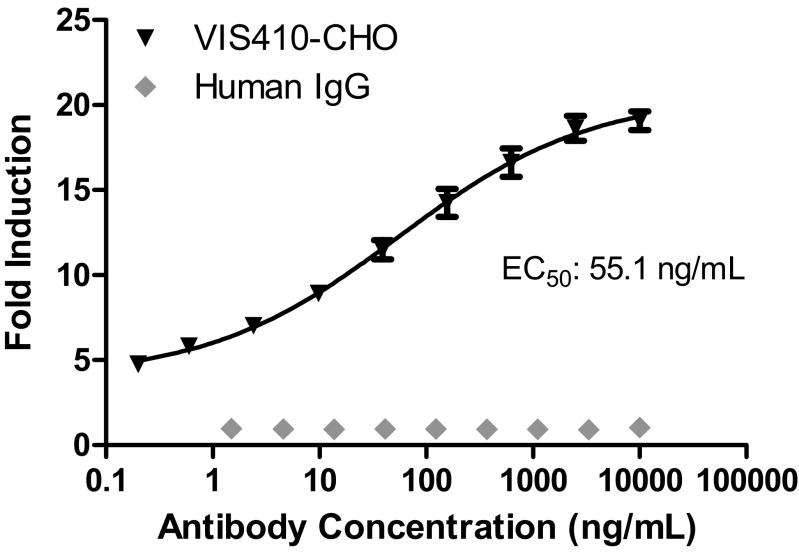
Fig. S3.
Antibody-dependent cell-mediated cytotoxicity (ADCC) activity of VIS410. To test the ability of VIS410 to engage with FcγRIIIa receptors to stimulate the cytotoxic signaling pathway, influenza-infected A549 cells were used as the target cells and the ADCC Reporter Bioassay (Promega) was performed as described by the manufacturer. Briefly, A549 cells were seeded the day before the ADCC assay at 15,000 cells per well in 50 μL of 5% FBS containing DMEM in sterile, white, flat-bottomed 96-well plates. A/Victoria/3/1975 (H3N2) influenza A virus stock was diluted to a multiplicity of infection of ∼1.0 in the 5% FBS-containing DMEM plus 4 μg/mL TPCK-trypsin (Sigma), and 50 μL of diluted virus was added to respective wells (or medium alone was added to wells representing uninfected A549 controls). Plates were incubated at 37 °C for ∼18 h, and the medium was exchanged with medium containing varying concentrations of either VIS410 or an irrelevant human IgG1 control mAb. Jurkat T cells engineered to express the high-affinity human FcγRIIIa (V/V 158) variant stably were used as effector cells and were added to wells containing antibody/influenza-infected cells. Following a 6-h incubation, luciferase induction as a measure of ADCC was assessed using Bio-Glo Luciferase Assay reagent (Promega) and a BioTek Luminometer. Data from the average of triplicate samples were run in two independent experiments, expressed as fold induction relative to no antibody. Data were fitted to a four-parameter curve using Prism software (version 5.03; GraphPad). The EC50 value calculated was 55.1 ng/mL for VIS410, whereas the irrelevant human IgG1 mAb did not demonstrate any ADCC activity against influenza virus-infected A549 cells.
Breadth of Binding to Heterosubtypic Influenza Virus HA.
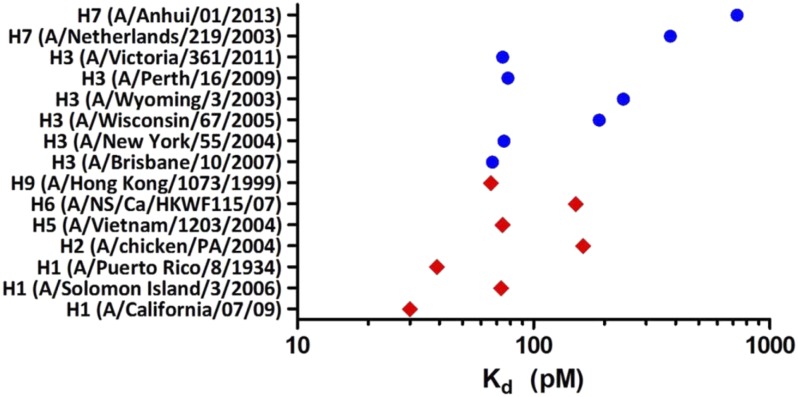
VIS410 was tested for binding to HA from representative group 1 and group 2 subtypes by ELISA. H1, H2, H5, H6, and H9 were chosen as representative HAs from group 1, and H3 and H7 HAs were chosen as group 2 representatives. To account for the high intrasubtypic diversity of H3, a broader panel of H3 HAs was used to assess VIS410 binding. Consistent with targeting a constrained epitope on HA, VIS410 bound all HAs with high avidity (measured apparent Kds of 50–730 pM; Fig. 1) as measured by ELISA. To confirm and extend these results, the affinity of VIS410 was measured by surface plasmon resonance on a sparsely HA-coated surface, where binding (Kd) was measured in the picomolar to single-digit nanomolar range. In vitro VIS410 demonstrated antiviral activity against group 1 and group 2 viruses, indicating that multivalent binding of VIS410 to trimeric HA likely mediates neutralization; this conclusion is supported by previous mechanistic studies conducted for stem-binding antibodies (10, 11) (Table 1). Consistent with its high-affinity binding, VIS410 was found to inhibit viral replication in MDCK cells with EC50s of ∼0.3–11 μg/mL, and it was 103- to 105-fold more potent than ribavirin on a molar basis.
Fig. 1.
Biochemical characterization of VIS410 binding to influenza HA. VIS410 binding affinity (Kd, apparent) to a panel of group 1 (red diamonds) and group 2 (blue circles) influenza A HA samples as determined by ELISA, covering the major subtypes that have been known to infect humans.
Table 1.
In vitro neutralization of group 1 and group 2 influenza A viruses by VIS410
| Virus | Inoculum* | VIS410 (μg/mL) | Ribavirin (μg/mL) | |||||
| EC50† | CC50‡ | SI§ | EC50† | CC50‡ | SI§ | |||
| H1N1 | California 04/2009 | 300 | 5.1 | >500 | >98 | 5.4 | >100 | >195 |
| H1N1 | Solomon Islands | 160 | 1.2 | >500 | >420 | 11 | >100 | >9.1 |
| H3N2 | Brisbane/10/2007 | 40 | 0.3 | >500 | >1,700 | 18 | >100 | >5.6 |
| H3N2 | Fujian/411/2003 | 65 | 0.3 | >500 | >1,800 | 8.7 | >100 | >12 |
| H3N2 | Panama/2007/99 | 13 | 11.0 | >500 | >45 | 8.7 | >100 | >12 |
| H3N2 | Shangdong/09/ 93 | 100 | 0.6 | >500 | >890 | 3.2 | >100 | >31 |
| H3N2 | Victoria/3/75 | 12 | 4.9 | >500 | >100 | 9.9 | >100 | >10 |
| H3N2 | Wyoming/03/2003 | 10 | 6.8 | >500 | >74 | 22 | >100 | >4.5 |
| H5N1 | Duck/MN/1525/81 | 160 | 1.5 | >500 | >330 | 5.3 | >100 | >19 |
Measured as cell culture infective dose (CCID50) of virus per well.
Measured as 50% toxic concentration (CC50) without virus (micrograms per milliliter).
Measured as EC50 (micrograms per milliliter).
Measured as selectivity index (SI) = CC50/EC50
Therapeutic Administration of VIS410 Rescues Mice Challenged with H3N2 Virus.
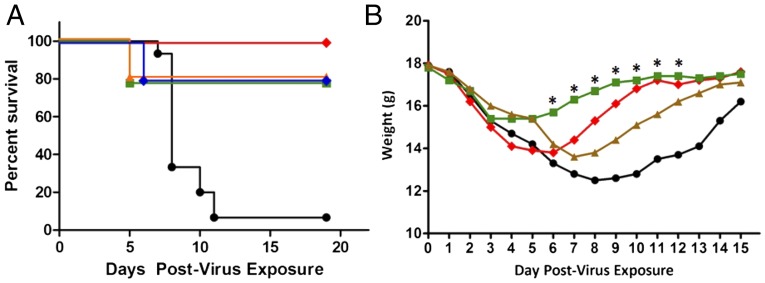
As a baseline for studies with H7N9, the prophylactic and therapeutic efficacy of VIS410 was initially evaluated in an H3N2 mouse model, as a representative group 2 strain. Upon intranasal exposure to H3N2, 93% of mock-treated mice succumbed to infection by day 11. VIS410 significantly protected mice against death as measured by total survivors (Fig. 2A; P < 0.001), and a single-treatment dose with VIS410 also profoundly affected weight loss (Fig. 2B), as well as timing of death.
Fig. 2.
In vivo effect of VIS410 and oseltamivir in a BALB/c mouse model of H3N2. Animals were infected intranasally with a lethal dose of mouse-adapted influenza A/Victoria/3/75 virus (2 LD50). Mice were weighed daily to day 15, or until death, and then once again on day 19. (A) Five groups of 10 mice each were administered VIS410 at 2.5 (blue diamonds), 5 (red diamonds), 10 (orange triangles), or 20 (green squares) mg/kg i.p. 48 h after virus exposure; 15 mice received placebo (black circle). Mice were euthanized on the day that 30% weight loss was observed. Statistical inferences were made by two-way ANOVA, followed by pairwise comparisons to compare each treatment group with the placebo group, considering the survival of both (Tukey’s multiple comparisons tests). VIS410 had a significant effect on survival (P < 0.001). (B) The combination of VIS410 at 5 mg/kg and oseltamivir at 10 mg/kg daily (green squares) had a greater effect on preventing weight loss than did 5 mg/kg VIS410 (red diamonds) or oseltamivir at 10 mg/kg daily (brown triangles) alone (*P < 0.001). The placebo is also shown (black circle).
VIS410 combination treatment with oseltamivir was also evaluated in an H3N2 sublethal model. The combination of VIS410 at 5 mg/kg (Fig. 2B) plus daily oseltamivir at 10 mg/kg significantly mitigated weight loss compared with either treatment alone from days 5–8 postvirus exposure; this period is the critical time frame in which weight loss is a strong predictor of later mortality due to virus infection. Mitigation in weight loss was also observed for other combinations of VIS410 and oseltamivir. At the highest doses of VIS410 (20 mg/kg) and daily oseltamivir (60 mg/kg), minimal weight loss was observed and the mice returned to their starting weight sooner compared with both placebo and either treatment alone. Thus, taken together, these data demonstrate that VIS410 alone, or in combination with oseltamivir, is efficacious in treating mice infected with a group 2 virus.
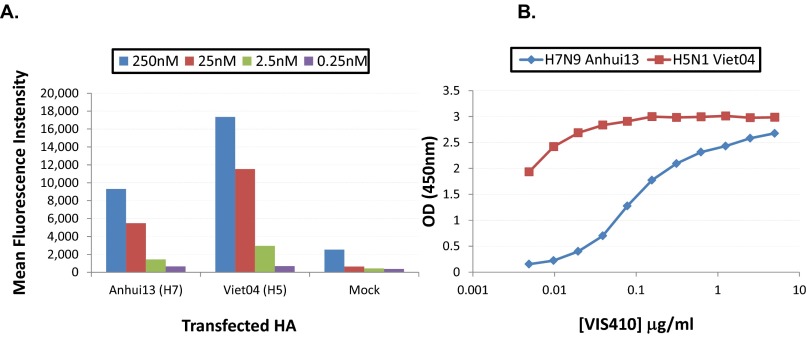
Given the in vivo data with H3N2, the ability of VIS410 to act as an effective treatment for H7N9 was evaluated. We initially examined the ability of VIS410 to bind to H7N9 HA. Binding was assessed by flow cytometry, as well as by ELISA, and demonstrated specific binding with a measured relative Kd of 0.73 nM (Fig. S4). In in vitro neutralization assays, VIS410 demonstrated neutralizing activity toward H7N9 at ∼50 μg/mL when virus and antibody were premixed, whereas no significant activity was observed when virus and antibody were added concurrently to target cells.
Fig. S4.
VIS410 binds HA from both H7 and H5 viruses. (A) HEK-293T cells were transfected with DNA constructs encoding full-length influenza HA. Cells were harvested and incubated with varying concentrations of antibody. Antibody binding was detected using mouse antihuman IgG conjugated to the Allophycocyanin fluorophore (Biolegend). Cells were washed and analyzed for fluorescence intensity using an Accuri C6 flow cytometer. Mean fluorescence intensity of staining was plotted. (B) H7 or H5 HA was coated onto ELISA plates, and VIS410 binding at varying concentrations was assessed as described in Materials and Methods.
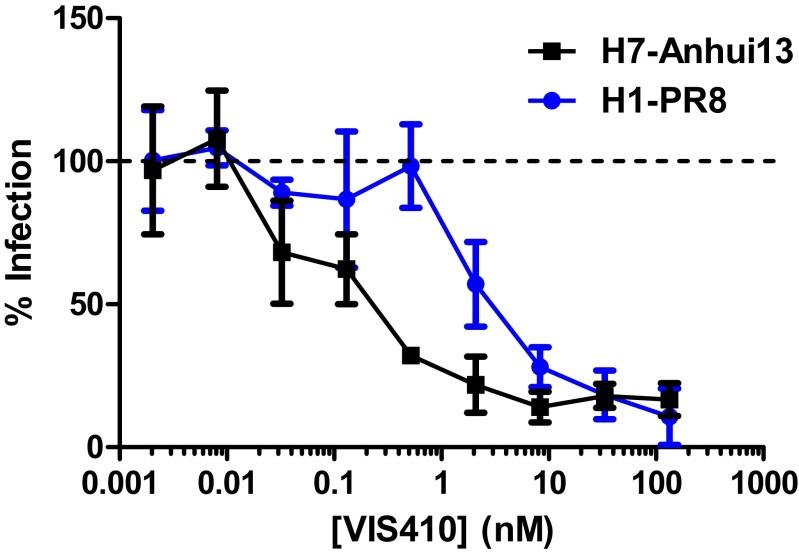
One reason for ambiguous results with the virus neutralization assays might be the system used to measure neutralization. In a standard neutralization assay, a small number of initially infected cells could release progeny virus that is amplified by subsequent infection events. Therefore, to assess VIS410 in a more controlled system, we examined the ability of VIS410 to inhibit H7N9 HA-typed pseudovirus. We found that VIS410 strongly inhibited pseudovirus with an IC50 of 1.2 nM, comparable to the IC50 observed for H1 (2.4 nM for A/Puerto Rico/08/1934) (Fig. S5).
Fig. S5.
VIS410 exhibits dose-dependent inhibition of H1- and H7-typed pseudovirus. As detailed in Materials and Methods, codon-optimized HA genes [either from A/Puerto Rico/8/1934 (H1N1) or A/Anhui/1/2013 (H7N9)] were cloned into pLP/VSV-G (Invitrogen) by replacing the VSV-G gene. Pseudotyped lentiviruses were generated by transient transfection of HEK-293T/17 cells with the pLP-HA and an HIV-1 backbone that contained a deletion (to prevent envelope glycoprotein expression) and an inserted luciferase gene (pNL Luc AM). Antibody/pseudovirus mixtures were applied to HEK-293T/17 or MDCK cells for 72 h at 37 °C, and HA-mediated cell fusion was assessed using One-Glo luciferase reagent (Promega) and measured using a BioTek Synergy 2 luminometer. Infection as a percentage of the virus-only control was plotted for each antibody concentration.
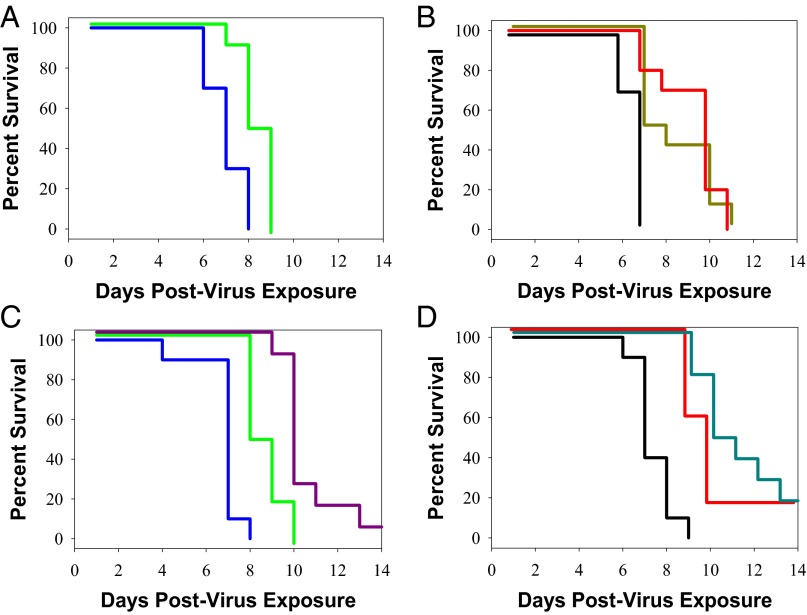
Confirming and extending these results, we examined the ability of VIS410 to protect DBA mice against a lethal challenge with A/Anhui/1/2013. Notably, DBA mice are more susceptible to influenza infection than traditional mouse models, such as BALB/c, and thus replicate some aspects of severe disease in humans (12). In an attempt to account for the severity of this model, and to provide a reasonable comparison in cohorts receiving oseltamivir, the dose of VIS410 and oseltamivir was increased to 50 mg/kg. Administration of VIS410 either once prophylactically or twice therapeutically resulted in a significant survival advantage for animals infected with H7N9 (P < 0.002). Administration of VIS410 before infection and again 48 h after infection demonstrated superior activity to oseltamivir, which was administered twice daily at 50 mg/kg for 8 d (Fig. 3 A and B; P = 0.015). Additionally, VIS410 either alone (P = 0.013) or in combination with oseltamivir (P < 0.001) resulted in a significant survival advantage compared with oseltamivir alone (Fig. 3 C and D). Finally, in a sublethal H7N9 animal model (2) using a distinct strain of H7N9 (A/Shanghai/2/2013), administration of VIS410 resulted in less viral-induced weight loss (Fig. 4) compared with either mock-treated or oseltamivir-treated animals. Furthermore, when VIS410 was administered in conjunction with oseltamivir, viral concentrations were significantly reduced compared to mock treatment or oseltamivir alone (Fig. 5A; P = 0.002 and P = 0.004 respectively).
Fig. 3.
In vivo effect of VIS410 and oseltamivir in a lethal DBA mouse model of H7N9. DBA mice were infected with 1.97 LD50 of A/Shanghai/2/2013 virus in 25 μL of PBS intranasally. (A) Animals treated by oral gavage with oseltamivir, 50 mg/kg daily (green line), had delayed death compared with animals administered water (blue line). (B) VIS410 administered at a dose of 50 mg/kg either once (gold line) or twice (red line) resulted in significantly delayed death compared with PBS-treated animals (black line). (C) Animals treated with a single injection of VIS410 plus daily administration of oseltamivir (purple line) had a significant survival advantage compared with placebo-treated animals (blue line) or animals treated only with oseltamivir (green line). (D) Addition of oseltamivir to two doses of VIS410 (cyan line) resulted in increased survival compared with VIS410 alone (red line) or PBS-treated animals (black line).
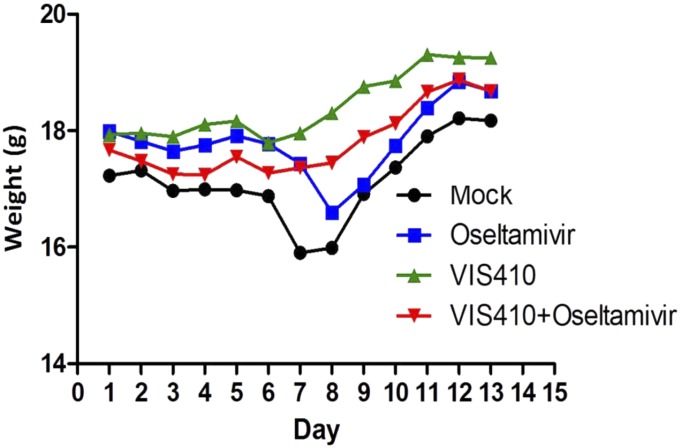
Fig. 4.
VIS410 protects against effects of H7N9 in a sublethal mouse model of H7N9. C57BL/6 mice were administered vehicle, oseltamivir, VIS410, or VIS410 plus oseltamivir before intranasal inoculation of A/Shanghai/2/2013 virus. Beginning at day 1 postinfection, weight loss was monitored for 12 consecutive days. Results are expressed as mean weight (n = 3).
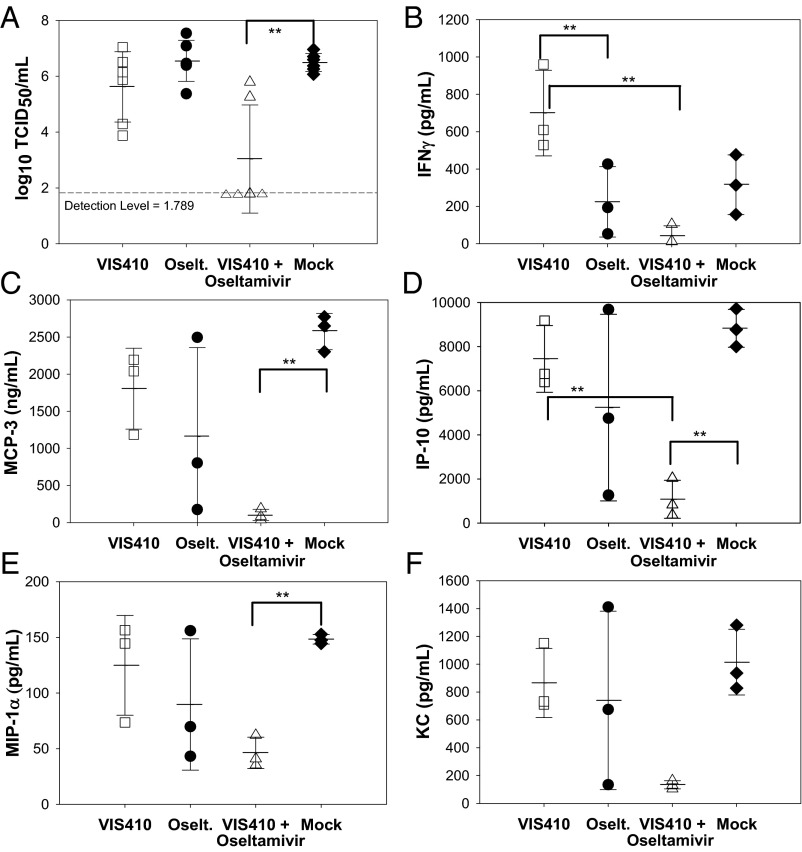
Fig. 5.
Analysis of virus and cytokine levels in mice administered H7N9. Therapy was initiated 12 h before infection as described in Materials and Methods. Animals were administered a sublethal dose of H7N9 and then monitored on day 5 for viral levels (A) and cytokines, including IFN-γ (B), MCP-3 (C), IP-10 (D), MIP-1α (E), and KC (F). Three animals were used per cohort, except for determination of viral titers (n = 6), and were mock-treated (♦), VIS410-treated (□), oseltamivir-treated (●), or cotreated with both oseltamivir and VIS410 (Δ). Tukey posttest analysis identified the groups that were significantly different from one another (**P < 0.05). Differences in IFN-γ, MCP-3, IP-10, and MIP-1α were significant, whereas the difference for KC trended toward significance (P < 0.1).
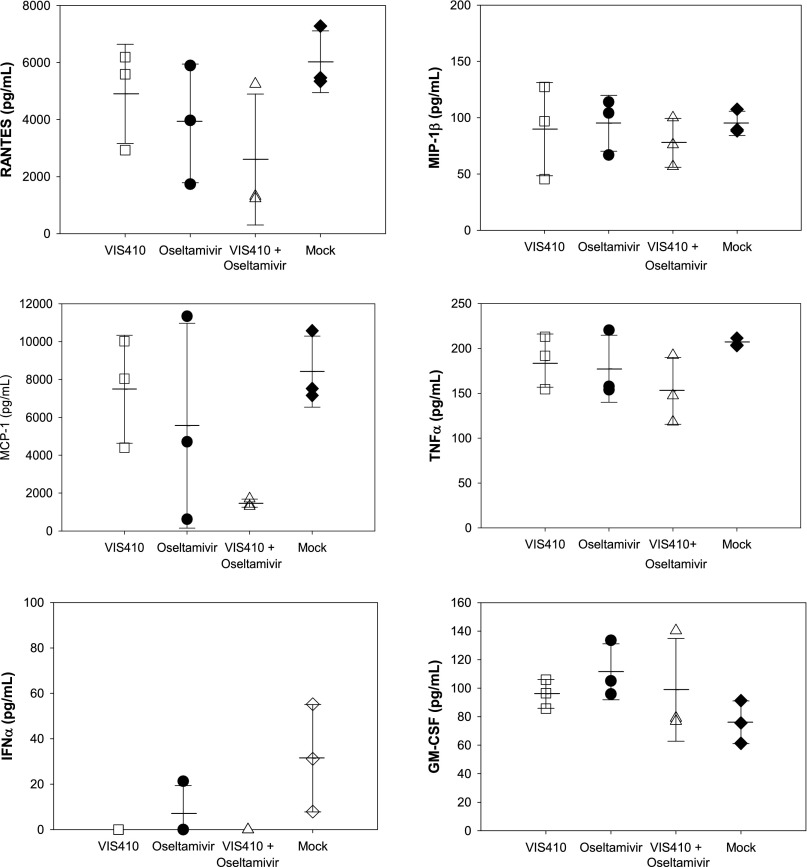
The sublethal H7N9 animal model replicates aspects of H7N9-induced pathology observed in human infections (2), including recent reports of hypercytokinemia in severely infected patients, which was associated with poor prognosis (4). An analysis of a panel of cytokines in H7N9-infected mice indicated an elevation in a number of cytokines, including MIP-1α/MIP-1β, IFN-γ, monocyte chemotactic protein 1/3 (MCP-1/MCP-3), keratinocyte chemoattractant (KC), and IFN-γ–induced protein 10 (IP-10), among others (Fig. 5 and Fig. S6). We note that administration of VIS410 is associated with an increase in IFN-γ. Elevation of IFN-γ has been associated with a significant protective antiviral effect and is likely one of the mechanisms by which VIS410 offers protection (13, 14). Additionally, when VIS410 was coadministered with oseltamivir, mean IFN-γ, MIP-1α, and IP-10 levels were significantly lower compared with mock-treated mice (P < 0.05) and KC trended toward significance (P < 0.10). Taken together, this data demonstrates that VIS410 plus oseltamivir can reduce viral replication and virus-induced inflammation in the lungs of H7N9-infected mice.
Fig. S6.
Analysis of additional cytokines in mice infected with H7N9. Cytokine levels were measured as described in Fig. 5 and in Materials and Methods.
SI Text
Information on VIS410: Epitope.
In terms of the epitope targeted by VIS410, we noted in a previous publication that amino acids within the trimeric interface of the stem of HA are highly networked, and therefore potentially limited in their ability to mutate (29). Additionally, the sequence conservation within the A helix is more similar to the sequence conservation within the core (nonsolvent accessible region) of HA than other surface residues (Fig. S1) for both H1 and H3 as representative subtypes of group 1 and 2, and mutations within this helix are associated with fitness loss (30). Therefore, our engineering efforts were focused on high-affinity engagement of anchor residues, specifically within the A helix.
VIS410 Design.
VIS410, targeting the trimeric interface, was engineered by iterations between in silico structure-based methods and experimentation. Starting with human antibody scaffolds (Table S1), in silico-predicted affinity-enhancing mutations were introduced either by site-directed mutagenesis or synthesis of the entire antibody construct. Seven rounds of iterations between in silico prediction and experimentation consisting of >500 constructs culminated in the optimized construct VIS410. In each case, the variable region of the heavy chain (VH) and variable region of the light chain (VL) were subcloned into pcDNA3.3-based vectors containing constant region of the heavy chain and constant region of the light chain, respectively. Recombinant expression of antibodies was carried out by transient transfection of 293F cells with heavy-chain and light-chain constructs.
VIS410 Direct Mechanism of Action.
Because VIS410 binds to a conformational epitope formed from the quaternary structure of influenza HA, the antibody was studied for its ability to inhibit HA-mediated cell–cell fusion. Viral HA, when exposed to low pH, undergoes a conformational change exposing its fusion peptide. When HA is expressed on the cell surface, the exposed fusion peptide is capable of inducing syncytia formation by mediating cell–cell membrane fusion of culture cells. VIS410 was tested for its ability to inhibit HA‐mediated cell fusion. Briefly, HA from H5N1 (A/Vietnam/1203/04) was expressed on the surface of HEK-293 cells by transient transfection, and the cells were induced by a transient 3-min pH change from pH 7.0 to pH 5.0 to allow HA to undergo a conformational change that would result in HA mediated cell–cell fusion. Antibody at varying dilutions was added to the culture wells either 1 h before or immediately after induction. After a 2- to 3-h recovery period, the cells were fixed and their nuclei were stained using HEMA3 (Fisher Healthcare). The degree of cell fusion was measured by the number of syncytia observed per field by microscopy (20× magnification). Pretreatment of HA-expressing cells with VIS410 inhibited syncytia formation in a dose-responsive manner (Fig. S2). Therefore, VIS410 most likely prevents the conformational change in HA required for viral membrane fusion with host cells.
Discussion
Given the public health and economic impacts of a regional epidemic or pandemic, significant effort has been focused on surveillance of emerging influenza strains and on vaccine development to counter emerging strains. In the context of controlling emerging influenza strains, such as H5N1 and H7N9, there are a number of challenges. First, as was documented in the case of the 2009 H1N1 pandemic, vaccine availability to the public will likely be delayed and limited. Furthermore, vaccination of immunologically naive individuals against a novel subtype likely requires a prime-boost regimen, further delaying onset of protective immunity. These shortcomings of vaccine strategies support that alternative, supplementary approaches should be considered as prophylactic and therapeutic measures.
Antibody administration to prevent viral infections is well established with licensed therapeutic antibodies for RSV, CMV, hepatitis B virus (HBV), varicella zoster virus, rabies, and vaccinia (15, 16). In the case of influenza, there is also clinical experience to support a potential therapeutic role for antibody. A meta-analysis of studies conducted during the 1918 pandemic using blood products strongly supports a benefit for treated patients (17). Overall, the six studies documented a 21% reduced risk of mortality in treatment groups that had received antiserum. Furthermore, a recent study evaluated the use of convalescent plasma in 93 patients with H1N1 2009 influenza in Hong Kong (18). In this prospective, multicenter, case-control study, mortality was significantly lower in the treatment groups that received convalescent plasma combined with standard of care compared with the controls who received only standard of care. These data support the potential for a mAb for use as treatment combined with oseltamivir or zanamivir for severe influenza A.
Given the diversity of the influenza virus, particularly in the HA gene, development of a neutralizing mAb that could provide a therapeutic adjunct for diverse strains that are both known and yet to emerge has been a daunting task. Recently, there has been a concerted effort to identify and characterize antibodies that bind to a number of influenza subtypes (so-called “broadly” neutralizing antibodies). Such antibodies have been identified through panning the B-cell repertoire of vaccinated or infected individuals (19, 20), and are infrequent, estimated to be ∼0.001–0.01% of the total antibody response (19). To supplement and expand antiinfective antibody discovery, recent developments in computational modeling and structural understanding of antibody–antigen engagement have enabled engineering human and humanized antibodies to increase the breadth of their binding, as well as increase their potency. Several recent examples have demonstrated the possibilities of this approach. In the case of anti-HIV antibodies that target the CD4-binding site, structure-based design of a single mutant, Gly54→Phe, resulted in a 10-fold enhancement in activity (21); additional changes resulted in decreasing possible pathways for resistance development (22, 23). Also, a recent study examined an antidengue antibody and engineered it to increase its affinity to serotype 4 resulting in increased potency to this serotype (24). Notably, this last study was completed in the absence of structural information and utilized an informatics-based approach, using existing information about antibody–antigen engagement. Taken together, these examples and others demonstrate the promise inherent in using engineering approaches to obtain optimized antibody candidates.
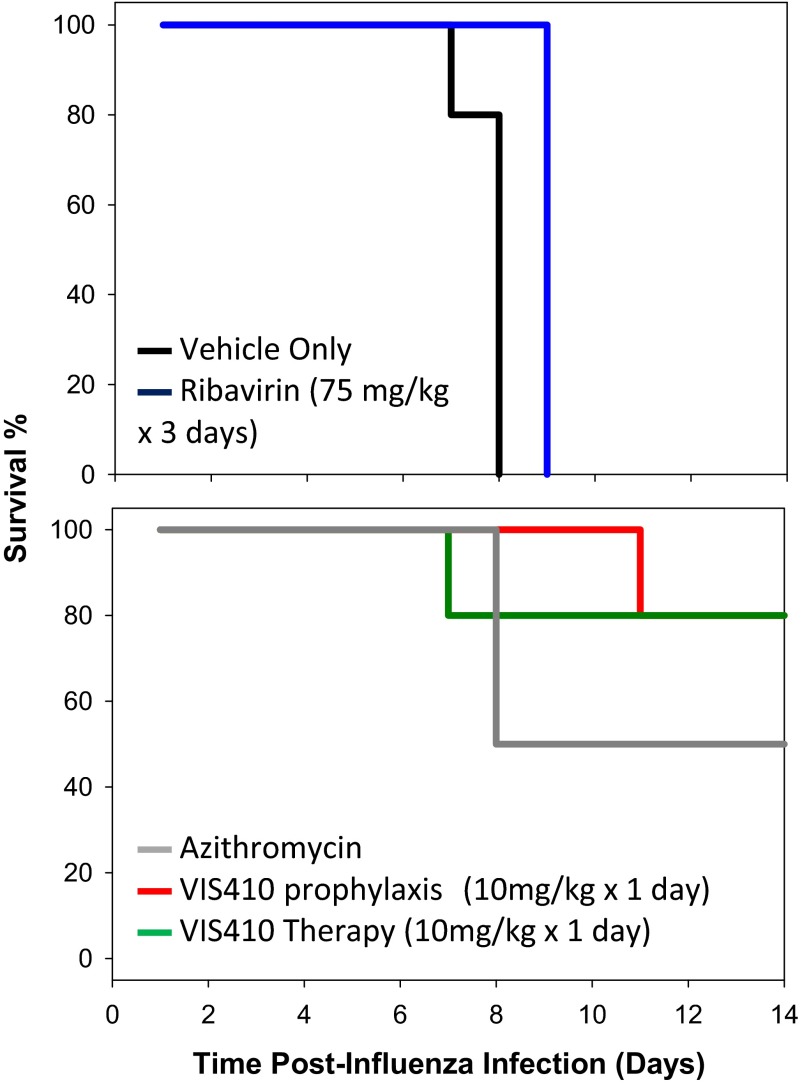
In the present study, we demonstrate that an engineered mAb, VIS410, can neutralize divergent group 1 and group 2 strains, including H1N1, H3N2, and H7N9. We show here that VIS410, alone or in combination with oseltamivir, can mitigate severe H7N9 infection. Finally, through its potent antiviral activity, VIS410 demonstrates the ability to protect against secondary bacterial infection in the mouse model, a common complication reported for influenza-infected patients, including those patients with H7N9 virus (1) (Fig. S7).
Fig. S7.
VIS410 inhibits bacterial coinfection after influenza insult. To study the effect of VIS410 on opportunistic bacterial infections in influenza-challenged animals (28), we used a mouse model to study viral-bacterial coinfection. Briefly, mice are infected with a sublethal dose of influenza virus [A/Puerto Rico/8/1934 (H1N1)], and 1 wk later, they are infected with a sublethal dose of Streptococcus pneumoniae. Together, the sequential infections are lethal and mice die within a few days of the bacterial challenge with a fulminant streptococcal lung infection. (Top) Administration of no therapeutic agent (black line) results in death of all animals at day 8. Administration of ribavirin (blue line), a commonly used antiinfluenza agent for in vitro experiments, was ineffective at rescuing mice from the bacterial challenge in the same model despite being dosed at 75 mg/kg daily for 3 d and reducing lung viral titer. (Bottom) Using this model, we determined that a single dose of our therapeutic antibody, VIS410, administered either prophylactically (red line) or as therapy 48 h postinfluenza virus challenge (green line) successfully rescues mice from subsequent bacterial infection. When delivered at the time of bacterial infection (+7 d), an antibacterial agent, such as azithromycin, reduces bacterial load and reduces death (gray line). Due to the fact that antibiotic is delayed until late in viral infection and to enable accurate comparison with other cohorts, the results presented for azithromycin treatment are the average of two separate experiments. Of note, through further in vitro assays, we have demonstrated that VIS410 does not have direct antibacterial activity. Thus, these data suggest that in a therapeutic setting, VIS410 can have not only an impact on viral-induced death, but also may potentially prevent complications from secondary opportunistic bacterial infections, such as infection from S. pneumonia.
Combination of different classes of molecules to control viral replication more effectively and reduce resistance emergence has proven to be a successful strategy for viral diseases, including HCV and HIV. In fact, specifically combining antibody with other antivirals has proved effective for preventing CMV and HBV infections. For influenza, it is clear that dual deployment of neuraminidase inhibitors is problematic from the standpoint of a shared mechanism of action, the potential for shared cross-resistance, and potential antagonism of antiviral activity (25). Furthermore, resistance development to oseltamivir and the adamantanes is widespread in H1N1 and has been already reported for H7N9, without having an impact on virus fitness (26). Therefore, development of a broadly neutralizing human mAb, such as VIS410, could provide an important addition to countermeasures for severe influenza, including H7N9, either alone or combined with other antiviral drugs.
Materials and Methods
Recombinant Expression of VIS410 Antibody.
Initial recombinant expression of antibody was carried out in HEK-293F FreeStyle suspension cells (Invitrogen) using recommended conditions. Cells were transfected with Poly-ethylene-imine Max (PEI-MAX; PolySciences) with equivalent amounts of heavy-chain– and light-chain–containing plasmids. Seven days posttransfection, cells were harvested by centrifugation and supernatants were sterile-filtered. Antibody was purified using Protein A resin (Pierce), buffer-exchanged with PBS, and concentrated as required. In lieu of transfection, later studies used a stable CHO cell line for the production of VIS410, and purification was performed as described above.
ELISA Binding Assays.
Recombinant HA (Protein Sciences Corporation) was coated on 96-well microtiter plates (Immuno Maxisorp; Nunc) and blocked with 5% (wt/vol) Blotto (Santa Cruz Biotechnology) in 1× PBS. Serially diluted antibody was added, and binding was detected using HRP-conjugated goat antihuman IgG (Fc-specific; Bethyl Laboratories), followed by development with 3,3′,5,5′-Tetramethylbenzidine solution (KPL). The absorbance at 450 nm was measured using a Spectramax plate reader (Molecular Devices), and data were analyzed with the Softmax software (Molecular Devices).
Pseudovirus Infection Assay.
Codon-optimized HA genes were synthesized (DNA2.0) and cloned into pLP/VSV-G (Invitrogen), replacing the VSV-G gene with the influenza HA gene. Pseudotyped lentivirus was generated by transient transfection of HEK-293T/17 cells with the pLP-HA and an HIV-1 backbone that contained a deletion to prevent envelope glycoprotein expression and an inserted luciferase gene (pNL Luc AM). Transfection medium consisted of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS. Twenty-four hours following transfection, the medium was replaced with DMEM/3% FBS containing 50 mU/mL neuraminidase purified from Vibrio cholerae (Sigma). Transfectants were incubated for an additional 48 h, and the medium was harvested, treated with 10 μg/mL TPCK-trypsin (Sigma) for 1 h at 37 °C, and frozen at −80 °C. A fixed volume of pseudovirus-containing supernatants that yielded ∼300,000 relative light units was incubated with varying concentrations of antibody for 1 h at 37 °C. Antibody/pseudovirus mixtures were applied to 2 × 104 HEK-293T/17 or MDCK cells in 96-well tissue culture plates and incubated for 72 h at 37 °C. One-Glo luciferase reagent (Promega) was added to each well and incubated for 4 min at room temperature. Light output was measured using a BioTek Synergy 2 luminometer.
Murine Lethal Challenge Model.
Studies with H3N2 (A/Victoria/3/75) and H7N9 (A/Anhui/1/2013) viruses were performed at the Institute of Antiviral Research at Utah State University. The animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of Utah State University in the American Association for the Accreditation of Laboratory Animal Care-accredited Laboratory Animal Research Center in accordance with the NIH Guide for the Care and Use of Laboratory Animals (27). Eight- to ten-week-old female DBA or BALB/c mice were obtained from Charles River Laboratories for the H7N9 (A/Anhui/1/2013) and H3N2 (A/Victoria/3/1975) experiments, respectively. Mice were maintained on Wayne Lab Blox and tap water ad libitum and quarantined for 24 h before use. Influenza A/Anhui/1/2013 (H7N9) virus was obtained from the CDC. A virus pool was prepared in MDCK cells and pretitrated in young adult DBA mice before its use. Mice were infected intranasally with concentrations of virus equal to one LD100 of A/Anhui/1/2013 or 2 LD50 of A/Victoria/3/1975. In all experiments, one group of 10 mice was administered VIS410 antibody i.p. at 2.5–50 mg/kg 24 h before virus exposure, 48 h after virus exposure, or both. An additional 10 mice received oral oseltamivir at 50 mg/kg twice daily for 8 d, beginning 24 h before virus exposure. Placebo groups receiving mock administration either i.p. or orally were also used. Mice were weighed daily up to day 13, or until death, beginning just before virus exposure. Adverse events for which observations were made included ruffling of fur, lethargy, paralysis, incontinence, repetitive circular motion, and aggression. Survival analyses were completed using the Kaplan–Meier graphing method, and pairwise comparisons of survivor curves (placebo vs. any treatment) were analyzed by the Mantel–Cox log rank test.
Sublethal Challenge Model.
Studies with H7N9 (A/Shanghai/2/2013) in the C57BL/6 mouse model were conducted at the biosafety level-3 facility at the Li Ka Shing Faculty of Medicine, the University of Hong Kong, under approved guidelines and ethics. Stock virus titer was determined to be 6 × 106 pfu/mL. Groups of 14 C57BL/6 mice obtained from the Laboratory Animal Unit at The University of Hong Kong were inoculated with 1,500 plaque-forming units (1.97 LD50) of A/Shanghai/2/2013 virus in 25 μL of PBS intranasally. Four different regimens were used starting 12 h before infection: (i) mock treatment using PBS via i.p. injection, (ii) a single dose of VIS410 at 50 mg/kg via i.p. injection, (iii) oseltamivir phosphate via oral gavage at 40 mg/kg twice daily for 7 d, or (iv) a single dose of VIS410 (50 mg/kg, i.p. injection) and oseltamivir phosphate via oral gavage at 40 mg/kg twice daily for 7 d. At day 5 postinoculation, six mice per group were killed and lung tissue was harvested to determine virus titers (n = 6 for each) and cytokine levels (n = 3 for each). For virus titration, mouse lungs were homogenized in 1 mL of PBS and titrated in MDCK cells to determine viral titers (log10 TCID50 per milliliter). For cytokine measurements, mouse lungs were homogenized in 1 mL of PBS and supernatant was clarified by centrifugation. Levels of cytokines were determined using FlowCytomix (eBioscience). Statistical comparisons of cytokine levels were made first using one-way ANOVA, followed by the Turkey posttest. Additional analysis using the nonparametric Kruskal–Wallis test was used to confirm this analysis.
Acknowledgments
We thank Ka-Tim Choy, Candy Chuk Kwan Ho, Ooiean Teng, and Sin Fun Sia at the University of Hong Kong for excellent technical assistance. We also thank Andrew Wollacott for his assistance with sequence and structural analysis and Hedy Adari and April Blodgett for their assistance with VIS410 characterization. This work was funded in part by an NIH Merit Award (R37 GM057073-13 to R.S.), and the National Research Foundation supported Interdisciplinary Research group in Infectious Diseases of SMART (Singapore MIT alliance for Research and Technology). In vitro and in vivo assessment of VIS410 was completed, in part, through the Preclinical Services for Researchers Program of the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (Contract HHSN272201000039I).
Footnotes
Conflict of interest statement: K.V., S.S., K.J.S., T.J.K., J.C.D., G.J.B., and Z.S. are employees of Visterra, Inc. and own equity. R.S. is a shareholder and member of the board of Visterra, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502374112/-/DCSupplemental.
References
- 1.Wang XF, et al. Clinical features of three avian influenza H7N9 virus-infected patients in Shanghai. Clin Respir J. 2013;8(4):410–416. doi: 10.1111/crj.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok CK, et al. Pathogenicity of the novel A/H7N9 influenza virus in mice. MBio. 2013;4(4):e00362–13. doi: 10.1128/mBio.00362-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao HN, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci USA. 2014;111(2):769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JI, et al. DBA/2 mouse as an animal model for anti-influenza drug efficacy evaluation. J Microbiol. 2013;51(6):866–871. doi: 10.1007/s12275-013-3428-7. [DOI] [PubMed] [Google Scholar]
- 6.Boon AC, et al. Host genetic variation affects resistance to infection with a highly pathogenic H5N1 influenza A virus in mice. J Virol. 2009;83(20):10417–10426. doi: 10.1128/JVI.00514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon AC, et al. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model. J Virol. 2010;84(15):7662–7667. doi: 10.1128/JVI.02444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pica N, et al. The DBA.2 mouse is susceptible to disease following infection with a broad, but limited, range of influenza A and B viruses. J Virol. 2011;85(23):12825–12829. doi: 10.1128/JVI.05930-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soundararajan V, et al. Networks link antigenic and receptor-binding sites of influenza hemagglutinin: Mechanistic insight into fitter strain propagation. Sci Rep. 2011;1:200. doi: 10.1038/srep00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messer WB, et al. Dengue virus envelope protein domain I/II hinge determines long-lived serotype-specific dengue immunity. Proc Natl Acad Sci USA. 2014;111(5):1939–1944. doi: 10.1073/pnas.1317350111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Brandenburg B, et al. Mechanisms of hemagglutinin targeted influenza virus neutralization. PLoS One. 2013;8(12):e80034. doi: 10.1371/journal.pone.0080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvier NM, Lowen AC. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses. 2010;2(8):1530–1563. doi: 10.3390/v20801530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolini R, Bernardini G, Molfetta R, Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26(2):113–120. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74(9):3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2(9):695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 16.ter Meulen J. Monoclonal antibodies for prophylaxis and therapy of infectious diseases. Expert Opin Emerg Drugs. 2007;12(4):525–540. doi: 10.1517/14728214.12.4.525. [DOI] [PubMed] [Google Scholar]
- 17.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 18.Hung IF, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashem AM, et al. Universal antibodies against the highly conserved influenza fusion peptide cross-neutralize several subtypes of influenza A virus. Biochem Biophys Res Commun. 2010;403(2):247–251. doi: 10.1016/j.bbrc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Ekiert DC, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333(6044):843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diskin R, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334(6060):1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diskin R, et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med. 2013;210(6):1235–1249. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi A, Tian J, Derdeyn CA, Korber B, Gnanakaran S. A mechanistic understanding of allosteric immune escape pathways in the HIV-1 envelope glycoprotein. PLOS Comput Biol. 2013;9(5):e1003046. doi: 10.1371/journal.pcbi.1003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tharakaraman K, et al. Redesign of a cross-reactive antibody to dengue virus with broad-spectrum activity and increased in vivo potency. Proc Natl Acad Sci USA. 2013;110(17):E1555–E1564. doi: 10.1073/pnas.1303645110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval X, et al. Bivir Study Group Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: A randomized placebo-controlled trial. PLoS Med. 2010;7(11):e1000362. doi: 10.1371/journal.pmed.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hai R, et al. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat Commun. 2013;4:2854. doi: 10.1038/ncomms3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Natl Inst Health; Bethesda: 1996. DHHS Publ. No. (NIH) 85-23. [Google Scholar]
- 28.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190(3):519–526. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- 29.Soundararajan V, et al. Networks link antigenic and receptor-binding sites of influenza hemagglutinin: Mechanistic insight into fitter strain propagation. Scientific Reports. 2011;1:200. doi: 10.1038/srep00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thyagarajan B, Bloom JD. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. eLife. 2014;3:e03300. doi: 10.7554/eLife.03300. [DOI] [PMC free article] [PubMed] [Google Scholar]