Significance
The importance of very early aquatic flowering plants is not well understood currently and is poorly documented. Here we present details of the morphology and reproductive biology of Montsechia, an extremely early fossil angiosperm that, because it is so ancient and is totally aquatic, raises questions centered on the very early evolutionary history of flowering plants. This paper challenges the paradigm of how we view the early evolution of basal angiosperms and particularly the role of aquatic habitats in the very early evolution and diversification of flowering plants.
Keywords: Montsechia, Ceratophyllum, Archaefructus, aquatic angiosperm, Lower Cretaceous
Abstract
The early diversification of angiosperms in diverse ecological niches is poorly understood. Some have proposed an origin in a darkened forest habitat and others an open aquatic or near aquatic habitat. The research presented here centers on Montsechia vidalii, first recovered from lithographic limestone deposits in the Pyrenees of Spain more than 100 y ago. This fossil material has been poorly understood and misinterpreted in the past. Now, based upon the study of more than 1,000 carefully prepared specimens, a detailed analysis of Montsechia is presented. The morphology and anatomy of the plant, including aspects of its reproduction, suggest that Montsechia is sister to Ceratophyllum (whenever cladistic analyses are made with or without a backbone). Montsechia was an aquatic angiosperm living and reproducing below the surface of the water, similar to Ceratophyllum. Montsechia is Barremian in age, raising questions about the very early divergence of the Ceratophyllum clade compared with its position as sister to eudicots in many cladistic analyses. Lower Cretaceous aquatic angiosperms, such as Archaefructus and Montsechia, open the possibility that aquatic plants were locally common at a very early stage of angiosperm evolution and that aquatic habitats may have played a major role in the diversification of some early angiosperm lineages.
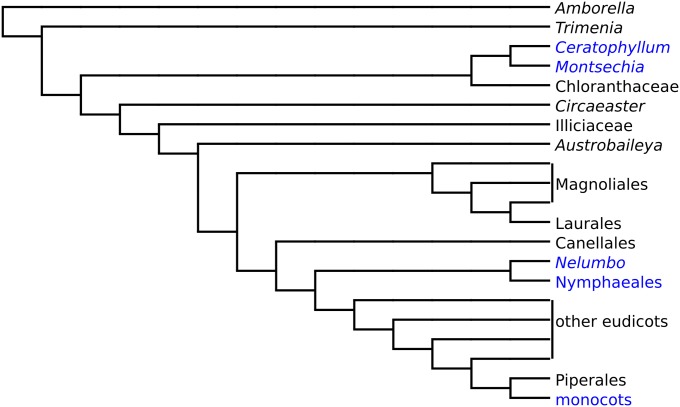
When did early angiosperms begin to diversify ecologically? This question is currently unanswered. Age estimates of the divergence of crown-group angiosperms using molecular clock data vary considerably, although it is in the range of (max. 210–) often accepted, 150–140 (min. 130) million years (1–7). Parsimony reconstruction of early angiosperm habit suggests that they may have been shrubs living in “damp, dark, and disturbed” habitats (8). In contrast, many living aquatic angiosperms are basal in angiosperm phylogenies [e.g., Nymphaeales in Amborella, Nymphaeales and Illiciales, Trimeniaceae-Austrobaileya (ANITA) or Ceratophyllales with the eudicots as commonly understood]. In the fossil record, we have found an aquatic angiosperm, Montsechia vidalii (Zeiller) Teixeira, which is an atypical plant fossil found in the Barremian (130–125 million years ago) freshwater limestone in the Pyrenees and Iberian Range in Spain. Montsechia (Fig. 1) lacks roots (no proximal or adventitious roots were found in more than 1,000 shoots examined) and shows flexible axes and two types of phyllotaxy and leaf morphology. The cuticle is very thin with rare stomata. The fruit is closed with a pore near the distal tip, indehiscent, and contains one unitegmic seed developed from an orthotropous and pendent ovule (Figs. 2 and 3). Cladistic analysis of these characters places Montsechia on the stem lineage basal to extant Ceratophyllum or a clade formed by Ceratophyllum and Chloranthaceae (Fig. 4) suggesting that mesangiosperms (non-ANITA angiosperms) existed 125 million years ago, as indicated by the tricolpate pollen record. Montsechia is well-adapted to a submerged aquatic habit. Montsechia is contemporaneous with another aquatic plant fossil, Archaefructus, indicating that some of the earliest angiosperms were fully aquatic very early in their ecological diversification.
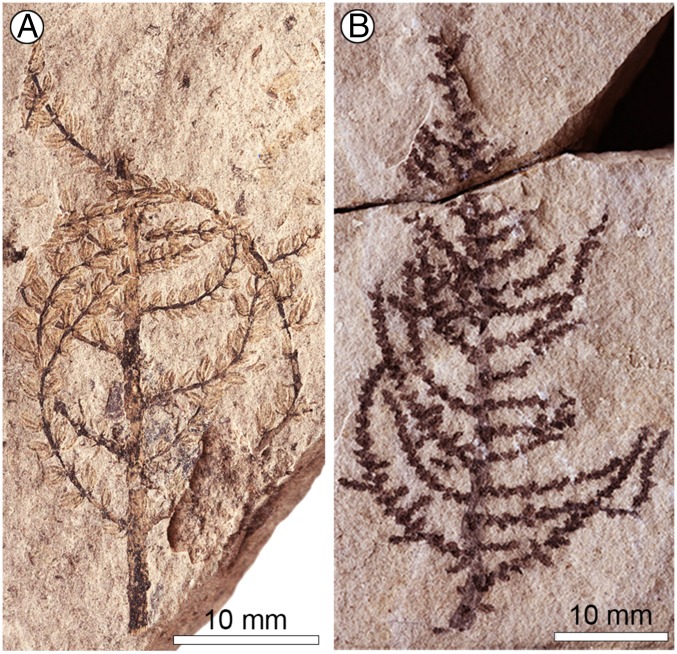
Fig. 1.
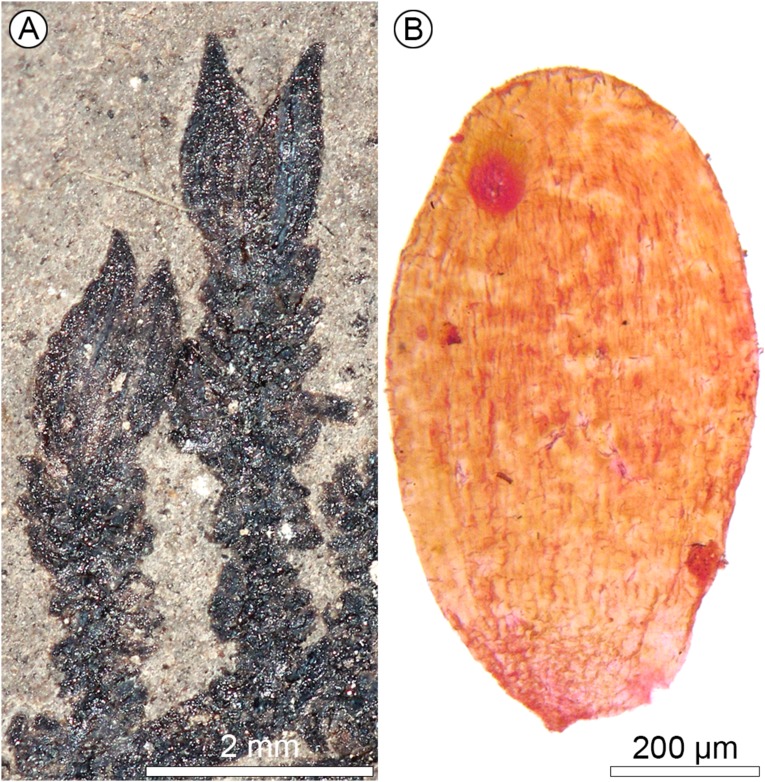
Long- and short-leaved forms of Montsechia vidalii. (A) The long-leaved specimen shows very flexuous branches and opposite, long leaves. LH02556. (Scale bar, 10 mm.) (B) The short-leaved specimen shows regularly developed lateral branches and tiny leaf rosettes. LH07198. (Scale bar, 10 mm.)
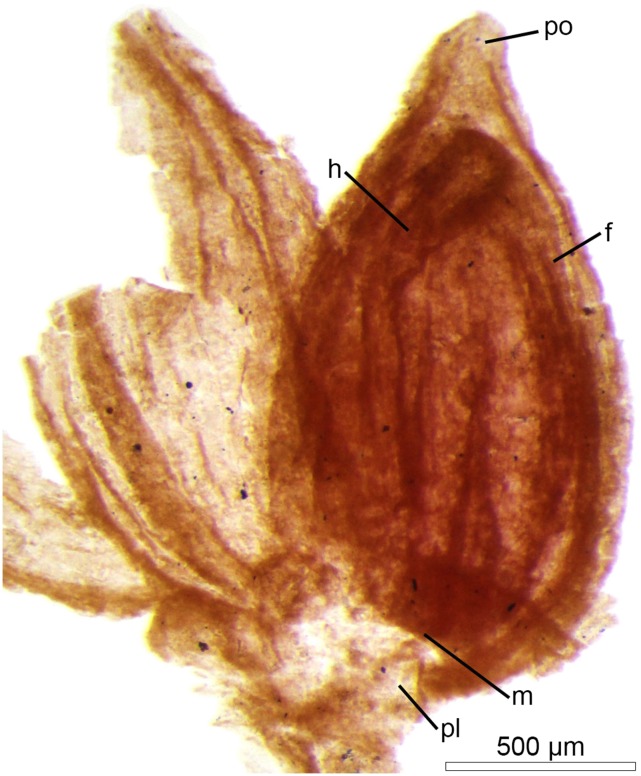
Fig. 2.
Fruit and seed of Montsechia vidalii. The fruit shows a small apical pore (po). The funicle (f) of the single, upside-down seed (orthotropous pendent) is attached from the hilum (h) to the placenta (pl). (Scale bar, 500 µm.)
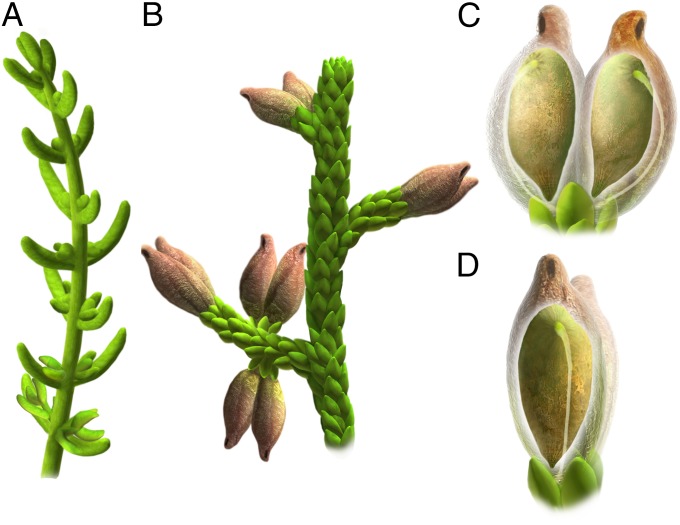
Fig. 3.
Reconstructions of Montsechia vidalii. (A) The long-leaved form shows the opposite leaves and branches. (B) The short-leaved form shows the alternate phyllotaxy of leaves and branches bearing pairs of ascidiate, nonornamented fruits. (C and D) The fruit shows a small apical pore and a single seed developed from an orthotropous pendent ovule. The funicle arises from the placenta (near the micropyle) to the hilum (near the pollination pore). (C) Lateral view. (D) Front view. Diagram by O. Sanisidro, B.G., and V.D.-G.
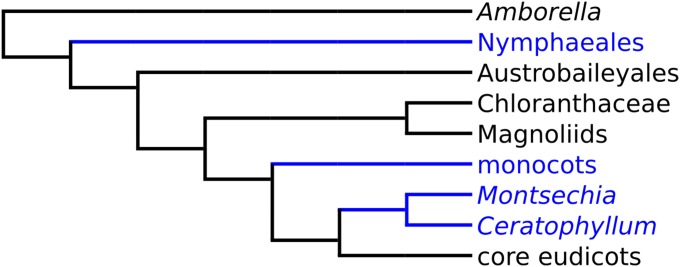
Fig. 4.
Most parsimonious position of Montsechia in a simplified tree derived from the matrix by Endress and Doyle (26) using the J & M backbone. Taxa in blue are considered ancestrally water-related (27). Diagram by C.C. and B.G.
Materials and Methods
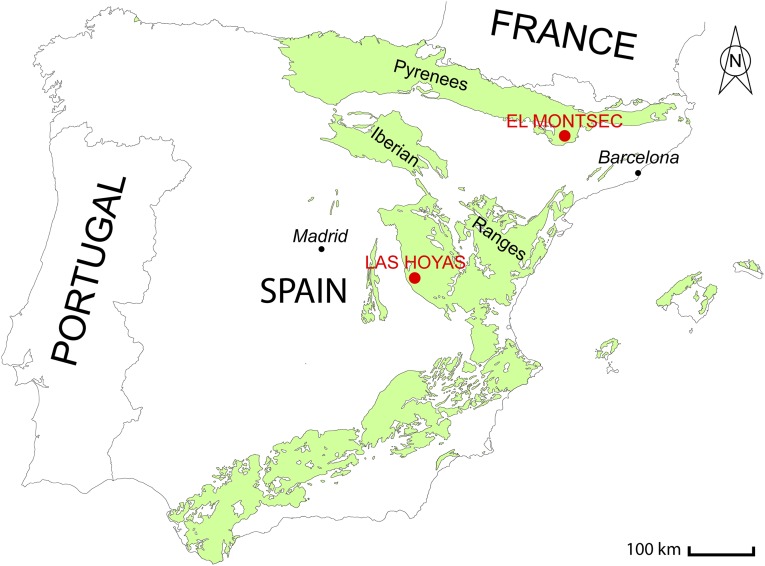
Two fossil areas yielding Montsechia are known (Supporting Information). From the late eighteenth century to the end of the 1990s, hundreds of specimens were collected from the lower Barremian limestones of the Pedrera quarry and the La Cabróa locality in the Montsec chain, western Spanish Pyrenees, Lleida Province, Spain. Another locality, Las Hoyas, was found in the early 1980s and is still excavated today in the Serranía de Cuenca, southwestern Iberian chain, Cuenca Province, Spain. Fossils are housed in various institutions in England, France, Germany, and Spain.
Some Montsechia shoots and fruits were removed from the rock by applying hydrochloric acid on a drop-by-drop basis. The cuticles were bleached using a mixture of nitric acid and potassium chlorate followed by a water bath with a few drops of ammonia (a two-step treatment commonly called “Schulze reagent”). Specimens were examined under a stereomicroscope, light microscope, and scanning electron microscope.
Cladistic analyses were performed with or without backbone using Mesquite. For more details see SI Materials and Methods.
SI Materials and Methods
Study Sites.
El Montsec.
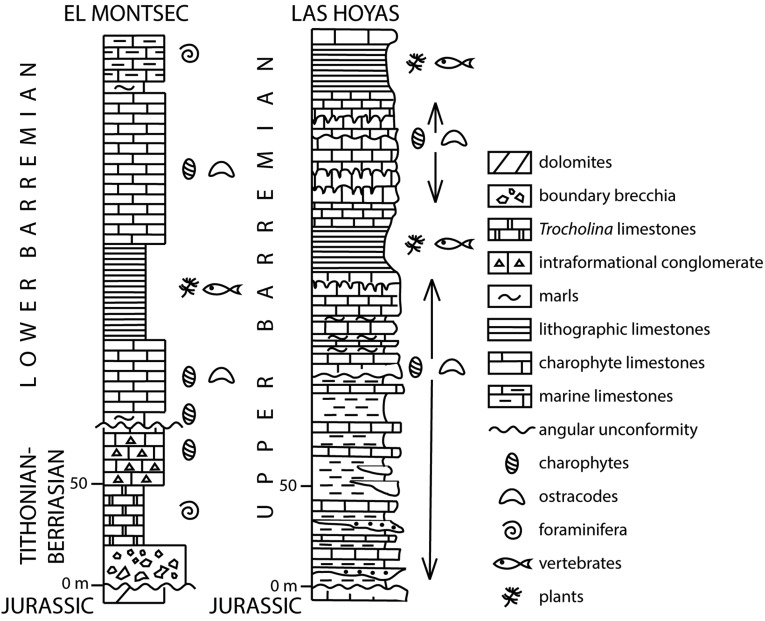
Montsechia vidalii was first described from the Pedrera quarry, Montsec chain, western Spanish Pyrenees, Lleida Province, Spain (Fig. S1) (10). This type locality had been reported a few years earlier (30), and for a few decades limestone slabs for lithography were extracted from the quarry. Later, beds containing animal and plant fossils also were found outcropping at other localities, especially at La Cabróa. These two main fossil localities were excavated by several international teams of scientists funded by western European institutions until the end of the 1990s. The fossils that were collected are housed in various institutions in France, Germany, Spain, and the United Kingdom and provided source material for extensive literature in all fields of paleontology [see the list of references given by Martínez-Delclòs (31, 32)]. Based on the local stratigraphy and fossil charophyte and ostracod assemblages, La Pedrera and La Cabróa animal and plant fossils have been attributed to the lower Barremian (Fig. S2) (33–35).
Fig. S1.
Fossil location. The map shows the El Montsec and Las Hoyas localities in the Pyrenees and Iberian Range where the fossil specimens of Montsechia vidalii were collected.
Fig. S2.
Fossil age. El Montsec plant and vertebrate beds are considered lower Barremian (130–127.2 million years ago). The La Huérguina Formation at the Las Hoyas locality is considered upper Barremian (127.2–25 million years ago). Diagram by C.M.-C.
Fig. S3.
Fruits and seeds of Montsechia vidalii. (A) The apices of short-leaved axes bear pairs of ascidiate fruits. LH 29265. (Scale bar, 2 mm.) (B) The seed is unitegmic and shows hilum and micropyle. (Scale bar, 200 µm.)
Fig. S4.
Most parsimonious position of Montsechia in a majority-rule tree derived from the matrix by Endress and Doyle (26) and rooted with Amborella. Taxa in blue are considered ancestrally water-related (27). Diagram by C.C. and B.G.
Las Hoyas.
The locality Las Hoyas was discovered in the early 1980s in the Serranía de Cuenca, southwestern Iberian chain, Cuenca Province, Spain (Fig. S1) (36). The animal and plant fossils of the La Huérguina Formation at Las Hoyas are contained in finely laminated lacustrine limestones deposited in an entirely freshwater wetland (37). Based on regional stratigraphy and charophyte and ostracod biostratigraphy, Las Hoyas animal and plant fossils are considered to be upper Barremian (Fig. S2) (38, 39).
Cladistic Analysis.
The data matrix by Endress and Doyle (40) was completed by adding the characters of Montsechia vidalii. We have coded 47 of 110 characteristics (43%) (see list below). The morphological dataset has been phylogenetically analyzed without backbone and using the two backbones, D & E and J & M, used by Endress and Doyle (40). These analyses were performed using Mesquite (41). The position of the fossil was investigated by linking the fossil to all possible branches of the backbone tree and recalculating the number of required parsimony changes (steps) in each alternative tree.
ED1 (DE 1). Habit: tree or shrub (0); rhizomatous, scandent, or acaulescent (1): Rhizomatous or scandent: 1
ED9 (DE 20 part). Phyllotaxis: alternate (spiral or distichous) (0); opposite or whorled (1): Opposite in the short-leafed form and alternate spiral in the long-leafed form: {01}
ED10 (DE 20 part). Distichous phyllotaxis: absent (0); present on some or all branches (1): Not distichous: 0
ED11 (DE 22 modified). First appendage(s) on vegetative branch: paired lateral prophylls (0); single distinct prophyll (adaxial, oblique, or lateral) (1): First pair of leaves in the short-leafed form and single leaf in the long-leafed form: {01}
ED12 (new). Leaf base: nonsheathing (0); sheathing (half or more of stem circumference) (1): Nonsheathing base: 0
ED13 (DE 23 modified). Stipules: absent (0); adaxial/axillary (1); interpetiolar (2); paired cap (3): Stipules absent: 0
ED14 (DE 24). Axillary squamules: absent (0); present (1): Nothing at the base: 0
ED15 (DE 25). Leaf blade: bifacial (0); unifacial (1): Leaf bifacial: 0
ED16 (DE 26). Leaf shape: obovate to elliptical to oblong (0); ovate (1); linear (2): Oblong in the short-leafed form and ovate in the long-leafed form: {01}
ED19 (DE 28 modified). Base of blade: not peltate (0); peltate in some or all leaves (1): Not peltate: 0
ED20 (DE 29 modified). Leaf dissection: simple (0); some or all leaves lobed or compound (1): Simple: 0
ED22 (DE 37 part). Inflorescence: solitary flower (or occasionally with one or two lateral flowers) (0); botryoid, panicle, or thyrsoid (monotelic) (1); raceme, spike, or thyrse (polytelic) (2): Unknown in the short-leafed form and solitary flowers in the long-leafed form: 0
ED23 (new). Inflorescence: partial units, single flowers (0); cymes (1): Unknown in the short-leafed form and single flowers in the long-leafed form: 0
ED24 (new). Pedicel: present in some or all flowers (0); absent or highly reduced (flower sessile or subsessile) (1): Unknown in the short-leafed form and absent or very reduced in the long-leafed form: 1
ED25 (new). Floral subtending bracts: present (0); present in female, absent in male flowers (1); absent in all flowers (2): Unknown in the short-leafed form and absent at least in female flowers in the long-leafed form: 2
ED26 (DE 38 modified). Sex of flowers: bisexual (0); unisexual (1): ? One specimen with female flowers and one unknown in the short-leafed form and female flowers in the long-leafed form: 1
ED27 (DE 39 modified). Floral base: hypanthium absent, superior ovary (0); hypanthium present, superior ovary (1); partially or completely inferior ovary (2): Unknown in the short-leafed form and hypanthium absent in the long-leafed form: 0
ED28 (new). Floral receptacle (female portion): short (0); elongate (1): Unknown in the short-leafed form and short floral receptacle in the long-leafed form: 0
ED30 (new). Floral apex: used up after production of carpels (0); protruding in mature flower (1); unicarpellate taxa scored as unknown: Unknown in the short-leafed form and no protruding floral apex in the long-leafed form: 0
ED31 (DE 41 part). Perianth: present (0); absent (1). See text for discussion: Unknown in the short-leafed form and no perianth in the long-leafed form: 1
ED36 (new). Petals: absent (0); present (1): Unknown in the short-leafed form and no petals in the long-leafed form: 0
ED37 (DE 45 modified). Nectaries on inner perianth parts: absent (0); present (1): Unknown in the short-leafed form and no nectaries in the long-leafed form: 0
ED39 (DE 44 part). Calyptra derived from the last one or two bracteate organs below the flower: absent (0); present (1): Unknown in the short-leafed form and no calyptra in the long-leafed form: 0
ED74 (DE 71). Carpel number: more than one (0); one (1): Unknown in the short-leafed form and two carpels in the long-leafed form: 0
ED75 (DE 72). Carpel form: ascidiate up to stigma (0); intermediate (both plicate and ascidiate zones present below the stigma) with ovule(s) on the ascidiate zone (1); completely plicate or intermediate with some or all ovule(s) on the plicate zone (2): Unknown in the short-leafed form and ascidiate morphology in the long-leafed form: 0
ED76 (DE 73 part). Postgenital sealing of carpel: none (0); partial (1); complete (2): Unknown in the short-leafed form and no postgenital sealing of carpels in the long-leafed form: 0
ED79 (DE 75). Style: absent (stigma sessile or capitate) (0); present (elongated apical portion of carpel distinctly constricted relative to the ovary) (1): Unknown in the short-leafed form and no style or style scar in the long-leafed form: 0
ED81 (DE 77 part). Multicellular stigmatic protuberances or undulations: (0) absent (0); present (1): Unknown in the short-leafed form and stigma undifferentiated in the long-leafed form: 0
ED82 (DE 77 part, modified). Stigma papillae (most elaborate type): absent (0); unicellular or with a single emergent cell and one or more small basal cells (1); uniseriate pluricellular with emergent portion consisting of two or more cells (2): Unknown in the short-leafed form and stigma undifferentiated in the long-leafed form: 0
ED84 (DE 79). Carpel fusion: apocarpous (including pseudo-syncarpous) (0); parasyncarpous (1); eusyncarpous (at least basally) (2): Unknown in the short-leafed form and two apocarpous carpels in the long-leafed form: 0
ED86 (new). Long unicellular hairs on and/or between carpels: absent (0); present (1): Unknown in the short-leafed form and no hair in the long-leafed form: 0
ED87 (new). Short, curved, appressed, unlignified hairs with up to two short basal cells and one long apical cell on carpels: absent (0); present (1) (42): Unknown in the short-leafed form and no hair carpels in the long-leafed form: 0
ED88 (new). Nectary on dorsal or lateral sides of carpel or pistillode: absent (0); present (1): Unknown in the short-leafed form and no nectaries in the long-leafed form: 0
ED89 (DE 81). Septal nectaries or potentially homologous basal intercapillary nectaries: absent (0); present (1): Unknown in the short-leafed form and no nectaries in the long-leafed form: 0
ED90 (DE 82 modified). Number of ovules per carpel: one (0); two or varying between one and two (1); more than two (2): Unknown in the short-leafed form and one ovule per carpel in the long-leafed form: 0
ED92 (DE 84). Ovule direction: pendent (0); horizontal (1); ascendent (3): Unknown in the short-leafed form and ovule pendent in the long-leafed form: 0
ED93 (DE 85). Ovule curvature: anatropous (or nearly so) (0); orthotropous (including hemitropous) (1): Unknown in the short-leafed form and orthotropous in the long-leafed form: 1
ED94 (DE 86). Integuments: two (0); one (1): Unknown in the short-leafed form and one tegument in the long-leafed form: 1
ED95 (DE 91). Chalaza: unextended (0); pachychalazal (1); perichalazal (2): Unknown in the short-leafed form and unextended chalaza in the long-leafed form: 0
ED97 (DE 93 part). Fruit wall: wholly or partly fleshy (0); dry (1): Unknown in the short-leafed form and fruit wall dry in the long-leafed form: 1
ED98 (DE 93 part). Lignified endocarp: absent (0); present (1): Unknown in the short-leafed form and no endocarp in the long-leafed form: 0
ED99 (DE 94 modified). Fruit dehiscence: indehiscent or dehiscing irregularly, dorsally only, or laterally (0); dehiscent ventrally or both ventrally and dorsally (1); horizontally dehiscent with vertical extensions (2): Unknown in the short-leafed form and dehiscing irregularly in the long-leafed form: 0
ED100 (DE 95). Testa: slightly or nonmultiplicative (0; multiplicative (1): Unknown in the short-leafed form and nonmultiplicative in the long-leafed form: 0
ED101 (DE 96). Exotesta: unspecialized (0); palisade or shorter sclerotic cells (1); tabular (2); longitudinally elongated, more-or-less lignified cells (3): Unknown in the short-leafed form and exotesta unspecialized in the long-leafed form: 0
ED102 (DE 100). Ruminations: absent (0); testal (1); tegminal and/or chalazal (3): Unknown in the short-leafed form and no ruminations in the long-leafed form: 0
ED103 (DE 101). Operculum: absent (0); present (1): Unknown in the short-leafed form and no operculum in the long-leafed form: 0
ED104 (DE 102). Aril: absent (0); present (1); Unknown in the short-leafed form and no aril in the long-leafed form: 0
Results
Montsechia vidalii (Zeiller) Teixeira is a locally abundant plant fossil found in the Barremian-age sediments of two well-known localities, El Montsec in the Pyrenees and Las Hoyas in the Iberian Range, Spain. Montsechia is an atypical aquatic fossil plant that was poorly understood systematically and morphologically. Its affinities have been suggested to be a liverwort (9), horsetail (10), conifer (11), Gnetales (12), and an angiosperm (13, 14). Basal angiosperms, as currently understood, are rooted in the terrestrial extant plant Amborella (refs. 15 and 16 and references therein). Extant Nymphaeales, containing mainly aquatic plants, often is considered a sister group. The aquatic nature of early angiosperms and how many times the angiosperms have moved into aquatic environments is unknown. However, the presence of the aquatic plant Archaefructus, contemporaneous with Montsechia, indicates that some early angiosperms were tied to aquatic environments and must be considered as significant elements with an important influence during early angiosperm evolution. Many of the characters of Montsechia are similar to, but are not exactly the same as, those found today in the extant aquatic, monotypic Ceratophyllum. It is generally accepted that aquatic angiosperms were derived from plants that previously lived in terrestrial environments (17). Only about 2% of angiosperms are aquatic today (18). The data presented here raise questions about the aquatic nature of some of the earliest angiosperms and their place in angiosperm history.
Montsechia shows no roots, consists of flexible axes, and has shoots of two types of phyllotaxy and leaf morphology (Figs. 1 and 3 A and B). Although there are thousands of Montsechia fragments in 10 beds, these two shoot types are never attached to each other in the field, but they are always collected together from the same bed and are about equally abundant in these beds. One type has opposite-decussate branches with awl-shaped linear leaves and is rarely fruit-bearing (Figs. 1A and 3A); the other has spiral branches and short scale-shaped leaves and is commonly fruit-bearing (Figs. 1B and 3B and Supporting Information). However, these two types of shoots probably belong to the same species because they have the same microstructure and female organs. The cuticle is thin with anomocytic stomata. Ascidiate, nonornamented fruits are borne in pairs on indeterminate inflorescences; each fruit has a pore near the distal tip (Figs. 2 and 3B and Supporting Information) and bears one unitegmic seed which is borne inverted with the micropyle proximal; a hilum is located ventrally with the funiculum running from the proximal placenta, at the base of the distal attachment, to the seed (Figs. 2 and 3 C and D). Thus the ovule is orthotropous and pendent. The fruits most often are borne in pairs, terminally on an axis.
No male reproductive organs or scars of such were found on any of the axes or dispersed with them.
Discussion
Montsechia and Ceratophyllum share many similarities, including hydrophily (water pollination), which is a rare event occurring in less than 5% of aquatic flowering plants (in nine families) (17, 19–21). Hydrophily is suggested because both genera have a pore in the fruit wall through which the pollen tube may enter, and other common features such as an orthotropous pendent ovule, a single-seeded fruit, and nonornamented unisexual flowers and lack roots. These characters when analyzed in a morphological cladistic dataset place the fossil on the stem group basal to extant Ceratophyllum or a clade formed by Ceratophyllum and Chloranthaceae (Fig. 4). Historically, based on molecular data, Ceratophyllum was placed basal to all angiosperms (22). Currently, based on molecular data, Amborella appears to be basal; the position of Ceratophyllum as basal to all eudicots is generally accepted (15, 23) but is not strongly supported (16). However, a few analyses (24, 25) propose that Ceratophyllum is basal to all angiosperms. In other analyses the Ceratophyllales are placed as sister to the eudicots (15, 16, 23) or as sister to Chloranthaceae in the analysis including Cretaceous fossils unrelated to Montsechia (26). This difference in placement depends on the backbone used for the analysis. All the analyses place Montsechia as sister to Ceratophyllum. The great age of Montsechia, and thus the great age of the Ceratophyllum clade, supports the molecular results placing Ceratophyllum in a rather basal position in angiosperm phylogeny.
The very ancient age of Montsechia and the close similarity of so many reproductive characters suggest that it is part of an early stem lineage of Ceratophyllales. Just as in extant Ceratophyllum, Montsechia has no roots and has a pore in the carpel wall that would allow pollen entry under water rather than a functional stigma typical of pollination in the majority of angiosperms. Aquatic plants often modify their vegetative form to accommodate an aquatic environment; however, the reproductive organs of aquatic plants are more conservative and often reflect remnants of the morphology of their terrestrial ancestry (17, 27). Many aquatic plants have emergent reproductive organs little modified from their terrestrial ancestors. Ceratophyllum is very different morphologically and also has a little understood long-branch molecular placement with extant angiosperm taxa. Therefore it has been difficult to place Ceratophyllum systematically. Even with the large dataset used by Ruhfel et al. (16), there is only 50–75% certainty in its relationships. The detailed analysis of Montsechia presented here demonstrates an extremely long and independent history for the stem lineage of Ceratophyllum. Montsechia, with characteristics typical of a plant living submerged in water, demonstrates that the ancestral stock of this clade lived and reproduced submerged in water more than 125 million years ago. The aquatic environments of the Lower Cretaceous probably were not very different from those in later ages, so there was little pressure for change over time. We find fossils even more similar to the extant Ceratophyllum in the late Albian (28). These fossils also support an ancient age for the Ceratophyllum lineage and suggest a more basal position for this lineage, as found in some recent phylogenetic analyses (24, 25).
Because of the differences between Montsechia and Ceratophyllum, we construct a new family, Montsechiaceae, for these fossils. Family Montsechiaceae Gomez, Daviero-Gomez, Coiffard, Martín-Closas et Dilcher fam. nov. Description: Herbaceous, aquatic/submerged plants with branching stems. Branches originate as axillary shoots terminating in reproductive organs. Stems are slender, flexible and dimorphic bearing simple, cylindrical leaves arranged in alternate or opposite phyllotaxy. Cuticle thin; stomata rare, anomocytic. Fruits paired on indeterminate inflorescences, ascidiate, closed except a pore near the distal tip. Seed unique, orthotropous and pendent, unitegmic. This new family should be included in the Ceratophyllales. This new family is extinct and basal in this order.
Montsechia, the fossil angiosperm presented here, raises questions centered on the very early evolutionary history of angiosperms. The importance of very early aquatic flowering plants, perhaps basal to all angiosperms, as previously proposed (29), merits serious consideration and reevaluation. Clearly, Montsechia was very well adapted to a submerged aquatic habit and lived during an early stage of angiosperm evolution. Now it is time for the fossil angiosperm families Montsechiaceae (30) and Archaefructaceae (29) to become a part of the phylogenies presented in our current angiosperm literature.
Supplementary Material
Acknowledgments
We thank Oscar Sanisidro for art reconstructions. Funding for this research was provided by Unité Mixte de Recherche 5276 of Centre National de la Recherche Scientifique (B.G. and V.D.-G.); Projects CGL2011-27869, CGL2012-35199, and CGL2013-42643-P of the Ministerio de Ciencia e Innovación of the Spanish government and Project 2014SGR-251 funded by the Catalan government (B.G., V.D.-G., and C.M.-C.); Grant CO 1060/3-1 from the German Funding Agency (to C.C.); European Community-funded Project SYNTHESYS Grants GB-TAF-1038, DE-TAF-1221, and ES-TAF-3066 (to B.G.); and the Indiana Geological Survey and the Department of Geological Sciences of Indiana University (D.L.D.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 10825.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509241112/-/DCSupplemental.
References
- 1.Sanderson MJ, Doyle JA. Sources of error and confidence intervals in estimating the age of angiosperms from rbcL and 18S rDNA data. Am J Bot. 2001;88(8):1499–1516. [PubMed] [Google Scholar]
- 2.Wikström N, Savolainen V, Chase MW. Evolution of the angiosperms: Calibrating the family tree. Proc Biol Sci. 2001;268(1482):2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies TJ, et al. Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc Natl Acad Sci USA. 2004;101(7):1904–1909. doi: 10.1073/pnas.0308127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell CD, Soltis DE, Soltis PS. The age of the angiosperms: A molecular timescale without a clock. Evolution. 2005;59(6):1245–1258. [PubMed] [Google Scholar]
- 5.Soltis DE, Bell CD, Kim S, Soltis PS. Origin and early evolution of angiosperms. Ann N Y Acad Sci. 2008;1133:3–25. doi: 10.1196/annals.1438.005. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc Natl Acad Sci USA. 2010;107(10):4623–4628. doi: 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol. 2015;207(2):437–453. doi: 10.1111/nph.13264. [DOI] [PubMed] [Google Scholar]
- 8.Feild TS, Chatelet DS, Brodribb TJ. Ancestral xerophobia: A hypothesis on the whole plant ecophysiology of early angiosperms. Geobiology. 2009;7(2):237–264. doi: 10.1111/j.1472-4669.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanc-Louvel C. Etude complémentaire de Montsechia vidali (Zeiller) Teixeira 1954: Nouvelle attribution systématique. Ann Paleontol. 1991;77(3):129–141. [Google Scholar]
- 10.Zeiller R. Sur quelques empreintes végétales du Kimméridgien de Santa Maria de Meya, province de Lérida en Catalogne (Espagne) Mem Real Acad Cienc Artes Barcelona. 1902;4(1902):345–356. [Google Scholar]
- 11.Lacasa-Ruiz A. 1975. Estudio del yacimiento portlandiense del Montsech de Rubiès. (Instituto de Estudios Ilerdenses, Lérida, Catalonia, Spain), 4 pp.
- 12.Krassilov VA. On Montsechia, an angiospermoid plant from the Lower Cretaceous of Las Hoyas, Spain: New data and interpretations. Acta Palaeobot. 2011;51(2):181–205. [Google Scholar]
- 13.Teixeira C. La flore fossile des calcaires lithographiques de Santa María de Meyá (Lérida, Espagne) Bol Soc Geol Portugal. 1954;11(2-3):139–152. [Google Scholar]
- 14.Blanc-Louvel C, Barale G. Montsechia vidali (Zeiller) Teixeira 1954. Nouvelles observations et réflexions sur son attribution systématique. Ann Paleontol. 1983;69(3):151–174. [Google Scholar]
- 15.Moore MJ, Bell CD, Soltis PS, Soltis DE. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proc Natl Acad Sci USA. 2007;104(49):19363–19368. doi: 10.1073/pnas.0708072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhfel BR, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG. From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol Biol. 2014;14:23. doi: 10.1186/1471-2148-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philbrick CT, Les DH. Evolution of Aquatic Angiosperm Reproductive Systems What is the balance between sexual and asexual reproduction in aquatic angiosperms? Bioscience. 1996;46(11):813–826. [Google Scholar]
- 18.Cook CDK. The number and dinds of embryo-bearing plants wish have become aquatic: A survey. Perspect Plant Ecol Evol Syst. 1999;2:79–102. [Google Scholar]
- 19.Cox PA. Water-pollinated plants. Sci Am. 1993;269(10):68–74. [Google Scholar]
- 20.Les DH. The origin and affinities of the Ceratophyllaceae. Taxon. 1988;37(2):326–345. [Google Scholar]
- 21.Philbrick CT. The evolution of hydrophily: Ecological and phylogenetic considerations. Rhodora. 1991;93(873):36–50. [Google Scholar]
- 22.Chase MW, et al. Phylogenetics of seed plants: An analysis of nucleotide sequences from the plastid gene rbcL. Ann Mo Bot Gard. 1993;80(3):528–580. [Google Scholar]
- 23.Jansen RK, et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc Natl Acad Sci USA. 2007;104(49):19369–19374. doi: 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morton CM. Newly sequenced nuclear gene (Xdh) for inferring angiosperm phylogeny. Ann Mo Bot Gard. 2011;98(1):63–89. [Google Scholar]
- 25.Goloboff PA, et al. Phylogenetic analysis of 73 060 taxa corroborates major eukaryotic groups. Cladistics. 2009;25(2009):211–230. doi: 10.1111/j.1096-0031.2009.00255.x. [DOI] [PubMed] [Google Scholar]
- 26.Doyle JA, Endress PK. Integrating Early Cretaceous Fossils into the phylogeny of living angiosperms: ANITA lines and relatives of Chloranthaceae. Int J Plant Sci. 2014;175(5):555–600. [Google Scholar]
- 27.Sculthorpe CD. The Biology of Aquatic Vascular Plants. St. Martin’s; New York: 1967. [Google Scholar]
- 28.Dilcher DL, Wang H. An Early Cretaceous fruit with affinities to Ceratophyllaceae. American Journal of Botany. 2009;96(12):2256–2269. doi: 10.3732/ajb.0900049. [DOI] [PubMed] [Google Scholar]
- 29.Sun G, Dilcher DL, Zheng S, Zhou Z. In search of the first flower: A Jurassic angiosperm, Archaefructus, from northeast China. Science. 1998;282(5394):1692–1695. doi: 10.1126/science.282.5394.1692. [DOI] [PubMed] [Google Scholar]
- 30.Vidal LM. Compte-rendu des excursions dans la province de Lérida du 11 au 15 octobre. Bull Soc Geol Fr. 1899;3(26):884–899. [Google Scholar]
- 31.Martínez Delclòs X. The Lower Cretaceous Lithographic Limestones of Montsec. Ten Years of Paleontological Expeditions. Institut d'Estudis Ilerdencs; Lleida, Spain: 1991. [Google Scholar]
- 32.Martínez-Delclòs X. Montsec and Montral-Alcover, Two Konservat-Lagerstätten, Catalonia, Spain. Institut d’Estudis Ilerdencs; Lleida: 1995. [Google Scholar]
- 33.Martín-Closas C. 1989. Els caròfits del Cretaci inferior de les conques perifèriques del Bloc de l’Ebre. PhD thesis (Universitat de Barcelona, Barcelona)
- 34.Martín-Closas C, López-Morón N. 1996. The Lower Cretaceous Charophyte Flora from El Montsec (Catalonia, Spain). El Patrimoni Natural del Montsec, 9–19, ed Fanló E (Institut d’Estudis Ilerdencs, Lleida, Spain)
- 35.Martín-Closas C, Clavel B, Charollais J, Conrad M-A. Charophytes from the Barremian-lower Aptian of the Northern Subalpine Chains and Jura Mountains, France: Correlation with associated marine assemblages. Cretac Res. 2009;30(1):49–62. [Google Scholar]
- 36.Sanz JL, et al. An early Cretaceous faunal and floral continental assemblage: Las Hoyas fossil site (Cuenca, Spain) Geobios. 1988;21(5):611–635. [Google Scholar]
- 37.Buscalioni AD, Fregenal-Martínez M. A holistic approach to the palaeoecology of Las Hoyas Konservat-Lagerstätte (La Huérguina Formation, Lower Cretaceous, Iberian Ranges, Spain) J Iber Geol. 2010;36(2):297–326. [Google Scholar]
- 38.Diéguez C, Trinção P, Martín-Closas C, López-Morón N. 1995. Paleobotany. Las Hoyas: A lacustrine Konservat-Lagerstätte, Cuenca, Spain, ed Meléndez N (Universidad Complutense de Madrid, 29–32, Madrid)
- 39.Vicente A, Martín-Closas C. Lower Cretaceous charophytes from the Serranía de Cuenca, Iberian chain: Taxonomy, biostratigraphy and palaeoecology. Cretac Res. 2013;40:227–242. [Google Scholar]
- 40.Endress PK, Doyle JA. Reconstructing the ancestral angiosperm flower and its initial specializations. Am J Bot. 2009;96(1):22–66. doi: 10.3732/ajb.0800047. [DOI] [PubMed] [Google Scholar]
- 41.Maddison WP, Maddison DR. 2011 Mesquite: A modular system for evolutionary analysis, version 2.75. Available at mesquiteproject.org. Accessed July 19, 2014.
- 42.Endress PK.2001The flowers in extant angiosperms and inferences on ancestral flowers International Journal of Plant Science16251111–1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.