Significance
Ectopic clusters of immune cells that mimic the structure and function of secondary lymphoid organs are defined as tertiary lymphoid organs (TLOs). They have been observed at sites of chronic inflammation for decades, but their formation and function have remained enigmatic. TLOs are thought to contribute to disease pathogenesis by promoting autoreactive lymphocyte survival and autoantibody production. In this study we identify a novel role for the cytokine IL-22 in TLO development and biology. We provide evidence that IL-22 expression within TLOs is instrumental for the production of the lymphoid chemokines, chemokine (C-X-C motif) ligand 13 and chemokine (C-X-C motif) ligand 12, which in turn orchestrate B-cell clustering, lymphoid aggregation, and autoantibody production. Our data provide a strong rationale for targeting IL-22 in TLO-associated autoimmune diseases.
Keywords: IL-22, tertiary lymphoid organs, chemokines, Sjogren's syndrome, autoimmunity
Abstract
The series of events leading to tertiary lymphoid organ (TLO) formation in mucosal organs following tissue damage remain unclear. Using a virus-induced model of autoantibody formation in the salivary glands of adult mice, we demonstrate that IL-22 provides a mechanistic link between mucosal infection, B-cell recruitment, and humoral autoimmunity. IL-22 receptor engagement is necessary and sufficient to promote differential expression of chemokine (C-X-C motif) ligand 12 and chemokine (C-X-C motif) ligand 13 in epithelial and fibroblastic stromal cells that, in turn, is pivotal for B-cell recruitment and organization of the TLOs. Accordingly, genetic and therapeutic blockade of IL-22 impairs and reverses TLO formation and autoantibody production. Our work highlights a critical role for IL-22 in TLO-induced pathology and provides a rationale for the use of IL-22–blocking agents in B-cell–mediated autoimmune conditions.
Tertiary lymphoid organs (TLOs) are organized clusters of immune cells that preferentially form in autoimmune diseases such as Sjogren’s syndrome and Hashimoto thyroiditis (1). TLOs’ cellular compartments, spatial organization, vasculature, and function are similar to those of secondary lymphoid organs (SLOs), providing a local hub for autoreactive B-cell proliferation and affinity maturation. TLOs appear to contribute to disease progression and to the emergence of malignant B clones responsible for lymphoma development (2). Within TLOs, the ectopic expression of lymphoid chemokines has been shown to correlate with the size or degree of organization of lymphoid aggregates and with the production of autoantibodies (3, 4). In the case of malignant transformation, lymphoid chemokine expression increases during disease progression (5). The pathways regulating chemokine expression in SLOs during embryonic life have been largely described and mainly involve the engagement of lymphotoxin β receptor on a family of gp38+ lymphoid tissue-organizer stromal cells (6). At sites of TLO formation, lymphotoxin and lymphotoxin-producing cells appear to be dispensable for the early production of lymphoid chemokines required during SLO formation, raising the possibility that signals other than lymphotoxin can regulate the formation of TLOs, stimulating the production of lymphoid chemokines during inflammation (7, 8). Some of these signals have been identified in the family of the IL-23/IL-17 cytokines (9), but other cytokines have been advocated in TLO formation; thus different molecules may play differential roles depending on site and nature of the etiological agent (10).
IL-22, a member of the IL-10 cytokine superfamily, regulates mucosal responses to danger and wound healing (11). IL-22 promotes tissue repair, inducing epithelial cell proliferation and survival, in both physiological and pathological conditions (12–15). Recently, an association between IL-22 expression and autoimmune B-cell activation has been proposed (16). In Sjogren’s syndrome, serum levels of IL-22 correlate with clinical manifestations including autoantibody production (17). Furthermore, B-cell–depleting treatment modulates salivary gland expression of IL-22, thus suggesting a potential functional relationship between IL-22 expression, B-cell infiltration, and local pathology (18). Previously we have demonstrated that direct cannulation of murine salivary glands with a replication-deficient adenovirus induces the formation of organized T-cell and B-cell aggregates, local expression of lymphoid chemokines, and the enzyme aicda [activation-induced cytidine deaminase (AID)] required for affinity maturation and isotype switching in B cells (19). Exploiting this model, we tested the hypothesis that IL-22 is involved in the generation of a humoral response within the TLOs (19). Here we demonstrate that early expression of IL-22 is instrumental for lymphoid chemokine production by stromal cells that, in turn, regulates B-cell aggregation. This work highlights a novel role for IL-22 in lymphoid chemokine expression and provides a mechanistic link between deranged mucosal responses and autoantibody production.
Results
Induction of IL-22–Expressing Cells in Murine Salivary Gland TLOs.
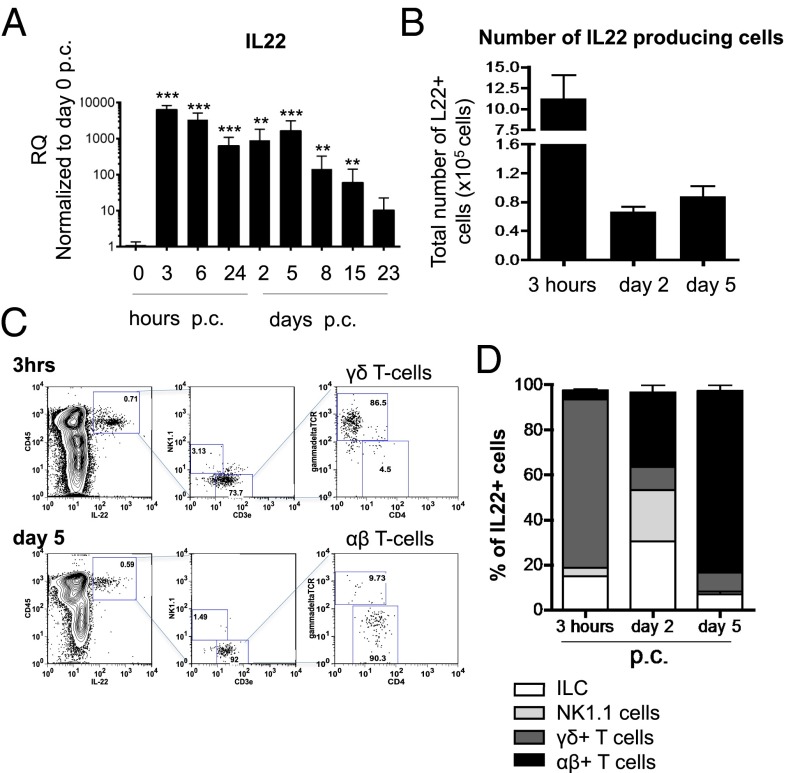
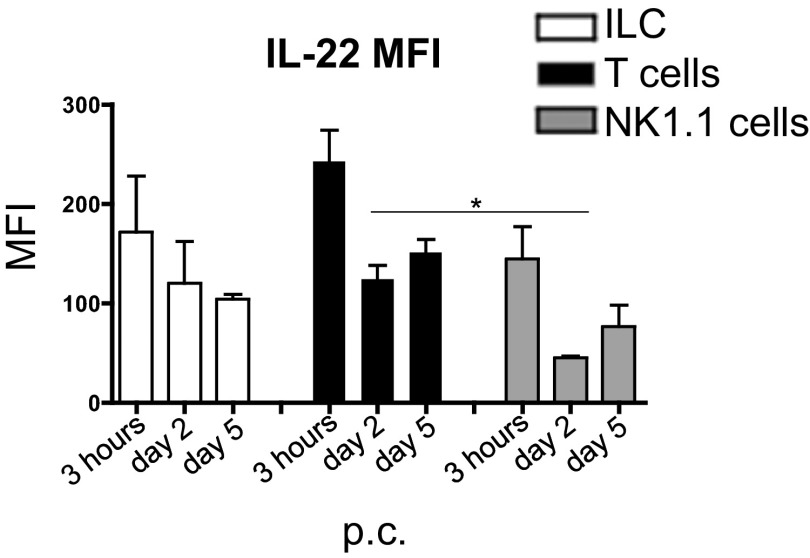
To determine whether IL-22 is produced in cannulated salivary glands, we analyzed mice early post viral cannulation (p.c.). In WT mice we detected early up-regulation of IL-22 mRNA transcript at 3 h p.c., with a second peak of expression at day 5 p.c. (Fig. 1A). These data were confirmed at the protein level by flow cytometry analysis of hematopoietic cells isolated from cannulated salivary glands. IL-22–producing cells were identified within the CD45+ cell population by 3 h p.c., with the largest source of IL-22 [both in terms of percentage of positive cells and mean fluorescence index (MFI)] deriving from T cells at all time points examined (Fig. 1 B–D and Fig. S1). At 3 h p.c. IL-22 production derived largely from γδ T cells, but by day 5 p.c. αβ T lymphocytes were the main producers of this cytokine (Fig. 1C). Other cell populations responsible for early IL-22 production were innate lymphoid cells (ILCs) and natural killer (NK) cells (Fig. 1D).
Fig. 1.
Source of IL-22 in TLO formation. (A) Quantitative RT-PCR analysis of mRNA transcript for Il22 in WT mice on day 0, at 3, 6, and 24 h p.c., and on days 2, 5, 8, 15, and 23 p.c., normalized to β-actin. Relative expression (RQ) was calibrated to 0 p.c. salivary glands. **P < 0.01; ***P < 0.001 versus day 0. Results represent two or three experiments with four glands analyzed per group. (B) Absolute number of IL-22+ cells in the CD45+ population from salivary glands at 3 h p.c. and on days 2 and 5 p.c. (C) Representative dot plots identifying IL-22–producing cells in γδ+ T cells (CD3e+gdTCR+NK1.1−CD4−) at 3 h p.c. and in αβ+ T cells (CD3e+gdTCR−NK1.1−CD4+) on day 5 p.c. Data present the mean ± SD of two different experiments with three salivary glands per experiment. (D) Distribution of IL-22+ cells in the CD45+ population at 3 h p.c. and on days 2 and 5 p.c. Data present the mean ± SD from two different experiments with three salivary glands per experiment.
Fig. S1.
Graph summarizing the MFI of IL-22 in the CD45+ leukocyte population at 3 h p.c. and days 2 and 5 p.c. Data present the mean ± SD of two different experiments with three salivary glands per experiment. *P < 0.05; GEE analysis followed by Sidak’s significance test.
TLO Formation Is Impaired in the Absence of IL-22.
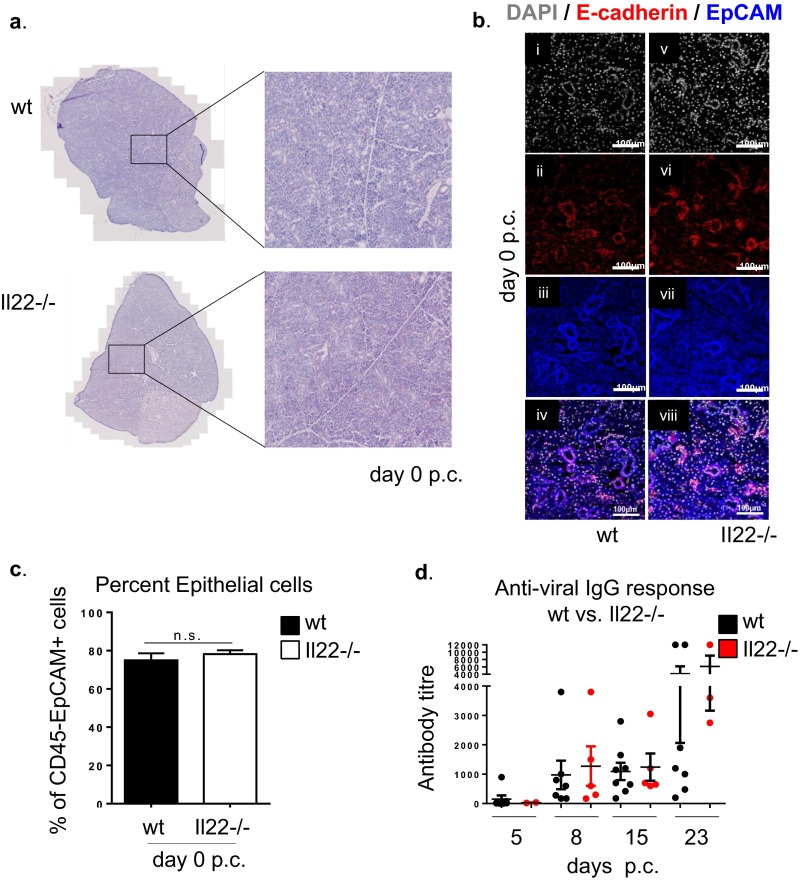
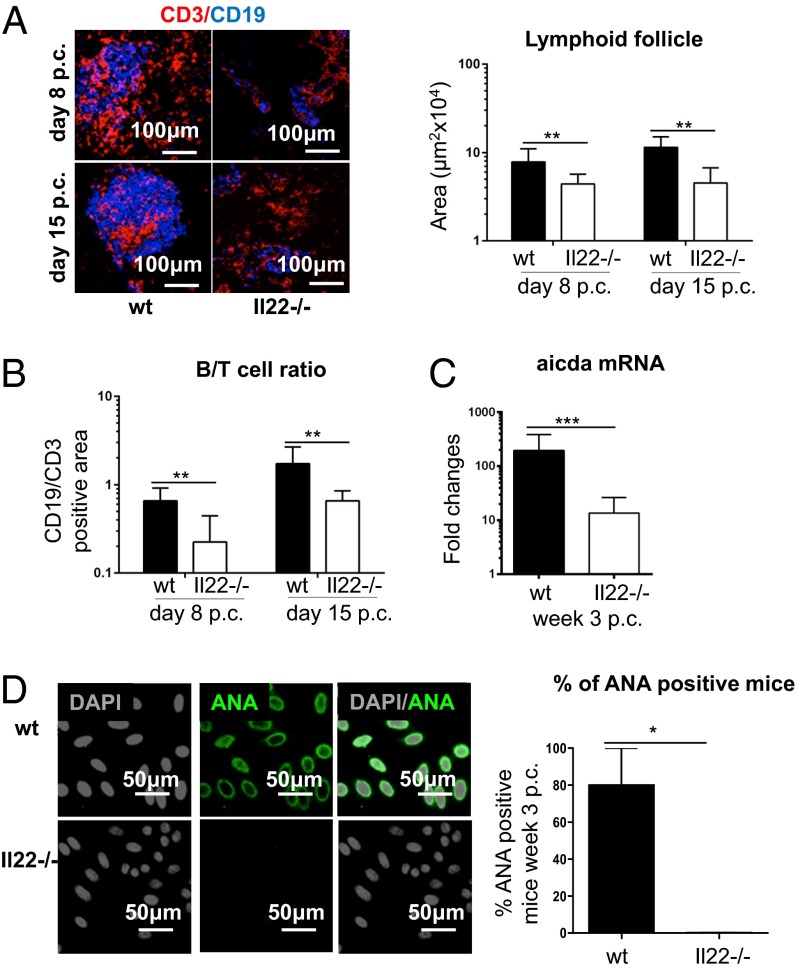
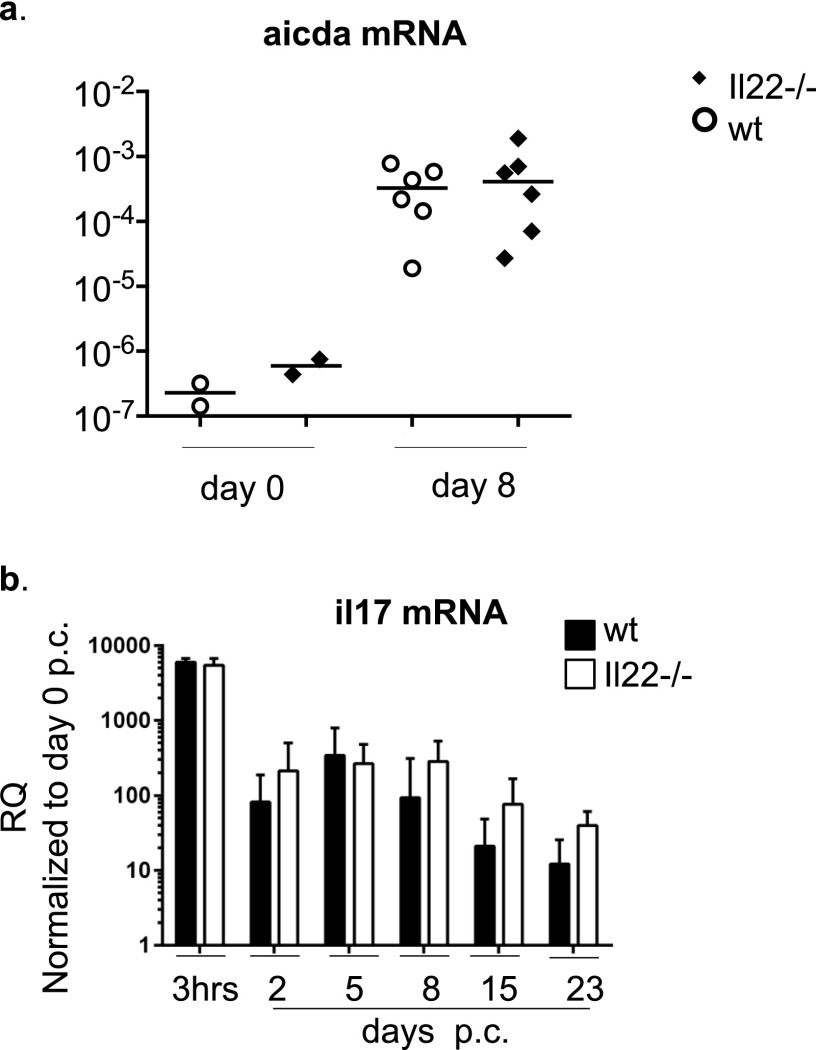
To evaluate the consequence of IL-22 deficiency in TLO formation and autoantibody production, we moved our analysis to Il22−/− mice. First, resting salivary glands from Il22−/− and WT mice were evaluated for the presence of potential anatomical or structural differences that could interfere with virus infectivity. No differences were found between WT and Il22−/− mice in resting condition by histological examination and flow cytometry analysis of the salivary gland epithelial component (Fig. S2 A–C). Moreover, antiviral responses measured by ELISA showed no significant differences that could interfere with the establishment of the model of TLO formation in Il22−/− mice (Fig. S2D). Histological examination revealed that Il22−/− mice develop significantly smaller salivary gland lymphocytic aggregates than WT mice (Fig. 2A). Aggregates in the Il22−/− mice were characterized by a defect in B-cell accumulation (shown as a decreased B-cell/T-cell ratio) and follicular organization (Fig. 2 A and B) as well as a strikingly lower expression of aicda transcripts (Fig. 2C). This defect was functionally associated with suppression in autoantibody production (Fig. 2D), suggesting that the lack of IL-22 impairs B-cell accumulation, organization, and maturation within TLOs. Interestingly, this defect was not observed in SLOs, where aicda expression was maintained post immunization (Fig. S3A). To evaluate the potential contribution of IL-17 expression to the overall effect observed in the Il22−/− mice, quantitative PCR was performed on cannulated WT and Il22−/− mice. Preserved IL-17 up-regulation was observed in Il22−/− mice p.c., suggesting that the effect on TLO formation in the Il-22−/− mice is not dependent on IL-17 (Fig. S3B).
Fig. S2.
(A) Representative microphotographs of H&E staining of salivary gland sections from WT and Il22−/− mice at day 0 p.c. (B) Salivary gland sections of WT (i–iv) and Il22−/− (v–viii) mice at day 0 p.c. were examined by immunofluorescence for E-cadherin (red), EpCAM (blue), and DAPI (gray). (Original magnification: 25×). (C) Graphs showing the percentage of CD45−EpCAM+ epithelial cells contained within the enzyme-digested salivary glands from WT (black bars) and Il22−/− (white bars) mice at day 0 p.c. (D) Expression of antiviral response quantified by ELISA on sera from WT (black circles) and Il22−/− (red circles) mice calculated at days 5, 8, 15, and 23 p.c.
Fig. 2.
Lack of IL-22 results in defective TLO formation and decreased autoantibody production. (A, Left) Salivary gland staining for CD3 (red) and CD19 (blue) in WT and Il22−/− mice at days 8 and 15 p.c. (Right) Lymphoid aggregate area calculated at days 8 and 15 p.c. for WT (black bars) and Il22−/− (white bars) mice. Data present the mean ± SD from two different experiments with two or three mice (four or six salivary glands) per group; **P < 0.01. (B) Ratio between the CD19+ and CD3+ area at days 8 and 15 p.c. in WT (black bars) and Il22−/− (white bars) mice. Data present the mean ± SD from two different experiments (four or six salivary glands); **P < 0.01. (C) mRNA transcripts of the aicda gene (normalized to β-actin), in week 3 p.c. salivary glands from WT and Il22−/− mice. RQ values are relative to day 0 p.c. aicda mRNA; data represent the mean ± SD of three independent experiments with four to six salivary glands analyzed in each experiment. ***P < 0.001. (D, Left) Autoantibody detection on Hep2 cells from WT and Il22−/− mice at week 3 p.c. (nuclear staining in gray, autoantibody reactivity in green) (serum dilution 1:80). ANA, antinuclear antibody. (Right) Detection of autoantibody reactivity (percentage of mice positive for autoantibody at 1:80 dilution or more) in WT and Il22−/− mice at day 23 p.c. Data present the mean ± SD of two experiments with five cannulated mice; *P < 0.05; unpaired t test.
Fig. S3.
(A) Quantitative PCR analysis for aicda mRNA obtained from the spleens of nonimmunized (day 0) and immunized (day 8) Il22−/− (black symbols) and WT (white symbols) mice. Results are presented as ΔΔCT value. (B) Quantitative RT-PCR analysis of mRNA transcript of Il-17α in salivary glands of WT (black bars) and Il22−/− (white bars) mice at 3 h p.c. and days 2, 5, 8, 15, and 23 p.c. Transcripts were normalized to β-actin. The relative expression values were calibrated to salivary gland values on day 0 p.c. Data present the mean and ± SD of two experiments with four or six mice analyzed per group. GEE analysis was followed by Sidak’s significance test.
IL22−/− Mice Fail to Induce Chemokine (C-X-C Motif) Ligand 13 and Chemokine (C-X-C Motif) Ligand 12 Expression Within TLOs.
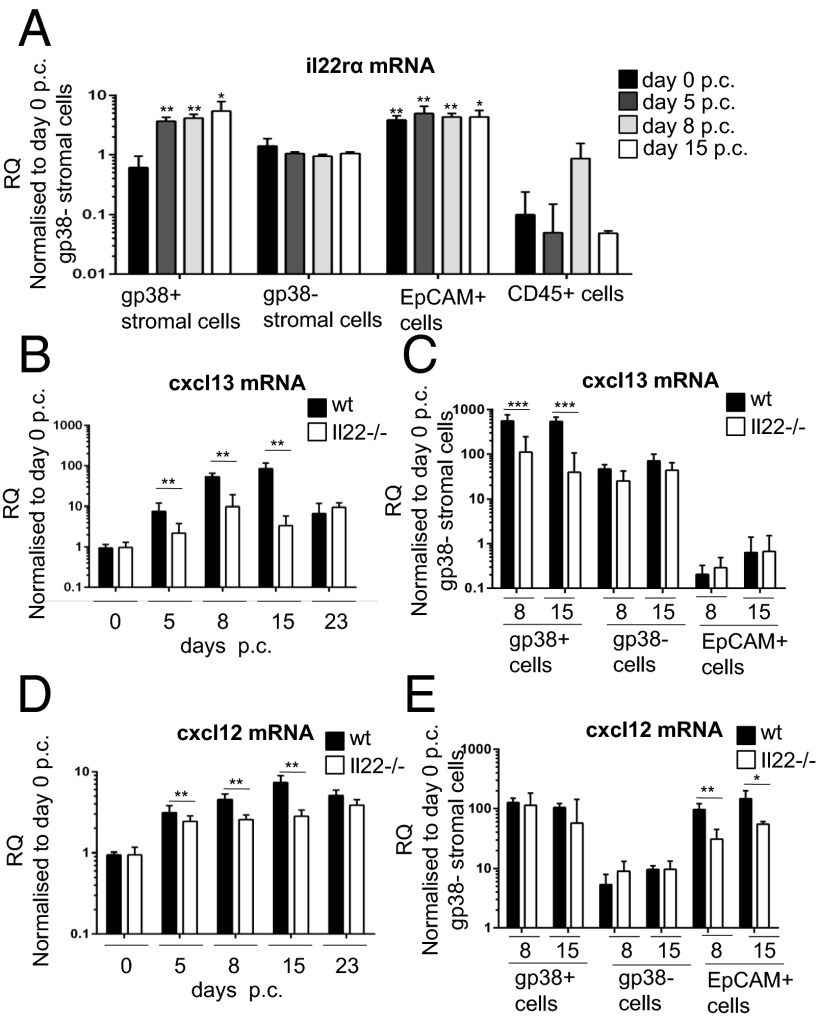
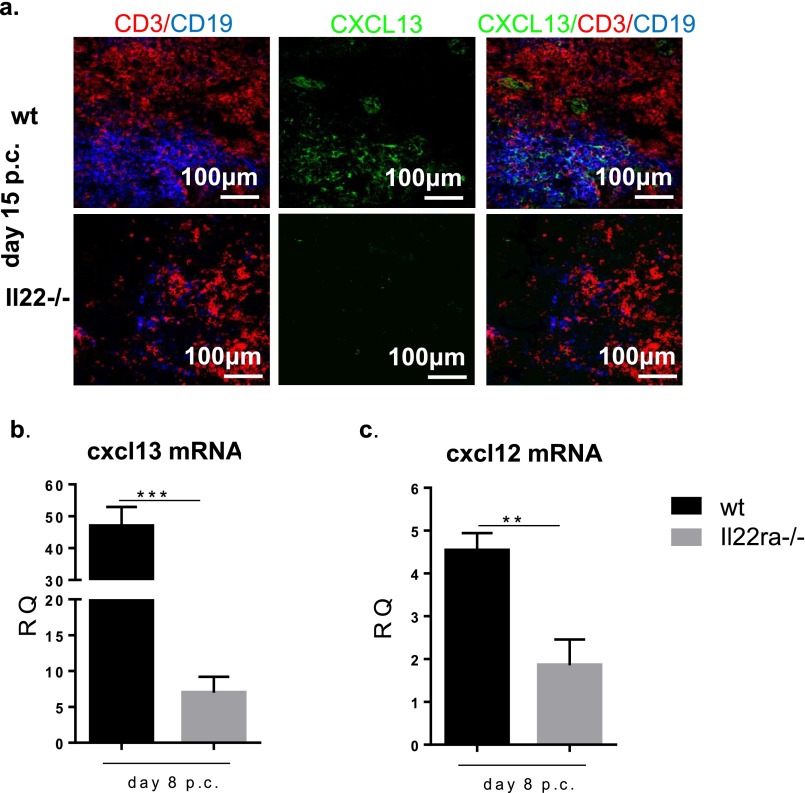
To dissect the mechanism by which IL-22 affects B-cell accumulation and response in TLOs, we first evaluated the distribution of Il-22 receptor α (IL-22Rα) on isolated cell populations from virus-cannulated salivary glands. As expected, IL-22Rα expression was restricted to nonhematopoietic (CD45−) cells (Fig. 3A). IL-22Rα mRNA expression was found on epithelial cells and on a nonendothelial, nonepithelial stromal cell population positive for gp38 [CD45−epithelial cell adhesion molecule (EpCAM)−CD31−gp38+], previously demonstrated to produce lymphoid chemokines during inflammation (20). We therefore hypothesized that IL-22Rα expression on stromal cells might contribute to chemokine (C-X-C motif) ligand 13 (CXCL13) expression in our model, accounting for the decreased B-cell recruitment observed in Il22−/− mice. Indeed, total mRNA transcript and protein expression for CXCL13 were significantly decreased in Il22−/− mice compared with WT mice (Fig. 3B and Fig. S4). These data were confirmed on sorted gp38+ stromal cells that showed a significant decrease in the transcript for CXCL13 in Il22−/− mice (Fig. 3C). In contrast, we observed no significant difference between WT and Il22−/− mice in CXCL13 expression on sorted epithelial cells or gp38− stromal cells (Fig. 3C). Next, we investigated another B-cell attractant, chemokine (C-X-C motif) ligand 12 (CXCL12). Whole-gland mRNA transcript analysis revealed impaired CXCL12 expression in Il22−/− mice (Fig. 3D). However, the decrease in CXCL12 expression was caused by the failure of CXCL12 up-regulation by epithelial cells rather than by gp38+ stromal cells (Fig. 3E). Taken together, these findings demonstrate that IL-22 promotes differential CXCL13 and CXCL12 expression in gp38+/EpCAM− stromal cells and epithelial stromal cells within TLOs. These findings also were confirmed in Il22rα−/− mice that showed decreased expression of the lymphoid chemokines CXCL13 and CXCL12 p.c., phenocopying the observation in Il22−/− mice (Fig. S4 B and C).
Fig. 3.
IL-22–deficient mice fail to induce CXCL13 and CXCL12 expression within stromal cells of TLOs. (A) Expression of Il22rα mRNA in FACS-sorted CD45−EpCAM−CD31−gp38+ cells (black bars) in comparison with CD45−EpCAM−CD31−gp38− cells (white bars), CD45−EpCAM+ epithelial cells (dark gray bars), and CD45+ cells (light gray bars). Il22rα mRNA transcripts were assessed by quantitative RT-PCR and normalized to β-actin. RQ was calculated with day 0 CD45−EpCAM−CD31−gp38− cells as calibrator. Results represent the mean ± SD of two independent experiments with four biological replicates. *P < 0.05; **P < 0.01. (B and D) Quantitative PCR analysis of cxcl13 and cxcl12 mRNA transcripts obtained from salivary glands at days 5, 8, 15, and 23 p.c. from Il22−/− (white bars) and WT (black bars) mice. Transcripts were normalized to pdgfrß mRNA; RQ values were calculated with day 0 salivary gland used as calibrator. **P < 0.01. Data present the mean ± SD of two independent experiments with two or three mice per group (four or six salivary glands per experiment). (C and E) mRNA expression of cxcl13 and cxcl12 mRNA on FACS-sorted CD45−EpCAM−CD31−gp38+, CD45−EpCAM−CD31−gp38−, and EpCAM+ epithelial cells from WT (black bars) and Il22−/− (white bars) mice at days 8 and 15 p.c. (normalized to β-actin), calculated with day 0 CD45−EpCAM−CD31−gp38− cells used as calibrator. Data present the mean ± SD of three biological replicates. *P < 0.05, **P < 0.01; ***P < 0.001.
Fig. S4.
(A) Microphotograph showing lack of CXCL13 protein expression (green) in cannulated salivary glands from Il22−/− mice but not WT mice at day 15 p.c. T cells (CD3 red) and B cells (CD19 green) are shown also. (B and C) Expression of cxcl13 (B) and cxcl12 (C) in Il22rα−/− mice (gray bars) and their WT counterparts (black bars) at day 8 p.c.; cxcl13 mRNA transcripts were normalized to pdgfrß mRNA, and results are presented as RQ values calculated with the day 0 salivary gland as calibrator. Data present the mean ± SD of two independent experiments with two or three mice per group. GEE analysis was followed by Sidak’s significance test; **P < 0.01; ***P < 0.001.
IL-22 Differentially Induces CXCL13 and CXCL12 Expression in Fibroblastic and Epithelial Cells in Vitro.
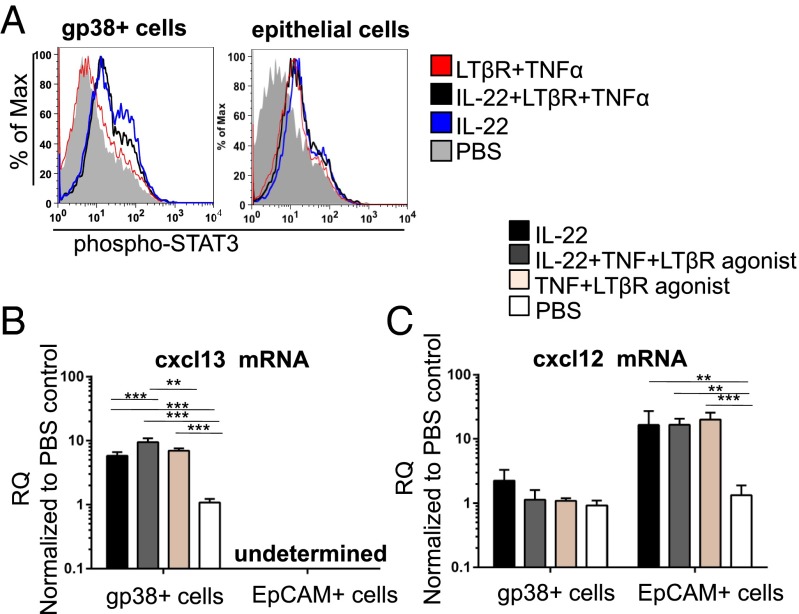
To confirm a direct role for IL-22 upstream of CXCL13 and CXCL12 production, we isolated gp38+ stromal cells and EpCAM+ epithelial cells from WT cannulated mice. Incubation of these cells with recombinant IL-22 protein induced the phosphorylation of Stat-3 (downstream of IL-22Rα signaling) (Fig. 4A). Recombinant IL-22 led to up-regulation of CXCL13 in gp38+ fibroblasts but not in epithelial cells (Fig. 4B). In contrast, IL-22 significantly stimulated CXCL12 expression in epithelial cells but not in fibroblasts (Fig. 4C). TNF and lymphotoxin, which are known to activate chemokine expression by stromal cells, also induced Stat3 phosphorylation as well as inducing efficient CXCL13 and CXCL12 expression in a manner comparable to IL-22 on its own (Fig. 4 B and C). Taken together, these data provide a molecular explanation for the defective B-cell accumulation and aberrant TLO formation observed in the Il22−/− mice, suggesting a novel role for IL-22 upstream of CXCL12 and CXCL13 expression in inflammatory conditions at mucosal sites.
Fig. 4.
IL-22 induces differential chemokine expression in fibroblasts and epithelial cells. (A) STAT3 phosphorylation assessed by flow cytometry in CD45−EpCAM−CD31−gp38+ stroma and EpCAM+ epithelial cells isolated from salivary glands at day 2 p.c. and stimulated in vitro with IL-22 (blue lines), IL-22, TNF-α and LTβR agonist (black lines), TNF-α and LTβR agonist (red lines), or PBS (gray lines). Two independent experiments were performed with three replicates per experiment. (B and C) Quantitative PCR analysis of cxcl13 and cxcl12 mRNA from CD45−EpCAM−CD31−gp38+ stromal and EpCAM+ epithelial cells isolated from salivary glands at day 2 p.c. stimulated in vitro with IL-22 (black bars), IL-22, TNF-α, and LTβR agonist (gray bars), TNF-α and LTβR agonist (light brown bars), or PBS (white bars). cxcl13 and cxcl12 mRNA transcripts were normalized to β-actin mRNA. Results are presented as RQ values compared with PBS-treated gp38+ stromal cells (B) or epithelial cells (C) as calibrator. Data present the mean ± SD of two independent experiments with three technical replicates per experiment. **P < 0.01; ***P < 0.001.
Therapeutic Blockade of IL-22 Inhibits Stromal Cell CXCL13 and CXCL12 Expression and Reduces Autoantibody Production.
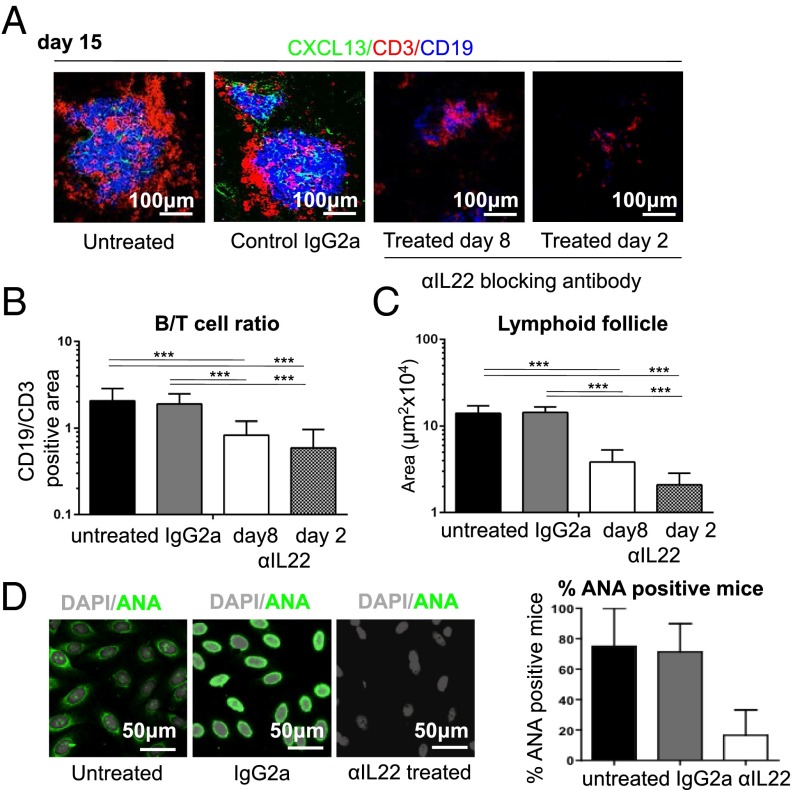
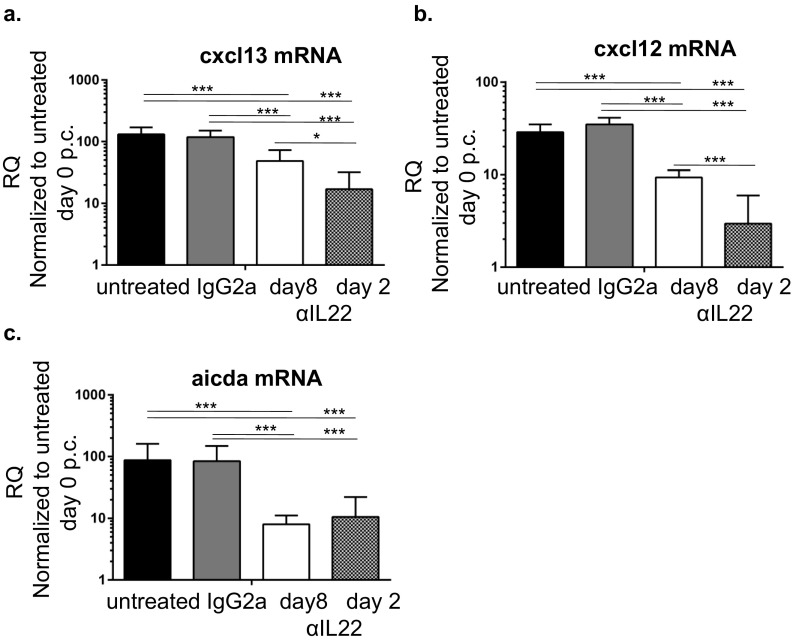
We next explored whether therapeutic antibody blockade of IL-22 could induce regression of TLOs, once formed, and abolish autoantibody production, thus reproducing the phenotype observed in Il22−/− mice. We treated cannulated mice with anti–IL-22 antibody blocking starting at either day 2 or day 8 p.c. Immunofluorescence analysis on day 15 p.c. revealed defective lymphoid aggregate formation, both in terms of TLO size (Fig. 5A) and B-cell accumulation (Fig. 5 A–C). This effect was more pronounced in mice that were treated from day 2 p.c. than in mice in which treatment began on day 8 p.c. (Fig. 5 A–C). Quantitative RT-PCR analysis of the salivary glands from treated mice showed a strong reduction in the production of both CXCL13 and CXCL12 (Fig. S5). This defect was accompanied by suppression of aicda mRNA transcripts (Fig. S5) that coincided with a significant decrease in autoantibody production (Fig. 5D), supporting the concept that IL-22–blocking agents might be therapeutically effective in clinical practice.
Fig. 5.
In vivo blockade of IL-22 reverses TLO formation and inhibits autoantibody production. (A) Lymphocytic aggregates from WT cannulated salivary glands from untreated mice, control IgG-treated mice, and mice treated with anti–IL-22 blocking antibody from day 8 or day 2 p.c. analyzed at day 15 p.c. for CD3 (red), CD19 (blue), and CXCL13 (green). (B) The ratio between the area covered by CD19+ B cells and CD3+ T cells at day 15 p.c. for the conditions above. Data present the mean ± SD of two independent experiments with two or three mice (four or six salivary glands) per group. ***P < 0.001. (C) Lymphoid follicle size for conditions above calculated as described in Methods for aggregates at day 15 p.c. Data present the mean ± SD of two independent experiments with two or three mice (four or six salivary glands) per group. ***P < 0.001. (D, Left) Immunofluorescent detection of autoantibody on Hep2 cells showing nuclear reactivity in sera at day 23 p.c. (nuclear staining in gray, autoantibody reactivity in green) (dilution 1:80). (Right) Graph summarizing autoantibody reactivity. The data present the mean ± SD of two experiments with four cannulated mice (biological replicates expressed as the percentage of mice positive for autoantibody at 1:80 dilution or more).
Fig. S5.
(A and B) Quantitative PCR analysis for cxcl13 (A) and cxcl12 (B) mRNA obtained from salivary glands at day 15 p.c. from untreated mice, mice treated with IgG2a isotope control, and mice treated with anti–IL-22 (αIL22) from day 8 and day 2 post cannulation. The cxcl13 and cxcl12 mRNA transcripts were normalized to pdgfrß mRNA, and results are presented as RQ values calculated with day 0 untreated salivary glands as calibrator. Data are presented as the mean ± SD of two independent experiments with two or three mice (four or six salivary glands) per group. *P < 0.05; ***P < 0.001 versus untreated or control Ig-treated cannulated salivary gland. (C) Quantitative PCR analysis for aicda mRNA obtained from salivary glands at day 23 p.c., for WT cannulated untreated mice (black bars), control Ig-treated mice (gray bars), and mice treated with IL-22–blocking antibodies from day 8 p.c. (white bars) or day 2 p.c. (patterned bars). Results are presented as RQ value compared with day 0 resting salivary glands. Data presented the mean ± SD of two independent experiments with two or three mice per group. GEE analysis was followed by Sidak’s significance test; *P < 0.05; ***P < 0.001.
Discussion
IL-22 is known to regulate mucosal homeostasis and promote epithelial repair following tissue damage (21, 22). Recently, Il-22 has been implicated in malignant epithelial cell proliferation (23), but its role in chronic inflammation remains enigmatic (15). Although observational studies have proposed a relationship between mucosal IL-22 expression and humoral immunity in humans (18, 24) a direct functional role for IL-22 in ectopic B-cell activation and TLO formation has not been established. In this study we explored the hypothesis that IL-22 regulates TLO development and autoantibody production, influencing the release of lymphoid chemokines such as CXCL13 and CXCL12 that are critical for TLO assembly. We used a recently described disease model in which cannulation of the salivary glands with a replication-deficient adenovirus leads to the formation of TLOs with breakage of local tolerance and autoantibody production (19). Here we demonstrate that absence of IL-22 results in strongly reduced B-cell accumulation within TLOs and significant reduction in autoantibodies. This defective autoimmune response correlates with a failure to induce the B-cell chemoattractants CXCL13 and CXCL12 by fibroblasts and epithelial cells, respectively. The role of IL-22 in a model of lymphoid organ development has been described previously (25). Ota et al. (25) reported the ability of IL-22 to restore the organization of gut-associated lymphoid tissue in mice lacking lymphotoxin signals. Nonetheless, the authors interpreted their results as suggesting that IL-22 expression is under the control of lymphotoxin (25). Here we provide evidence that, in vitro, IL-22 can regulate the production of lymphoid chemokines in fibroblasts, independently of lymphotoxin. IL-22 is known to regulate RANKL expression on synovial fibroblasts from patients with rheumatoid arthritis via activation of p38 MAPK/NF-κB or JAK-2/STAT-3 signaling (26). Similarly, in our model we demonstrated that treatment of isolated salivary gland fibroblasts and epithelial cells with recombinant IL-22 was sufficient to induce STAT3 phosphorylation and production of CXCL13 and CXCL12 in these two stromal cell populations. Our work and that of Ota et al. (25) do not exclude the possibility that in vivo, during the dynamic formation of TLOs, IL-22 and lymphotoxin synergistically contribute to chemokine production, thus coregulating the enlargement, organization, and maintenance of the inflammatory aggregates. Strikingly, the induction of IL-22 expression occurs within hours of salivary gland cannulation and in different innate immune cell populations. Because Il22−/− mice are characterized by profound defects in early TLO maturation, with significant loss in lymphoid chemokine expression, we propose that first resident innate cells and later γδ and αβ T cells, sensing tissue damage, produce IL-22 to activate stromal cell expression of B-cell chemokines, thereby allowing the development of B-cell–rich infiltrates and autoantibody production. The recruitment of IL-22–producing retinoid-related orphan receptor γ t (RORγt)+natural killer p46 (NKp46)−CD3− CD4+ lymphoid tissue inducer cells in a CXCL13 transgenic model that mimics isolated lymphoid follicle formation in the gut has been described (27). Taken together, these data suggest that IL-22 plays a regulatory role in the early phases of lymphoid tissue development at mucosal sites. Transgenic expression of lymphoid chemokines is known to induce TLO formation (8, 28–30). However, TLOs can form in the absence of RORγt lymphoid tissue inducer cells (9, 31, 32). This observation suggests that cytokines present within the inflammatory microenvironment provide alternative pathways of activation, different from those conventionally associated with physiological lymphoid tissue formation. A role for IL-17 has been shown in two models of TLO formation in the lungs (9, 33). Peters et al. (34) also established the requirement for gp38+ Th17 cells for the development of experimental allergic encephalomyelitis in the brain. We demonstrated in vitro and in vivo that IL-22 is sufficient to induce chemokine expression. Moreover in Il22−/− mice the signal for IL-17 is unaffected, thus excluding any significant contribution of IL-17 in the defect we observed in Il22−/− mice. Nonetheless, we cannot exclude a combined effect of IL-17 and IL-22 in TLO development at other sites and under different conditions. It has been elegantly demonstrated that CXCL13 expression is necessary and sufficient, both in the embryonic life and at ectopic sites, for the establishment of lymphoid follicles (28, 29) and the regulation of functional germinal centers (35). CXCL12 plays a role both in germinal center development and plasma cell attraction (30, 36). More recently, CXCL12 expression in nonepithelial stromal cells also has been implicated in B-cell recruitment to bronchial-associated lymphoid tissue (33). Carefully dissecting the source of lymphoid chemokines within TLOs, we have demonstrated that IL-22 exerts an unexpected differential role in the induction of these two chemokines on different stromal cell populations. On EpCAM−gp38+ fibroblasts, IL-22 stimulation induces CXCL13 expression, both independently from and additively with TNF- and lymphotoxin β (LTβ)-receptor signals (known regulators of CXCL13 expression) (37). Conversely, on EpCAM+ gp38+ epithelial cells, IL-22Rα engagement by IL-22 is able to increase CXCL12 but not CXCL13 production. Autoantibody production is the hallmark of many autoimmune diseases. Autoantibodies have been shown to precede disease onset, define prognosis, and stratify response to therapy (38–40). There is convincing evidence that AID expression on locally activated B cells within TLOs is able to support class-switch recombination and somatic hypermutation, thus sustaining affinity maturation of autoreactive B cells to tissue autoantigens (41, 42). Strikingly, therapeutic blockade of IL-22 by function-blocking antibodies is sufficient to impair TLO formation, by preventing organized B-cell aggregation and significantly affecting autoantibody production. In this context it is important to highlight the absence of a defect in aicda up-regulation in SLOs, further emphasizing the differences between SLO and TLO biology. Recently it has been shown that exposure of salivary gland epithelial cells to anti-Ro/SSA and La/SSB antibodies can induce IL-22 production (43). In agreement with these in vitro observations, there is evidence that B-cell depletion following treatment with rituximab leads to decreased expression of IL-22 in the salivary glands (18). This report suggests the possibility of a feed-forward loop involving IL-22 expression, survival of autoreactive B cells, and autoantibody production. Such an amplificatory loop, dependent on IL-22, also may be relevant to the well-known but poorly understood association between Sjogren’s syndrome and the predisposition to B-cell mucosal-associated lymphoma (41). Previously we have shown that lymphoma-associated epithelial cells preferentially express CXCL12, potentially contributing to ductal infiltration and B-cell survival (5). It is tempting to speculate that, just as excess production of IL-22 leads to uncontrolled epithelial proliferation and tumor development in the intestine (42), overproduction of IL-22 in exocrine glands leads to excessive CXCL12 and CXCL13 production, contributing to B-cell persistence in TLOs and lymphoma development. The relationship between TLO persistence and cancer development is well recognized; for example, in the gut the formation of mucosa-associated lymphoid tissue lymphoma has been associated with excessive CXCL13 expression (44). Our data suggest that IL-22, a critical cytokine activated in response to mucosal danger signals, provides a previously unidentified functional link between mucosal autoimmunity, TLO development, and cancer. Our findings suggest that targeting the IL-22 pathway might be an effective treatment in autoimmune diseases characterized by TLO formation. A translational program involving the use of anti–IL-22 in Sjogren’s syndrome is currently in development in the Rheumatology Unit at the University of Birmingham.
Methods
Mice and Salivary Gland Cannulation.
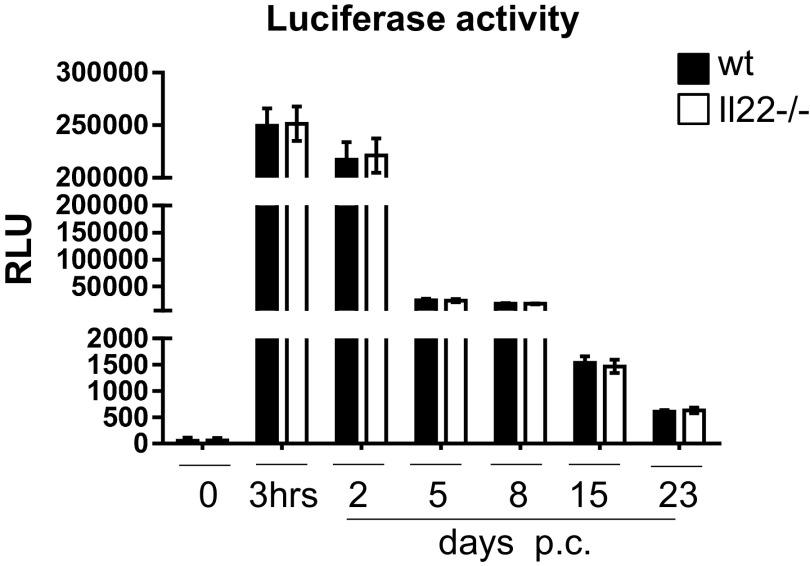
C57BL/6 mice were purchased from Harlan. Il22−/− mice were generated at Lexicon Genetics in collaboration with Pfizer. Il22Rα1−/− mice were sourced from the European Mouse Mutant Archive, Sanger Center, United Kingdom. Mice were maintained in the Biomedical Service Unit at the University of Birmingham according to Home Office and local ethics committee regulations (University of Birmingham). Submandibular glands of 8- to 12-wk-old female C57BL/6 and knockout mice were intraductally cannulated with 108–109 pfu of luciferase-encoding replication-defective adenovirus (Ad5) under anesthesia, as described (19). Animals were culled at specific time points by terminal anesthesia and cardiac puncture. Salivary glands and whole blood were harvested. Mice were excluded from the analysis on the basis of suboptimal virus delivery (Fig. S6) (19).
Fig. S6.
Expression of luciferase activity in salivary glands of WT (black bars) versus Il22−/− (white bars) mice cannulated with AdV5 replication-deficient virus containing the luciferase transgene at day 0, at 3 h p.c., and days 2, 5, 8, 15, and 23 p.c. Data present the mean ± SD of four to six mice for each p.c. time point. GEE analysis was followed by Sidak’s significance test.
In Vivo Blocking of Salivary Glands with Anti–IL-22.
Rat anti-mouse IL-22 Ab-03 was used as described (45). Starting at either day 2 or day 8 p.c., mice were administered a dose of 200 μg of anti–IL-22 antibody via i.p injection followed by injections three times per week.
Histology and Immunofluorescence.
Seven-micrometer sections of salivary gland were cut by cryosectioning and were stained with directly conjugated or unconjugated antibodies (SI Flow Cytometry Antibodies). Sections were mounted using Prolong Gold Antifade reagent (Invitrogen Life Technologies). Images were acquired on a Zeiss LSM 510 laser-scanning confocal head with a Zeiss Axio Imager Z1 microscope; LSM510 Image Examiner Software was used to process the images.
Isolation of Stromal Cells.
Harvested salivary glands were cut into small pieces and tissue digested for 40 min at 37 °C with stirring in 1.5 mL RPMI 1640 medium containing collagenase D (3.7 mg/mL) (Roche), DNase I (30 μg/mL) (Sigma), and 2% (vol/vol) FCS. The suspension was gently pipetted at 15-min intervals to break up cell aggregates. Remaining fragments were digested further for 20 min at 37 °C with medium containing collagenase dispase (3.7 mg/mL) and DNase I (30 μg/mL). EDTA was added to a final concentration of 5 mM; then cells were passed through a 70-μm mesh, washed twice, and resuspended in RPMI 1640 medium containing 10% (vol/vol) FCS.
Isolation of Leukocytes and in Vitro Stimulation for Cytokine Production.
After digestion, cells were resuspended in DMEM [with 10% (vol/vol) FCS, 1% Glutamine-Penicillin-Streptomycin (GPS) solution, 1% nonessential amino acids (NEAA), 1% Hepes, and 50 µM β-mercaptoethanol] for in vitro stimulation culture. Isolated cells were incubated with 50 ng/mL phorbol-12-myristate-13-acetate, 750 ng/mL ionomycin, 10 μg/mL Brefeldin A (all from Sigma Aldrich), 10 μg/mL recombinant IL-23 (BioLegend), and 10 μL of 108–109 pfu of adenovirus for 4 h at 37 °C.
Flow Cytometry.
Single-cell suspensions were incubated with antibodies on ice for 30 min in PBS (with 0.5% BSA and 2 mM EDTA) with mixtures of antibodies (SI Flow Cytometry Antibodies). Intracellular staining was performed according to the manufacturer’s protocol (BD Cytofix/Cytoperm). After surface staining with mixtures of desired antibodies, cells were washed in PBS (with 0.5% BSA and 2 mM EDTA), resuspended in 150 µL Cytofix/Cytoperm (BD Pharmingen), and incubated overnight at 4 °C. Cells were washed twice with the BD Perm/Wash Buffer; then intracellular antibody was added, and cells were incubated at 4 °C for 30–40 min. Cells were washed twice, resuspended, and then analyzed using a Cyan-ADP (Dako) with forward/side scatter gates set to exclude nonviable cells. Data were analyzed with FlowJo software (Tree Star). For cell sorting, stained cells were sorted using MoFlo-XDP (Beckman Coulter, Inc.). Sorted cell purity exceeded 96%.
In Vitro Stimulation of Sorted Stromal Cells with Recombinant Cytokines.
Isolated stromal cell subsets were resuspended at the same cell density in 500 µL of DMEM [with 10% (vol/vol) FCS, 1% GPS, 1% NEAA, 1% Hepes, and 50 µM β-mercaptoethanol] in 48-well plates. Recombinant cytokines were reconstituted in Dulbecco's phosphate-buffered saline (DPBS) (Sigma Aldrich). Cells were incubated with 2 μg/mL of recombinant IL-22 (Peprotech) alone, 2 μg/mL LTβR agonist antibody (Novus Biologicals) plus 500 ng/mL recombinant TNF-α (Peprotech), 2 μg/mL IL-22 μg/mL plus LTβR agonist antibody plus 500 ng/mL TNF-α, or DPBS alone (Sigma Aldrich) for 24 h at 37 °C. Cells were harvested after 24 h and taken for quantitative PCR analysis.
RNA Isolation and Quantitative PCR.
Total RNA was isolated from salivary glands with an RNeasy mini kit (Qiagen) and reverse-transcribed using the high-capacity reverse transcription cDNA synthesis kit (Applied Biosystems) on a Techne 312 Thermal Cycler PCR machine. Quantitative RT-PCR (Applied Biosystems) was performed on cDNA samples for cxcl13, cxcl12, aicda, il22rα, il22, and il17 mRNA expression. β-actin and pdgfrß were used as an endogenous control. PCR products were detected using an ABI PRISM 7900HT instrument and were analyzed with the Applied Biosystems SDS software (SDS 2.3) as described (46). CT values above 34 were not accepted, nor were technical replicates with more than two cycle differences between them.
Detection of Autoantibodies and Antiviral Response.
Hep-2 cells cultured on slides (IMMCO Diagnostics) were incubated with murine serum diluted in PBS (1:80). Sera were incubated for 1 h at room temperature, washed twice in PBS, and incubated with FITC anti-mouse IgG (Sigma) for 30 min. Slides were washed in PBS, mounted, and analyzed blindly by two different investigators. Antiviral titer was calculated as already described (19).
Statistical Analysis.
Statistical significance was determined by Generalized Estimating Means (GEE analysis) followed by Sidak’s significance test. In Figs. 3 A and C and 4 B and C statistical significance was determined by two-way ANOVA. In Fig. 2D an unpaired t test was used for statistical significance.
SI Flow Cytometry Antibodies
CD31-FITC clone 390, gp38-PE clone 8.1.1, CD45 PERCPCY5.5 or CD45 PECy7 clone 30-F11, NK1.1 FITC clone PK136, CD3e PE or FITC clone 145-2C11, IL7Ra APC clone A7R34, CD4 PB clone RM4-5, CD11b FITC clone M1/70, Gr1 FITC clone RB6-8C5, B220 FITC clone RA3-6B2, CD11c FITC clone N418, and CD5 FITC clone 53-7.3 were obtained from eBioscience. EpCAM PECy7 or EpCAM APC clone G8.8 and IL-22 PE were obtained from BioLegend. Phosphorylated STAT3 was obtained from Cell Signaling.
Acknowledgments
We thank Dr. T. Cupedo and Dr. M. Coles for useful discussions during manuscript preparation, Dr. P. Nightingale for professional statistical advice, and the Biomedical Services Unit facility at the University of Birmingham. F.B. and D.R.W. are funded by the Wellcome Trust. C.D.B. is supported by Arthritis Research UK Grant 19791.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503315112/-/DCSupplemental.
References
- 1.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 2.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 3.Barone F, et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005;52(6):1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 4.Salomonsson S, et al. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren’s syndrome. Scand J Immunol. 2002;55(4):336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 5.Barone F, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren’s syndrome and MALT lymphoma: Association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180(7):5130–5140. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 6.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10(9):664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 7.Krautler NJ, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150(1):194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin AP, et al. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol. 2004;173(8):4791–4798. doi: 10.4049/jimmunol.173.8.4791. [DOI] [PubMed] [Google Scholar]
- 9.Rangel-Moreno J, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley CD, Barone F, Nayar S, Bénézech C, Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715–745. doi: 10.1146/annurev-immunol-032713-120252. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantelyushin S, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122(6):2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andoh A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129(3):969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 14.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31(1):15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: Immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puel A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavoie TN, Stewart CM, Berg KM, Li Y, Nguyen CQ. Expression of interleukin-22 in Sjögren’s syndrome: Significant correlation with disease parameters. Scand J Immunol. 2011;74(4):377–382. doi: 10.1111/j.1365-3083.2011.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciccia F, et al. Rituximab modulates the expression of IL-22 in the salivary glands of patients with primary Sjogren’s syndrome. Ann Rheum Dis. 2013;72(5):782–783. doi: 10.1136/annrheumdis-2012-202754. [DOI] [PubMed] [Google Scholar]
- 19.Bombardieri M, et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol. 2012;189(7):3767–3776. doi: 10.4049/jimmunol.1201216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peduto L, et al. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182(9):5789–5799. doi: 10.4049/jimmunol.0803974. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 23.Kirchberger S, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210(5):917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciccia F, et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 2012;64(6):1869–1878. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- 25.Ota N, et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12(10):941–948. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 26.Kim KW, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64(4):1015–1023. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- 27.Marchesi F, et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2(6):486–494. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 28.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12(5):471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 29.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 30.Luther SA, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 31.Marinkovic T, et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116(10):2622–2632. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furtado GC, et al. TNFα-dependent development of lymphoid tissue in the absence of RORγt⁺ lymphoid tissue inducer cells. Mucosal Immunol. 2014;7(3):602–614. doi: 10.1038/mi.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleige H, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med. 2014;211(4):643–651. doi: 10.1084/jem.20131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters A, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltman JB, Allen CD, Cyster JG, de Boer RJ. B cells within germinal centers migrate preferentially from dark to light zone. Proc Natl Acad Sci USA. 2011;108(21):8755–8760. doi: 10.1073/pnas.1101554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen CD, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5(9):943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 37.Lötzer K, et al. Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin beta-receptor NF-kappaB signaling. Arterioscler Thromb Vasc Biol. 2010;30(3):395–402. doi: 10.1161/ATVBAHA.109.191395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rantapää-Dahlqvist S, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 39.Bos WH, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: A prospective cohort study. Ann Rheum Dis. 2010;69(3):490–494. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 40.Barra L, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013;65(6):1439–1447. doi: 10.1002/art.37911. [DOI] [PubMed] [Google Scholar]
- 41.Bombardieri M, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren’s syndrome. J Immunol. 2007;179(7):4929–4938. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 42.Humby F, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisto M, et al. Autoantibodies from Sjögren’s syndrome trigger apoptosis in salivary gland cell line. Ann N Y Acad Sci. 2007;1108:418–425. doi: 10.1196/annals.1422.044. [DOI] [PubMed] [Google Scholar]
- 44.Mazzucchelli L, et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104(10):R49–R54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118(2):597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barone F, Patel P, Sanderson JD, Spencer J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2009;2(6):495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]