Significance
RNA silencing-mediated virus interference or cross-protection generally occurs between closely related strains of a single virus species. Here, we show strong virus interference between unrelated RNA viruses in the filamentous ascomycetous fungus, Cryphonectria parasitica. Lateral transmission and replication of a totivirus with an undivided dsRNA genome was severely inhibited by a silencing suppressor deletion mutant of the prototype hypovirus with a positive-strand RNA genome or the prototype mycoreovirus with an 11-segmented dsRNA genome, and even by transgenic expression of hairpin RNA of an endogenous fungal gene. This interference required high-level expression of the key RNA silencing gene, dicer-like 2 (dcl2), but not necessarily argonaute-like 2 (agl2). This study provides insight into broad-spectrum virus control.
Keywords: RNA silencing, dicer, argonaute, virus, Cryphonectria parasitica
Abstract
Viruses often coinfect single host organisms in nature. Depending on the combination of viruses in such coinfections, the interplay between them may be synergistic, apparently neutral with no effect on each other, or antagonistic. RNA silencing is responsible for many cases of interference or cross-protection between viruses, but such antagonistic interactions are usually restricted to closely related strains of the same viral species. In this study, we present an unprecedented example of RNA silencing-mediated one-way interference between unrelated viruses in a filamentous model fungus, Cryphonectria parasitica. The replication of Rosellinia necatrix victorivirus 1 (RnVV1; Totiviridae) was strongly impaired by coinfection with the prototypic member of the genus Mycoreovirus (MyRV1) or a mutant of the prototype hypovirus (Cryphonectria hypovirus 1, CHV1) lacking the RNA silencing suppressor (CHV1-Δp69). This interference was associated with marked transcriptional induction of key genes in antiviral RNA silencing, dicer-like 2 (dcl2) and argonaute-like 2 (agl2), following MyRV1 or CHV1-Δp69 infection. Interestingly, the inhibition of RnVV1 replication was reproduced when the levels of dcl2 and agl2 transcripts were elevated by transgenic expression of a hairpin construct of an endogenous C. parasitica gene. Disruption of dcl2 completely abolished the interference, whereas that of agl2 did not always lead to its abolishment, suggesting more crucial roles of dcl2 in antiviral defense. Taken altogether, these results demonstrated the susceptible nature of RnVV1 to the antiviral silencing in C. parasitica activated by distinct viruses or transgene-derived double-stranded RNAs and provide insight into the potential for broad-spectrum virus control mediated by RNA silencing.
RNA silencing is a homology-dependent RNA degradation mechanism that is conserved in eukaryotic organisms across kingdoms. Briefly, double-stranded RNA (dsRNA), generated from transgenes, endogenous genes, or molecular parasites such as viruses and transposable elements, is processed into small interfering RNAs (siRNA) of 21–26 nucleotides by Dicer or Dicer-like proteins (DCLs). siRNAs are recruited into an RNA-induced silencing complex (RISC), whose major constituent is Argonaute (AGO) or Argonaute-like protein (AGL), an effector molecule (1–3). RISC cleaves target RNAs using siRNAs as guides. RNA silencing is part of the host defense mechanism operating primarily against viruses (2, 4, 5). As a counterdefense tool, viruses encode RNA silencing suppressors (RSSs) that inhibit different steps of the silencing pathway (6, 7). Therefore, any defects in RNA silencing components typically make hosts supersusceptible or nonhosts susceptible to infection (8–11).
There are three types of interplay between coinfecting viruses in a single host—synergistic, neutral (with no effects on each other), and antagonistic interactions—many of which involve RNA silencing. In synergistic infections, the RSS(s) of one virus plays an important role in facilitating the multiplication of unrelated coinfecting viruses. This is exemplified by the observation that potyviruses (HC-Pro RSS) increased potexvirus multiplication and pathogenicity in plants (12, 13). In antagonistic interactions, a preinfecting virus, usually a very mild strain, incites RNA silencing and interferes with the replication of a closely related severe strain of the same virus (14). This phenomenon is also referred to as cross-protection, and some attenuating plant viruses have been commercialized as biological control agents (15). Most, if not all, cross-protection phenomena in plants are shown to be due to RNA silencing (14, 16). Therefore, RNA silencing-mediated cross-protection in plants is reminiscent of acquired immunity-mediated cross-protection between closely related viruses in vertebrates (17).
The Cryphonectria parasitica (the chestnut blight fungus) has been established as a model filamentous fungus for studying virus/virus and virus/host interactions (18). This C. parasitica/virus system provides a unique platform for the exploration of RNA silencing and virus replication (18–20). The fungus supports diverse fungal viruses within six different families: Hypoviridae, Narnaviridae, Reoviridae, Partitiviridae, Megabirnaviridae, and Totiviridae. These viruses are from not only the homologous host fungus but also heterologous fungi belonging to different orders (18, 21). The fungus has two Dicer-like genes (dcl1 and dcl2), four Argonaute-like genes (agl1 to agl4), and four RNA-dependent RNA polymerase (RDR) genes (rdr1 to rdr4) (22, 23). Among these RNA silencing-related genes, only dcl2 and agl2 play roles in antiviral defense at the cellular level (4, 24). No redundant roles have been observed in other dcl or agl homologs, unlike those in plants (25). Interestingly, dcl2 and agl2 were considerably up-regulated (over 10-fold) by transgenic expression of exogenous dsRNA and infection by mutants of the prototype hypovirus Cryphonectria hypovirus 1 (CHV1) (24, 26), a member of the expanded picornavirus superfamily (27). A multifunctional protein encoded by CHV1, p29, inhibits the up-regulation of these host genes as an RSS (24). This protein shows phylogenetic affinity to and plays a role similar to potyvirus HC-Pro as a protease, a symptom determinant, and an RSS (28–31). It remains unknown whether the up-regulation of agl2 and dcl2 is transgene sequence-specific or virus-specific and to what degree these transcriptional inductions contribute to antiviral defense.
During the course of studying antiviral RNA silencing in C. parasitica, we have found distinct induction patterns of the key RNA silencing genes upon infection by different viruses. That is, some viruses markedly induce the expression of dcl2 and agl2, whereas others do not. In this study, we provide solid evidence for RNA silencing-mediated, sequence-independent virus interference among unrelated RNA viruses. Lateral transmission and the replication of a totivirus, Rosellinia necatrix victorivirus 1 (RnVV1) (32), which has an undivided dsRNA genome, was abolished by an RSS-deletion mutant of CHV1 or a reovirus with an 11-segmented dsRNA genome, and even by transgenic expression of hairpin RNA of an endogenous fungal gene. This interference coincided with high expression of two key genes for RNA silencing, dcl2 and agl2. A difference was also noted in the level of contribution to the interference between the two host genes.
Results
Behavior of CHV1, MyRV1, and RnVV1 in Wild-Type and RNA Silencing-Deficient C. parasitica.
Wild-type (WT) CHV1 (CHV1-wt) infection of C. parasitica caused reduced pigmentation and reduced asexual sporulation in the WT standard strain EP155, whereas MyRV1 and a CHV1 mutant lacking most of 5′-proximal ORF A (CHV1-∆p69) induced a similar subset of symptoms, reducing the growth of aerial hyphae and increasing brown pigmentation without affecting sporulation considerably on potato dextrose agar (PDA) media (33, 34) (Fig. 1A). Infection of C. parasitica by RnVV1, originally isolated from another phytopathogenic ascomycete (Rosellinia necatrix), did not induce macroscopic alterations (32) (Fig. 1A). In antiviral RNA silencing-deficient mutants of C. parasitica EP155 (∆dcl2 and ∆agl2), CHV1-wt, CHV1-∆p69, and MyRV1 commonly showed more severe symptoms than those in EP155 (4, 24). In contrast, RnVV1-infected mutant hosts exhibited similar colony morphology to their virus-free counterparts (Fig. 1A). Note that RnVV1 initially induced symptoms in ∆dcl2, which, however, was mitigated during repeated subculture (32).
Fig. 1.
Infections of C. parasitica WT (EP155) and mutant strains (∆dcl2 or ∆agl2) by RnVV1, MyRV1, CHV1-wt, and CHV1-∆p69. (A) Phenotypes of virus-free and -infected C. parasitica strains. Colonies were grown on PDA for 8 d on the bench-top and photographed. (B) Northern analyses of mRNAs of viruses and host dcl2 and agl2 genes. ssRNA fractions were extracted from virus-infected C. parasitica strains and subjected to the assays. Counterpart virus-free strains were analyzed in parallel. Upper panels show accumulation of viral genomic and/or messenger RNAs (g/mRNAs or mRNAs) (CHV1, MyRV1, and RnVV1), and Lower panels show host gene expression levels (dcl2 and agl2). Significant transcriptional induction of the dcl2 gene is marked by a red arrowhead. Loading control is the ethidium bromide (EtBr)-stained ribosomal RNAs (rRNAs) in this and all the following figures for Northern analyses.
In these antiviral RNA silencing-deficient hosts, viruses were consistently accumulated at considerably or slightly higher levels relative to those in RNA silencing-competent EP155 (Fig. 1B, upper blots). EP155 induced dcl2 and agl2 expression upon infection by MyRV1 and CHV1-∆p69 significantly compared with RnVV1 infection (Fig. 1B, lower blots). CHV1-wt carrying the p29 RSS canceled such induction (26). The agl2 induction was never observed in the ∆dcl2 background, at least following infection by CHV1-wt, CHV1-∆p69, and MyRV1, consistent with previous observations that agl2 induction depends upon dcl2 (24) (Fig. 1B, lower blots). However, RnVV1-infected ∆dcl2 showed a marked increase in agl2 mRNA level. Furthermore, although Sun et al. (24) reported the requirement of agl2 for the induction of dcl2 upon CHV1-wt infection (thus vice versa), dcl2 induction apparently occurred in the ∆agl2 host infected by MyRV1, and relatively weakly in hosts infected by CHV1-∆p69 and RnVV1. Therefore, dcl2 transcription could be induced independently of AGL2. CHV1-wt with the p29 RSS successfully interfered with dcl2 and agl2 induction in all host backgrounds tested (Fig. 1B).
These combined results showed that dcl2 and agl2 induction are regulated by mycovirus infections; they are highly activated by MyRV1 and CHV1-∆p69 but apparently less so by CHV1-wt (through p29 RSS activities) and RnVV1 in EP155. It is striking that the highest induction of dcl2 was observed in MyRV1-infected EP155 and ∆agl2 and in CHV1-∆p69–infected EP155 (Fig. 1B, red arrowheads).
Interference in RnVV1 Multiplication by MyRV1 and CHV1-∆p69 in C. parasitica.
We have previously found virus strain-specific synergistic and apparently antagonistic interactions: CHV1-wt enhances the multiplication of RnVV1, and CHV1-∆p69 represses RnVV1 in EP155 (32). To further analyze this phenomenon, we devised an assay as shown in Fig. 2, which was used throughout the study. Two fungal mycelia were inoculated on a single PDA medium side-by-side and grown for 7–10 d (coculture) to allow viruses to move between two fungal strains (horizontal transmission) during hyphal fusion. In this system, either side (L or R) of the two colonies harbored RnVV1, whereas the other side usually carried virus(es) that might affect RnVV1 replication and transmission. Mycelial plugs were taken from designated positions (N, near; M, middle; F, far from the hyphal contact junction), placed on new PDA media, and further subcultured in potato dextrose broth (PDB) media for single-stranded RNA (ssRNA) extraction and subsequent Northern blot and RT-PCR analyses.
Fig. 2.
Experimental procedure used in the virus transmission assay. Experimental flow is schematically represented. For virus transmissions, two mycelial plugs (original paired strains) selected from fungal strains such as those shown in Fig. 1A were inoculated on a large PDA plate, side by side, and grown on the bench-top for 7∼10 d. The left-side (L side) colony was mostly ∆dcl2 carrying RnVV1 to be transmitted to the other side, and the right-side (R side) colony was WT, ∆dcl2, or ∆agl2 in which virus accumulation and host responses were analyzed. After coculture (horizontal virus transmission period), mycelial plugs were taken from the growing edge positioned in the F, M, or N position relative to the hyphal border between the L and R side colonies, and subcultured onto new small PDA plates. These subisolates were further cultured in PDB liquid media in flasks in the dark for 7 d, and ssRNA fractions were recovered for Northern blotting and RT-PCR analyses.
When ∆dcl2/RnVV1 (L side) was fused with either the recipient EP155 fungus (R side) uninfected or infected by MyRV1 or CHV1-∆p69, Northern blotting of RnVV1 showed different RnVV1 detection profiles (Fig. 3). RnVV1 was successfully transmitted to and accumulated in originally virus-free EP155 (Fig. 3, set 1, RnVV1). However, RnVV1 was undetectable in MyRV1-infected or CHV1-∆p69–infected EP155 by Northern blotting and was only faintly detected or undetected by RT-PCR (Fig. 3, sets 2 and 3, RnVV1). Note that all subcultures from the three positions—N, M, and F—in recipient EP155 showed a similar detection pattern. Importantly, MyRV1 and CHV1-∆p69 moved to ∆dcl2/RnVV1 (L side), indicating the occurrence of proper hyphal fusion that would allow for RnVV1 transmission in the reverse direction (Fig. 3, sets 2 and 3, MyRV1 and CHV1-∆p69). Another important observation is that mRNA levels of dcl2 were substantially increased in EP155 infected by MyRV1 and CHV1-∆p69 but not in virus-free or RnVV1-transmitted EP155 (Fig. 3, dcl2). These combined results suggest the involvement of activated host antiviral RNA silencing in the inhibitory effects on RnVV1 multiplication and transmission.
Fig. 3.
Inhibition of RnVV1 accumulation by MyRV1 and CHV1-Δp69 in the WT strain (EP155) of C. parasitica. The RnVV1-carrying C. parasitica ∆dcl2 strain (L side) was cocultured with virus-free (set 1), MyRV1-infected (set 2), or CHV1-∆p69–infected (set 3) EP155 (R side). Their subculture strains were obtained from the N, M, and F positions of the R sides and from the M position of the L side (∆dcl2/RnVV1). The original paired strains were also included in the assay. Northern blotting was performed in this and subsequent figures to detect mRNAs of RnVV1, CHV1-∆p69, given segment(s) of MyRV1 (segment S1 in this figure), and the host dcl2 and/or agl2 gene (Northern blotting). Detection targets are specified on the right of each panel. Supporting RT-PCR analysis to detect RnVV1 and host actin mRNAs is presented at the bottom (RT-PCR). Sizes of DNA fragments are shown on the left. Arrows above the lanes indicate the theoretical direction of virus movement, and bars refer to originally virus-free strains in this and subsequent figures. DI-RNA, defective-interfering RNA.
Invasive MyRV1 and CHV1-∆p69 Eliminate RnVV1.
To further confirm the above observations, we took an inverse approach: MyRV1 and CHV1-∆p69 were transmitted from ∆dcl2 (L side) into EP155 infected by RnVV1 (R side). As shown in Fig. 4, RnVV1 was detectable by Northern blotting in EP155 (set 2, R side) and was transmitted to ∆dcl2 (set 2, L side), when cocultured with virus-free ∆dcl2. In contrast, when cocultured with ∆dcl2 carrying MyRV1 (set 1, L side), RnVV1 in EP155 was no longer detectable by Northern blotting but faintly detectable or undetectable by RT-PCR (set 1, R side). In this combination, MyRV1 invaded EP155 preinfected by RnVV1 and strongly interfered with RnVV1 replication. Although we expected RnVV1 transmission to the L side (MyRV1-carrying ∆dcl2) given the coinfection established in the previous experiment in the ∆dcl2 background (Fig. 3, set 2, L side), such transmission was not observed (Fig. 4, set 1, L side, RnVV1). Similar results were obtained using CHV1-∆p69 as an invader (Fig. S1). These suggest that the lateral transfer of RnVV1 from EP155 to ∆dcl2 was blocked before the establishment of coinfection with CHV1-∆p69 or MyRV1 in the ∆dcl2 background. It seems that RNA silencing was activated upon virus infection in heterokaryotic cells with the EP155 and ∆dcl2 karyons during anastomosis and led to RnVV1 clearance. It should be noted that during coculture karyons move from one colony to another more extensively than previously thought (32).
Fig. 4.
Clearance of preinfected RnVV1 from EP155 by invasive MyRV1. The MyRV1-infected (set 1) or virus-free (set 2) C. parasitica ∆dcl2 strain (L side) was cocultured with the RnVV1-infected EP155 strain (R side, EP155/RnVV1), and subcultures were obtained from the N and F positions of the R sides. The subcultures from the L side (∆dcl2/MyRV1 and ∆dcl2, M position) and original cocultured strains were included as controls. Northern blot and RT-PCR analyses were performed as described in Fig. 3.
Fig. S1.
Clearance of preexisting RnVV1 from EP155 by invasive CHV1-∆p69. CHV1-∆p69–infected (set 1) or virus-free (set 2) C. parasitica ∆dcl2 (L side) was cocultured with the RnVV1-infected WT fungus EP155 (R side, EP155/RnVV1). After 7 d of coculture, substrains were obtained from the N and F positions of the R sides and from the M position of the L sides (∆dcl2/CHV1-∆p69 and ∆dcl2, M). The original cocultured strains were also included in the assay. RNA blot analyses were performed to detect mRNAs of RnVV1 and CHV1-∆p69 (Upper) and the host dcl2 gene (Lower) (Northern blotting). Detection targets are specified on the right of each panel. Overexposed film is shown for RnVV1 detection (overexpose). The EtBr-stained rRNAs are shown as loading controls. Arrows above the lanes indicate the theoretical directions of virus movement (bar; virus-free) in the two sets of transmission. Successful RnVV1 transmission (L ← R) in set 2 was observed, and the RnVV1 in set 1 was eliminated by invading CHV1-∆p69 (R side). In ∆dcl2/CHV1-∆p69, relatively low levels of dcl2 mRNA were observed after coculture with EP155/RnVV1 (set 1, ∆dcl2/CHV1-∆p69, dcl2). This unexpected result was most likely because EP155 karyons were transferred to the L side, as reported by Chiba et al. (32), and dcl2 was transcriptionally induced by CHV1-∆p69. The abnormally slow growth of ∆dcl2/CHV1-∆p69 may have facilitated the accumulation of EP155 karyons in the L side (see Fig. 1A for fungal growth phenotype). An arrow below the panel indicates the probable movement of EP155 karyons, and an asterisk indicates substantial transcriptional induction of dcl2 with contaminated EP155 karyons. DI-RNA, defective-interfering RNA; g/mRNA, genomic and messenger RNA.
We also examined interference between CHV1-∆p69 and MyRV1, but these viruses established coinfection in EP155 with similar accumulation levels to those of single infections (Fig. S2). Moreover, dcl2 expression levels were comparable between single and double infections, indicating that the antiviral RNA silencing was fully activated by these viruses and that each virus tolerates it.
Fig. S2.
No interference between MyRV1 and CHV1-Δp69. (A) Northern blotting of ssRNA fractions from C. parasitica EP155 singly infected by MyRV1 or CHV1-∆p69 and doubly infected by these (CHV1-∆p69 + MyRV1), to determine their accumulation levels (Upper) and host dcl2 expression levels (Lower). Detection targets are shown on the right of each panel. The EtBr-stained rRNAs are shown as loading controls. (B) Detection of viral dsRNAs in agarose gel electrophoresis. Total RNA fractions from the same set of fungal strains as in A were analyzed. Sample loading amount was normalized against EtBr-stained rRNAs (asterisks). Size standards of DNA fragments are loaded in the lefthand lane. There was no apparent interference between MyRV1 and CHV1-∆p69. Almost equal dcl2 expression levels were observed in single infections and coinfections. DI-RNA, defective-interfering RNA; g/mRNA, genomic and messenger RNA.
DCL2 but Not AGL2 Is Essential for the RnVV1 Interference.
We were interested in defining the functional roles of AGL2 and DCL2 in this RnVV1 interference. In cocultures, RnVV1-infected ∆dcl2 and ∆agl2 (L side) were allowed to undergo hyphal fusion with virus-free, MyRV1-infected, or CHV1-∆p69–infected counterparts. In the ∆dcl2 background, RnVV1 and each of the two other viruses were bidirectionally transmitted and established coinfections (Fig. 5A). RnVV1 accumulated at high levels regardless of single or double infections. The expression levels of agl2 varied, suggesting that AGL2 has no effect on RnVV1 accumulation levels in the absence of DCL2. The same experiment in the ∆agl2 background showed interesting differences. Firstly, RnVV1 failed to establish coinfection with MyRV1 in ∆agl2 (Fig. 5B, set 2), unlike in ∆dcl2, but RnVV1 was successfully transmitted to virus-free and CHV1-∆p69–infected ∆agl2 (Fig. 5B, sets 1 and 3). Secondly, the dcl2 expression level in ∆agl2/CHV1-∆p69 was considerably lower than that in ∆agl2/MyRV1 (Fig. 5B and Fig. 1B, dcl2). Thus, maximal up-regulation of the dcl2 expression appears to be required for RnVV1 interference, and this can occur in the absence of agl2 (Fig. 5B, set 2). This observation was accordingly confirmed by a cotransmission analysis in which ∆dcl2 coinfected by RnVV1 and MyRV1 was fused with virus-free EP155, ∆dcl2, or ∆agl2. Consequently, ∆dcl2 allowed for cotransmission, but not EP155 or ∆agl2 recipients, in which dcl2 expression was highly induced (Fig. S3).
Fig. 5.
Bidirectional transmission of RnVV1 and MyRV1 or CHV1-∆p69 in ∆dcl2 and ∆agl2 hosts. (A) Transmission in ∆dcl2. The RnVV1-infected C. parasitica ∆dcl2 (L sides, ∆dcl2/RnVV1) was cocultured with virus-free (set 1), MyRV1-infected (set 2), or CHV1-∆p69–infected (set 3) ∆dcl2 (R sides) and allowed to undergo horizontal transmission. The subcultures were obtained from the M positions of both the L and R sides and subjected to Northern analysis. The original cocultured strains were analyzed in parallel. (B) Transmission in ∆agl2. The same experiment as in A (∆dcl2) was conducted with ∆agl2.
Fig. S3.
Clearance of RnVV1 during cotransmission with MyRV1. C. parasitica ∆dcl2 strain doubly infected by MyRV1 and RnVV1 (L side) was cocultured with virus-free EP155, ∆dcl2, or ∆agl2 strain (R side). Subisolates were obtained from the M position (two independent transmissions) of the R sides. The original paired strains used for these cocultures were included in the assay as controls. Northern blotting of ssRNA samples was performed to detect mRNAs of RnVV1, segments 1 and 4 (S1 and S4) of MyRV1, and the host dcl2 gene. Detection targets are specified on the right of each panel. Overexposed image for RnVV1 detection is provided. Coinfection was maintained only in ∆dcl2 (sets 3 and 4), and interference with RnVV1 by MyRV1 occurred in EP155 and ∆agl2 (sets 1, 2, 5, and 6). The results support those shown in Figs. 3–5.
Nonviral dsRNA-Induced dcl2/agl2 Expression Reduced RnVV1 Accumulation.
Transgene-generating dsRNAs (hairpin RNAs) have been reported to induce dcl2 and agl2 expression in this fungus (24). We examined whether the transgene-induced dcl2 and agl2 result in the reduction of RnVV1 transmission/accumulation as observed above. A well-characterized endogenous, mitogen-activated protein kinase (CpMK1) gene (35) was targeted with a hairpin construct in EP155, and we obtained CpMK1-knockdown lines (EP155/CpMK1-KD) in which CpMK1 expression levels were reduced compared with WT EP155 (Fig. S4 A and B). The dcl2 mRNA levels in the EP155/CpMK1-KD were higher than those in EP155, and thus, dcl2 induction was triggered by the hairpin RNAs (Fig. S4B, dcl2). Using one of these nonvirally dcl2-induced strains, we performed RnVV1 transmission assay from ∆dcl2/RnVV1 (L side) into EP155/CHV1-wt, EP155/Twtp29 (the CHV1 p29-expressing strain), EP155, EP155/CpMK1-KD, and EP155/MyRV1 (R sides) to analyze the behavior of RnVV1. As shown in Fig. 6, Northern analysis revealed various levels of RnVV1 accumulation and virus levels declined basically in inverse proportion to host dcl2 mRNA accumulation. The RnVV1-mRNAs were most abundantly accumulated in the ∆dcl2 background, slightly less in EP155 under CHV1-wt infection, even less in the p29-expressing host, and much less in EP155 overall. In EP155/CpMK1-KD and EP155/MyRV1, RnVV1 was no longer detectable and these fungi consistently showed elevated dcl2 and agl2 expression. Note that RnVV1 can replicate in a knockout mutant of CpMK1 (CpMK1-KO) (35) to a comparable extent as in EP155 (Fig. S4C), thus eliminating the possibility that the RnVV1 interference in CpMK1-KD was mediated by reduced expression levels of CpMK1.
Fig. S4.
Effects of knockdown of endogenous CpMK1 gene on dcl2 induction and RnVV1 infection. (A) Schematic representation of a knockdown construct. The 5′ half of CpMK1 ORF was targeted for transgene-mediated RNA silencing using a hairpin construct pCPXHY2-CpMK1-IR. Blue boxes illustrate CpMK1 exon sequences. Thick red arrows indicate silencing target sequence (sense/antisense orientation). Promoters (Pgpd-1, Pcpmk1) and terminators (Tgpd-1, Tcpmk1) are indicated by thick gray bars. (B) Selection of CpMK-KD lines. The virus-free (EP155, control) and candidate CpMK1-KD strains (CpMK1-KD lines; IR2-1, -4, -5, -6, -7, and -8) were analyzed by Northern blotting of ssRNAs. The mRNAs of CpMK1 and dcl2 were detected. EtBr-stained rRNAs are shown as loading controls. The IR2-1 strain (red) was used for further analysis. A DIG-labeled PCR product (nt positions 100–954 on cDNA) was used as a probe for CpMK1. (C) Effect of CpMK1 on the RnVV1 infection. The accumulation levels of RnVV1 and transcription of CpMK1 and dcl2 in EP155 and in the CpMK1-KO (CpMK1-KO) strain (35) were compared by Northern blotting as performed in Fig. 1 and Fig. S4B. There was no obvious difference in the virus accumulation levels between EP155 and CpMK1-KO, indicating no overt effect of CpMK1on RnVV1 replication.
Fig. 6.
Hierarchic reduction of the RnVV1 accumulations in inverse proportion to dcl2 expression levels. Northern analysis of mRNAs for RnVV1 and the host dcl2 and agl2 in EP155 variants. RnVV1 was horizontally transmitted from ∆dcl2 (∆dcl2/RnVV1, donor) into the EP155 variants (RnVV1 recipients): CHV1-wt–infected strain (EP155/CHV1-wt), p29-expressing strain (EP155/Twtp29), virus-free strain (EP155), CpMK1-KD strain (EP155/CpMK1-KD), and MyRV1-infected strain (EP155/MyRV1). Two independent transmission assays (two lanes per recipient) were conducted for each EP155 variant, and subcultures from the M positions were analyzed for RnVV1 accumulation (RnVV1) and dcl2 and agl2 expression levels (dcl2 and agl2).
Overall, we conclude that the strong interference with RnVV1 by CHV1-∆p69, MyRV1, and transgenically expressed dsRNA specifically occurs in conjunction with massive dcl2 induction.
Discussion
Various interactions have been reported in the C. parasitica/virus system: one-way synergism between CHV1 [nonsegmented (+) RNA genome] and MyRV1 (11-segmented dsRNA genome) (36), and the generation of MyRV1 genome rearrangements by CHV1 coinfection; transgenic expression of its RSS, p29; or deletion of host dcl2 or agl2 (37, 38). This study focused on different virus/virus interplays involving RnVV1 (undivided dsRNA genome) and showed that (i) MyRV1 and an RSS-lacking CHV1 mutant, CHV1-Δp69, interfere with the replication and lateral transmission of unrelated RnVV1 in WT C. parasitica; (ii) this interference is also mediated by transgene-derived dsRNA; (iii) disruption of dcl2 completely abolished the interference, whereas disruption of agl2 did not always do so; and (iv) the interference coincided with high levels of accumulation of the dcl2 and agl2 transcripts. Taken together, these results indicate that RnVV1 interference is mediated by RNA silencing activated by the interfering viruses or transgenic hairpin RNA, in which the primary player is DCL2. Such virus interference appears to operate at the intracellular replication level, as supported by the observation that invasive MyRV1 and CHV1-Δp69 inhibit replication of the preexisting RnVV1 in the WT EP155 host background (Fig. 4 and Fig. S1). The apparent inhibition of RnVV1 transmission (Figs. 3 and 5B and Fig. S3) is considered to be a manifestation of blocked RnVV1 replication.
The mechanisms for RNA silencing suppression by CHV1 are well-studied, but there is little understanding of how MyRV1 and RnVV1 avoid their host RNA silencing systems. Segment S10 of MyRV3, a member of the genus Mycoreovirus with a 12-segment genome, encodes an RSS (39). Although the suppressor activities of MyRV3 S10-coded VP10 against transgene silencing have been validated, those against virus infection remain enigmatic. Interestingly, MyRV1 (11-segment genome) has no counterpart to MyRV3 VP10. In addition, no synergistic interactions were found between MyRV1 and CHV1-Δp69 (Fig. S2), unlike between CHV1-wt and RnVV1 (Fig. 6). These results suggest that MyRV1 lacks an RSS. RnVV1 appears to be targeted by a steady-state level of RNA silencing (Figs. 1 and 3), but there is no evidence that RnVV1 encodes an RSS. In support of this, no RSS activities were detected in another totivirus, Magnaporthe oryzae virus 2 (MoV2) (40). The same authors identified MoV2 siRNAs derived from both the virus negative and positive strands. This suggests that genomic dsRNA serves as a template for DCL in the host fungus, Magnaporthe oryzae, but it then remains unclear how virus dsRNA, believed to be confined within particles, is exposed to DCL. Highly structured regions of viral mRNA appear to serve as DCL templates (41). Whether the same is true for totivirus mRNA also remains elusive.
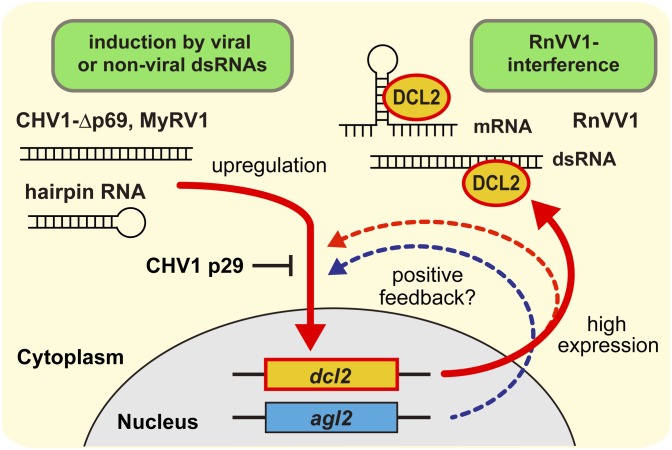
Considering the aforementioned points, we propose a model to account for the RnVV1 interference observed here in view of RNA silencing levels. In this model (Fig. 7), RNA silencing levels are determined based primarily on expression levels of AGL2 and DCL2, which are influenced by infection with CHV1-Δp69 and MyRV1. There are several key unanswered questions. Firstly, how do DCL2 and AGL2 exert differential effects on RnVV1 interference? Different levels of contribution of key RNA silencing players to antiviral defense were noted earlier in Drosophila melanogaster in which AGO2 and Dicer-2 (DCR2) play major roles in antiviral defense. AGO2 appears to make a greater contribution to defense against cricket paralysis virus (CrPV) than DCR2, when examined according to survival rate (42), whereas mutation of DCR2 has a slightly greater positive impact on flock house virus accumulation (10). In C. parasitica, RNA silencing-mediated RnVV1 interference did not always require AGL2 (Fig. 5 and Fig. S3), a phenomenon more prominent than the precedents in insect hosts. As long as dcl2 expression was sufficiently elevated, RnVV1 multiplication was impaired even in the absence of AGL2. AGL2 appears to indirectly contribute to RnVV1 interference by enhancing dcl2 transcription levels in certain conditions rather than by directly degrading RnVV1 mRNA. CHV1-Δp69 requires AGL2 for full-scale dcl2 elevation, but not MyRV1 (Fig. 5B, compare sets 2 and 3). The requirement for AGL2 for induction of dcl2 mRNA by CHV1-wt was reported by Sun et al. (24). An investigation of the mechanism underlying the augmentation of agl2 and dcl2 transcription in C. parasitica is a future challenge.
Fig. 7.
A model for the RnVV1 interference mediated by host RNA silencing. Activities of host (C. parasitica) RNA silencing are influenced by viruses. CHV1-∆p69, MyRV1, and transgenic dsRNA activate antiviral RNA silencing by transcriptional induction (up-regulation) of the dcl2 and agl2 genes. The total mass of DCL2 and AGL2 is correspondingly increased or decreased along with transcription levels, and thus silencing activity should be significantly different. DCL2 and AGL2 may be involved in the positive feedback regulation for activation of RNA silencing. RnVV1 infection does not induce such events as in the case of the virus-free state (basal RNA silencing). RnVV1 is highly susceptible to the C. parasitica RNA silencing pathway. RnVV1 dsRNAs and possibly structured mRNAs are effectively digested by DCL2, and this results in strong interference. This interference is possibly independent of the ssRNA cleavage activity by AGL2. The whole picture illustrates that RnVV1 replication is influenced by host RNA silencing activity, which is modulated by other viruses and dsRNAs.
Secondly, is the virus interference specific for certain virus/virus combinations? This study showed RnVV1 to be severely affected by RNA silencing, which was activated by the other two viruses. It is obvious that MyRV1 and CHV1-Δp69 can survive and establish coinfection at highly activated RNA silencing levels (Fig. S2). However, RnVV1 is highly susceptible to RNA silencing such that its accumulation was often below the detection limit for RT-PCR (Figs. 3 and 4). This may be associated with the apparent lack of an RnVV1 RSS, as discussed earlier. In this regard, how CHV1-Δp69 and MyRV1, apparently lacking RSSs, tolerate the activated antiviral state remains elusive. The high susceptibility of RnVV1 to RNA silencing may also be related to the fact that RnVV1 is from a heterologous fungus, R. necatrix (43), and has not adapted to the new host, C. parasitica. To examine this possibility, it would be interesting to compare the genome sequences and tolerance to RNA silencing of RnVV1 maintained for a long term by repeated subculturing in the natural and experimental hosts. Alternatively, RnVV1 may by nature be highly susceptible to RNA silencing. Support for this comes from the observation by Drinnenberg et al. (44) that Saccharomyces cerevisiae virus l-A (SsV-l-A) is highly susceptible to RNA silencing restored in S. cerevisiae. SsV-l-A is one of the best studied fungal viruses, which belongs to the same family, Totiviridae, as RnVV1 (45, 46). It should also be determined whether other viruses of C. parasitica origin are subject to RNA silencing-mediated interference in the same way as RnVV1.
Similarly, whether this interference is specific for the fungal host C. parasitica is another interesting question to address. The responsiveness of dcl2 and agl2 homologs upon virus infection has been poorly explored in other fungus/virus systems. In Neurospora crassa, these genes are induced by transgenically expressed hairpin RNAs. However, no virus has been isolated as an infectious entity in this fungus (18). Plants generally carry larger numbers of DCL and AGO genes than fungi, and some of them are functionally redundant. Plants have RDR genes to amplify sequence-specific silencing signals by converting aberrant RNAs into dsRNA as substrates for DCLs. These genes might be regulated transcriptionally and posttranscriptionally. However, only some genes have been reported to be up-regulated, generally by less than 10-fold, by infections by specific viruses (47, 48). This smaller magnitude of up-regulation of genes in plants may be compensated by the RDR6/DCL4-mediated amplification of viral siRNA that is functionally substitutable for RNA silencing activation in C. parasitica. Insects operate antiviral RNA silencing with a similar number of genes (DCR2, AGO2, R2D2) to those of C. parasitica (49). Insect genes responsible for antiviral RNA silencing are generally up-regulated by some viruses or hairpin RNAs (50–52). Importantly, there are, however, no examples of RNA silencing-mediated interference among unrelated viruses in plants or insects.
In plants, the RNA silencing-mediated cross-protection requires sequence homology between a preexisting, protective mild strain and a challenging severe strain (14, 16). siRNAs derived from an interfering virus and integrated into RISC-AGO target challenging viruses for degradation. Thus, the protective and challenge viruses must be closely related at the nucleotide sequence level, and the spectrum of this interference is rather narrow. However, a wide spectrum of RNA silencing-mediated antiviral defenses in plants was recently hypothesized by Ding and colleagues (53). These authors showed that virus infection activates the production of various siRNAs targeting over 1,000 host genes that include defense-related genes. As mentioned earlier, the virus interference in C. parasitica differs in several ways. In C. parasitica, as long as the dcl2 transcription level is sufficiently elevated by infection by heterologous viruses or transgenic expression of dsRNA (Fig. 7), no sequence homology is required between interfering elements and susceptible viruses. Viral inhibition most probably relies on the activities of DCL2 to cleave viral dsRNA or structured ssRNA sequence independently and does not always require AGL2 (Figs. 5B and 7). However, such RNA silencing-based effects can be impaired by viral counterdefense via RSSs such as CHV1 p29, which is able to suppress dcl2 and agl2 expression (Figs. 6 and 7). There are many plant genes whose transcription is responsive to and up-regulated by virus infection. The use of their promoters to generate transgenic plants overexpressing major antiviral RNA silencing genes may also result in tolerance against a wide range of virus infections.
Materials and Methods
Fungal and Viral Materials.
C. parasitica standard strain EP155 WT and its RNA silencing-deficient derivatives ∆dcl2 and ∆agl2 were used. The RnVV1 transfectant strain of C. parasitica ∆dcl2 (∆dcl2/RnVV1) was previously named A12 (54). The EP155 strains infected with CHV1-wt, CHV1-∆p69, or MyRV1 were regenerated from freeze stocks (EP155/CHV1-wt, EP155/CHV-∆p69, and EP155/MyRV1) (33, 34). These viruses were horizontally transferred into either EP155, ∆dcl2, or ∆agl2 from respective donor strains at least three times sequentially to eliminate possible heterokaryon formation (32). The host and viral strains used in this study are summarized in Table S1. The fungi were cultured at 22–27 °C on PDA plates (6 cm in diameter) on the bench-top for maintenance and phenotypic observations and in PDB liquid media (20 mL) in the dark for RNA preparation.
Table S1.
Viral and fungal strains used in this study
| Strain | Description | Reference or source |
| Viral | ||
| CHV1 | Prototype of the family Hypoviridae (strain EP713) | Shapira et al. (1991) (57) |
| CHV1-∆p69 | Version “b” of the ORF-A deletion mutants of CHV1 (∆p69b) | Suzuki et al. (2002) (33) |
| Mycoreovirus 1 (MyRV1) | Prototype of the genus Mycoreovirus (strain 9B21) | Suzuki et al. (2004) (58) |
| RnVV1 | A totivirus from R. necatrix (strain W1029) | Chiba et al. (2013) (54) |
| Fungal | ||
| EP155 | Virus-free standard strain of C. parasitica | ATCC 38755 |
| ∆dcl2 | dcl2 KO mutant of EP155 | Segers et al. (2007) (4) |
| ∆agl2 | agl2 KO mutant of EP155 | Sun et al. (2009) (24) |
| EP155/Twtp29 | EP155 expressing CHV1 p29 (RSS) | Sun et al. (2006) (36) |
| EP155/CpMK1-KD | EP155 knocked down for CpMK1 (a map kinase gene) | This study |
| EP155/CpMK1-KO | EP155 knocked out for CpMK1 | Park et al. (2004) (35) |
Plasmid Construction and Artificial RNA Silencing Induction.
The CpMK1 gene (accession no. AY166687, coding region 492–1773 consisting of four exons and three introns) was knocked down by transformation of EP155 using a hairpin construct, pCPXHY2-CpMK1-IR, which was constructed based on a conventional expression vector pCPXHY2 (55). This construct expresses an inverted-repeat messenger of the CpMK1 gene (targeting the 5′ region of the ORF) carrying sense (1–1748/Nco I) and directly connected antisense (492–1200) sequences (Fig. S4A). Transformation of C. parasitica protoplasts and subsequent selection with hygromycin B were performed as described previously (56). All transgenic strains were subjected to single conidial spore isolation, and representative homokaryon strains were selected for use (Fig. S4B).
Horizontal Virus Transmission.
Horizontal viral transmission via hyphal anastomosis was allowed by coculture of viral-donor and -recipient fungal strains (Fig. 2). Mycelial plugs from paired colonies were inoculated on a new PDA plate (9 cm in diameter) side-by-side and cultured for 7–10 d. Mycelial plugs from the growing edge of the donor and recipient sides were transferred to new PDA plates and grown for 3–5 d. These were further subjected to coculture when required.
RNA Extraction.
Mycelia-containing PDB media (20 mL) were cultured in the dark at 24 °C for 7 d, and fungal flora were obtained by filtration with Miracloth (Calbiochem). These were homogenized in liquid nitrogen, and the resultant powders were mixed with 2.5 mL of the RNA extraction buffer [200 mM NaCl, 100 mM Tris∙HCl (pH 8.0), 4 mM EDTA-2Na, 2% SDS (wt/vol)] and an equal volume of phenol/chloroform in a mortar. Aqueous layers were clarified with phenol/chloroform and subsequently with chloroform once each, followed by 2-propanol (equal volume) or lithium chloride (final 2 M) precipitation to obtain total RNA or ssRNA fractions, respectively. The resulting pellets were washed in 70% (vol/vol) ethanol and dissolved in 50 μL of sterilized water, and their concentrations were calculated by NanoDrop 2000 (Thermo Fisher Scientific). The ssRNA samples were subjected to Northern hybridization and RT-PCR analyses, whereas the total RNA samples were simply analyzed by agarose gel electrophoresis with or without nuclease treatments.
Northern Blotting.
Northern blot assays were performed as described by Chiba et al. (54). We heat-denatured 1–3 μg (for viruses) and 8–12 μg (for host gene and transgene messages) of ssRNA samples (68 °C for 10 min) and electrophoresed them in agarose gels containing formaldehyde in Mops buffer system. RNAs were capillary transferred onto nylon membranes (Hybond N+, Amersham) and fixed by UV Crosslinker CL-1000 (UVP). Specific DIG-labeled cDNA probes were prepared by PCR (PCR DIG Labeling mix, Roche) and hybridized with target RNAs in DIG easy Hyb buffer (Roche) at 42 °C overnight in a hybridization oven HB-500 Minidizer (UVP). The membranes were sequentially washed and treated with anti-DIG antibody (Roche) at a 1/10,000 concentration. Alkaline phosphatase-based chemiluminescent detection was then conducted using CDP-Star (Roche) in a dark room with film development or through digital imaging in the ImageQuant LAS 4000 system (GE Healthcare Life Sciences), or by (nitro-blue tetrazolium chloride)/BCIP (5-bromo-4-chloro-3′-indolylphosphate p-toluidine)-based coloring.
RT-PCR.
The first cDNA strands were synthesized by M-MLV reverse transcriptase (Takara) with virus-specific primers and oligo-(dT) from 1 μg of ssRNA as templates. PCR fragments of target sequences were amplified by QuickTaq (Takara) polymerase in optimal conditions in 25–30 cycles and were analyzed by agarose gel electrophoresis. Gels were stained by ethidium bromide (EtBr) and photographed under UV illumination in a Dolphin-Chemi system (Wealtec). All oligonucleotide primers used in this study are available upon request.
Acknowledgments
We are grateful to Drs. Donald L. Nuss, Bradley I. Hillman, Satoko Kanematsu, and Dae-Hyuk Kim for the generous gift of the fungal strains (EP155, Δdcl2, Δagl2, Cp9B21, W1029, and CpMK1-KO). We thank Drs. Ida Bagus Andika, Liying Sun, and Hideki Kondo for plasmid pCPXHY2-CpMK1-IR and their helpful comments on the manuscript. This study was supported in part by Yomogi Inc. and a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (KAKENHI 25252011 to N.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509151112/-/DCSupplemental.
References
- 1.Aliyari R, Ding SW. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol Rev. 2009;227(1):176–188. doi: 10.1111/j.1600-065X.2008.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bologna NG, Voinnet O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol. 2014;65:473–503. doi: 10.1146/annurev-arplant-050213-035728. [DOI] [PubMed] [Google Scholar]
- 3.Baulcombe D. RNA silencing in plants. Nature. 2004;431(7006):356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 4.Segers GC, Zhang X, Deng F, Sun Q, Nuss DL. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc Natl Acad Sci USA. 2007;104(31):12902–12906. doi: 10.1073/pnas.0702500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csorba T, Pantaleo V, Burgyán J. RNA silencing: An antiviral mechanism. Adv Virus Res. 2009;75:35–71. doi: 10.1016/S0065-3527(09)07502-2. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, Wang X, Ding SW. Viral suppressors of RNA-based viral immunity: Host targets. Cell Host Microbe. 2010;8(1):12–15. doi: 10.1016/j.chom.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csorba T, Kontra L, Burgyan J. 2015. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 479-480:85–103. [DOI] [PubMed]
- 8.Andika IB, et al. Differential contributions of plant Dicer-like proteins to antiviral defences against potato virus X in leaves and roots. Plant J. 2015;81(5):781–793. doi: 10.1111/tpj.12770. [DOI] [PubMed] [Google Scholar]
- 9.Jaubert M, Bhattacharjee S, Mello AF, Perry KL, Moffett P. ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol. 2011;156(3):1556–1564. doi: 10.1104/pp.111.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliyari R, et al. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe. 2008;4(4):387–397. doi: 10.1016/j.chom.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mourrain P, et al. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell. 2000;101(5):533–542. doi: 10.1016/s0092-8674(00)80863-6. [DOI] [PubMed] [Google Scholar]
- 12.Anandalakshmi R, et al. A viral suppressor of gene silencing in plants. Proc Natl Acad Sci USA. 1998;95(22):13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasschau KD, Carrington JC. A counterdefensive strategy of plant viruses: Suppression of posttranscriptional gene silencing. Cell. 1998;95(4):461–470. doi: 10.1016/s0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 14.Ziebell H, Carr JP. Cross-protection: A century of mystery. Adv Virus Res. 2010;76:211–264. doi: 10.1016/S0065-3527(10)76006-1. [DOI] [PubMed] [Google Scholar]
- 15.Kosaka Y, et al. Effectiveness of an attenuated Zucchini yellow mosaic virus isolate for cross-protecting cucumber. Plant Dis. 2006;90(1):67–72. doi: 10.1094/PD-90-0067. [DOI] [PubMed] [Google Scholar]
- 16.Ratcliff FG, MacFarlane SA, Baulcombe DC. Gene silencing without DNA. rna-mediated cross-protection between viruses. Plant Cell. 1999;11(7):1207–1216. doi: 10.1105/tpc.11.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterhouse PM, Wang MB, Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411(6839):834–842. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- 18.Eusebio-Cope A, et al. The chestnut blight fungus for studies on virus/host and virus/virus interactions: From a natural to a model host. Virology. 2015;477:164–175. doi: 10.1016/j.virol.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Hillman BI, Suzuki N. Viruses of the chestnut blight fungus, Cryphonectria parasitica. Adv Virus Res. 2004;63:423–472. doi: 10.1016/S0065-3527(04)63007-7. [DOI] [PubMed] [Google Scholar]
- 20.Nuss DL. Hypovirulence: Mycoviruses at the fungal-plant interface. Nat Rev Microbiol. 2005;3(8):632–642. doi: 10.1038/nrmicro1206. [DOI] [PubMed] [Google Scholar]
- 21.Kondo H, Kanematsu S, Suzuki N. Viruses of the white root rot fungus, Rosellinia necatrix. Adv Virus Res. 2013;86:177–214. doi: 10.1016/B978-0-12-394315-6.00007-6. [DOI] [PubMed] [Google Scholar]
- 22.Nuss DL. Mycoviruses, RNA silencing, and viral RNA recombination. Adv Virus Res. 2011;80:25–48. doi: 10.1016/B978-0-12-385987-7.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang DX, Spiering MJ, Nuss DL. Characterizing the roles of Cryphonectria parasitica RNA-dependent RNA polymerase-like genes in antiviral defense, viral recombination and transposon transcript accumulation. PLoS One. 2014;9(9):e108653. doi: 10.1371/journal.pone.0108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q, Choi GH, Nuss DL. A single Argonaute gene is required for induction of RNA silencing antiviral defense and promotes viral RNA recombination. Proc Natl Acad Sci USA. 2009;106(42):17927–17932. doi: 10.1073/pnas.0907552106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deleris A, et al. Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science. 2006;313(5783):68–71. doi: 10.1126/science.1128214. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Nuss DL. A host dicer is required for defective viral RNA production and recombinant virus vector RNA instability for a positive sense RNA virus. Proc Natl Acad Sci USA. 2008;105(43):16749–16754. doi: 10.1073/pnas.0807225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koonin EV, Wolf YI, Nagasaki K, Dolja VV. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat Rev Microbiol. 2008;6(12):925–939. doi: 10.1038/nrmicro2030. [DOI] [PubMed] [Google Scholar]
- 28.Koonin EV, Choi GH, Nuss DL, Shapira R, Carrington JC. Evidence for common ancestry of a chestnut blight hypovirulence-associated double-stranded RNA and a group of positive-strand RNA plant viruses. Proc Natl Acad Sci USA. 1991;88(23):10647–10651. doi: 10.1073/pnas.88.23.10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segers GC, van Wezel R, Zhang X, Hong Y, Nuss DL. Hypovirus papain-like protease p29 suppresses RNA silencing in the natural fungal host and in a heterologous plant system. Eukaryot Cell. 2006;5(6):896–904. doi: 10.1128/EC.00373-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki N, Maruyama K, Moriyama M, Nuss DL. Hypovirus papain-like protease p29 functions in trans to enhance viral double-stranded RNA accumulation and vertical transmission. J Virol. 2003;77(21):11697–11707. doi: 10.1128/JVI.77.21.11697-11707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki N, Chen B, Nuss DL. Mapping of a hypovirus p29 protease symptom determinant domain with sequence similarity to potyvirus HC-Pro protease. J Virol. 1999;73(11):9478–9484. doi: 10.1128/jvi.73.11.9478-9484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. A novel victorivirus from a phytopathogenic fungus, Rosellinia necatrix, is infectious as particles and targeted by RNA silencing. J Virol. 2013;87(12):6727–6738. doi: 10.1128/JVI.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki N, Nuss DL. Contribution of protein p40 to hypovirus-mediated modulation of fungal host phenotype and viral RNA accumulation. J Virol. 2002;76(15):7747–7759. doi: 10.1128/JVI.76.15.7747-7759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillman BI, Supyani S, Kondo H, Suzuki N. A reovirus of the fungus Cryphonectria parasitica that is infectious as particles and related to the coltivirus genus of animal pathogens. J Virol. 2004;78(2):892–898. doi: 10.1128/JVI.78.2.892-898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SM, et al. Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus-mediated perturbation of its phosphorylation in response to hypertonic stress. Mol Microbiol. 2004;51(5):1267–1277. doi: 10.1111/j.1365-2958.2004.03919.x. [DOI] [PubMed] [Google Scholar]
- 36.Sun L, Nuss DL, Suzuki N. Synergism between a mycoreovirus and a hypovirus mediated by the papain-like protease p29 of the prototypic hypovirus CHV1-EP713. J Gen Virol. 2006;87(Pt 12):3703–3714. doi: 10.1099/vir.0.82213-0. [DOI] [PubMed] [Google Scholar]
- 37.Sun L, Suzuki N. Intragenic rearrangements of a mycoreovirus induced by the multifunctional protein p29 encoded by the prototypic hypovirus CHV1-EP713. RNA. 2008;14(12):2557–2571. doi: 10.1261/rna.1125408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eusebio-Cope A, Suzuki N. Mycoreovirus genome rearrangements associated with RNA silencing deficiency. Nucleic Acids Res. 2015;43(7):3802–3813. doi: 10.1093/nar/gkv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaegashi H, Yoshikawa N, Ito T, Kanematsu S. A mycoreovirus suppresses RNA silencing in the white root rot fungus, Rosellinia necatrix. Virology. 2013;444(1-2):409–416. doi: 10.1016/j.virol.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Himeno M, et al. Significantly low level of small RNA accumulation derived from an encapsidated mycovirus with dsRNA genome. Virology. 2010;396(1):69–75. doi: 10.1016/j.virol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Molnár A, et al. Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J Virol. 2005;79(12):7812–7818. doi: 10.1128/JVI.79.12.7812-7818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Rij RP, et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20(21):2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaegashi H, et al. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol Ecol. 2013;83(1):49–62. doi: 10.1111/j.1574-6941.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 44.Drinnenberg IA, Fink GR, Bartel DP. Compatibility with killer explains the rise of RNAi-deficient fungi. Science. 2011;333(6049):1592. doi: 10.1126/science.1209575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickner RB, Fujimura T, Esteban R. Viruses and prions of Saccharomyces cerevisiae. Adv Virus Res. 2013;86:1–36. doi: 10.1016/B978-0-12-394315-6.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickner RB, Ghabrial SA, Nibert ML, Patterson JL, Wang CC. In: Family Totiviridae. Virus Taxonomy: Ninth Report of the International Committee for the Taxonomy of Viruses. King AMQ, Adams MJ, Carstens EB, Lefkowits EJ, editors. Elsevier, Academic Press; New York: 2011. pp. 639–650. [Google Scholar]
- 47.Du P, et al. Viral infection induces expression of novel phased microRNAs from conserved cellular microRNA precursors. PLoS Pathog. 2011;7(8):e1002176. doi: 10.1371/journal.ppat.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivaprasad PV, et al. The CaMV transactivator/viroplasmin interferes with RDR6-dependent trans-acting and secondary siRNA pathways in Arabidopsis. Nucleic Acids Res. 2008;36(18):5896–5909. doi: 10.1093/nar/gkn590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronkhorst AW, van Rij RP. The long and short of antiviral defense: Small RNA-based immunity in insects. Curr Opin Virol. 2014;7:19–28. doi: 10.1016/j.coviro.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Garbutt JS, Reynolds SE. Induction of RNA interference genes by double-stranded RNA; implications for susceptibility to RNA interference. Insect Biochem Mol Biol. 2012;42(9):621–628. doi: 10.1016/j.ibmb.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Kolliopoulou A, Smagghe G, Swevers L. Modulation of the transcriptional response of innate immune and RNAi genes upon exposure to dsRNA and LPS in silkmoth-derived Bm5 cells overexpressing BmToll9-1 receptor. J Insect Physiol. 2014;66:10–19. doi: 10.1016/j.jinsphys.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Xu Y, Zhou W, Zhou Y, Wu J, Zhou X. Transcriptome and comparative gene expression analysis of Sogatella furcifera (Horváth) in response to southern rice black-streaked dwarf virus. PLoS One. 2012;7(4):e36238. doi: 10.1371/journal.pone.0036238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao M, et al. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in Arabidopsis. Proc Natl Acad Sci USA. 2014;111(40):14613–14618. doi: 10.1073/pnas.1407131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. Effects of defective interfering RNA on symptom induction by, and replication of, a novel partitivirus from a phytopathogenic fungus, Rosellinia necatrix. J Virol. 2013;87(4):2330–2341. doi: 10.1128/JVI.02835-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craven MG, Pawlyk DM, Choi GH, Nuss DL. Papain-like protease p29 as a symptom determinant encoded by a hypovirulence-associated virus of the chestnut blight fungus. J Virol. 1993;67(11):6513–6521. doi: 10.1128/jvi.67.11.6513-6521.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi GH, Nuss DL. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992;257(5071):800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- 57.Shapira R, Choi GH, Nuss DL. Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991;10(4):731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki N, Supyani S, Maruyama K, Hillman BI. Complete genome sequence of Mycoreovirus-1/Cp9B21, a member of a novel genus within the family Reoviridae, isolated from the chestnut blight fungus Cryphonectria parasitica. J Gen Virol. 2004;85(Pt 11):3437–3448. doi: 10.1099/vir.0.80293-0. [DOI] [PubMed] [Google Scholar]