Significance
The molecular explanation for lactate or ethanol production by cells under aerobic conditions has remained a puzzle since first identified by Otto Warburg almost a century ago. To address this question, we reanalyzed 13C NMR measurements of yeast cells under aerobic conditions immediately after they were exposed to high glucose levels. We showed that the large amount of ethanol produced is due to glycogen and trehalose synthesis requiring glycolytic derived ATP despite aerobic conditions. This glycogen shunt maintains metabolic homoeostasis by preventing accumulation of glycolytic intermediates and glycolytic ATP production. The similarities between yeast and cancer cell metabolism suggest that the model can, under comparable conditions, explain the Warburg effect of lactate formation in aerobic cancer cells.
Keywords: Warburg effect, glycogen synthesis, Pasteur effect, glycolysis, homeostasis
Abstract
Aerobic glycolysis in yeast and cancer cells produces pyruvate beyond oxidative needs, a paradox noted by Warburg almost a century ago. To address this question, we reanalyzed extensive measurements from 13C magnetic resonance spectroscopy of yeast glycolysis and the coupled pathways of futile cycling and glycogen and trehalose synthesis (which we refer to as the glycogen shunt). When yeast are given a large glucose load under aerobic conditions, the fluxes of these pathways adapt to maintain homeostasis of glycolytic intermediates and ATP. The glycogen shunt uses glycolytic ATP to store glycolytic intermediates as glycogen and trehalose, generating pyruvate and ethanol as byproducts. This conclusion is supported by studies of yeast with a partial block in the glycogen shunt due to the cif mutation, which found that when challenged with glucose, the yeast cells accumulate glycolytic intermediates and ATP, which ultimately leads to cell death. The control of the relative fluxes, which is critical to maintain homeostasis, is most likely exerted by the enzymes pyruvate kinase and fructose bisphosphatase. The kinetic properties of yeast PK and mammalian PKM2, the isoform found in cancer, are similar, suggesting that the same mechanism may exist in cancer cells, which, under these conditions, could explain their excess lactate generation. The general principle that homeostasis of metabolite and ATP concentrations is a critical requirement for metabolic function suggests that enzymes and pathways that perform this critical role could be effective drug targets in cancer and other diseases.
A challenge to contemporary biological research was sounded by Steve McKnight, who noted (1) that “the low lying fruit that could be picked by molecular biologists without considering the metabolic state of the cell, tissue or organism is largely gone.” He proposed that now we must seek an understanding of the reciprocity between the regulatory state driven by signals from transcription factors and the dynamics of metabolic control. This was a timely call because the interactions of genetics and metabolism, developed in yeast research (2), was becoming paradigmatic in cancer studies (3–5). Because both cancer cells and yeast derive metabolites and energy from glucose, the relations of yeast metabolism with genetic expression, rather well understood in the glucose pathways, are providing an informative parallel for the study of cancer cells (6, 7).
The traditional approach attributes cellular metabolism to the kinetic properties of the component enzymes, as summarized in the comprehensive biochemistry textbooks, and then correlates changes in enzyme activity with differences in cell phenotype (3, 4). In our opinion, this approach neglects the interconnected nature of biochemical pathways that can be measured noninvasively, using magnetic resonance spectroscopy (MRS) (8–13), and the ability to quantitatively understand the sharing of biochemical control between these pathways using metabolic modeling methods such as metabolic control analysis (MCA). Of particular relevance to the present analysis, MCA has shown that maintaining metabolite homeostasis is of critical importance for cell survival and depends upon all of the pathways that produce and consume the metabolites in question, and not only on the one considered the “primary” pathway (14–18).
This paper analyzes carbon flows from glucose measured by 13C MRS in yeast to address the long-standing question as to why respiratory-competent yeast convert most of their glucose to ethanol rather than following the more efficient energy production of mitochondrial oxidation. This question originated in the mid-1920s when Otto Warburg (19) measured in yeast the fermentation of glucose to ethanol in fully oxidized, respiratory-competent yeast. Warburg also found that tumors, metabolizing glucose aerobically, similarly fermented most of their glucose to lactate, which, like the ethanol produced by yeast, is a measure of the yield of pyruvate by aerobic glycolysis (20–22). We find that the production of ethanol is driven by a coupling of the metabolic pathways of glycolysis, futile cycling, and glycogen/trehalose synthesis (which we refer to as the glycogen shunt). The glycogen shunt acts in concert with glycolysis and futile cycling to maintain homeostasis of glycolytic intermediates and to match ATP consumption and glycolytic production, which prevents the high glucose levels from creating excess ATP. The excess pyruvate produced is beyond the yeast’s other metabolic needs, resulting in ethanol accumulation.
We previously were able to explain aerobic lactate production in active muscle and brain through an analysis of how the glycogen shunt interacts with glycolysis during and between muscle contractions and neuronal firing. Although there are substantial differences from yeast, in all cases, the glycogen shunt acts to maintain metabolic and ATP homeostasis during quasi-steady-state conditions in which there are periodic large increases in glycolysis (23–25). Results in cancer cells suggest that, under at least transient conditions of high glucose, the glycogen shunt may play a similar important role for maintaining homeostasis and provide a basis for explaining excess lactate production.
Results
The measurements we analyzed (10) were made on respiratory-competent derepressed yeast cells, grown on acetate, soon after they were exposed to a high level of glucose in a nongrowth medium. These conditions are a variation of the conditions studied by Pasteur (20), often referred to as the Crabtree effect (22) or simply as the Warburg effect in yeast. The addition of glucose triggers both transcriptional and posttranscriptional changes in yeast that drastically affect metabolism (26). After several hours, due to both genetic transcription and posttranscriptional changes, the enzyme activities (27) in the cells reach a steady-state value appropriate for the new environment. However, before these changes, the cells adapt to the increased glucose level using allosteric and other nontranscriptional mechanisms. The data we use, shown in Fig. 1, are from previous measurements by 13C MRS of the metabolic fluxes and metabolite concentrations measured in vivo during the first 20 min after the introduction of glucose (8–13), before significant transcriptional modification of enzyme activities. Concentrations of relevant metabolites were generally measured in vivo by 13C MRS but, for a few end products, were measured by chemical analysis after extraction.
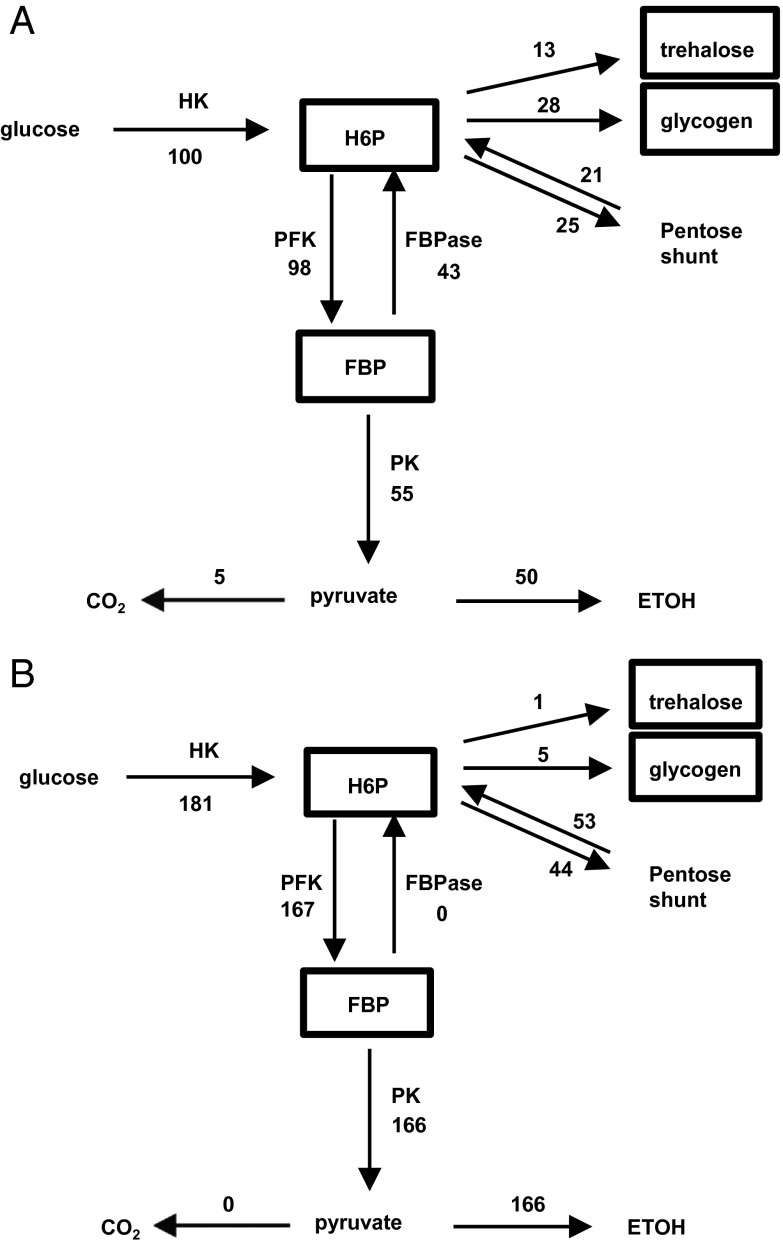
Fig. 1.
Metabolic fluxes of glucose consumption in yeast after exposure to high glucose levels under aerobic (A) and anaerobic (B) conditions. The fluxes are normalized to glucose uptake in the aerobic case, which is set to 100 (A). Respiratory-competent yeast grown on acetate were exposed to 50 mM glucose in a nongrowth medium. FBP, fructose 1, 6, bis phosphate; FBPase, fructose biphosphatase; HK, hexokinase; HMP, sum of hexose monophosphates glucose 6 phosphate, fructose 1 phosphate, and trehalose 6 phosphate; PFK, phosphofructokinase; PK, pyruvate kinase. Of particular note in the aerobic case is the high amount of glucose uptake (100) relative to the rate of oxidation in the TCA cycle to CO2 (5), with a large fraction converted to ETOH.
During the measurement period, the rate of glucose uptake was constant, indicating metabolic steady state (8, 9). Separate studies showed that the rate of glucose metabolism was constant throughout the 20 min after glucose addition until levels dropped below 2–3 mM (8, 9), indicating the ability of the system to maintain metabolic flux steady state and homeostasis of intermediate levels over a wide range of glucose concentration. Due to the ability of 13C MRS to follow the positional 13C enrichment in metabolites, measurements included metabolic pathway rates, as opposed to the majority of yeast studies to date, which only have measured the rate of glucose uptake and lactate/ethanol production. Fig. 1 compares the fluxes measured in the glycolytic and glycogen/trehalose synthesis pathways calculated from the 13C MRS data during the sudden exposure to high glucose in aerobic and anaerobic environments. In the aerobic state, in contrast to the anaerobic state, there is an almost twofold reduction in total glucose uptake and a significant storage of glucose as trehalose and glycogen. In both states, there is an active pentose shunt, measured by the loss of [1 − 13C] glucose label, which is larger in the anaerobic state, but which does not create a significant loss of glucose moieties because of flow back into the glycolytic pathway.
The Net Production per Glucose of ATP, NADH, and Pyruvate by the Glycogen Shunt.
We perform a mass balance calculation of the carbon, redox, and ATP flows in the glycogen shunt, glycolysis, and futile cycling to determine the fractional production of pyruvate, NADH, and glycogen/trehalose per glucose. Our previous application of this analysis to muscle and brain (23–25) was able to explain aerobic lactate production as a consequence of the relative fluxes of the glycogen shunt (both synthesis and degradation) and glycolysis.
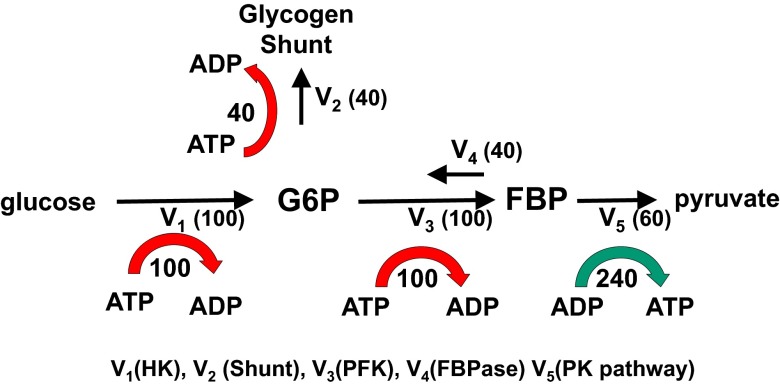
The model system for yeast under aerobic conditions is shown in Fig. 2. For clarity, we omit the effect of the pentose shunt, which removes only a small amount of carbon from the system and does not require ATP. We performed mass balance analysis on the reactions catalyzed by hexokinase (HK), phosphofructokinase (PFK), fructose bis phosphatase (FBPase), the distal to FBP segment of glycolysis that contains pyruvate kinase (PK), and the glycogen shunt, which is the combined synthetic flow of G6P into glycogen and trehalose via glycogen synthase (GS) and trehalose 6 phosphate synthase (TPS1). In the equations below, the flux through each step in Fig. 2 is expressed as a fraction of total glucose uptake. The glycogen shunt flux is expressed as the fractional rate X2 = V2/V1. The fractional rate of PFK is given by X3 = V3/V1 and of PK is given by X5 = V5/V1. The reverse rate from FBP back to F6P by FBPase is given by X4 = V4/V1. The overall carbon, redox, and ATP balance per glucose for the system can be obtained by writing balance equations for HK, PFK, FBPase, the glycogen shunt, and PK and substituting for the fractional PK flux based on mass balance X5 = 1 − X2. Similarly, by mass balance, the difference between the fractional flux through PFK and FBPase is given by (X3 − X4), which is the same as the fractional flux through PK, which equals (1 − X2). Making those substitutions and adding the balance equations yields
| [1] |
The experimentally measured fractional flux through PFK (X3) was ∼1. Substituting into Eq. 1 yields
| [2] |
Substituting the measured value of the glycogen shunt (X2 ≈ 0.4) into Eq. 3 yields the net stoichiometry of the system,
| [3] |
The canonic production of ATP, NADH, and pyruvate by glycolysis is recovered when FBPase and GS/TPS1 are set to 0, as is the case during anaerobic glycolysis (Fig. 1B). Examination of Eq. 3 shows that net ATP produced by glycolysis is completely used to support the glycogen shunt. Other energetic needs of the cell are met by the oxidation of ∼5% of the total glucose uptake (see Fig. 1A). The small fraction of glucose that is completely oxidized, based on the standard stoichiometry for ATP oxidation, produces ATP at a rate similar to that of glycolysis during the anaerobic conditions (Fig. 1B).
Fig. 2.
The glycogen shunt maintains ATP, G6P, and FBP homeostasis. The diagram shows the relative fluxes calculated for glycolytic pathway, futile cycling, and the glycogen shunt required to maintain glycolytic intermediate and ATP homeostasis. The rate of ATP production or consumption for HK (V1), glycogen shunt (V2), PFK (V3), and the PK segment of glycolysis (V5) are shown. Comparison with Fig. 1 shows that within measurement error, the relative fluxes measured in yeast under aerobic conditions are the same as the theoretical ideal values. The red arrows indicate the consumption of ATP by the demand portion of the pathway, and the green arrow indicates production by the supply portion. Adding the supply and demand together shows that there is no net production or consumption of ATP in the system.
In Vivo Fluxes During Aerobic Glycolysis Ensure ATP, FBP, and G6P Homeostasis.
Eq. 2 indicates that when the fractional glucose storage by the glycogen shunt (X2) is larger than the measured value of 0.4, then external ATP is needed to fuel the glycogen shunt. Similarly, if X2 is smaller than 0.4, then ATP is generated by the system. Therefore, when futile cycling (X4) equals the glycogen shunt, only the measured value of X2 of ∼0.4 does not produce or consume net ATP. In the Discussion, we will describe how net ATP production by the system could be deleterious to cell survival under the conditions of a sudden glucose load.
The flux ratios calculated above can also be derived from the principle of maintaining steady state of G6P, FBP, and ATP. The resultant steady-state equations are given by.
| [4] |
| [5] |
| [6] |
| [7] |
Solving the above equations for V2 in terms of V1 and V4 and setting V1 to 100 yields
| [8] |
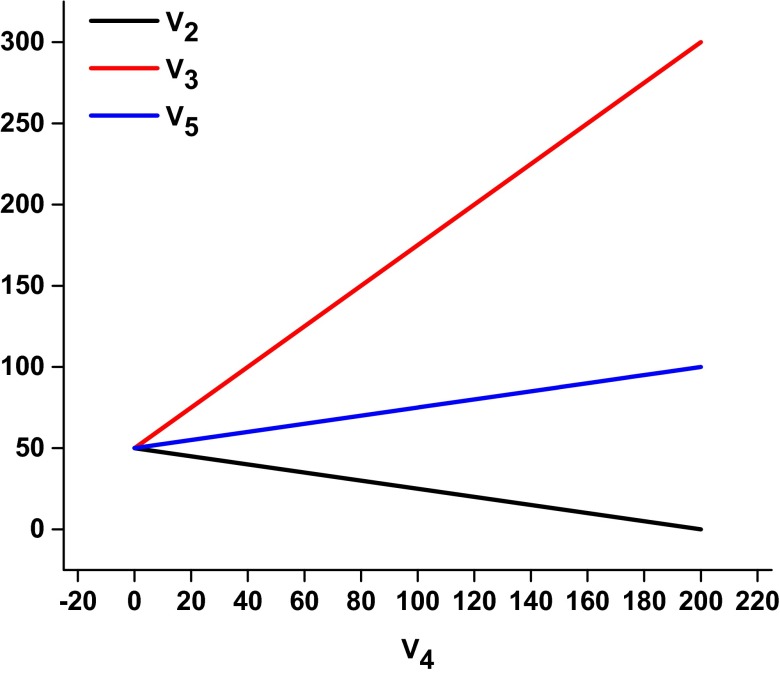
Eq. 8 shows that the FBPase flux (V4) determines the rate of V2. For the in vivo case of V2 = V4 the only value that satisfies Eq. 8 is V2 = V4 = 40 (X2 = X4 = 0.4). Similar equations can be derived relating V2 and V5 to the rates of V1 and V4. These equations are plotted in Fig. 3. Although a higher rate of glycogen/trehalose synthesis would occur if there was no FBPase flux (V4 = 0, V2 = 50), as described in the Discussion, the FBPase flux may be necessary for maintaining metabolic control of the system.
Fig. 3.
Calculated relative flux values (with glucose phosphorylation set to 100 units) for the glycogen shunt (V2), PFK (V3), and PK (V5) as a function of the futile cycling flux through FBPase (V4). It is seen that when the system is constrained so that there are no changes in HMP, FBP, and ATP concentrations, the rate of futile cycling and the requirement for homeostasis determine the other fluxes in the system.
Discussion
The Glycogen Shunt Maximizes Glucose Storage Without Requiring Oxidative ATP.
The glycogen shunt stores 40% of the glucose as readily available future energy in glycogen/trehalose. As shown by the equations, and for the measured condition of FBPase being the same as glycogen/trehalose storage, this is the only value for V2 that will maintain homeostasis of G6P, FBP, and ATP for the isolated system. The use of glycolytic ATP to store glycogen/trehalose results in the production of excess pyruvate and NADH beyond the needs of the cell, which are then converted to ethanol and NAD+. The small fraction of the pyruvate that is oxidized via the TCA cycle is similar to the rate of the TCA cycle measured before the addition of glucose when the carbon source was acetate (28). This fermentation to ethanol in yeast, and similarly aerobic lactate production in cancer cells, has been described as an inefficient energy pathway compared with complete oxidation. These results show that fermentation stores carbon as glycogen/trehalose for future use, not simply burning it to CO2. From this perspective, fermentation is not a wasteful pathway but a valuable storage of potential energy from glucose, resolving Warburg’s paradox in yeast.
The Glycogen Shunt Maintains Homeostasis of Glycolytic Intermediates.
Examination of Fig. 2 and the mass balance and steady-state equations shows that the glycogen shunt, consistent with the activation of yeast GS by G6P (29), takes up excess G6P that would otherwise accumulate due to the mismatch of the HK and PK segments of the pathway. We (30, 31) and others (32) have shown that glycogen synthesis in muscle similarly prevents G6P accumulation in mammalian muscle when glucose entry is increased rapidly by high postmeal insulin levels. The importance of the homeostatic role of the glycogen shunt has been shown dramatically in yeast by a lethal mutant of the cif1 gene (33) that led to cell death in a glucose medium despite normal enzymatic activity in the glycolytic pathway. Studies of the cif1 mutant and trehalose by Thevelein and Hohmann (7) showed that the mutations were in the TPS1 enzyme (trehalose phosphate synthase), considered a paradoxical finding at the time, because that enzyme has no direct role in the glycolytic pathway. However, a 31P MRS study of yeast with cif mutations (33) grown in a glucose-free medium found that under aerobic conditions, when they were challenged with a glucose load, they had large increases in G6P relative to wild type (0.1 mM WT, 5.4 mM cif). There were also larger increases in FBP (6.8 mM WT, 48.9 mM cif), and a drop in the ATP concentration to 1/5 of WT levels due to sequestration of phosphate in the glycolytic intermediate pools.
Blocking the flow of hexose monophosphates (HMP) into trehalose by a mutation illustrates how the shunt stores the glycolytic intermediates resulting from the mismatch between fluxes in the HK and PK regions. In addition, the cif mutation blocks the synthesis of trehalose-6-phosphate (T6P), which is an allosteric inhibitor of yeast HK (7), furthering the loss of homeostasis by preventing feedback inhibition of the supply flux into the HMP pool. The mismatch between HK and PK (shown in Fig. 1 by the glycolytic flux of 100 units through HK being reduced to ∼60 through PK) would, in the absence of a shunt, lead to a continuous increase of phosphorylated, glycolytic intermediates until ATP levels collapsed, leading to cell death. This example illustrates the limitations of examining metabolic pathways based only on the kinetics of enzymes in a single pathway of interest without taking interactions with pathways sharing common intermediates into account. As shown theoretically by MCA (34) and experimentally by this and many other examples, to retain viability, the cell needs to maintain metabolite homeostasis throughout the cellular metabolic network.
The Glycogen Shunt Protects Against Glucose-Driven Excessive ATP Production.
Respiratory-competent yeast switched from acetate to high glucose have the capacity to use glycolysis to generate an abundance of additional ATP, beyond that generated by mitochondrial oxidation of acetate in their initial condition. In anaerobic conditions, where there is little glycogen and trehalose synthesis, as shown in Fig. 1B, the lethality of cif mutations has been analyzed by Westerhoff and Teusink and colleagues (35). They found that the excessive glycolytic intermediate accumulation could be partially explained by the loss of inhibition of HK due to the block in T6P synthesis. However, they also found that the rate of accumulation of glycolytic intermediates was worsened by an increase in the ATP/ADP ratio due to excessive ATP production by glycolysis, further accelerating the rate of glucose phosphorylation, which they called the turbo effect.
Recently (36), Teusink and colleagues showed that although it was not possible, under all conditions, to explain the properties of the system based upon HK and TPS1 kinetics, the behavior of the system could still be explained by supply and demand pathway analysis. With the additional pathways of futile cycling and glycogen/trehalose synthesis revealed by the rich NMR data, the balance of supply and demand pathways also explains the ability to maintain homeostasis under the conditions studied here of yeast being challenged with glucose under aerobic conditions, which was first studied by Crabtree (22). ATP supply from the PK pathway is balanced by ATP demand in the HK, futile cycling (PFK, FBPase), and the glycogen/trehalose synthesis pathways. This balance, which suggests that the local ATP/ADP level is sensed within the system, protects against excess, glucose-driven ATP production. ATP for other cellular functions is supplied by glucose oxidation, and, within experimental error, the production of oxidative ATP from glucose was similar to what was measured experimentally when the cells were in an acetate-containing medium (28). In principle, the yeast cell could support glycogen/trehalose synthesis more efficiently by supplying the ATP needed via glucose oxidation in the TCA cycle, which would only require a small increase in the TCA cycle rate. However, the isolation of glycolytic ATP production to meet the needs of glycogen/trehalose synthesis suggests that preventing the risk of excess glycolytic ATP production is more important for survival than maximizing efficiency of glucose use.
Potential Metabolic Control Points of the System.
Although complete MCA studies under the conditions described remain to be performed, some insight into control can be obtained by examining the relative fluxes measured in the different segments of the pathways involved. Fig. 3 shows that if homeostasis is maintained, there are only fixed ranges of relative values allowed for the glycogen shunt, futile cycling, and the PK portion of glycolysis. From the standpoint of the supply and demand pathway analysis of Hofmeyr and colleagues (15–17), around the conservation of the ATP moiety (see steady-state equations), the pathway in Fig. 2 can be broken up into a demand side (HK/PFK/GS/TPS1) and a supply side consisting of the PK branch. However, the control mechanisms coupling these branches remain to be elucidated. Some insight into control may be obtained based upon previous studies that have shown that PFK under similar conditions has minimal control over the glycolytic flux, as does GS and TPS1 over the shunt flux (29, 31). Based on elimination, the relative fluxes between the pathways in the system are most likely determined by PK and FBPase. In yeast, the predominant form of pyruvate kinase, Pyk1p, is controlled by the PYK1 gene. Yeast defective in this gene fail to grow on glucose, but they can grow on ethanol or other gluconeogenic carbon sources (37). Boles et al. (37) discovered the existence of an isoform of PK, Pyk2p, which they reported “is subject to glucose repression and… is catalytically almost insensitive to FBP.” They suggested that this inactive form of PK was needed to prevent further glycolytic flux and to support the glycogenesis that prevailed before glucose addition. The present results suggest that the low sensitivity of Pyk2p to FBP allows it to maintain flux control during the high-glucose transition period and send 40% of the flux entering FBP back to glycogen/trehalose synthesis.
Evidence from Previous Studies for Lack of Flux Control by PFK.
Because the activity of PFK exhibits extensive allosteric regulation, it has traditionally been assumed to control the glycolytic flux. Although formal flux control studies were not performed in the present case, several studies have shown, under similar conditions, that PFK exerts little flux control (38–40). For example, Schaaff et al. (39) changed the amount of enzyme expressed in Escherichia coli, and Davies and Brindle (40), using plasmids, overexpressed PFK in yeast, all of which showed no change in the rate of glycolysis. The absence of flux control by PFK, and its role in the absence of such control, can be understood from the quantitative experimental evaluation of the allosteric effectors of PFK made under the conditions reported here showing how PFK adapts to match the flux input from HK rather than controlling the glycolytic flux (13).
Potential Role of Futile Cycling at FBPase in Maintaining FBP Homeostasis.
In principle, steady state conditions can be met with no futile cycling, resulting in greater glycogen and trehalose storage. The lower efficiency due to futile cycling may reflect the inability of the yeast to inactivate FBPase by transcriptional regulation over the short time period after glucose addition during which the measurements took place. However, as shown in Fig. 3, the fluxes in the system are determined by the flux through FBPase. In addition, futile cycling may be important for allowing feedback from the PK supply pathway to the proximal demand pathways because, in the absence of FBPase, there is no way to divert the flux from FBP to glycogen/trehalose synthesis. Instead, it would be necessary for accumulation of FBP to inhibit PFK, resulting in a greater fraction of glucose carbon going directly from G6P to glycogen. The resultant high level of FBP, even under homeostatic conditions, may negatively impact metabolic regulation.
The Glycogen Shunt Under Conditions of Anaerobic Glycolysis.
Fig. 1B shows the fluxes measured under anaerobic conditions where there is no futile cycling and a low synthesis of glycogen/trehalose. Approximately half of the ATP produced by the supply PK branch of glycolysis is fueling ATP needs outside of glycolysis and the glycogen shunt. As shown by Teusink et al. (35), under these conditions, yeast protect themselves from excessive ATP production and G6P accumulation via inhibition of HK by T6P (and potentially trehalose and glycogen synthesis, as in the aerobic glycolysis case). However, the intrinsic protection against ATP overproduction provided by the match between supply and demand fluxes observed under aerobic conditions is lost.
The Glycogen Shunt in Yeast, Brain, and Muscle.
We have previously used the glycogen shunt to explain aerobic lactate production in activated muscle and brain cells (23–25). In brain and muscle, glycogen supplies the substrate for rapid glycolytic energy needed during contraction (muscle) or glutamate uptake (brain). During the intercontraction/firing period, glucose uptake and glycogen synthesis rebuilds the glycogen lost in the process. By using glycogen to produce G6P during the contraction, glucose transport activity can be kept low and low intercontraction levels of glucose and G6P can be maintained. Because little energy is needed for glycogen resynthesis, only a small amount of the lactate generated from glycogen is oxidized to produce ATP, resulting in excess lactate that can be shuttled to other cells for oxidation (41). Although energetically unfavorable, the glycogen shunt allows muscle and brain to have relatively low levels of glucose, allowing tight control of G6P concentration, a critical first step for several pathways.
In yeast, the glycogen shunt also provides an important homeostatic function. By storing excess G6P, which accumulates due to the sudden large increase in glucose, it buffers the G6P pool, similar to what we have observed with insulin-stimulated muscle glycogen synthesis (31, 32). ATP homeostasis is maintained, and therefore the turbo effect is prevented (35), by the match between ATP consumption and production. The excess ethanol that is produced in yeast from pyruvate is then released or, in the case of lactate in mammalian cells, is oxidized by neighboring cells or goes to the liver to be converted to glucose in the Cori cycle (41). The required stoichiometry for having no net ATP production or consumption by the system results in pyruvate production beyond that needed for oxidative ATP generation, a production that is converted to ethanol. The balance between glycolytic ATP production and consumption supports glycogen/trehalose synthesis as the primary purpose of the excess glycolytic pyruvate/ethanol production. That conclusion differs from previous proposals that the purpose of excess pyruvate/ethanol generation is to increase intermediates for biosynthesis [e.g., the pentose phosphate shunt (3, 4)] or from the suggestion that it is needed for specialized cellular reactions that require glycolytic ATP (42).
Potential for a Similar Role of PK in Cancer as in Yeast.
The presence in yeast of forms of PK sensitive and insensitive to FBP potentially allows PK to exert flux control over both the glycogen shunt (the excess energy storage of the backward flow) and the demand portion (the excess energy production to pyruvate) of the glycolytic pathway. The form of PK found in mammalian cells with a high growth rate, such as cancer cells, is PKM2, which exists either as an active tetramer with a high activity for its substrate, phosphoenol pyruvate (PEP), or as a low-affinity, inactive dimer. The activity and fractional existence of the tetramer are regulated by early-stage metabolic intermediates such as FBP, amino acids, and possibly genetic factors (43). FBP at high concentrations can convert the enzyme from the inactive dimer to the active tetramer. However, if FBP concentrations are not allowed to rise to high levels, as is the case in yeast due to the combined action of the glycogen shunt and futile cycling, PK can potentially play an important role in controlling pyruvate and ATP production and glycogen synthesis. The presence of a high fraction of inactivated PK in cancer cells is consistent with this possibility, although it would be paradoxical from the viewpoint of cancer cells wanting maximal glycolytic energy production. The quandary has been summarized as “these studies support a model in which the ability of PKM2 to be inactivated is important for cancer cell proliferation” (44).
The additional function of PK to divert glucose carbons into glycogen storage fueled by glycolytic ATP would resolve the quandary of Mazurek (43) that “Due to the high rate of lactate production in tumor cells it would at first glance appear paradoxical that in tumor cells PKM2 was found to be mainly in the inactive dimeric form.” The active form of PK would allow the formation of glycolytic ATP that would provide energy for the formation of glycogen, whose carbon molecules were formed from the back flux through the inactive form of PK. This differs from the suggestion by Eigenbrodt and Glossman in 1980 (42) that the low-PEP-affinity dimeric form leads to an accumulation of glycolytic intermediates ahead of the PK reaction, to provide metabolic precursors for biosynthesis. Although these explanations are not mutually exclusive, the activities of the glycogen shunt must be evaluated before any of the back flow can be assigned to biosynthesis.
Answering Warburg’s Paradox for Yeast and Cancer Cells.
Warburg’s puzzlement came from his assumption that rapidly dividing cells, such as cancer cells and yeast, would maximize their energy production by full oxidation to support their enhanced biosynthesis. However, it was an assumption about what would be valuable for a cell that we now see is trumped by the fundamental cellular need to maintain metabolite homeostasis during changing conditions. Homeostasis of glycolytic intermediates and ATP is maintained by the coupling of the glycogen shunt to glycolysis and futile cycling (Fig. 2). The importance of homeostasis is shown by the glucose toxicity induced in the cif mutants by the loss of trehalose synthase activity, in which glycolytic intermediates continue to rise until ATP levels collapse due to lack of available Pi for synthesis. Homeostasis is also maintained by the ratios of the glycogen shunt flux to the fluxes of glycolysis and futile cycling leading to no net ATP synthesis, despite the surge in glucose supply, preventing accelerated loss of homeostasis and cell death via the turbo effect (35). Furthermore, the consequences of the shunt make a valuable contribution to the future energy needs of the cell.
Potential Role of the Glycogen Shunt in Cancer Cells.
Due to similarities of yeast and cancer cells, the glycogen shunt observed in yeast could play a similar mechanistic role for maintaining homeostasis in cancer cells exposed to changes in substrate. There are a number of papers that have shown that glycogen is important for cancer cell growth (5, 45). Schwartz et al., in 1975 (46), reported the time course of the disappearance of a 5-mM glucose load followed by the lagging formation and disappearance of glycogen in a suspension of C-6 glioma cells. These results, in which the glycogen was first formed from glucose and then served as an energy source, are in accord with the observations in yeast and the expectation of subsequent glycogenolysis. These examples cannot be considered to be universal explanations of the different conditions of the Warburg effect. However, they do illustrate that the glycogen shunt provides a valuable concept for studying the conditions of the Warburg effect where cancer cells face a glucose load.
Conclusions
We have analyzed the extensive flux measurements by 13C and 31P MRS in yeast during the aerobic glycolysis to address the long-standing questions of why the yeast only partially oxidize the glucose they take up and why mutations in the glycogen/trehalose pathway are lethal under these conditions. We summarize here the conclusions of this study. (i) The explanation of excess ethanol production noted by Warburg and others in aerobic yeast exposed to high glucose is the coupling of the energy requirements of the glycogen shunt to net ATP production by glycolysis. Due to the inefficiency of glycolytic ATP production, excess pyruvate above the cell’s need is made, which is converted to ethyl alcohol (ETOH). (ii) As shown by the lethality of the cif mutation, the glycogen shunt prevents the cellular toxicity that would have been the consequence of simply catabolizing the glycolytic load, and it does so without perturbing cellular energetic needs. (iii) The relative rates of the glycogen shunt, glycolysis, and futile cycling ensure that close to the maximum fraction of glucose taken up is stored for future energy needs without requiring ATP generated by oxidation. (iv) The flux through the glycogen shunt is most likely determined by the enzymes PK and FBPase. (v) The many similarities between glucose metabolism in cancer cells and yeast suggest a similar role for PK and the glycogen shunt in tumors under conditions of high glucose. (vi) The general principle that homeostasis of metabolite concentrations is a critical requirement for metabolic function suggests that enzymes and pathways that perform this role could be effective drug targets in cancer and other diseases.
Acknowledgments
We thank Jannie Hofmeyr for significant comments on the presentation and analysis of the results and contribution of the steady-state analysis equations and Fig. 3. We also thank Kevin Brindle, Henk De Feyter, Graeme Mason, Fahmeed Hyder, and Kevin Behar for helpful suggestions on the manuscript and Henk De Feyter for assistance in manuscript preparation. The experimental data used in this paper were obtained approximately 30 years ago by a dedicated group of R.G.S.’s colleagues at Bell Telephone Laboratories and then at Yale. Jan den Hollander had a guiding presence in a group that included continuing, significant contributions from Gil Navon, Kamil Ugurbil, Jeffrey Alger, Truman R. Brown, Jacqueline K. Barton, and Sharon Campbell-Burk, and included valuable, specific contributions from David Reibstein, Johan M. Thevelein, and John J. Hopfield. The early research was supported by the Bell Telephone Laboratories, and the later research at Yale was supported by National Institutes of Health Grant 5R01DK027121.
Footnotes
The authors declare no conflict of interest.
References
- 1.McKnight SL. On getting there from here. Science. 2010;330(6009):1338–1339. doi: 10.1126/science.1199908. [DOI] [PubMed] [Google Scholar]
- 2.Thevelein JM. Fermentable sugars and intracellular acidification as specific activators of the RAS-adenylate cyclase signalling pathway in yeast: The relationship to nutrient-induced cell cycle control. Mol Microbiol. 1991;5(6):1301–1307. doi: 10.1111/j.1365-2958.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011;14(4):443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favaro E, et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012;16(6):751–764. doi: 10.1016/j.cmet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Ruiz R, Rigoulet M, Devin A. The Warburg and Crabtree effects: On the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta. 2011;1807(6):568–576. doi: 10.1016/j.bbabio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Thevelein JM, Hohmann S. Trehalose synthase: Guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20(1):3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 8.den Hollander JA, Shulman RG. 2005. Futile cycling in yeast: How to control gluttony in the midst of plenty. Metabolomics by In Vivo NMR, eds Shulman RG, Rothman DL (John Wiley, New York), pp 137–148.
- 9.den Hollander JA, Shulman RG. 13C NMR studies of in vivo kinetic rates of metabolic processes. Tetrahedron. 1983;39(21):3529–3538. [Google Scholar]
- 10.denHollander JA, et al. Studies of anaerobic and aerobic glycolysis in Saccharomyces cerevisiae. Biochemistry. 1986;25(1):203–211. doi: 10.1021/bi00349a029. [DOI] [PubMed] [Google Scholar]
- 11.den Hollander JA, Ugurbil K, Shulman RG. 31P and 13C NMR studies of intermediates of aerobic and anaerobic glycolysis in Saccharomyces cerevisiae. Biochemistry. 1986;25(1):212–219. doi: 10.1021/bi00349a030. [DOI] [PubMed] [Google Scholar]
- 12.Campbell-Burk SL, den Hollander JA, Alger JR, Shulman RG. 31P NMR saturation-transfer and 13C NMR kinetic studies of glycolytic regulation during anaerobic and aerobic glycolysis. Biochemistry. 1987;26(23):7493–7500. doi: 10.1021/bi00397a044. [DOI] [PubMed] [Google Scholar]
- 13.Reibstein D, den Hollander JA, Pilkis SJ, Shulman RG. Studies on the regulation of yeast phosphofructo-1-kinase: Its role in aerobic and anaerobic glycolysis. Biochemistry. 1986;25(1):219–227. doi: 10.1021/bi00349a031. [DOI] [PubMed] [Google Scholar]
- 14.Fell DA. Understanding the Control of Metabolism. Portland Press; London: 1997. [Google Scholar]
- 15.Hofmeyr JH, Westerhoff HV. Building the cellular puzzle: Control in multi-level reaction networks. J Theor Biol. 2001;208(3):261–285. doi: 10.1006/jtbi.2000.2216. [DOI] [PubMed] [Google Scholar]
- 16.Hofmeyr JH, Rohwer JM. Supply–demand analysis: A framework for exploring the regulatory design of metabolism. Methods Enzymol. 2011;500:533–554. doi: 10.1016/B978-0-12-385118-5.00025-6. [DOI] [PubMed] [Google Scholar]
- 17.Hofmeyr JS, Cornish-Bowden A. Regulating the cellular economy of supply and demand. FEBS Lett. 2000;476(1-2):47–51. doi: 10.1016/s0014-5793(00)01668-9. [DOI] [PubMed] [Google Scholar]
- 18.Kascer H, Burns JA. 1973. The control of flux. Symp Soc Exp Biol 27:65–104.
- 19.Warburg O. Uber die Wirkung von Blausaureathylester (Athylcarbylamin) auf die Pasteurische Reaktion. Biochem Z. 1926;172:432–444. [Google Scholar]
- 20.Racker E. 1974. History of Pasteur effect and its pathobiology. Mol Cell Biochem 5(1-2):17–23.
- 21.Meyerhof O. Uber den Einfluss des Sauerstoffs auf die alkoholische Garung der Herfe. Biochem Z. 1925;162:43–86. [Google Scholar]
- 22.Crabtree HG. The carbohydrate metabolism of certain pathological overgrowths. Biochem J. 1928;22(5):1289–1298. doi: 10.1042/bj0221289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98(11):6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001b;14(7-8):389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- 25.Shulman RG, Rothman DL. The “glycogen shunt” in exercising muscle: A role for glycogen in muscle energetics and fatigue. Proc Natl Acad Sci USA. 2001;98(2):457–461. doi: 10.1073/pnas.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daran-Lapujade P, et al. The fluxes through glycolytic enzymes in Saccharomyces cerevisiae are predominantly regulated at posttranscriptional levels. Proc Natl Acad Sci USA. 2007;104(40):15753–15758. doi: 10.1073/pnas.0707476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolland F, Winderickx J, Thevelein JM. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002;2(2):183–201. doi: 10.1111/j.1567-1364.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 28.den Hollander JA, Behar KL, Shulman RG. 13C NMR study of transamination during acetate utilization by Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1981;78(5):2693–2697. doi: 10.1073/pnas.78.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskaran S, Roach PJ, DePaoli-Roach AA, Hurley TD. Structural basis for glucose-6-phosphate activation of glycogen synthase. Proc Natl Acad Sci USA. 2010;107(41):17563–17568. doi: 10.1073/pnas.1006340107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shulman RG, Rothman DL. 13C NMR of intermediary metabolism: Implications for systemic physiology. Annu Rev Physiol. 2001;63:15–48. doi: 10.1146/annurev.physiol.63.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Schafer JR, Fell DA, Rothman D, Shulman RG. Protein phosphorylation can regulate metabolite concentrations rather than control flux: The example of glycogen synthase. Proc Natl Acad Sci USA. 2004;101(6):1485–1490. doi: 10.1073/pnas.0307299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palm DC, Rohwer JM, Hofmeyr JH. Incorporating covalent and allosteric effects into rate equations: The case of muscle glycogen synthase. Biochem J. 2014;462(3):525–537. doi: 10.1042/BJ20140196. [DOI] [PubMed] [Google Scholar]
- 33.Navon G, et al. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- 34.Kacser H, Acerenza L. A universal method for achieving increases in metabolite production. Eur J Biochem. 1993;216(2):361–367. doi: 10.1111/j.1432-1033.1993.tb18153.x. [DOI] [PubMed] [Google Scholar]
- 35.Teusink B, Walsh MC, van Dam K, Westerhoff HV. The danger of metabolic pathways with turbo design. Trends Biochem Sci. 1998;23(5):162–169. doi: 10.1016/s0968-0004(98)01205-5. [DOI] [PubMed] [Google Scholar]
- 36.van Heerden JH, Bruggeman FJ, Teusink B. Multi-tasking of biosynthetic and energetic functions of glycolysis explained by supply and demand logic. BioEssays. 2015;37(1):34–45. doi: 10.1002/bies.201400108. [DOI] [PubMed] [Google Scholar]
- 37.Boles E, et al. Characterization of a glucose-repressed pyruvate kinase (Pyk2p) in Saccharomyces cerevisiae that is catalytically insensitive to fructose-1,6-bisphosphate. J Bacteriol. 1997;179(9):2987–2993. doi: 10.1128/jb.179.9.2987-2993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauf J, Zimmermann FK, Müller S. Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb Technol. 2000;26(9-10):688–698. doi: 10.1016/s0141-0229(00)00160-5. [DOI] [PubMed] [Google Scholar]
- 39.Schaaff I, Heinisch J, Zimmermann FK. Overproduction of glycolytic enzymes in yeast. Yeast. 1989;5(4):285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- 40.Davies SE, Brindle KM. Effects of overexpression of phosphofructokinase on glycolysis in the yeast Saccharomyces cerevisiae. Biochemistry. 1992;31(19):4729–4735. doi: 10.1021/bi00134a028. [DOI] [PubMed] [Google Scholar]
- 41.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587(Pt 23):5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eigenbrodt E, Glossman H (1980) Glycolysis—One of the keys to cancer? Trends Pharmacol Sci 1:240–245.
- 43.Mazurek S. Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Israelsen WJ, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155(2):397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zois CE, Favaro E, Harris AL. Glycogen metabolism in cancer. Biochem Pharmacol. 2014;92(1):3–11. doi: 10.1016/j.bcp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz JP, Lust WD, Lauderdale VR, Passonneau JV. 1975. Glycolytic metabolism in cultured cells of the nervous system. II. Regulation of pyruvate and lactate metabolism in the C-6 glioma cell line. Mol Cell Biochem 9(2):67–72. [DOI] [PubMed]