Significance
The guanine nucleotide exchange factor (GEF) GIV/Girdin has previously been shown to trigger noncanonical activation of trimeric G proteins in response to multiple chemical stimuli at the plasma membrane and at various locations within cells. In this work we identified a single phosphoevent and pinpointed cyclin-dependent kinase 5 (CDK5) as the responsible kinase that activates GIV's GEF function and initiates noncanonical G protein signaling via GIV. These studies provide evidence that CDK5 is essential for maximal activation of GIV-GEF in cells and insights into a hitherto unrecognized crosstalk between CDK5 and G proteins via GIV. Such crosstalk may shape migration–proliferation dichotomy during several physiological and pathological scenarios such as wound healing and cancer metastasis.
Keywords: migration–proliferation dichotomy, heterotrimeric G protein, growth factor receptor tyrosine kinase, GIV/Girdin, guanine nucleotide exchange factor
Abstract
Signals propagated by receptor tyrosine kinases (RTKs) can drive cell migration and proliferation, two cellular processes that do not occur simultaneously—a phenomenon called “migration–proliferation dichotomy.” We previously showed that epidermal growth factor (EGF) signaling is skewed to favor migration over proliferation via noncanonical transactivation of Gαi proteins by the guanine exchange factor (GEF) GIV. However, what turns on GIV-GEF downstream of growth factor RTKs remained unknown. Here we reveal the molecular mechanism by which phosphorylation of GIV by cyclin-dependent kinase 5 (CDK5) triggers GIV's ability to bind and activate Gαi in response to growth factors and modulate downstream signals to establish a dichotomy between migration and proliferation. We show that CDK5 binds and phosphorylates GIV at Ser1674 near its GEF motif. When Ser1674 is phosphorylated, GIV activates Gαi and enhances promigratory Akt signals. Phosphorylated GIV also binds Gαs and enhances endosomal maturation, which shortens the transit time of EGFR through early endosomes, thereby limiting mitogenic MAPK signals. Consequently, this phosphoevent triggers cells to preferentially migrate during wound healing and transmigration of cancer cells. When Ser1674 cannot be phosphorylated, GIV cannot bind either Gαi or Gαs, Akt signaling is suppressed, mitogenic signals are enhanced due to delayed transit time of EGFR through early endosomes, and cells preferentially proliferate. These results illuminate how GIV-GEF is turned on upon receptor activation, adds GIV to the repertoire of CDK5 substrates, and defines a mechanism by which this unusual CDK orchestrates migration–proliferation dichotomy during cancer invasion, wound healing, and development.
Upon growth factor stimulation, cells initiate signaling cascades favoring either migration or proliferation (migration–proliferation dichotomy) depending on the extracellular environmental cues and/or cellular needs. This dichotomy (also known as “go-or-grow mechanism”) plays a crucial role during a variety of normal and pathophysiologic processes, including development, wound healing, and cancer progression (1–5).
Of the multiple molecular mechanisms implicated in the orchestration of migration–proliferation dichotomy, Gα-interacting vesicle associated protein (GIV) (also known as Girdin) is one such player that helps tilt the signaling network to favor migration over proliferation downstream of stimulated growth factor receptors as well as G protein-coupled receptors (GPCRs) (6–12). In the case of EGF stimulation, GIV is recruited to the plasma membrane (PM) where it directly binds ligand-activated EGFR via its SH2-like domain (13) and serves as a platform for the assembly of receptor tyrosine kinase (RTK)-GIV-Gαi complexes and transactivation of Gαi via its guanine exchange factor (GEF) motif (8, 14). Such transactivation of Gi in the vicinity of RTKs at the PM enhances RTK autophosphorylation, prolongs RTK signaling from the PM, enhances PI3K/Akt signals and actin reorganization, and triggers cell migration (7–9, 13). Besides its role in the assembly of RTK-GIV-Gαi complexes at the PM, GIV also assembles EGFR-GIV-Gαs complexes on early endosomes where it facilitates down-regulation of EGFR via endosomal maturation, ensures finiteness of mitogenic signaling from that compartment, and limits cell proliferation (15).
Much of the experimental evidence supporting GIV’s ability to skew the phenotypic response toward migration has been generated using a GEF-deficient mutant (F1685A, FA) of GIV, which cannot bind either Gαi at the PM or Gαs on endosomes (8, 15). Without the formation of RTK-GIV-Gαi/s complexes, ligand-activated EGFR spends a shorter time at the cell surface, but takes longer to transit through endosomes due to delayed endosomal maturation. Consequently, cells expressing GIV-FA suppress promigratory PI3K-Akt signals at the PM and enhance mitogenic signals from endosomes to preferentially trigger proliferation (8, 15). Despite the insights gained, the GIV-FA mutant did not illuminate how GIV-GEF may be reversibly switched “on/off” in physiology until recently, when that question was partially answered by the discovery of a key phosphoevent triggered by PKCθ at S1689 on GIV, which turns GIV-GEF “off” (16). However, what turns it “on” upon receptor activation still remained unknown.
Here, we identify a key phosphoevent triggered by cyclin-dependent kinase 5 (CDK5) which turns on GIV's GEF activity by phosphorylating it at Ser1674 and thereby increases GIV's ability to bind Gαi/s and enhances its ability to activate Gαi. We provide mechanistic insight into how GIV-GEF is activated and also define a previously unidentified substrate by which CDK5 triggers cell migration. Thus, this study illustrates a physiologic event to activate GIV’s GEF function via a promigratory kinase, CDK5, which in turn dictates the orchestration of migration–proliferation dichotomy.
Results
Identification of a Key Phosphosite That Enhances GIV’s GEF Activity.
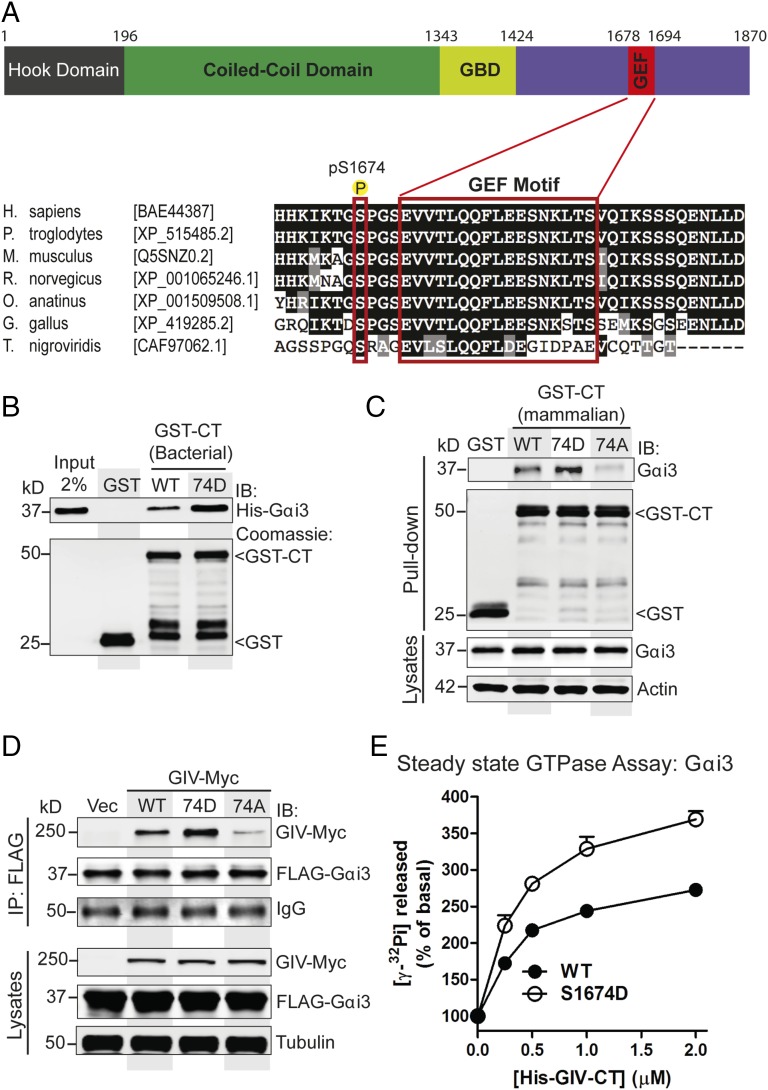
We have previously shown that GIV binds Gαi/s and activates Gαi via an evolutionarily conserved short stretch of residues (amino acids 1678–1694), i.e., the GEF motif within its C terminus (Fig. 1A) (9, 15). We noted that there are 10 Ser/Thr within or flanking the GEF sequence, of which 9 are conserved, and several of them are phosphorylated as shown by multiple high-throughput phosphoproteomic studies (www.phosphosite.org). Because phosphorylation at one such site, S1689, is known to turn off GIV-GEF (16), we hypothesized that similar phosphoevents may serve to turn on GIV-GEF. To explore what those phosphosites may be, we performed a mass spectrometric analysis on GIV’s C terminus (amino acids 1660–1870, expressed and purified from Cos7 cells as GST-tagged protein; GST-GIV-CT). We used this construct because prior studies have demonstrated that this fragment of GIV (tagged with an equally bulky CFP-tag) is sufficient for assembly of RTK-GIV-Gαi complexes at the PM and transactivation of Gi (14). We found that S1674, an evolutionarily conserved residue that lies adjacent to the GEF motif, is the most abundantly phosphorylated residue (∼30–35% of all peptides), both at steady state in 10% (vol/vol) FBS and after EGF stimulation (Fig. 1A).
Fig. 1.
Phosphomimetic S1674D (74D) mutation enhances GIV's ability to bind and activate Gαi3, whereas the nonphosphorylatable S1674A (74A) mutation abolishes binding. (A) Schematic of the domain architecture of GIV and sequence alignment of its C-terminal GEF motif. (Top) Various domains of GIV are shown including the N-terminal microtubule-binding hook domain, coiled-coil domain, Gα-binding domain (GBD), and the C-terminal domain containing the GEF motif. The residue numbers marking the boundaries of each domain are shown. (Bottom) The sequence encompassing the GEF motif (in red rectangle) and surrounding residues was aligned among various species (accession numbers are shown in brackets) using ClustalW. Conserved residues are shaded in black and similar residues in gray. The most heavily phosphorylated residue in the C terminus as determined by mass spectrometry (S1674 in human GIV) is boxed in red. (B) Equimolar amounts of purified His-Gαi3 were incubated with purified GST or GST-GIV-CT proteins immobilized on glutathione-Sepharose beads. Bound His-Gαi3 was analyzed by immunoblotting with anti-His antibody. (C) Lysates of Cos7 cells expressing WT or mutant (74D or 74A) GST-GIV-CT were incubated with glutathione-Sepharose beads. Bound proteins (Upper) were analyzed by immunoblotting for endogenous Gαi3. Equal loading was confirmed by immunoblotting the pull-downs for GST and GST-GIV CT and the lysates for Gαi3 and actin. (D) Immunoprecipitation (IP) was carried out with anti-FLAG antibody on equal aliquots of lysates of Cos7 cells coexpressing FLAG-Gαi3 and full-length Myc-tagged GIV-WT, 74D, or 74A followed by incubation with protein-G Sepharose beads. Bound immune complexes (IP) were analyzed for FLAG (Gαi3) and Myc (GIV) by immunoblotting, and equal loading of lysates was confirmed by immunoblotting for tubulin. (E) The steady-state GTPase activity of His-Gαi3 (50 nM) was determined in the presence of increasing amounts (0–2 μM) of purified His-GIV-CT WT (solid circles) and 74D (open circles). Gαi3 activation is expressed as percent of the steady-state GTPase activity of Gαi3 alone. Results are shown as mean ± SEM of three independent experiments. Activation of Gαi3 by GIV-CT is enhanced in the case of the 74D mutant compared with its WT counterpart.
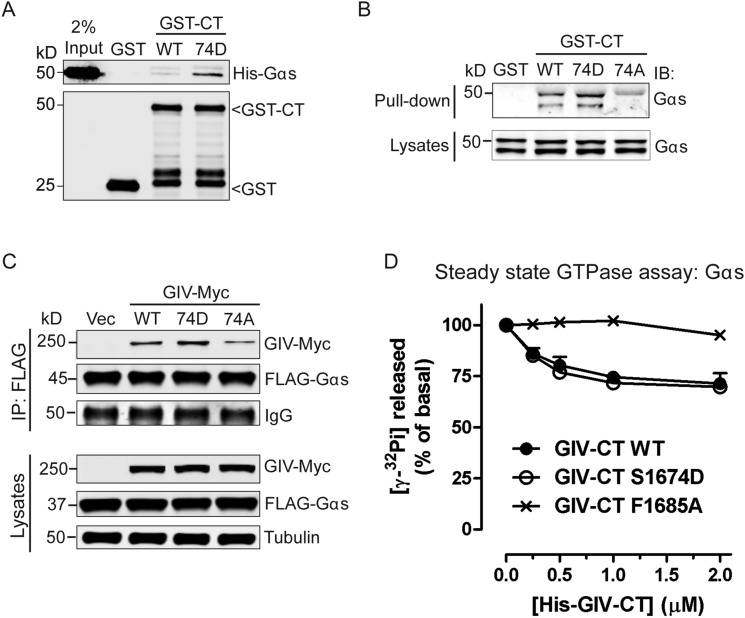
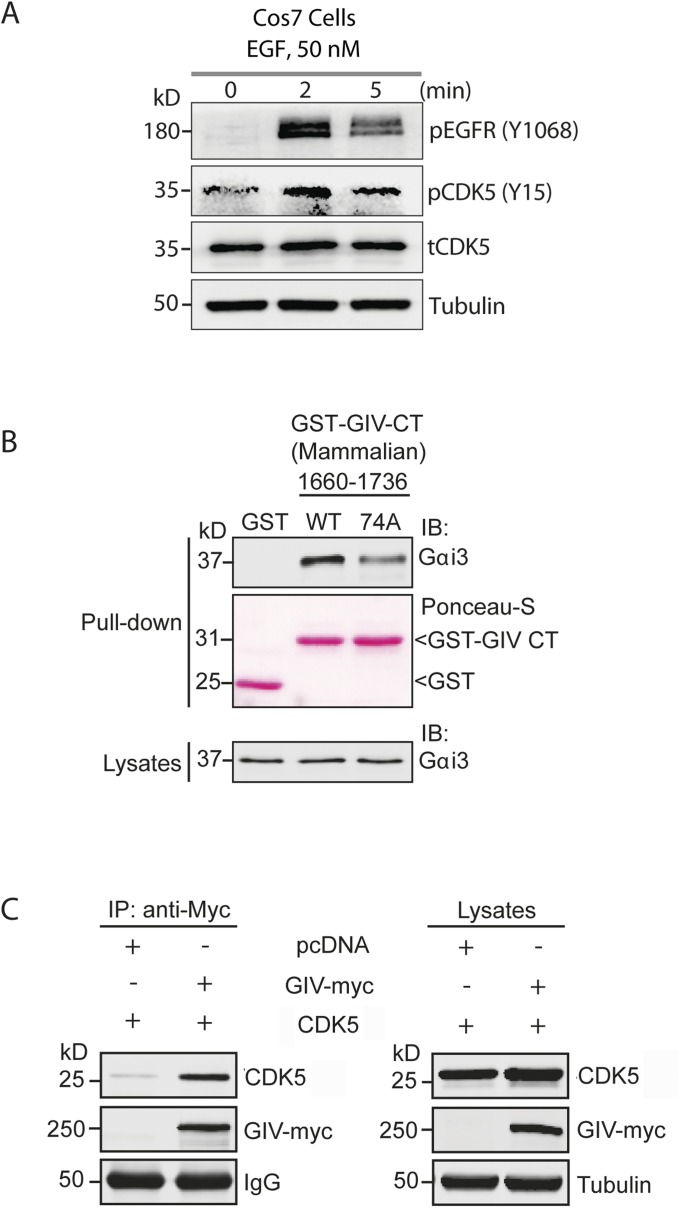
Given its proximity to the GEF motif, next we asked if phosphorylation of S1674 affects GIV’s ability to bind Gαi/s in vitro. We generated a phosphomimetic mutation at S1674 (74D) in GST-GIV-CT and carried out in vitro pulldown assays with recombinant 6×His-tagged Gαi3 and Gαs. Compared with GIV-CT-WT, the phosphomimetic 74D mutant bound Gαi3 (Fig. 1B) and Gαs (Fig. S1A) with approximately three- to fivefold increased affinity. To determine how this phosphosite affects GIV-G protein interactions in cells, we generated both phosphomimetic (S1674D) and nonphosphorylatable (S1674A) GST-GIV-CT mutants, expressed them in mammalian cells, and tested their ability to bind endogenous Gαi3 and Gαs. Compared with GIV-WT, binding of GIV-74D to both Gαi3 (Fig. 1C) and Gαs (Fig. S1B) was enhanced, but the increase was not as robust as observed in in vitro binding assays carried out with recombinant proteins (Fig. 1B and Fig. S1A). A likely explanation for such a discrepancy came from mass spectrometry data, which showed that the S1674 residue in GIV-CT-WT is abundantly phosphorylated (∼35%) in cells at steady state. The nonphosphorylatable GIV-74A mutant, on the other hand, showed little to no binding to either Gαi3 (Fig. 1C) or Gαs (Fig. S1B). These results indicate that S1674 is a key determinant of GIV's ability to bind Gαi/s subunits and that phosphorylation at that site significantly improves GIV–Gα interactions. Similar results were obtained when we immunoprecipitated FLAG-tagged Gαi3 or Gαs and looked for binding to Myc-tagged full-length GIV-74D, or 74A mutants compared to GIV-WT. Binding of the phosphomimetic 74D mutant was increased, and binding of the nonphosphorylatable 74A mutant to both Gαi3 (Fig. 1D) and Gαs (Fig. S1C) was decreased.
Fig. S1.
Phosphomodification of S1674 enhances GIV–Gαs interaction. (A) Phosphomimetic mutation at S1674 (74D) in GIV enhances its ability to bind Gαs in vitro. Equimolar amounts of purified His-tagged Gαs were incubated with bacterially expressed and purified GST, WT, or 74D GST-GIV-CT (1660–1870) immobilized on glutathione-Sepharose beads. Bound proteins were analyzed by immunoblotting. Input represents 2% of the total His-tagged Gαs used in the binding reactions. n = 3. (B) Nonphosphorylatable GIV-74A mutant (74A) shows impaired binding to Gαs in cells. Cos7 cells were transiently transfected with WT, 74D, or 74A GST-GIV-CT, and clarified cell lysates were incubated with glutathione-Sepharose beads. Bound proteins and lysates were analyzed by immunoblotting for endogenous Gαs. n = 3. (C) Gαs preferentially coimmunoprecipitates with the phosphomimetic but not the nonphosphorylatable form of full-length GIV. Immunoprecipitation was carried out with anti-FLAG antibody on equal aliquots of Cos7 lysates coexpressing FLAG-Gαs and full-length Myc-tagged GIV-WT, 74D, or 74A followed by incubation with protein G-Sepharose beads. Bound immune complexes were analyzed for FLAG and Myc by immunoblotting, and equal loading was confirmed by immunoblotting for tubulin (lysates). n = 3. (D) His-GIV-CT WT and 74D, but not His-GIV-CT F1685A, decrease the steady-state GTPase activity of His-Gαs in a dose-dependent manner. The steady-state GTPase activity of purified His-Gαs (50 nM) was determined in the presence of the indicated amounts of purified His-GIV-CT WT (closed circles) or mutants (74D, open circles; F1685A, cross symbol) by quantification of the amount of [γ-32P]GTP (0.5 μm, ∼50 cpm/fmol) hydrolyzed in 15 min. Data are expressed as percent of GTP hydrolyzed by the G protein alone (0 μM). Results are shown as mean ± SD of two independent experiments.
To determine if enhanced binding of the phosphomimetic mutant to Gαi3 translates into higher GEF activity, we measured the steady-state GTPase activity of Gαi3 in the presence of increasing amounts of recombinant His-GIV-CT-WT and 74D proteins as described previously (9). The 74D mutant was significantly more potent than GIV-CT-WT as a GEF at each concentration tested (Fig. 1E), suggesting that phosphorylation at S1674 augments GIV's ability to bind and activate Gαi. As for Gαs, no significant difference was observed between WT and 74D mutant His-GIV-CT proteins; both showed a weak inhibitory effect on the steady-state GTPase activity of Gαs in vitro compared with the G protein binding-deficient FA mutant (Fig. S1D).
Phosphorylation of S1674 Regulates EGFR Trafficking and Signaling.
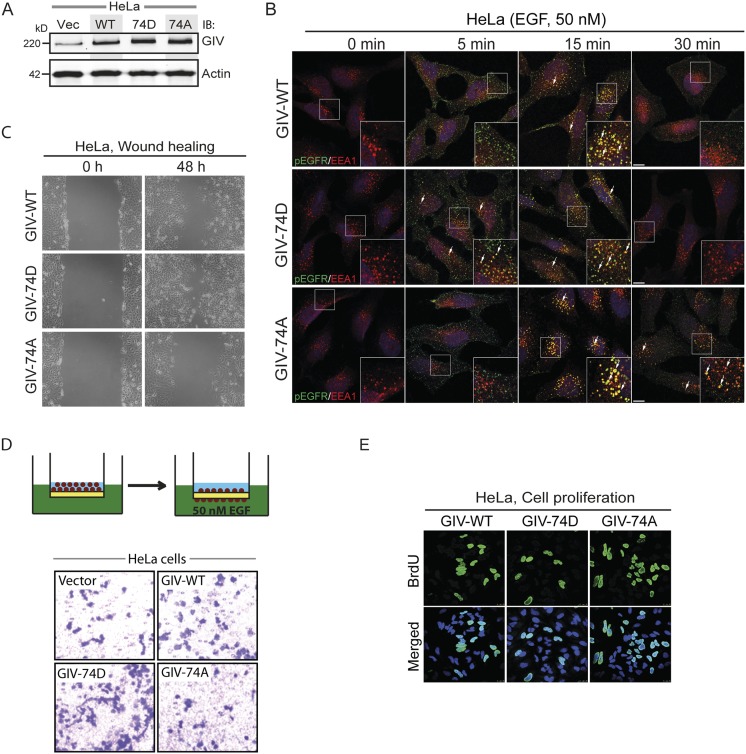
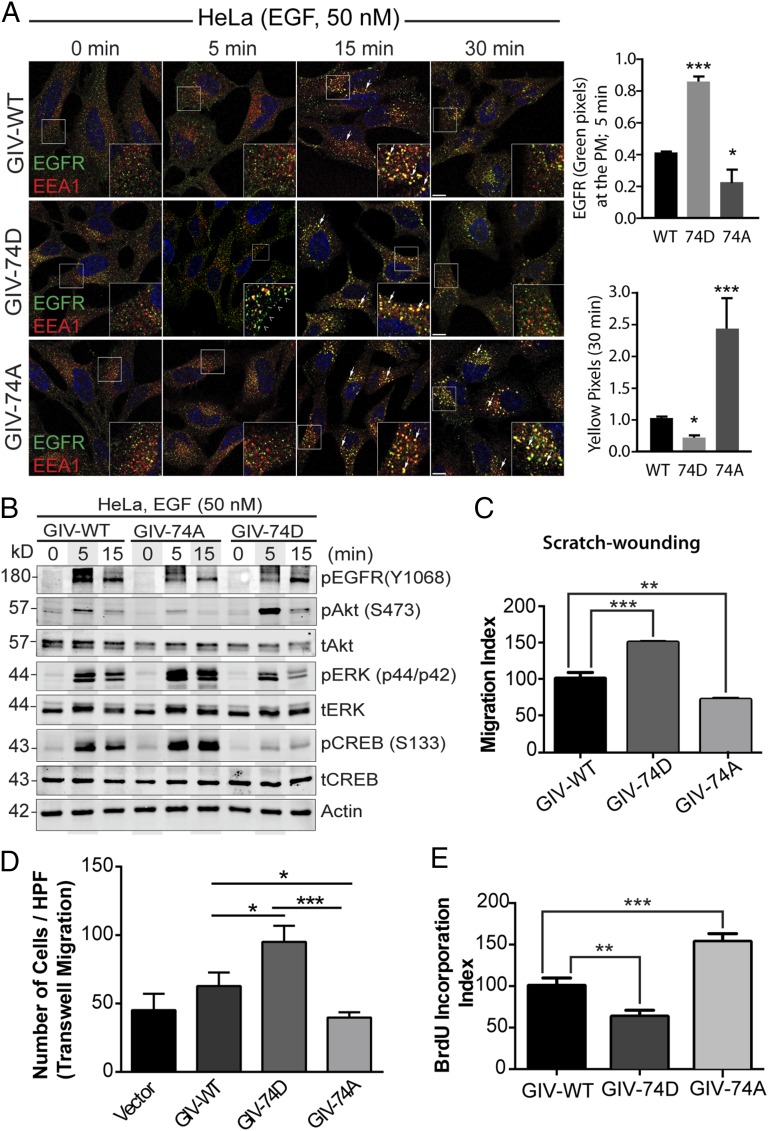
Because GIV-Gαi/s interactions have been shown to shape RTK trafficking, which is a key determinant of downstream signals (8, 13, 15), next we asked if phosphorylation of GIV at S1674 affects EGFR trafficking. To answer this question, we generated GIV-depleted HeLa cell lines stably expressing full-length GIV-FLAG (WT, 74D, and 74A) at levels ∼1.5- to 2-fold higher than endogenous GIV (Fig. S2A). Previous studies using this approach have characterized the phenotypic effects of various mutants of GIV in a clean background devoid of endogenous GIV (8, 13). Upon serum starvation, EGFR localized at the PM in all three cell lines (Fig. 2A, 0 min), but after EGF stimulation they showed differential responses. At 5 min after ligand stimulation, the amount of EGFR on the cell surface was higher in GIV-74D cells compared with GIV-WT, but little or no EGFR was on the cell surface in GIV-74A cells (Fig. 2A), indicating that the duration EGFR spends at the PM is increased when GIV is phosphorylated at S1674 and can effectively enhance Gi activity. At 15 min, EGFR colocalized with early endosome antigen 1 (EEA1) endosomes in GIV-WT and in both the mutants. By 30 min, the receptor was no longer seen in EEA1 endosomes in GIV-WT and GIV-74D cells, but in GIV-74A cells, EGFR still persisted on EEA1-positive endosomes. We further confirmed that such differences in transit time through EEA1-positive endosomes are associated with differential rates of down-regulation of receptor autophosphorylation. Autophosphorylated receptor as determined by anti-EGFR pY1068 (Fig. S2B) was rapidly cleared from endosomes in 74D cells (fewer pixels by 15 min, compared with GIV-WT and GIV-74A cells), but lingered on until 30 min in GIV-74A cells. Together, these findings indicate that the phosphomimicking GIV-74D mutant, which enhances Gαi activation and binds more strongly to Gαs, prolongs receptor stay at the PM, but once endocytosed, ensures rapid clearance of the ligand-activated receptor through endosomes and the finiteness of signaling from that site. By contrast, the nonphosphorylatable GIV-74A mutant, which impairs binding to Gαs and Gαi, shortens receptor stay at the PM, delays endosomal maturation, and prolongs the transit time and signaling from ligand-activated EGFR on EEA1 endosomes.
Fig. S2.
Phosphorylation of S1674 affects EGFR trafficking to and signaling from early endosomes as well as the balance of migration–proliferation dichotomy in HeLa cells. (A) GIV expression in HeLa stable cell lines expressing WT, 74D, and 74A constructs. Equal aliquots of whole-cell lysates of parental (vector alone; Vec), GIV-WT, GIV-74D, and GIV-74A HeLa cell lines were analyzed for GIV and actin by immunoblotting (IB). (B) The nonphosphorylatable GIV mutant prolongs EGFR activation at EEA1 endosomes. HeLa cells stably expressing GIV-WT, 74D, and 74A were treated with 50 nM EGF for the indicated time points, fixed, and stained for activated (phosphorylated) pY1068-EGFR (green) and EEA1 (red). Arrows pointing to yellow pixels in the images denote colocalization of pEGFR and EEA1. (Insets) Enlargement (3×) of boxed regions. (Scale bars, 10 μm.) n = 3. (C) Phospho-status of S1674 on GIV influences cell migration. HeLa cells stably expressing GIV-WT, GIV-74D, and GIV-74A were subjected to scratch wounding and examined immediately after wounding (0 h) and 48 h later (n = 3). The GIV-74D cells showed enhanced migration in scratch-wound assays compared with cells expressing GIV-WT, whereas the GIV-74A showed decreased cell migration. (D) GIV-74D enhances cell migration in response to EGF. Chemotaxis of HeLa cells toward EGF supplemented serum-free medium was evaluated using a Transwell migration assay (schematic shown on Top). Cells were allowed to migrate for 6 h and were fixed and stained with Toluidine Blue. Representative images are shown for vector, WT, 74D, and 74A cells. (E) GIV-74A mutation enhances cell proliferation. HeLa cells stably expressing GIV-WT, GIV-74D, and GIV-74A were grown in low serum [2% (vol/vol) FBS] overnight, incubated in BrdU for 30 min, fixed [3% (wt/vol) paraformaldehyde], stained for BrdU, and analyzed by confocal microscopy (n = 3). Representative images are shown for WT, 74D, and 74A cells. Green, BrDU; blue, DAPI.
Fig. 2.
Phosphorylation of GIV at S1674 influences EGFR trafficking, signaling, and preference for migration versus proliferation of HeLa cells. (A) Serum starved (0.2% FBS, overnight) GIV-depleted HeLa cells stably expressing GIV-WT, 74D, and 74A (Fig. S2A) were stimulated with 50 nM EGF before fixation. Fixed cells were stained for total EGFR (green), the early endosome marker EEA1 (red), and DAPI (nucleus; blue) and examined by confocal microscopy. (Insets) Enlargement (3×) of boxed regions. (Scale bars, 10 μm.) Phosphomimetic 74D mutant GIV enhances the duration EGFR spends at the PM (arrowheads, green pixels at 5 min), whereas the nonphosphorylatable 74A mutant GIV prolongs the duration EGFR spends on early endosomes (arrow, yellow pixels at 30 min). Bar graphs on the Right show quantification of pixels in each set of cells at the indicated time points. (B) Serum starved, GIV-depleted (by shRNA) HeLa cell lines stably expressing shRNA-resistant GIV-WT, 74D, and 74A were stimulated with 50 nM EGF before lysis. Equal aliquots of whole-cell lysates were analyzed for phospho(pY1068)EGFR, total(t) and phospho(p)-Akt, -ERK, and -CREB and actin by immunoblotting. (C) HeLa cells in B were subjected to scratch wounding and examined immediately (0 h) and after 24 h by light microscopy (Fig. S2C). The migration index (y axis) was calculated as described in Experimental Procedures, normalized to cells expressing GIV-WT (set at 100%) and plotted as a bar graph. (D) HeLa cells in B were analyzed for chemotaxis toward EGF using a Transwell migration assay (Fig. S2D). Cells were allowed to migrate for 6 h, fixed, and stained with Toluidine Blue. The number of migrating cells was averaged from 20 field-of-view images per experiment. Data are presented as mean ± SEM; n = 3. HPF, high power field. (E) HeLa cells in B were grown in low serum [2% (vol/vol) FBS] overnight, incubated in BrdU for 30 min, fixed [3% (wt/vol) paraformaldehyde], stained for BrdU, and analyzed by confocal microscopy (Fig. S2E). Bar graph shows percentage of cells with BrdU uptake (y axis). Data are presented as mean ± SEM; n = 3.
Phosphorylation of GIV at S1674 Orchestrates Migration–Proliferation Dichotomy During Epithelial Wound Healing and Morphogenesis.
The distribution of ligand-activated EGFR between the PM and endosomes is known to affect EGFR signaling (8, 17) in that promigratory PI3K-Akt signals are initiated by ligand-activated receptor largely or exclusively at the PM, whereas mitogenic MAPK-ERK1/2 signals can be propagated from endosomes (7, 18). Because trafficking of EGFR is closely intertwined with signals downstream of the receptor, next we asked if phosphomodulation of GIV's GEF motif at S1674 also affects signals that originate from the PM versus endosomes. Compared with the GIV-WT cells, those expressing the GIV-74A mutant, in which EGFR spends less time at the PM but stays longer on endosomes, suppress PM-based promigratory PI3K/Akt signals (as determined by phosphorylation of Akt at S473) and enhance endosome-based mitogenic MAPK/ERK signals [as determined by phosphorylation of ERK1/2 and cAMP response element-binding protein (CREB)] (Fig. 2B). By contrast, a mirror image response (high PI3K/Akt, low ERK1/2/CREB) was seen in cells expressing GIV-74D, in which EGFR spends more time at the PM but less on endosomes.
Next we hypothesized that the contrasting promigratory and promitotic signaling profiles in cells expressing GIV-74D and GIV-74A may translate into a differential preference for cell migration versus proliferation. We found that indeed such was the case, because cells expressing GIV-74D, which enhance promigratory PI3K/Akt signals, displayed a higher migration index (∼50% faster than WT), as determined by scratch-wound assays (Fig. 2C and Fig. S2C). Conversely, cells expressing GIV-74A, which suppress promigratory signals, displayed a lower migration index (∼25–30% slower compared with WT, and ∼55% slower than GIV-74D). These cells also showed contrasting chemotaxis toward EGF, as determined by Transwell migration assay using a modified Boyden chamber apparatus (Fig. S2D) (19). GIV-74D cells showed a ∼65% increase, whereas GIV-74A cells showed a ∼30% decrease compared with GIV-WT cells (Fig. 2D and Fig. S2D). Noteworthy, the GIV-74A cells were ∼50–60% less motile than GIV-74D cells. Consistent with the fact that GIV-74A cells enhanced mitogenic MAPK/ERK/CREB signals, the rate of proliferation of these cells, as determined by BrDU uptake assays (Fig. 2E and Fig. S2E) was also increased (∼50% compared with GIV-WT cells). Conversely, cells expressing GIV-74D, which suppressed mitogenic signals, proliferated less (∼40% less than WT, and ∼60% less than GIV-74A). Together, these observations demonstrate that phosphorylation of GIV at S1674 not only alters receptor localization, but also alters the profile of downstream signals propagated and the resultant phenotype. Phosphorylation at S1674 and activation of GIV-GEF led to increased migratory and suppressed mitogenic signals, and cells preferentially migrated. By contrast, dephosphorylation at S1674 and inactivation of GIV-GEF led to increased mitogenic and suppressed migratory signals, and cells preferentially proliferated.
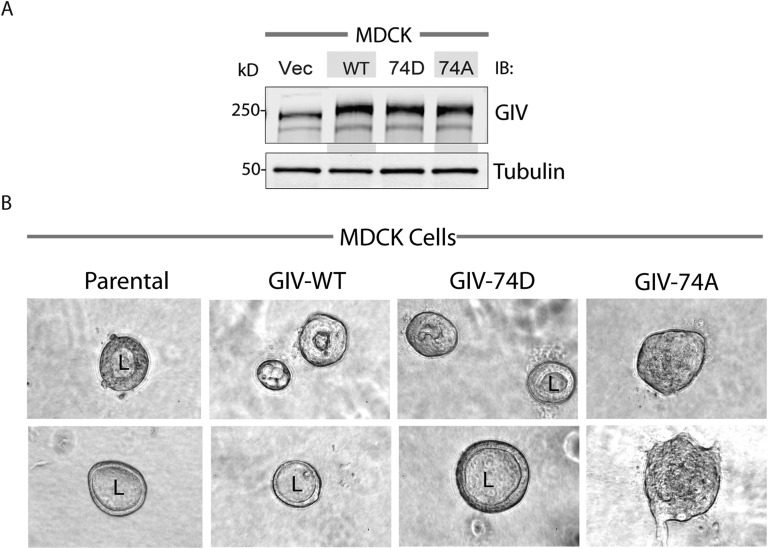
Next we asked how phosphomodification of GIV at S1674 affects epithelial morphogenesis, which relies on many cellular processes, including a delicate balance between migration and proliferation. To study GIV's effect on epithelial morphogenesis, we generated Madin–Darby canine kidney (MDCK) cell lines stably expressing GIV-WT or 74A/74D mutants (Fig. S3A) and used them in 3D cultures of MDCK epithelial cysts, which mimic epithelial organization in vivo. Consistent with the need for directional cell migration during epithelial morphogenesis (20, 21), GIV-WT cells and the promigratory GIV-74D cells, but not the proproliferative GIV-74A cells formed mature cysts with a central lumen (Fig. S3B). In the case of GIV-74A cells, cells grew as solid masses, without a central lumen. Together, these results demonstrate that phosphorylation of GIV-GEF at S1674, which is a key determinant of whether cells preferentially migrate or divide, is also required for the formation of MDCK epithelial cysts and suggest a role for this phosphoevent during epithelial morphogenesis.
Fig. S3.
Phosphomimetic mutation at S1674 in GIV promotes cyst formation in MDCK cells. (A) GIV expression in type II MDCK cell lines expressing WT, 74D, and 74A constructs. Equal aliquots of whole-cell lysates of parental (vector alone; Vec), GIV-WT, GIV-74D, and GIV-74A MDCK cell lines were analyzed for GIV and tubulin by immunoblotting (IB). (B) MDCK cells in A were seeded in collagen gels and monitored for cyst formation by phase contrast microscopy. Representative images are shown for each cell line. MDCK parental cells and those expressing GIV-WT and GIV-74D, but not those expressing GIV-74A mutant, form cysts with a distinct central lumen (L).
Phosphorylation of GIV at S1674 Orchestrates Migration–Proliferation Dichotomy in Invasive Cancer Cells.
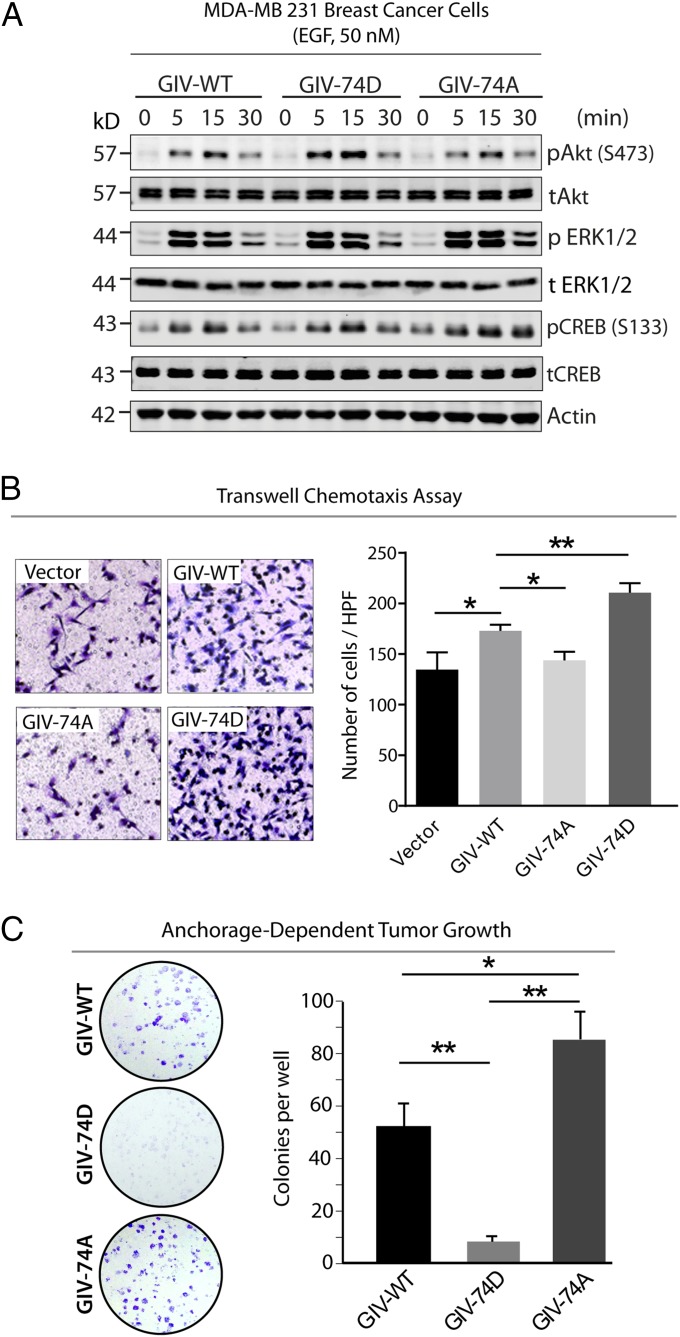
Migration–proliferation dichotomy was first described in invasive tumors that exhibit high and spatially heterogeneous cell proliferation and motility rates (1), and the ability to switch between the two phenotypes is a hallmark of invasive cancer cells. We asked if phosphorylation of GIV at S1674 can affect differential activation of signaling pathways in highly metastatic breast cancer cell line MDA-MB231. MDA-MB231 cells stably expressing GIV-74D (Fig. S4) enhanced promigratory PI3K/Akt signals as determined by Akt phosphorylation at 5 and 15 min after EGF stimulation, whereas 74A cells enhanced and sustained promitotic ERK and CREB signals (as determined by phosphorylation of ERK1/2 and CREB at 15 and 30 min) (Fig. 3A). Consistent with their opposing signaling programs, GIV-74D cells preferentially migrated, as determined by Transwell chemotaxis assays (Fig. 3B), but failed to proliferate into colonies as determined by anchorage-dependent cell growth assays (Fig. 3C). By contrast, GIV-74A cells were less motile (Fig. 3B) and instead, preferentially grew into colonies (Fig. 3C). These results demonstrate that reversible phosphorylation of GIV at S1674, which directly impacts GIV's ability to bind Gαi/s, is sufficient to impart migration–proliferation dichotomy in invasive cancer cells.
Fig. S4.
GIV expression in MDA-MB231 stable cell lines expressing WT, 74D, and 74A constructs. Equal aliquots of whole-cell lysates of parental (vector alone; Vec), GIV-WT, GIV-74D, and GIV-74A cell lines were analyzed for GIV and actin by immunoblotting (IB).
Fig. 3.
Phosphorylation of GIV at S1674 influences EGF signaling and influences preference for migration versus proliferation of MDA-MB231 cancer cells. (A) Serum-starved MDA-MB231 cells stably expressing GIV-WT or 74D/A mutants were stimulated with EGF before lysis. Whole-cell lysates were analyzed for total(t) and phospho(p) Akt, ERK, CREB, and actin by immunoblotting. (B) MDA-MB231 cells used in A were subjected to chemotaxis toward EGF, and migrating cells were visualized and analyzed as in Fig. 2D. Results are expressed as mean ± SEM; n = 3. HPF, high power field. (Magnification: 10×.) (C) MDA-MB231 cells used in A were analyzed for their ability to form adherent colonies on plastic plates for 2–3 wk before fixation and staining with crystal violet. (Left) Photograph of the crystal violet-stained wells. (Right) Bar graphs showing the number of colonies/cell line counted by ImageJ (colony counter).
CDK5 Binds and Phosphorylates GIV at S1674.
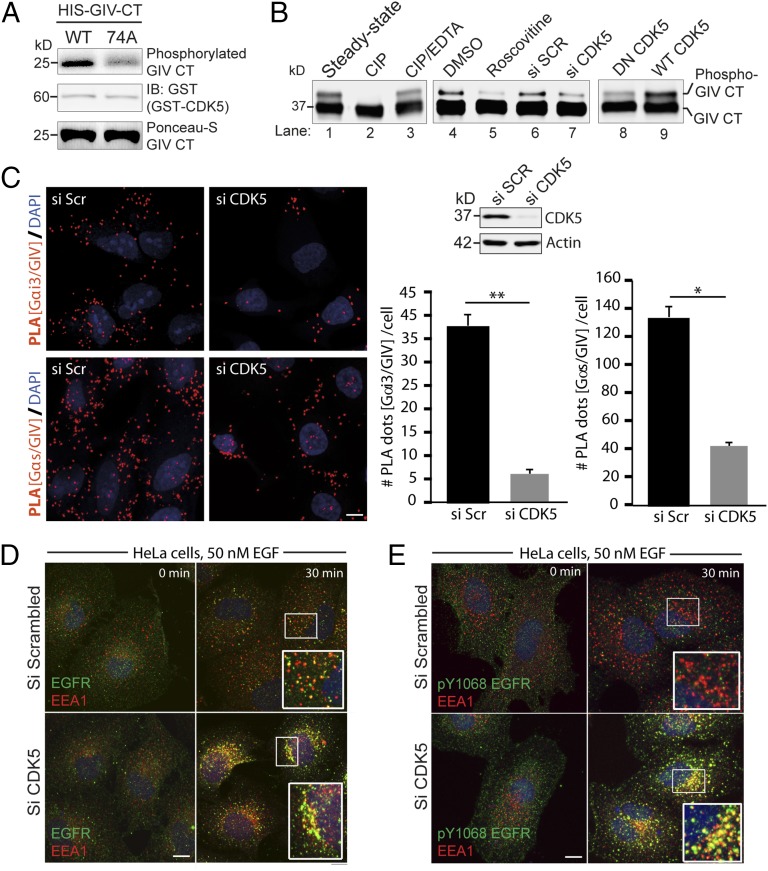
To identify the kinase responsible for phosphorylating GIV at S1674, we analyzed the sequence flanking it for known consensus sites of kinases. Because the sequence resembled the consensus for multiple kinases, i.e., PKA/B/C (K/RXXS/T consensus), MAPK/ERK and CDKs (SP/TP consensus) (22), we screened multiple recombinant kinases (PKA, PKB, all PKC isoforms, CDK1-6, ERK/MAPK, Braf kinase) in in vitro kinase assays for their ability to phosphorylate His-GIV-CT at S1674. We found that among all of the kinases tested, CDK5 emerged as the only kinase that could specifically phosphorylate GIV-CT-WT, but not 74A, indicating that S1674 is a substrate site for CDK5 in vitro (Fig. 4A).
Fig. 4.
CDK5 phosphorylates GIV at S1674. (A) In vitro kinase assays were carried out on bacterially expressed and purified His-GIV-CT (WT) or nonphosphorylatable 74A mutant with recombinant CDK5 kinase in the presence of [32P]ATP followed by autoradiography (Upper). Equal loading of active kinase and His-GIV-CT substrate was analyzed by immunoblotting (Middle) and Ponceau-S staining (Lower), respectively. (B) GST-GIV CT 1660–1736 expressed in Cos7 cells was subjected to different treatments as indicated and analyzed by immunoblotting using anti-GST antibody. The faster migrating form of the doublet represents the unmodified form, and the slower migrating form represents the phosphorylated form of GIV-CT 1660–1736 as determined by treatment of bead-bound GIV-CT with calf intestinal phosphatase (CIP) (lanes 1–3). CDK5 inactivation using Roscovitine (lanes 4 and 5), siRNA mediated depletion (lanes 6 and 7), and expression of a dominant negative (DN) form of CDK5 (lanes 8 and 9) reduced phosphorylation of GIV-CT 1660–1736. (C) Control or CDK5-depleted HeLa cells were fixed and analyzed for interactions between GIV and Gαi3 (Upper Left) or GIV and Gαs (Lower Left) by in situ PLA (red). Nuclei, DAPI (blue). Whole cell lysates of HeLa cells used for PLA assays were analyzed for efficiency of CDK5 depletion by immunoblotting (Upper Right). PLA dots were quantified per cell from a total of 25–30 cells per experiment (Lower Right). Results are expressed as mean ± SEM; n = 3. (D and E) Serum-starved control (si Scr) or CDK5-depleted (si CDK5) HeLa cells were stimulated with 50 nM EGF, fixed, and stained for total EGFR (green; D) or pY1068-EGFR (green; E), EEA1(red), and DAPI (nucleus; blue), and analyzed by confocal microscopy as in Fig. 2A. Yellow pixels, indicative of total (D) and active EGFR (E) in endosomes were increased ∼2.8-fold in D and ∼8.3-fold in E, respectively, as determined using ImageJ. (Scale bar, 10 μm.)
Because CDK5 is known to be activated early (within seconds) after EGF stimulation (23) and confirmed by us to be the case (Fig. S5A), we hypothesized that this kinase should be able to phosphorylate S1674 on GIV and activate its GEF function for subsequent coupling of Gαi proteins to RTKs and trigger G-protein activation at the PM by 5 min (14). To determine if CDK5 indeed phosphorylates GIV-GEF in cells and modulates its ability to bind Gαi/s, we generated a truncated form of GST-GIV-CT (1660–1736), which contains only one “SP” motif (S1674P1675) as the potential site for CDK5 and retains its ability to interact with Gαi3 (Fig. S5B). Moreover, when the S1674A mutation was introduced into this construct, it reduced the binding between GIV and Gαi3 (Fig. S5B). When expressed in Cos7 cells, immunoblots revealed that GIV-CT1660–1736 appears as two differentially migrating bands—one faster major band and one slower minor band (Fig. 4B, lane 1). Because phosphorylation of proteins tends to retard their electrophoretic mobility, we hypothesized that the slower migrating band may represent phosphorylated GIV-CT. We found such is indeed the case because the band disappeared when the samples were treated with Calf intestinal alkaline phosphatase (CIP) (Fig. 4B, lane 2) but persisted when a similar CIP treatment was carried out in the presence of its inhibitor, EDTA (Fig. 4B, lane 3). The phosphorylated form also decreased when cells expressing GST-GIV-CT1660–1736 were either treated with Roscovitine, a CDK5-specific inhibitor (Fig. 4B, lanes 4 and 5) or depleted of CDK5 by siRNA (Fig. 4B, lanes 6 and 7). Finally, the relative abundance of the phosphorylated band was enhanced when GST-GIV-CT1660–1736 was coexpressed with WT CDK5, but not a kinase inactive/dominant negative mutant of CDK5(D144N) (24) (Fig. 4B, lanes 8 and 9). Although these data confirmed that CDK5 phosphorylates GIV at S1674 in vivo, we noted that the extent of phosphorylation was modest. The substoichiometric phosphorylation we observe in cells likely represents an underestimation due to overexpression related artifact (saturated system) or due to the synthetic nature of a suboptimal substrate that does not resemble endogenous protein. It is also possible that kinases other that CDK5 may phosphorylate GIV at that site. Regardless, these results strongly suggest that CDK5 is able to phosphorylate GIV at S1674 in cells. CDK5 also coimmunoprecipitated with full-length GIV (Fig. S5C), indicating that CDK5 binds its substrate GIV.
Fig. S5.
CDK5 is activated within minutes after EGF stimulation and binds and phosphorylates GIV. (A) EGF stimulation activates CDK5. Serum-starved Cos7 cells expressing CDK5 WT were stimulated with EGF before lysis. Whole-cell lysates were analyzed for phospho(pY1068)EGFR, total(t) and phospho(pY15) CDK5 and tubulin by immunoblotting. (B) Nonphosphorylatable GIV CT 1660–1736 mutant shows impaired binding to Gαi3 in cellulo. Cos7 cells were transiently transfected with WT or 74A mutant GST-GIV CT 1660–1736, and clarified cell lysates were incubated with glutathione-Sepharose beads. Bound proteins were analyzed by immunoblotting for endogenous Gαi3. Equal loading of GST and GST-GIV-CT proteins was confirmed by Ponceau-S staining (pulldown) and immunoblotting for Gαi3 (lysates). (C) CDK5 interacts with GIV. Cleared lysates obtained from Cos7 cells coexpressing pcDNA or GIV-myc and CDK5 were immunoprecipitated with anti-myc antibody. The immunoprecipitates were analyzed for bound CDK5 by immunoblotting with anti-CDK5 antibody. The expression of proteins was also checked by immunoblotting the lysates for the indicated proteins (Right).
To determine if CDK5-dependent phosphorylation of GIV-GEF translates to increased binding of GIV to Gαi/s, we analyzed the abundance of GIV–Gαi and GIV–Gαs complexes in control vs. CDK5-depleted cells by the in situ proximity ligation assay (PLA). Depletion of CDK5 (∼95% efficacy; Fig. 4C, Right) significantly reduced the interaction of endogenous GIV with Gαi3 (Fig. 4C, Top Left) and Gαs (Lower Left). These data confirm that phosphorylation of GIV-GEF by CDK5 at S1674 is required for enhanced interactions between GIV and Gαi/s subunits.
Because phosphorylation of GIV at S1674 plays a role in trafficking of EGFR through early endosome compartments, we hypothesized that CDK5 may be required for efficient trafficking of EGFR. Depletion of CDK5 by siRNA resulted in a phenotype identical to that observed in cells expressing the nonphosphorylatable GIV-74A mutant, i.e., ligand-activated EGFR stayed longer in EEA1-positive endosomes (at 30 min), indicating a delay in transit (Fig. 4 D and E). Taken together, these data suggest that phosphorylation of GIV at S1674 by CDK5, which enhanced GIV–Gαs interaction, is required for efficient EGFR trafficking through the endosomal system.
CDK5 Triggers Cell Migration via Phosphoactivation of GIV-GEF.
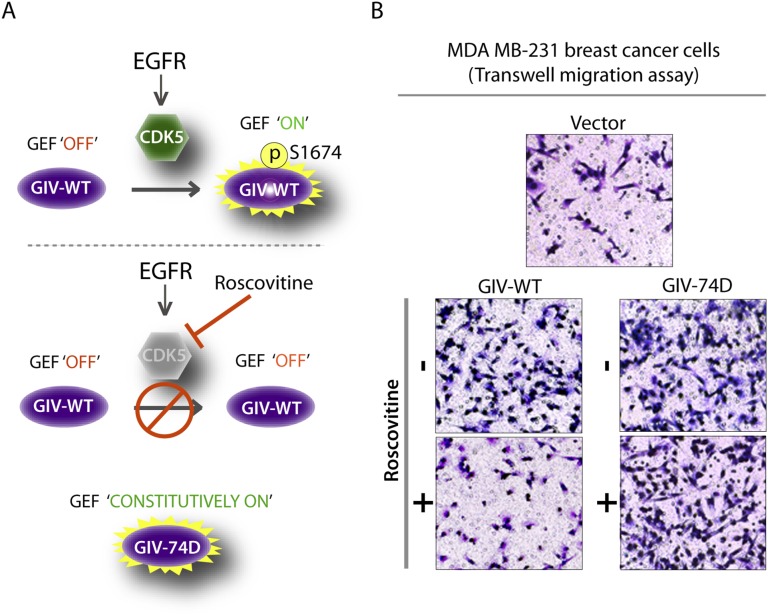
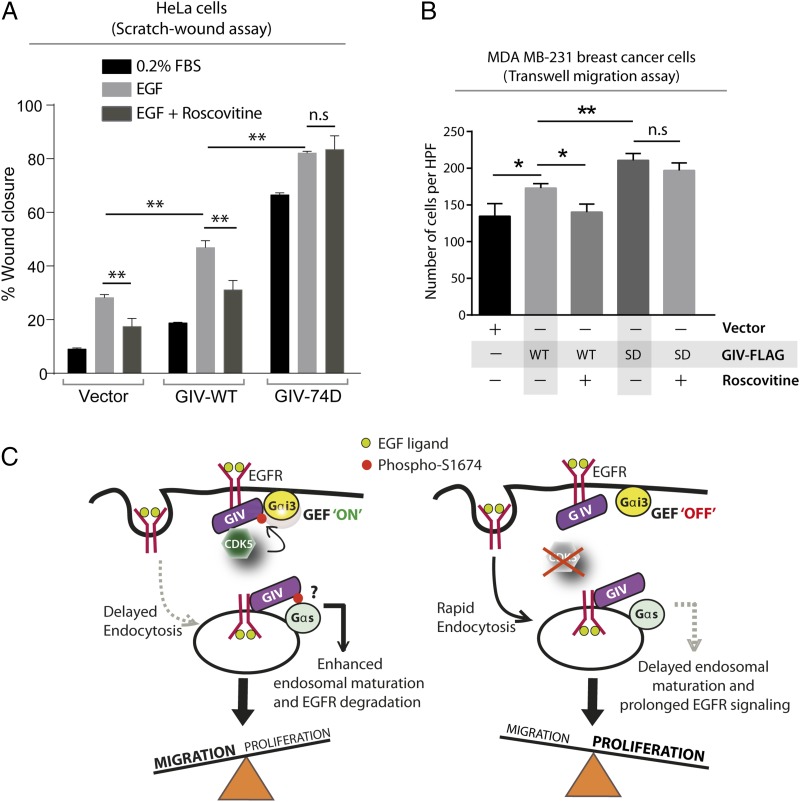
Prior studies have implicated CDK5 in directional cell migration via phosphoregulation of multiple substrates (25–28). We asked if phosphorylation and activation of its newly identified substrate GIV-GEF contributes to the promigratory role of CDK5, and if so, to what extent. To answer this question, we analyzed the effect of the CDK5 inhibitor Roscovitine on cells expressing GIV-WT or GIV-74D. If GIV is a major substrate and a promigratory effector of CDK5, we predicted that inhibition of CDK5 with Roscovitine will inhibit phosphorylation of GIV at S1674 and thereby inhibit migration of GIV-WT cells. However, similar treatment with Roscovitine should spare the promigratory, constitutively activated GIV-74D cells, which essentially “bypass” the kinase inhibition (schematically depicted in Fig. S6A). If GIV is not a major substrate/effector of CDK5, Roscovitine should inhibit cell migration in both cell lines. We found that Roscovitine inhibited wound healing in control and GIV-WT HeLa cells (Fig. 5A), but did not have any effect on GIV-74D cells. Similarly, Roscovitine impaired the migration of MDA-MB231 cells expressing GIV-WT in Transwell chemotaxis assays, but had no significant effect on GIV-74D cells (Fig. 5B and Fig. S6B). These results indicate that phosphorylation of GIV is a key mechanism by which CDK5 triggers cell migration.
Fig. S6.
CDK5 mediates its role in cell migration in part through phosphorylation of GIV S1674. (A) Schematic showing role of CDK5 in activating GIV’s GEF function. In control cells (Upper), EGF stimulation leads to activation of CDK5, which in turn activates the GEF function of GIV by phosphorylating S1674. In cells treated with Roscovitine (Middle), a CDK5-specific inhibitor, S1674, is not phosphorylated and GIV-GEF remains “off.” Cells harboring a phosphomimetic GIV (74D) (Lower) bypass the need for CDK5-mediated activation and are in a constitutively GEF “on” mode. (B) Phosphomimetic mutation at S1674 can compensate for CDK5 inactivation in MDA-MB231 cell migration in response to EGF. Chemotaxis of MDA-MB231 (vector, WT, and 74D) cells toward EGF supplemented serum-free medium was evaluated using a Transwell migration assay with or without Roscovitine treatment. Cells were allowed to migrate for 6 h and were fixed and stained with Toluidine Blue. Representative images are shown. (Magnification: B, 10×.)
Fig. 5.
Cell migration after scratch-wounding and cancer cell chemotaxis were inhibited by Roscovitine in cells that express GIV-WT, but not in those expressing the phosphomimic 74D GIV mutant. (A) GIV-depleted HeLa cell lines stably expressing vector control, GIV-WT, and GIV-74D were treated or not with EGF in the presence or absence of Roscovitine and analyzed for cell migration after scratch wounding as in Fig. 2C. Bar graph shows the migration index (wound closure) of each cell line under each condition. Results are expressed as mean ± SEM; n = 3. (B) MDA-MB231 cells were evaluated for chemotaxis toward EGF as in Fig. 3B in the presence (+) or absence (−) of Roscovitine. The number of migrating cells was averaged from 20 field-of-view images per experiment. Results are expressed as mean ± SEM; n = 3; n.s., not significant. (C) Schematic of a working model. (Left) EGF stimulation activates CDK5, which turns GIV’s GEF activity “on” by phosphorylating it at S1674. This phosphoevent enhances GIV's ability to bind and activate Gαi at the PM, which in turn prolongs motogenic EGF signaling at the PM, likely via a delay in endocytosis. Such phosphorylation also enhances GIV's ability to bind Gαs (and perhaps modulate its activation; “?”) on endosomes, which speeds up endosomal maturation and suppresses mitogenic EGF signaling from endosomes. Overall, phosphorylation of GIV by CDK5 at S1674 triggers preferential cell migration over proliferation. (Right) Inhibition or depletion of CDK5 maintains GIV's GEF function in the “off” state, reducing GIV's ability to bind and modulate Gαi/s at both locations. Consequently, EGF triggers an opposite response, such that inhibition of CDK5 favors proliferation over cell migration.
Discussion
Summary and Working Model.
The major finding in this work is the identification of CDK5 as a kinase that phosphoactivates GIV-GEF and initiates noncanonical G protein signaling downstream of growth factor RTKs. Whether or not such activation occurs determines if cells migrate or proliferate. Based on our findings here and the literature, we propose the following model (Fig. 5C, Left): EGF stimulation activates CDK5 within seconds (23), which in turn phosphorylates GIV-GEF at S1674 and turns on its ability to bind Gαi/s and activate Gαi. Binding and activating Gαi prolongs the duration that EGFR spends on the cell surface and enhances PM-based promigratory PI3K-Akt signals (8). Binding Gαs, on the other hand, shortens the duration EGFR spends in endosomes and diminishes endosome-based promitotic MAPK/ERK signals (15). Consequently, when CDK5 phosphoactivates GIV-GEF and enhances its ability to bind both Gα-subunits, cells preferentially migrate while suppressing proliferation. Inhibition or depletion of CDK5 or targeted inhibition of this phosphoevent (Fig. 5C, Right) renders GIV-GEF unresponsive to growth factors and maintains it in an off state: each of the above responses are reversed, i.e., GIV does not bind either Gαi/s, EGFR stays shorter at the PM and longer in endosomes, PI3K-Akt signals are suppressed, MAPK/ERK signals are enhanced, and cells preferentially divide.
Dynamic Phosphorylation of GIV at Ser1674 Provides a Physiologic Mechanism for Modulating GIV’s GEF Activity.
Previously, using a battery of synthetic mutants generated on the basis of a structure-guided approach we showed that the potency of GIV's GEF activity has an in vivo threshold effect on cellular response (29), that its presence triggers cell migration, and its absence triggers mitosis (8). Together these results provided a potential explanation of how growth factor signals undergo bifurcation at the immediate postreceptor level to choose between two distinct fates (go or grow) in response to a single stimulus and how their activation levels may be modulated. However, both studies used an approach involving artificial mutations to irreversibly modulate the GEF function of GIV. Here we show that phosphorylation of GIV at S1674 is a physiologic mechanism by which GIV's GEF function overcomes the previously described in vivo threshold effect because phosphorylation was essential to trigger cell migration. We also show that dynamic phospho-dephosphorylation events at a single site can enable or disable, respectively, GIV's GEF motif and dictate whether cells migrate or divide. Although the kinase-substrate relationship between CDK5 and GIV is specifically responsible for triggering the phosphoevent, the identity of the antagonistic phosphatase remains unknown.
Phosphorylation of GIV at Ser1674 Modulates EGFR Signaling and Trafficking via Two Gα Subunits at Two Distinct Locations.
We previously showed that GIV's ability to bind and activate Gαi at the PM and bind Gαs on endosomes are key determinants of when and where the activated receptor is compartmentalized, for how long, and which downstream signals are propagated and which are attenuated (8, 15, 30). Here we show that the phosphorylation status of S1674 enhances GIV's ability to bind both Gαi and Gαs. Based on our prior work and the findings of this study, the consequences of enhanced GIV–Gαi interaction triggered by CDK5 are expected to increase the assembly of EGFR–GIV–G protein complexes and transactivation of Gαi in the vicinity of ligand-activated EGFR (as shown recently by live cell FRET studies) (14), increase PM-association of EGFR, enhance PM-based PI3K-Akt signals, and enhance cell migration. Therefore, CDK5-dependent phosphorylation of GIV-GEF may influence both G protein and growth factor signaling.
In the case of Gαs, we previously showed that the GIV–Gαs interaction is critical for endosomal maturation and clearance of ligand-activated receptors (15). In order for the GIV–Gαs complex to promote rapid clearance of EGFR out of early endosomes and impose finiteness to promitotic signaling from that compartment, Gαs must remain inactive (15). Here we show that GIV's GEF motif has a weak inhibitory effect on nucleotide exchange by Gαs in vitro. Whether this weak effect is responsible for keeping Gαs inactive on early endosomes and whether additional posttranslational modifications on either GIV or Gαs further enhance the inhibitory effect remain unclear. What is clear are the consequences of enhanced GIV–Gαs interaction triggered by CDK5 on EGFR signaling and trafficking: the duration of EGFR on early endosomes is shortened and the endosome-based promitotic MAPK/ERK1/2/CREB signals are suppressed. When CDK5 cannot phosphorylate GIV, and GIV cannot bind Gαs, EGFR stays longer in early endosomes and endosome-based promitotic signals are enhanced. Together, the GIV–Gαi and GIV–Gαs interactions promoted by CDK5 coordinately enhance promigratory signals from the PM while dampening the mitogenic signals from endosomes with the net outcome being persistent cell migration. Despite these insights, how GIV may interact with two Gα subunits (that have opposing effects on adenylyl cyclase) at two subcellular locations and whether such interactions are simultaneous or sequential remain unknown.
The Functional Interplay Between GIV and CDK5 Unravel the Molecular Mechanisms for Their Previously Known Functions.
Since its discovery two decades ago, CDK5, the atypical member of the CDK family, has emerged as a trigger for cell migration in diverse cell types during angiogenesis, neurite outgrowth, epithelial wound healing, cancer cell invasion, etc., and is known to simultaneously arrest cell cycle progression via unclear mechanisms (31). Consistent with our finding that phosphorylation of GIV by CDK5 enhances Akt phosphorylation and cell migration, others have shown that Cdk5-null mice die prenatally due to massive failure of neuronal cell migration, and the embryonic extracts show reduced levels of phospho-Akt (32–34). Like GIV, which is a bona fide metastasis-related protein (35), CDK5 has also been found to be overexpressed in malignant human cancers (prostate, pancreatic, and breast) where it has been implicated in promoting metastasis (36–40). The enhanced migration of MDA-MB231 cells containing a constitutively phosphorylated GIV mutant (S1674D) suggests that the degree of phosphorylation of GIV at S1674 may provide an additive value as a biomarker (besides the use of GIV-positivity score) (41) to accurately prognosticate outcome in patients who have a GIV-positive tumor. Furthermore, inhibition of the CDK5-GIV axis may be a viable/promising strategy to curb the aggressive behavior of highly metastatic cancers.
Although CDK5 is known to trigger cell migration via phosphorylation of several substrates (31), how this kinase suppresses the cell cycle is poorly understood. Some reports have indicated that CDK5 inhibits cell cycle entry via suppression of MAPK/ERK signaling (42, 43); however, little or nothing is known about how this might occur. We showed that CDK5 suppresses mitogenic MAPK/ERK1/2/CREB signals in part by phosphorylating GIV's GEF motif and enhancing GIV–Gαs interaction, which speeds up endosomal maturation and thereby limits the duration of EGFR signals from endosomes.
This work also illuminates a few spatiotemporal aspects of G protein activation by GIV. Using FRET studies in living cells, we have recently shown (14) that within 3–5 min after EGF stimulation, GIV gets recruited to ligand-activated EGFR. Within 5 min EGFR–GIV–Gαi ternary complexes are assembled at the PM, which is followed by activation of Gi by ∼5 min and suppression of cAMP by ∼6 min. Because CDK5 is activated within seconds after EGF stimulation (23), it is likely that once activated, CDK5 can promptly phosphorylate GIV at S1674 before or during the latter’s recruitment to the activated receptor at the PM, ensuring maximal coupling to and activation of Gαi within 5 min after ligand stimulation. Because GIV-GEF has also been found to be functional on two types of intracellular membranes, e.g., triggers secretion from the Golgi (44) and inhibits the formation/maturation of autophagosomes (45), and because activation of CDK5 exerts similar effects on both processes (46, 47), it is tempting to speculate that activation of GIV-GEF on internal membranes is also via CDK5. Further studies are required to determine if such is the case.
In conclusion, we provide insights into a hitherto unrecognized crosstalk between CDK5 and Gαi/s proteins via GIV and how such crosstalk may shape migration–proliferation dichotomy during several physiological and pathological scenarios such as development, wound healing, and cancer metastasis.
Experimental Procedures
Detailed methods are described in SI Experimental Procedures.
Cell Culture, Transfection, Immunoblotting, Immunofluorescence, and Protein–Protein Interaction Assays (GST Pulldowns and Immunoprecipitations).
These assays were carried out exactly as described before (7, 8, 15). All transfections were performed using TransIT-LT1 reagent (Mirus). All Western blotting (Odyssey-LICOR) images were processed and assembled for presentation using Image Studio Lite, Photoshop, and Illustrator software (Adobe).
Scratch Wounding, BrdU Incorporation, and Steady-State GTPase Activity Assays.
These assays were performed as described previously (8, 9, 15).
PLA.
In situ interactions of endogenous GIV with Gαi3 or Gαs were detected using a proximity ligation assay kit Duolink (Olink Biosciences) as per the manufacturer’s instructions.
Anchorage-Dependent Colony Formation Assay.
Anchorage-dependent growth was monitored on solid (plastic) surface as described previously (48). Briefly, 1,000 MDA-MB231 cells stably expressing WT, 74D, and 74A GIV-FLAG constructs were grown in six-well tissue culture plates at 37 °C for 2 wk in medium supplemented with 0.2% FBS before staining with 0.005% crystal violet for 1 h. Images were acquired by light microscopy.
MDCK Cyst Formation Assay.
This assay was performed as previously described (49). Briefly, 3 × 104 MDCK type II cells stably expressing GIV constructs were added to a collagen solution at pH 7 and placed in a well of a four-well chamber slide. After the collagen was polymerized, culture media [DMEM 1×, FBS 2% (vol/vol)] was added, and the plate was incubated at 37 °C in a CO2 incubator. The medium was changed every 2 d for 2 wk. Cyst formation was monitored by phase-contrast microscopy.
Statistical Analysis.
Each experiment presented in the figures is representative of at least three independent experiments. Statistical significance between the differences of means was calculated by unpaired Student’s t test. A two-tailed P value of <0.05 at 95% confidence interval is considered as statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. All graphical data presented were prepared using GraphPad or Matlab software.
SI Experimental Procedures
Antibodies and Reagents.
All reagents and chemicals were of analytical grade and were purchased from Sigma unless otherwise indicated. Cell culture media, epidermal growth factor (EGF), and DAPI were purchased from Life Technologies. Bromodeoxyuridine (BrdU) was obtained from Sigma. Recombinant purified kinases were purchased from SignalChem. Pfu Ultra DNA polymerase was purchased from Agilent and γ32P-GTP and γ[32P]ATP were purchased from Perkin-Elmer. Control scrambled and GIV siRNA were purchased from Ambion and CDK5 siRNA was purchased from Santa Cruz Biotechnology. Anti-mouse His antibody was from Abgent and anti-goat GST was from GE Healthcare. Mouse anti-FLAG (M2), β-actin, and α-tubulin were from Sigma. Rabbit anti-CREB (total and phospho), Gαs, pEGFR (Y1068), pAkt (S473), pERK (p44/42), mouse anti-Myc, and Akt were from Cell Signaling. Rabbit anti-GIV CT, Gαi3, CDK5, pCDK5 (Y15), EGFR, and ERK antibodies were from Santa Cruz Biotechnology. Mouse anti-EEA1 and anti-BrdU were from BD Biosciences. Goat anti-rabbit and goat anti-mouse Alexa Fluor 680 and IRDye 800 F(ab′)2 used for Odyssey infrared imaging were from LICOR Biosciences. Goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 594 for immunofluorescence were purchased from Life Technologies.
Plasmid Constructs and Mutagenesis.
Cloning of GIV, Gαi3, and Gαs into p3×FLAG-CMV-14 plasmid (GIV-FLAG, Gαi3-FLAG, and Gαs-FLAG), pET28b plasmid (6×His-GIV-CT 1660–1870, 6×His-Gαi3 and 6×His-Gαs short) and pGEX-4T1-GIV CT 1660–1870 (GST-GIV CT) has been described previously (7–9, 12, 15). Full-length GIV cDNA with a myc tag at the C terminus was cloned into the NotI and KpnI sites of pcDNA3.1. GST-tagged GIV-CT proteins for mammalian expression and use in protein–protein interaction assays were generated by inserting C-terminal amino acids 1660–1870 and 1660–1736 of human GIV into pCEFL plasmid (a generous gift from Pedro A. Lazo, Universidad de Salamanca, Salamanca, Spain) between EcoRI and NotI restriction sites (14). CDK5 (WT and D144N) and p35-HA constructs were gifts from David S. Park (University of Ottawa, Ottawa, Ontario) and Edward Giniger (NIH/NINDS), respectively. GIV constructs harboring S1674D and S1674A mutations (hereafter referred to as GIV-74D and GIV-74A) were created using the QuikChange II site-directed mutagenesis kit (Agilent Technologies). All mutations and the integrity of the rest of the insert were confirmed by sequencing.
Cell Culture, Transfection, and Stable Cell Line Preparation.
Cells were cultured according to American Type Culture Collection (ATCC) guidelines. They were grown in the recommended medium—HeLa and MDCK type II in DMEM (Invitrogen) and MDA-MB231 in RPMI plus 1% sodium pyruvate (Invitrogen) supplemented with 10% (vol/vol) FBS (HyClone) and penicillin-streptomycin-glutamine (Invitrogen). All pDNA and siRNA transfections were performed using TransIT-LT1 (Mirus Bio) and Oligofectamine (Life Technologies), respectively. HeLa cells were stably depleted of endogenous GIV by shRNA as described previously (14). HeLa, MDCK type II, and MDA-MB231 cell lines stably expressing shRNA resistant full-length GIV-WT, GIV-74D, and GIV-74A were generated by transfecting cells with the desired GIV-FLAG construct and selection in the presence of G418 (800 μg/μL) for 4–6 wk. The resultant multiclonal pool was subsequently maintained in the presence of 500 μg/mL G418. GIV expression was verified by immunoblotting using anti-GIV antibody.
Recombinant Protein Expression and Purification.
Protein expression and purification were carried out as described previously (7, 9). Briefly, plasmids encoding His-Gαi3, His-Gαs, or GST-GIV-CT (1660–1870; WT and 74D) fusion constructs were used to express these proteins in Escherichia coli strain BL21 (DE3) (Invitrogen). Cells were grown in LB medium containing appropriate selection antibiotic at 37 °C until an OD600 of ∼0.5–0.7, and protein expression was induced by adding 0.5 mM IPTG and growing cultures overnight at 25 °C. For His-tagged proteins, bacteria pelleted from 1 L of culture were resuspended in 10 mL His-lysis buffer (50 mM NaH2PO4, pH 7.4, 300 mM NaCl, 10 mM Imidazole, 1% Triton X-100 (vol/vol), 1× Complete Protease Inhibitor Mixture; Roche), sonicated four times (50% power, 20 s on/20 s off) and centrifuged at 12,000 × g for 20 min at 4 °C. Solubilized protein was purified using a cobalt resin (Pierce), eluted with 250 mM imidazole, and dialyzed in 1× PBS. Likewise for GST-tagged proteins, bacteria were pelleted from 500 mL of culture, resuspended in 20 mL of lysis buffer (50 mM Tris⋅HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 30 μM GDP, 2 mM DTT, 0.5% Triton X-100, 1× Complete Protease Inhibitor Mixture; Roche), sonicated, and centrifuged to remove insoluble material. Affinity purification of solubilized proteins was carried out using glutathione-Sepharose 4B beads (GE Healthcare).
In Vitro GST Pulldown Assays.
Equimolar amounts of purified GST-GIV-CT (amino acids 1660–1870; WT or 74D) or GST alone immobilized on glutathione-Sepharose beads were incubated with purified His-tagged Gαi3 or Gαs in binding buffer (50 mM Tris⋅HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 30 μM GDP, 2 mM DTT, 0.5% Triton X-100, 1× Complete Protease Inhibitor Mixture; Roche) for 4 h with constant tumbling at 4 °C. Beads were washed (three times) with 1 mL of binding buffer and eluted by heating to 95 °C in 1× Laemmli sample buffer for 5 min. The binding between GIV-CT and His-Gα proteins was analyzed by immunoblotting.
In Cellulo GST Pulldown Assays.
Cos7 cells were grown in 10-cm plates and transfected with pCEFL-GST-GIV-CT wild type and mutants (74D and 74A). After 48 h, cells were washed once with cold 1× PBS and harvested by scraping in 0.5 mL of binding buffer (25 mM Hepes, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 30 μM GDP, 2 mM DTT, 0.5% Triton X-100, 1× Complete Protease Inhibitor Mixture (Roche), 1× Phosphatase Inhibitor Mixtures 1 and 2; Sigma). Cell lysates were incubated with glutathione-Sepharose beads with constant tumbling at 4 °C for 4 h. Beads were washed three times with 1 mL of lysis buffer, eluted in 1× Laemmli sample buffer by heating to 95 °C for 5 min, and binding of endogenous Gα proteins was analyzed by immunoblotting.
Coimmunoprecipitation Assays.
Coimmunoprecipitation assays were performed as described before (8). Briefly, Cos7 cells grown on 10-cm plates were cotransfected with either FLAG-Gαi or FLAG-Gαs and pcDNA3.1, GIV-Myc WT, 74D, or 74A constructs. After 48 h, cells were washed once with cold 1× PBS and harvested by scraping into 0.5 mL of IP buffer [20 mM Hepes, pH 7.4, 5 mM Mg-acetate, 125 mM K-acetate, 0.4% Triton X-100, 1 mM DTT, 1× Complete Protease Inhibitor Mixture (Roche), 1× Phosphatase Inhibitor Mixtures 1 and 2 (Sigma)] on ice. Cell lysates were incubated for 10 min at 4 °C and centrifuged at 12,000 × g for 10 min. Clarified cell lysates were incubated with anti-mouse FLAG-M2 antibody overnight at 4 °C, followed by incubation with equilibrated protein G-Sepharose beads for 1 h at 4 °C. Beads were washed three times with cold IP buffer, eluted by heating at 95 °C for 5 min in 1× Laemmli sample buffer, and bound proteins were analyzed by SDS/PAGE and immunoblotting.
EGF Stimulation.
For EGF stimulation experiments, 65,000 cells stably expressing either GIV-WT, GIV-74D, or GIV-74A were seeded in a six-well plate in DMEM with 10% (vol/vol) FBS. Twenty-four hours postseeding, cells were starved for ∼18 h in DMEM with either 0.2% FBS (HeLa) or 0% FBS (MDA-MB231). Cells were stimulated with 50 nM EGF for 0–30 min. For signaling assays, cells were directly lysed in 1× Laemmli sample buffer by heating at 95 °C for 5 min and analyzed by SDS/PAGE and immunoblotting. For immunofluorescence, cells were prepared as below.
Immunofluorescence Microscopy.
Trafficking of activated EGFR was tracked as previously described (15). Briefly, cells grown on coverslips were fixed at room temperature with 3% (wt/vol) paraformaldehyde (PFA) for 30 min, washed (three times) with 1× PBS, blocked and permeabilized for 45 min, and then incubated for 1 h each with primary and then secondary antibodies. For staining with anti-pEGFR (pY1068), coverslips were incubated in the primary antibody overnight at 4 °C. DAPI at 1:10,000 (Invitrogen) was used to stain the nucleus. Images were taken using a Leica DMI400B confocal microscope.
Scratch-Wound Healing Assay.
Wound healing assays were performed as described previously (8). HeLa stable cell lines (WT, 74D, and 74A) were grown on six-well plates to 90% confluency and starved in 0.2% FBS (DMEM) overnight before the day of wounding. The wound was made by scratching the plate with a sterile p200 pipette tip. Plates were rinsed once with 0.2% FBS (DMEM) to remove floating cells, and cells were maintained in 0.2% FBS containing DMEM throughout the experiment. Images were taken using the Canon Powershot A630 camera with lens adapter for Zeiss Axiovert 40 CFL microscope every 12 h. To quantify cell migration (migration index is expressed as percentage of wound area covered), images were analyzed using ImageJ (National Institutes of Heath) to calculate the difference between area covered at 0 min and at the end of the assay divided by the area of the wound at 0 min multiplied by 100.
BrdU Incorporation Assay.
BrdU incorporation assay was performed as described previously (15). HeLa cells stably expressing GIV-WT, GIV-74D, and GIV-74A were grown on coverslips to 90% confluency in low serum (2%) overnight. Cells were incubated with 10 μM BrdU for 30 min at 37 °C and fixed with 70% (vol/vol) ethanol for 30 min at room temperature. Cells were then washed (three times) with 1× PBS, treated with 2 M HCl for 20 min at room temperature, followed by 0.1 M Na2B407 (pH 8.5) for 2 min at room temperature, and washed again in 1× PBS (three times). The coverslips were incubated with anti-BrdU for 30 min at room temperature, washed (three times) with 1× PBS, and then incubated with goat anti-mouse 594 secondary antibody for 30 min at room temperature. DAPI at 1:10,000 was used to stain the nucleus. Images were taken using a Leica DMI140 confocal microscope. Cells (300–400) from 10 randomly chosen fields were counted (DAPI stained) from three independent experiments and quantitated by dividing BrdU positive cells by total cells and normalizing to GIV-WT (100%).
Steady-State GTPase Assay.
This assay was performed as described previously (12) with minor modifications. Briefly, His-Gαi3 (100 nM) was preincubated with different concentrations of His-GIV-CT (1660-1870) WT or mutants for 60 min at 4 °C in assay buffer [20 mM Na-Hepes, pH 8, 100 mM NaCl, 1 mM EDTA, 2 mM MgCl2, 1 mM DTT, 0.05% (w:v) C12E10]. GTPase reactions were initiated at 30 °C by adding an equal volume of assay buffer containing 1 µM [γ-32P]GTP (∼50 cpm/fmol). Duplicate aliquots (25 μL) were removed at different time points, and reactions were stopped with 975 μL ice-cold 5% (wt/vol) activated charcoal in 20 mM H3PO4, pH 3. Samples were then centrifuged for 10 min at 10,000 × g, and 500 μL of the resultant supernatant was scintillation counted to quantify released [32P]Pi. To determine the specific Pi produced, the background [32P]Pi detected in the absence of G protein was subtracted from each reaction. In experiments with His-Gαs the conditions were identical except that the reactions were carried out at 20 °C. Data were expressed as raw counts or percentage of the basal G protein activity in the absence of His-GIV-CT.
Anchorage-Dependent Growth Assay.
Anchorage-dependent growth was monitored on a solid (plastic) surface as described previously (48). Briefly, 1,000 MDA-MB231 cells stably expressing WT, 74D, and 74A GIV-FLAG constructs were grown in six-well tissue culture plates at 37 °C for 2 wk in media supplemented with 0.2% FBS before staining with 0.005% crystal violet for 1 h.
MDCK Cyst Formation Assay.
The 3D collagen matrix procedure was performed as described previously (49). Briefly, 3 × 104 MDCK cells were added to a collagen solution at pH 7 [GlutaMAX 24 mM, NaHCO3 2.35 mg/mL, DMEM 1× FBS 2% (vol/vol), Hepes 20 mM, Collagen I 2 mg/mL] and placed in a well of a four-well chamber slide. After the collagen was polymerized, culture media [DMEM containing 2% (vol/vol) FBS] was added to the plate, which was placed in a 37 °C CO2 incubator. Media was changed every 2 d for up to 2 wk. Cyst formation was monitored by phase-contrast microscopy.
In Vitro Kinase Assays.
In vitro kinase assays were carried out as described previously (16) using purified His-GIV-CT (WT and 74A; 3–5 μg per reaction) and commercially obtained recombinant kinases (100 ng per reaction). The reactions were started by addition of ATP (5 μCi per reaction γP32-ATP and 6 μM cold ATP and carried out at 30 °C in 30 μL of kinase buffer (20 mM Tris·HCl, pH 7.5, 2 mM EDTA, 10 mM MgCl2, and 1 mM DTT) for 60 min. Phosphorylated proteins were separated on 12% (wt/vol) SDS/PAGE and detected by autoradiography.
Proximity Ligation Assay.
HeLa cells seeded on glass coverslips were grown to 60–70% confluency, washed two times with PBS, and fixed 20 min at room temperature (RT) with 4% (wt/vol) PFA. After washing with PBS, cells were processed for proximity ligation assay (PLA) using the Duolink In Situ PLA kit (Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, coverslips were blocked for 1 h at RT, incubated with primary antibodies to GIV and Gαi3 or Gαs for 1 h. Cells were then incubated with PLA probes for 1 h in a humidified chamber at 37 °C. If the two oligo probes are in close proximity, ligation occurs and further amplification steps allow for fluorescence detection of the reaction as bright red dots (566–594 nm). Cells were counterstained with DAPI to visualize nuclei.
Acknowledgments
We thank Karen Sykes for technical assistance. This work was supported by NIH grants CA100768 (to M.G.F.), and CA160911 and DK099226 (to P.G.). D.B. was supported by the California State University Program for Education and Research in Biotechnology (CSUPERB) New Investigator Grant GF00631143. P.G. was also supported by the Burroughs Wellcome Fund [Career Awards for Medical Scientists (CAMS) award], the American Cancer Society (ACS-IRG 70-002), and by the University of California at San Diego Moores Cancer Center. M.G.-M. was supported by the American Cancer Society (RGS-13-362-01-TBE) and NIH (R01GM108733). I.L.-S. was supported by a fellowship from the American Heart Association (AHA 14POST20050025) and I.-C.L. by a fellowship (NSC 100-2917-1-564-032) from the National Science Council of Taiwan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514157112/-/DCSupplemental.
References
- 1.Fedotov S, Iomin A. Migration and proliferation dichotomy in tumor-cell invasion. Phys Rev Lett. 2007;98(11):118101–118104. doi: 10.1103/PhysRevLett.98.118101. [DOI] [PubMed] [Google Scholar]
- 2.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaylarde PM, Sarkany I. Cell migration and DNA synthesis in organ culture of human skin. Br J Dermatol. 1975;92(4):375–380. doi: 10.1111/j.1365-2133.1975.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonneton C, Sibarita JB, Thiery JP. Relationship between cell migration and cell cycle during the initiation of epithelial to fibroblastoid transition. Cell Motil Cytoskeleton. 1999;43(4):288–295. doi: 10.1002/(SICI)1097-0169(1999)43:4<288::AID-CM2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Chung EH, Hutcheon AE, Joyce NC, Zieske JD. Synchronization of the G1/S transition in response to corneal debridement. Invest Ophthalmol Vis Sci. 1999;40(9):1952–1958. [PubMed] [Google Scholar]
- 6.Enomoto A, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9(3):389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh P, Garcia-Marcos M, Bornheimer SJ, Farquhar MG. Activation of Galphai3 triggers cell migration via regulation of GIV. J Cell Biol. 2008;182(2):381–393. doi: 10.1083/jcb.200712066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh P, et al. A Gαi-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol Biol Cell. 2010;21(13):2338–2354. doi: 10.1091/mbc.E10-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc Natl Acad Sci USA. 2009;106(9):3178–3183. doi: 10.1073/pnas.0900294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang P, et al. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008;68(5):1310–1318. doi: 10.1158/0008-5472.CAN-07-5111. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10(3):329–337. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Marcos M, Ghosh P, Ear J, Farquhar MG. A structural determinant that renders G α(i) sensitive to activation by GIV/girdin is required to promote cell migration. J Biol Chem. 2010;285(17):12765–12777. doi: 10.1074/jbc.M109.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C, et al. Structural basis for activation of trimeric Gi proteins by multiple growth factor receptors via GIV/Girdin. Mol Biol Cell. 2014;25(22):3654–3671. doi: 10.1091/mbc.E14-05-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Midde KK, et al. Multimodular biosensors reveal a novel platform for activation of G proteins by growth factor receptors. Proc Natl Acad Sci USA. 2015;112(9):E937–E946. doi: 10.1073/pnas.1420140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beas AO, et al. Gαs promotes EEA1 endosome maturation and shuts down proliferative signaling through interaction with GIV (Girdin) Mol Biol Cell. 2012;23(23):4623–4634. doi: 10.1091/mbc.E12-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Sánchez I, et al. Protein kinase C-theta (PKCθ) phosphorylates and inhibits the guanine exchange factor, GIV/Girdin. Proc Natl Acad Sci USA. 2013;110(14):5510–5515. doi: 10.1073/pnas.1303392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haugh JM. Localization of receptor-mediated signal transduction pathways: The inside story. Mol Interv. 2002;2(5):292–307. doi: 10.1124/mi.2.5.292. [DOI] [PubMed] [Google Scholar]
- 18.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: A legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106(42):17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards KL, McCullough J. A modified microchamber method for chemotaxis and chemokinesis. Immunol Commun. 1984;13(1):49–62. doi: 10.3109/08820138409025449. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Girdin is an intrinsic regulator of neuroblast chain migration in the rostral migratory stream of the postnatal brain. J Neurosci. 2011;31(22):8109–8122. doi: 10.1523/JNEUROSCI.1130-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohara K, et al. Involvement of Girdin in the determination of cell polarity during cell migration. PLoS One. 2012;7(5):e36681. doi: 10.1371/journal.pone.0036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amanchy R, et al. A curated compendium of phosphorylation motifs. Nat Biotechnol. 2007;25(3):285–286. doi: 10.1038/nbt0307-285. [DOI] [PubMed] [Google Scholar]
- 23.Lee HY, Jung H, Jang IH, Suh PG, Ryu SH. Cdk5 phosphorylates PLD2 to mediate EGF-dependent insulin secretion. Cell Signal. 2008;20(10):1787–1794. doi: 10.1016/j.cellsig.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Nikolic M, Dudek H, Kwon YT, Ramos YFM, Tsai L-H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10(7):816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 25.Hosoi T, et al. Evidence for cdk5 as a major activity phosphorylating tau protein in porcine brain extract. J Biochem. 1995;117(4):741–749. doi: 10.1093/oxfordjournals.jbchem.a124771. [DOI] [PubMed] [Google Scholar]
- 26.Veeranna SKT, et al. Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. J Neurochem. 1995;64(6):2681–2690. doi: 10.1046/j.1471-4159.1995.64062681.x. [DOI] [PubMed] [Google Scholar]
- 27.Beffert U, et al. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J Neurosci. 2004;24(8):1897–1906. doi: 10.1523/JNEUROSCI.4084-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, et al. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol. 2009;11(5):624–630. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Marcos M, et al. Functional characterization of the guanine nucleotide exchange factor (GEF) motif of GIV protein reveals a threshold effect in signaling. Proc Natl Acad Sci USA. 2012;109(6):1961–1966. doi: 10.1073/pnas.1120538109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh P, Garcia-Marcos M, Farquhar MG. GIV/Girdin is a rheostat that fine-tunes growth factor signals during tumor progression. Cell Adhes Migr. 2011;5(3):237–248. doi: 10.4161/cam.5.3.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhavan R, Tsai L-H. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2(10):749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 32.Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18(16):6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93(20):11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li BS, et al. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem. 2003;278(37):35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV/Girdin transmits signals from multiple receptors by triggering trimeric G protein activation. J Biol Chem. 2015;290(11):6697–6704. doi: 10.1074/jbc.R114.613414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strock CJ, et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66(15):7509–7515. doi: 10.1158/0008-5472.CAN-05-3048. [DOI] [PubMed] [Google Scholar]
- 37.Hsu FN, et al. Regulation of androgen receptor and prostate cancer growth by cyclin-dependent kinase 5. J Biol Chem. 2011;286(38):33141–33149. doi: 10.1074/jbc.M111.252080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldmann G, et al. Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res. 2010;70(11):4460–4469. doi: 10.1158/0008-5472.CAN-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggers JP, et al. Cyclin-dependent kinase 5 is amplified and overexpressed in pancreatic cancer and activated by mutant K-Ras. Clin Cancer Res. 2011;17(19):6140–6150. doi: 10.1158/1078-0432.CCR-10-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang Q, et al. CDK5 is essential for TGF-β1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep. 2013;3:2932. doi: 10.1038/srep02932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Marcos M, et al. Expression of GIV/Girdin, a metastasis-related protein, predicts patient survival in colon cancer. FASEB J. 2011;25(2):590–599. doi: 10.1096/fj.10-167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modi PK, Komaravelli N, Singh N, Sharma P. Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol Biol Cell. 2012;23(18):3722–3730. doi: 10.1091/mbc.E12-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng YL, et al. Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell. 2007;18(2):404–413. doi: 10.1091/mbc.E06-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo IC, et al. Activation of Gαi at the Golgi by GIV/Girdin imposes finiteness in Arf1 signaling. Dev Cell. 2015;33(2):189–203. doi: 10.1016/j.devcel.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Marcos M, Ear J, Farquhar MG, Ghosh P. A GDI (AGS3) and a GEF (GIV) regulate autophagy by balancing G protein activity and growth factor signals. Mol Biol Cell. 2011;22(5):673–686. doi: 10.1091/mbc.E10-08-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paglini G, Peris L, Diez-Guerra J, Quiroga S, Cáceres A. The Cdk5-p35 kinase associates with the Golgi apparatus and regulates membrane traffic. EMBO Rep. 2001;2(12):1139–1144. doi: 10.1093/embo-reports/kve250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuya T, et al. Negative regulation of Vps34 by Cdk mediated phosphorylation. Mol Cell. 2010;38(4):500–511. doi: 10.1016/j.molcel.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 49.Elia N, Lippincott-Schwartz J. 2009. Culturing MDCK cells in three dimensions for analyzing intracellular dynamics. Curr Protoc Cell Biol. Chapter 4:Unit 4.22.