Abstract
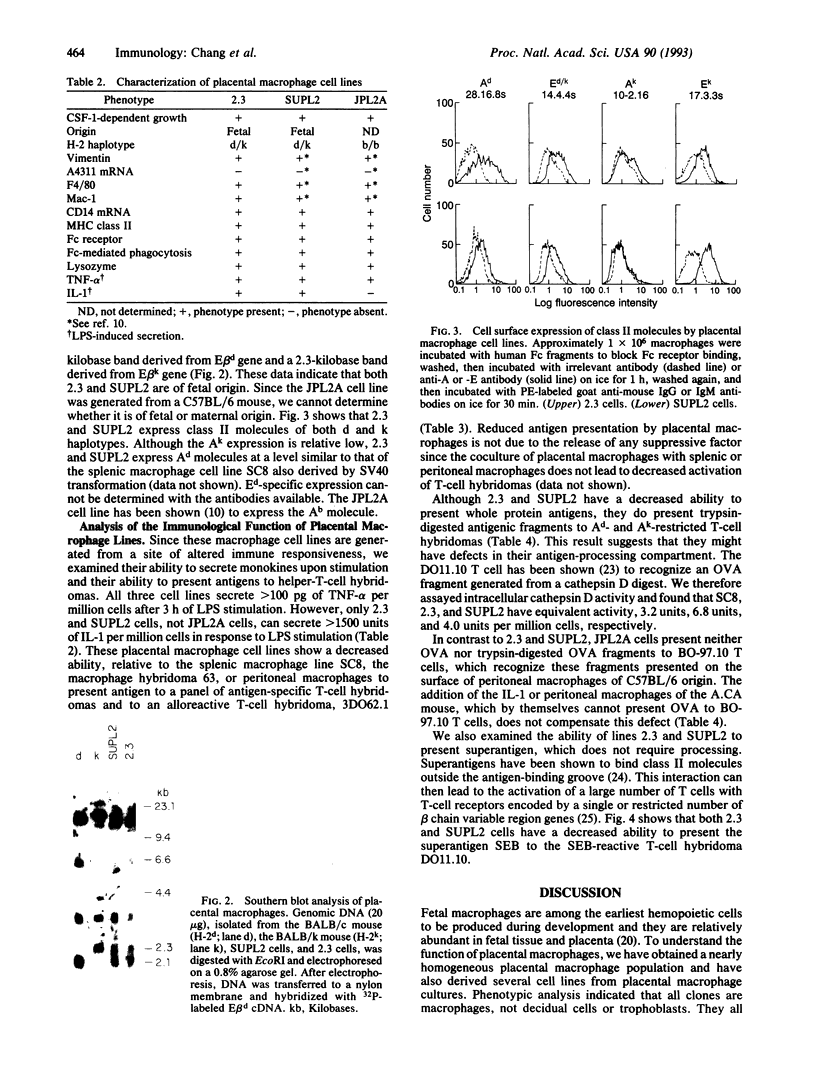
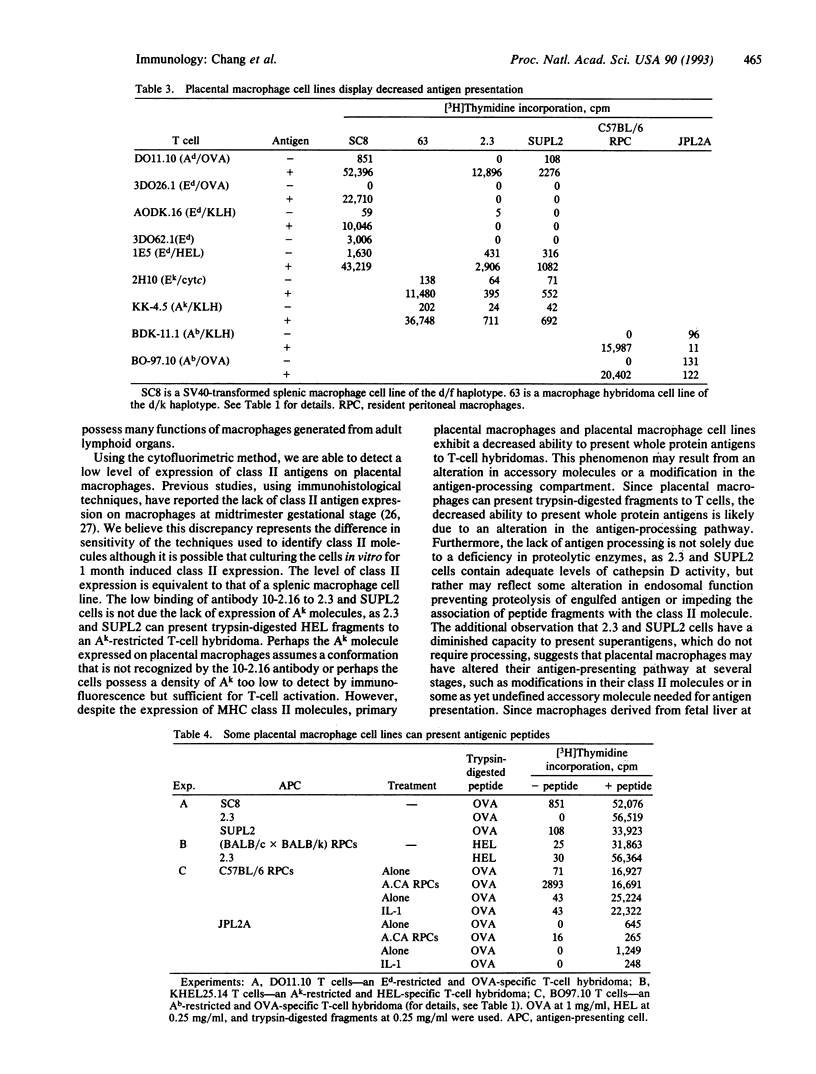
Large numbers of macrophages can be found in an animal's uteroplacental unit. This high concentration of macrophages suggests they must play an important role during placental development. To gain a better understanding of the functional capacity of placental macrophages, we have obtained a highly enriched placental macrophage culture and have derived several cell lines from this population. Both placental macrophages and cell lines show colony-stimulating factor 1-dependent growth, express Fc receptors, and can perform Fc-receptor-mediated phagocytosis. In addition, they express macrophage markers Mac-1, F4/80, and CD14. Although placental macrophages express major histocompatibility complex class II molecules constitutively, they display a decreased ability to present protein antigens to T cells. Since primary fetal liver macrophages of the same gestational stage also show a decreased ability to present antigens, this phenomenon may reflect a developmental stage of macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Shanahan F., Stanley E. R., Pollard J. W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock C. A., Hayes M., Chang M. Y., Pollard J. W. Isolation and characterization of four CSF-1-dependent placental macrophage cell lines. J Leukoc Biol. 1992 Jun;51(6):535–545. doi: 10.1002/jlb.51.6.535. [DOI] [PubMed] [Google Scholar]
- Chang M. Y., Kowal C., Marzullo L. R., Briner T. J., Gefter M. L., Diamond B. Genetic recombination in the alpha 2 domain of the E alpha chain yields an Ed molecule with altered T cell activation. Eur J Immunol. 1990 Dec;20(12):2571–2576. doi: 10.1002/eji.1830201207. [DOI] [PubMed] [Google Scholar]
- Chen H. L., Yang Y. P., Hu X. L., Yelavarthi K. K., Fishback J. L., Hunt J. S. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991 Aug;139(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- De M., Wood G. W. Analysis of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from implantation through parturition. J Leukoc Biol. 1991 Oct;50(4):381–392. doi: 10.1002/jlb.50.4.381. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Diment S. Different roles for thiol and aspartyl proteases in antigen presentation of ovalbumin. J Immunol. 1990 Jul 15;145(2):417–422. [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Ferrero E., Hsieh C. L., Francke U., Goyert S. M. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J Immunol. 1990 Jul 1;145(1):331–336. [PubMed] [Google Scholar]
- Goyert S. M., Ferrero E. M., Seremetis S. V., Winchester R. J., Silver J., Mattison A. C. Biochemistry and expression of myelomonocytic antigens. J Immunol. 1986 Dec 15;137(12):3909–3914. [PubMed] [Google Scholar]
- Head J. R., Drake B. L., Zuckermann F. A. Major histocompatibility antigens on trophoblast and their regulation: implications in the maternal-fetal relationship. Am J Reprod Immunol Microbiol. 1987 Sep;15(1):12–18. doi: 10.1111/j.1600-0897.1987.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989 Sep;16(1):1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Pollard J. W. Macrophages in the uterus and placenta. Curr Top Microbiol Immunol. 1992;181:39–63. doi: 10.1007/978-3-642-77377-8_2. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Ashwell J. D., Schwartz R. H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988 May 15;140(10):3324–3330. [PubMed] [Google Scholar]
- Lattime E. C., Stutman O. Tumor growth in vivo selects for resistance to tumor necrosis factor. J Immunol. 1989 Dec 15;143(12):4317–4323. [PubMed] [Google Scholar]
- Lescisin K. R., Varmuza S., Rossant J. Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev. 1988 Dec;2(12A):1639–1646. doi: 10.1101/gad.2.12a.1639. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Miyagawa Y., Matsuoka T., Baba A., Nakamura T., Tsuno T., Tamura A., Agematsu K., Yabuhara A., Uehara Y., Kawai H. Fetal liver T cell receptor gamma/delta+ T cells as cytotoxic T lymphocytes specific for maternal alloantigens. J Exp Med. 1992 Jul 1;176(1):1–7. doi: 10.1084/jem.176.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L., Graham C. F., Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991 Jun;112(2):517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest. 1989 Jul;61(1):27–36. [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987 Apr;79(4):1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbaum S., Diamond B. Generation of functional I-Ed variants from an antigen-presenting macrophage cell line. J Immunol. 1985 Jul;135(1):141–146. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Naito M., Katabuchi H., Higashi K. Development, differentiation, and maturation of macrophages in the chorionic villi of mouse placenta with special reference to the origin of Hofbauer cells. J Leukoc Biol. 1991 Jul;50(1):57–68. doi: 10.1002/jlb.50.1.57. [DOI] [PubMed] [Google Scholar]
- Uchida T., Ju S., Fay A., Liu Y., Dorf M. E. Functional analysis of macrophage hybridomas. I. Production and initial characterization. J Immunol. 1985 Feb;134(2):772–778. [PubMed] [Google Scholar]




