Abstract
In light of the emerging interplay between redox and metabolic signaling pathways we investigated the potential cross talk between nuclear factor E2-related factor 2 (Nrf2) and AMP-activated kinase (AMPK), central regulators of the cellular redox and energy balance, respectively. Making use of xanthohumol (XN) as an activator of both the AMPK and the Nrf2 signaling pathway we show that AMPK exerts a positive influence on Nrf2/heme oxygenase (HO)-1 signaling in mouse embryonic fibroblasts. Genetic ablation and pharmacological inhibition of AMPK blunts Nrf2-dependent HO-1 expression by XN already at the mRNA level. XN leads to AMPK activation via interference with mitochondrial function and activation of liver kinase B1 as upstream AMPK kinase. The subsequent AMPK-mediated enhancement of the Nrf2/HO-1 response does not depend on inhibition of the mammalian target of rapamycin, inhibition of glycogen synthase kinase 3β, or altered abundance of Nrf2 (total and nuclear). However, reduced endoplasmic reticulum stress was identified and elaborated as a step in the AMPK-augmented Nrf2/HO-1 response. Overall, we shed more light on the hitherto incompletely understood cross talk between the LKB1/AMPK and the Nrf2/HO-1 axis revealing for the first time involvement of the unfolded protein response as an additional player and suggesting tight cooperation between signaling pathways controlling cellular redox, energy, or protein homeostasis.
Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, AMP-activated kinase; ARE, antioxidant response element; βTrcP1, β-transducin-repeat containing protein 1; CaMKK, calcium calmodulin-dependent kinase kinase; CHO, Chinese hamster ovary; DCF, dichlorofluorescein; DMSO, dimethyl sulfoxide; ER, endoplasmic reticulum; FCS, fetal calf serum; GSH, glutathione (reduced); GSK3β, glycogen synthase kinase 3β; GSSG, oxidized glutathione; HO-1, heme oxygenase 1; Hrd1, synoviolin/Hrd1 (HMG-CoA reductase degradation)-ubiquitin ligase; Keap1, Kelch-like ECH-associated protein; LKB1, liver kinase B1; Maf, small musculoaponeurotic fibrosarcoma; MEF, mouse embryonic fibroblasts; mTOR, mammalian target of rapamycin; NADPH, nicotinamide adenine dinucleotide phosphate; Nrf2, nuclear factor E2-related factor 2; OCR, oxygen consumption rate; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ROS, reactive oxygen species; TAK, transforming growth factor β-activated kinase; UPR, unfolded protein response; WT, wild type; XN/Xn, xanthohumol
Keywords: AMPK, ER stress, HO-1, LKB1, MEF, Nrf2, PERK, Xanthohumol
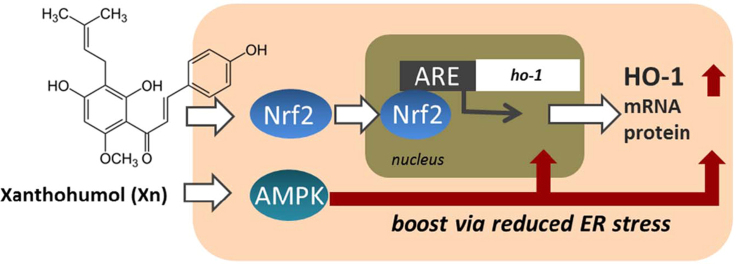
Graphical abstract
Highlights
-
•
Activated AMPK boosts the Nrf2/HO‐1 signaling axis in xanthohumol‐treated cells.
-
•
Xanthohumol leads to AMPK activation in an LKB1‐dependent manner.
-
•
The AMPK boost hits on a step prior to or at transcription of HO‐1 mRNA.
-
•
Inhibition of GSK3β or mTOR is not involved in the observed AMPK boost.
-
•
Higher ER stress accounts for the lower Nrf2/HO1 response in AMPK‐deficient cells.
Introduction
Generation and detoxification of reactive oxygen species (ROS) are tightly linked with the nutrient and metabolic status of a cell. Likewise, oxidation of nutrients is linked with superoxide formation. Reduced nicotinamide adenine dinucleotide phosphate (NADPH), mainly derived from the pentose phosphate pathway, is a crucial cosubstrate for enzymes involved in detoxification (e.g., glutathione reductase, thioredoxin reductase) and production (NADPH oxidases, NO synthases) of reactive (oxygen) species or oxidation products. ROS-forming enzymes alter their activity on phosphorylation, glycosylation, or acetylation, all posttranslational modifications lastly dependent on the cellular nutrient status (e.g., [1], [2], [3]).
On the level of individual players within the cellular redox balance, nuclear factor E2-related factor 2 (Nrf2) is an accepted master regulator. Nrf2 is a ubiquitously expressed transcription factor of the cap´n´collar basic leucine zipper family, and constitutes a major part of the cellular defense against oxidative but also other harmful insults. In unstressed cells the level of Nrf2 protein is low which is usually brought about by binding of Nrf2 to its most prominent inhibitor Kelch-like ECH-associated protein (KEAP) 1 in a 1:2 molar ratio. Keap1, being an adapter for Cul3/Rbx1 E3 ubiquitin ligases, facilitates constant proteasome-dependent degradation of Nrf2. On exposure to oxidative or electrophilic agents cysteine residues of Keap1 are oxidized or covalently modified. This alters the conformation of the Nrf2/ Keap1 complex, stops ubiquitination and degradation of Nrf2, and favors its accumulation and subsequent nuclear translocation. In the nucleus, Nrf2 heterodimerizes with small musculoaponeurotic fibrosarcoma (Maf) proteins and binds to antioxidant response element (ARE) consensus sequences (TCAG/CXXXGC) in promoters of Nrf2-regulated genes. Those comprise approximately 200 genes including heme oxygenase 1 (HO-1) and promote detoxification of the initial insult. Besides the canonical Keap1-dependent regulation Nrf2 abundance is further controlled by degradation via the glycogen synthase kinase 3β (GSK3 β)/β-transducin-repeat containing protein (βTrcP) 1 pathway or synoviolin/Hrd1-ubiquitin ligase (Hrd1), by altered transcription of the Nrf2 gene or microRNAs targeting Nrf2. Posttranslational modifications at the Nrf2 protein fine-tune nuclear residence and activity of Nrf2 and include phosphorylation or acetylation of Nrf2 or interaction of the transcription factor with distinct binding partners [4], [5], [6], [7]. Thus, the Nrf2-dependent cytoprotection pathway is able to receive and integrate cues from multiple different signal transduction pathways.
AMP-activated kinase (AMPK) is a central hub for cellular energy homeostasis. The heterotrimeric serine/threonine kinase is formed by the catalytic alpha and the regulatory beta and gamma subunits. Its enzymatic activity is highly responsive to the cellular energy load. Binding of AMP to the gamma subunit in cases of low energy alters conformation and favors phosphorylation of Thr172 of the alpha subunit mainly by liver kinase B1 (LKB1), resulting in a massive increase of enzymatic activity of AMPK. Largely AMP-independent phosphorylation and activation of AMPK can be mediated by calcium calmodulin-dependent kinase kinase (CaMKK)β and transforming growth factor β-activated kinase (TAK)1, rendering AMPK also susceptible to stimuli beyond energy stress, such as increased intracellular calcium levels [8], [9], [10], [11]. Accordingly, activated AMPK elicits pleiotropic effects including generally increased catabolism and decreased anabolism, improved endothelial function, reduced inflammation, or improved redox balance [12], [13], [14]. Notably, those phenotypic readouts were also observed in numerous studies on activation of Nrf2 [15], [16], [17], [18], suggesting that Nrf2 and AMPK may cooperate within their signaling networks.
Given the link between the cellular metabolic and redox state [4] and the widely overlapping cellular responses on Nrf2 or AMPK activation we set out to investigate a potential AMPK/Nrf2 cross talk in molecular detail. We used wild-type (WT) and isogenic Nrf2 or AMPK knockout mouse embryonic fibroblasts (MEF) as well as xanthohumol (XN), a prenylated chalcone from hops, as small molecular probe mediating activation of both AMPK and Nrf2 [19], [20], [21]. Induction of HO-1, an enzyme decomposing heme to biliverdin, iron, and CO2 and accounting for many beneficial outcomes on Nrf2 activation (e.g., [22], [23], [24]), served as readout for Nrf2 activity.
Materials and methods
Cells, chemicals, and antibodies
Isogenic Nrf2-/- and WT mouse embryonic fibroblasts (MEF) were kindly provided from Thomas Kensler (University of Pittsburgh, PA, USA), isogenic AMPKα-/- and WT MEF from Benoit Viollet (Institute Cochin INSERM, Paris, France), and the LKB1-/- and WT MEF from Reuben J. Shaw (Salk Institute, La Jolla, CA, USA) [25], [26], [27]. The clone of Chinese hamster ovary (CHO) cells stably expressing the ARE-dependent luciferase reporter (CHO-ARE-Luc) has been previously described [28]. The primary antibodies directed against AMPKα (No. 2532), phospho-AMPKα (Thr172) (No. 2535), ACC (No. 3662), phospho-ACC (Ser79) (No. 3661), LKB1 (No. 3047), lamin B1 (No. 13435), BiP (No. 3183), and α,β-tubulin (No. 2148) were from Cell Signaling Technology (Danvers, MA, USA). The antibody against Nrf2 was from Santa Cruz (C-20; No. sc-722) (Dallas, TX, USA), the antibody against HO-1 (No. ADI-SPA-896) from Enzo Life Sciences (Farmingdale, NY, USA), and the antibody against actin (No. 69100) from MP Biomedicals (Santa Ana, CA, USA). The secondary HRP-linked antibody against rabbit was from Cell Signaling, the antibody against mouse from MP Biomedicals. Xanthohumol, compound C, chloroquine, phenylbutyrate, tunicamycin, and thapsigargin were purchased from Sigma-Aldrich (St. Louis, MO, USA), rapamycin from Cell Signaling, and CHIR99021 from Santa Cruz. The PERK inhibitor GSK2606414 came from EMD Millipore (Darmstadt, Germany).
Cell cultivation and treatment
MEF and CHO-ARE-Luc were cultured in Dulbecco’s modified essential medium (DMEM; Lonza, Braine-L’Alleud, Belgium) supplemented with 10% heat inactivated fetal calf serum (FCS, Lonza) and 2 mM glutamine (Invitrogen, Carlsbad, CA, USA) under a humidified atmosphere of 5% CO2 at 37 °C. For all experiments cells were reseeded in appropriate plates and grown to approximately 80–90% confluence. During treatment with XN the serum concentration was reduced to 2% in all wells (including control wells) in order to avoid major adsorption of XN to serum proteins [29]. Unless otherwise stated, all compounds were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO did not exceed 0.2%.
ARE-luciferase reporter gene assay
CHO-ARE-LUC cells were treated as indicated for 8 h before their lysates were subjected to assessment of luciferase activity as previously described [28].
Preparation of whole cell lysates, SDS-PAGE, and Western blot
Cells were seeded in 6-well culture plates. After being treated as indicated cells were lysed, and total cell lysates were subjected to SDS polyacrylamide electrophoresis and immunoblot as described elsewhere [30]. Detailed protocols can be found in the supplemental information.
Fractionated extraction of nuclear and cytosolic proteins
Cells were grown in 6- or 10-cm dishes. Treated as indicated cells were subjected to extraction of cytosolic and nuclear proteins as described previously [16]. Detailed information can be found in the supplemental material. Successful fractionation was confirmed by a lacking signal for lamin B1 in the cytosolic fraction and a missing signal for tubulin in the nuclear fraction in the respective immunoblot.
Real-time quantitative polymerase chain reaction (RTqPCR)
Extraction of total RNA, reverse transcription, and quantitative PCR and evaluation were performed as previously described [30]. Detailed information can be found in the supplemental material. Primers for murine HO-1 and the reference gene, murine hypoxanthine-phosphoribosyl-transferase (HPRT), came as QuantiTect Primer Assays from Qiagen (Venlo, Netherlands).
Determination of the total intracellular ROS load
Levels of intracellular ROS were determined by flow cytometric assessment of dichlorofluorescein (DCF) fluorescence as described previously [30].
Determination of the reduced (GSH) and oxidized glutathione (GSSG)
The cellular ratio of GSSG/GSH was determined with the luminescent GSSG-GSH Glo Assay from Promega (Madison, WI, USA) according to the manufacturers’ instructions.
Determination of mitochondrial superoxide production
Cells were incubated with 5 µM MitoSox Red (Invitrogen) for 15 min and then treated with vehicle, XN (5 µM), or antimycin A (10 µM, positive control) for different periods of time. Then the red fluorescence (FL2 channel, ex 488 nm; em 585/42 nm) of 10,000 cells was determined by flow cytometry (FACS Calibur, BD Biosciences, San Jose, CA, USA) and corrected for autofluorescence. The obtained arbitrary mean was taken as readout for produced mitochondrial superoxide.
Determination of mitochondrial function by extracellular flux analysis
MEF were seeded in appropriate collagen-coated 24-well cell culture plates (from Seahorse Biosciences, Copenhagen, Denmark) at a cell density of 2.7 × 104 cells/well. Cells were kept in serum-free medium (glucose free DMEM plus 2 mM glutamine, 2 mM pyruvate, pH 7.35–7.40) at 37 °C and ambient CO2 for 1 h before they were subjected to mitochondrial (readout: oxygen consumption rate (OCR) in pmol O2/ min) stress tests. Appropriate test kits came from Seahorse Biosciences, were performed according to the manufacturers´ instructions, and analyzed on a Seahorse 24XFe extracellular flux analyzer and Wave software (www.seahorsebio.com) as described elsewhere [31] and in detail in the supplemental information.
Statistics
Experiments were performed at least three times. Error bars in the pictures represent the SD (standard deviation) of the mean. Statistical significance was determined by using Student’s t test or by one-way ANOVA followed by Bonferroni’s post test. All statistical analyses were done with GraphPad Prism. P values <0.05 were considered as significant and are designated with ⁎ in the figures.
Results
AMPK boosts the Nrf2-mediated HO-1 induction by XN
In order to confirm the previously reported XN-triggered Nrf2 and AMPK activation in our hands we determined ARE-dependent luciferase expression and AMPK activation on exposure to XN, respectively. XN significantly activated Nrf2 and induced ARE-dependent luciferase expression at a concentration of 5 µM (Fig. 1A). Moreover, 5 µM XN led to AMPK activation as evident by elevated phosphorylation of AMPK at Thr172 and of acetyl-CoA-carboxylase (ACC) at Ser79, a downstream target of AMPK (Fig. 1B). Administration of XN to MEF cells revealed a time-dependent increase of HO-1 protein expression in WT cells. Upregulation of the protein started already after 3 h, reached a maximum after 7 h, and declined after 24 h. Isogenic Nrf2-/- MEF completely failed to induce HO-1 on exposure to XN, indicating that HO-1 induction by XN is a strictly Nrf2-dependent process (Fig. 1C). The Nrf2 dependency could be confirmed at the level of HO-1 mRNA (Fig. 1D). XN led to a vigorous induction of HO-1 mRNA in WT, but not at all in Nrf2-/- cells. These data validated our model system based on XN as activator of the AMPK and Nrf2 signaling pathway and on HO-1 as exclusively Nrf2-dependent readout.
Fig. 1.
XN leads to activation of AMPK and Nrf2 and triggers HO-1 induction in a strictly Nrf2-dependent manner in MEF. (A) CHO-ARE-Luc cells were treated with vehicle or 5 µM xanthohumol (XN, Xn; upper panel) for 8 h before their luciferase expression was assessed. Bar graph depicts compiled data from three independent experiments expressed as fold activation compared to vehicle control (mean + SD; ⁎P<0.05; ANOVA). (B) WT MEF cells were treated with vehicle or XN (5 µM) for 30 min before total cell lysates were subjected to immunoblot analyses for pAMPK (T172), total AMPK, pACC (S79), and total ACC. Representative blots and compiled densitometric analyses of three independent experiments are depicted (mean + SD; ⁎P<0.05; Student’s t test). (C) WT and Nrf2-/- MEF were treated with XN (5 µM) for the indicated periods of time before total cell lysates were subjected to immunoblot analysis for HO-1 and actin, respectively. Respective blots of three independent experiments are depicted with the bar graph showing compiled densitometric data from all performed experiments (mean + SD; ⁎P< 0.05; ANOVA, to vs tx). (D) WT and Nrf2-/- MEF were treated with vehicle (DMSO, D) or XN (5 µM) for 4 h before their mRNA was isolated and subjected to qPCR analysis for quantification of HO-1 (target) and HPRT (reference) transcripts. The bar graph shows compiled data of three independent experiments expressed as fold HO-1 induction (normalized to HPRT levels) on XN exposure (mean + SD; ⁎P<0.05; Student´s t test).
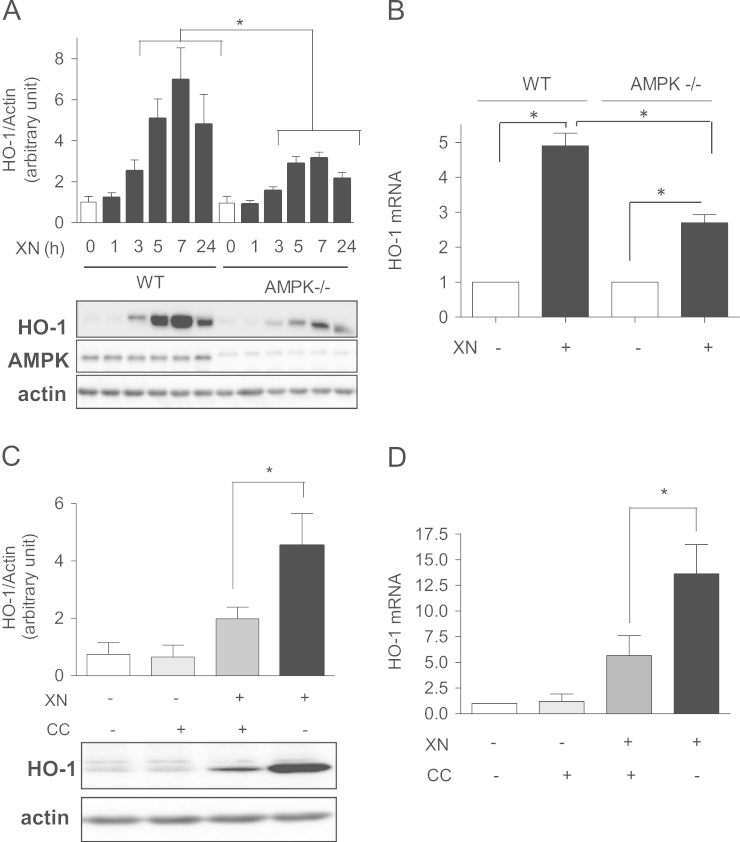
We then addressed the question whether activated AMPK influences Nrf2-dependent HO-1 induction using WT and AMPK-/- MEF. XN time dependently induced HO-1 in WT cells, again with a maximum at around 7 h after exposure. AMPK-/- cells also responded to XN with induction of HO-1, with similar kinetics but reduced amplitude compared to WT cells. Thus, AMPK is not pivotal for, but increases, the strength of the cellular Nrf2/HO-1 response after XN treatment. The reduced response in AMPK-/- cells became evident on both the protein and the mRNA level (Fig. 2A and B). Experiments using actinomycin D, a transcriptional inhibitor, revealed that the half-life of HO-1 mRNA is not markedly different between WT and AMPK-/- cells (Suppl. Fig. S1), excluding a major impact of AMPK on HO-1 mRNA stability. In line with the genetic knockout of AMPK cotreatment of WT cells with the AMPK inhibitor compound C significantly reduced the XN-triggered HO-1 induction on the protein (Fig. 2C) and mRNA level (Fig. 2D). These findings indicate that activated AMPK exerts a positive impact on the Nrf2-mediated HO-1 induction at a step prior to or at mRNA production.
Fig. 2.
AMPK boosts the Nrf2-mediated HO-1 induction by XN. (A) WT and AMPK-/- MEF were treated with XN (5 µM) for the indicated periods of time before total cell lysates were subjected to immunoblot analysis for HO-1, AMPK, and actin, respectively. Representative blots of three independent experiments are depicted with the bar graph showing compiled densitometric data from all performed experiments (mean + SD; ⁎P< 0.05; ANOVA). (B) WT and AMPK-/- MEF were treated with vehicle (DMSO, D) or XN (5 µM) for 4 h before their mRNA was isolated and subjected to qPCR analysis for quantification of HO-1 (target) and HPRT (reference) transcripts. The bar graph shows compiled data of three independent experiments expressed as fold HO-1 induction (normalized to HPRT levels) on XN exposure (mean + SD; ⁎P<0.05; ANOVA). (C and D) WT MEF were treated with the AMPK inhibitor compound C (10 µM; CC) for 30 min and then with XN (5 µM) for 7 h (C) or 4 h (D) before their HO-1 expression was determined by immunoblot (C) or qPCR (D). Bar graphs depict compiled data from three independent experiments (mean + SD; ⁎P<0.05; ANOVA). representative blots are shown.
XN activates AMPK and boosts Nrf2 signaling in an LKB1-dependent manner
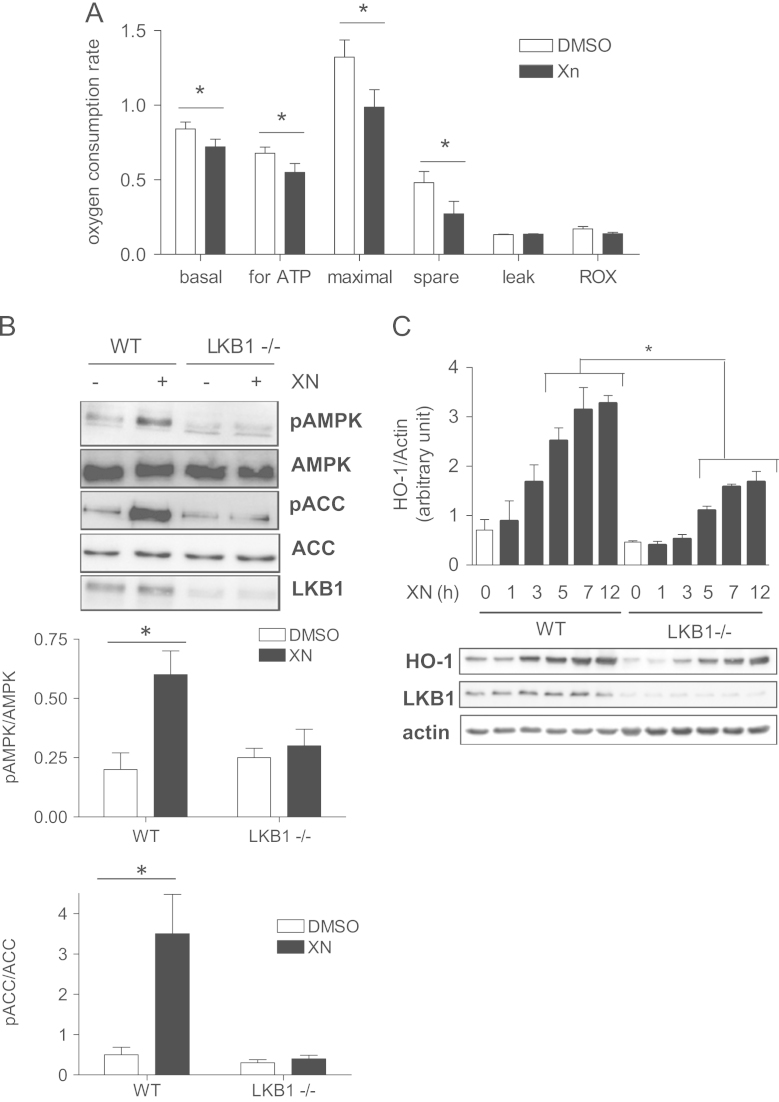
Before investigating in more detail the AMPK-mediated boost of Nrf2 signaling we examined how XN leads to AMPK activation. XN has been reported to interfere with proper mitochondrial function and ATP production in various cell systems [32], [33]. Using extracellular flux analysis we observed reduced basal respiration and reduced oxygen consumption for ATP synthesis, as well as decreased maximal and spare respiratory capacities in XN-treated MEF compared to control cells (Fig. 3A, Suppl. Fig S2A (raw data)). XN per se did not act as FCCP-like uncoupler (i.e., direct dissipator of the mitochondrial proton gradient) in MEF (Suppl. Fig. S2B), but led to an elevated production of mitochondrial superoxide (Suppl. Fig. S2C). These data suggest that XN at 5 µM acts as inhibitor of the electron transport chain in MEF rather than as uncoupler as suggested by Kirkwood and coauthors using myo-, hepato-, and preadipocytes [32]. Reduced mitochondrial function can go along with a reduced ATP/AMP ratio, activation with LKB1, and subsequent phosphorylation and activation of AMPK [10], [34]. We therefore examined the involvement of LKB1 in the XN-induced AMPK activation. Using WT and LKB1-/- MEF revealed that XN increases the phosphorylation of both AMPK at Thr172 and ACC at Ser79 in WT cells but not in the LKB1-/- cells (Fig. 3B). Furthermore, compared to WT the XN-dependent upregulation of HO-1 is blunted in LKB1-/- cells (Fig. 3C), reminiscent of the observations made in AMPK-/- cells. These data indicate that LKB-1 is upstream of AMPK to boost Nrf2 signaling, and XN is likely to interfere with the mitochondrial electron transport chain in MEF.
Fig. 3.
XN impairs mitochondrial function and activates AMPK in an LKB-1-dependent manner. (A) Mitochondrial function was assessed in vehicle and XN (5 µM)-treated WT MEF based on the oxygen consumption rate (OCR) on addition of mitochondrial stressors and subsequent extracellular flux analysis (as described in detail under Materials and methods). The graph depicts data from three independent experiments (mean + SD; ⁎P<0.05; Student’s t test (DMSO vs Xn)). (B) WT and LKB1-/- cells were treated with vehicle or XN (5 µM) for 30 min before total cell lysates were subjected to immunoblot analyses for pAMPK (T172), total AMPK, pACC (S79), total ACC and LKB1. Representative blots and compiled densitometric analyses of three independent experiments are depicted (mean + SD; ⁎P<0.05; Student’s t test). (C) WT and LKB1-/- MEF were treated with XN (5 µM) for the indicated periods of time before total cell lysates were subjected to immunoblot analyses for HO-1, LKB1, and actin. Representative blots of three independent experiments as well as compiled densitometric data of all performed experiments are depicted (mean + SD; ⁎P<0.05; ANOVA).
WT and AMPK-/- MEF do not differ in their level of oxidative stress under basal and XN-exposed conditions
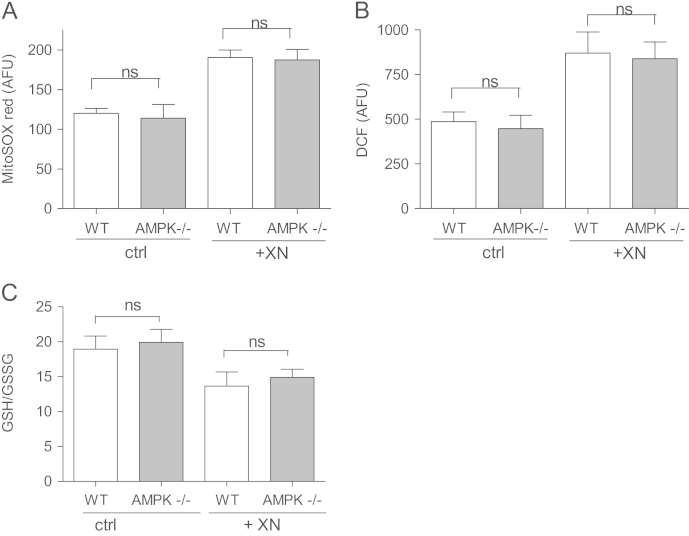
AMPK has already been reported as a positive upstream regulator of Nrf2 in C. elegans [35]. In that study AMPK activation was shown to lead to an elevated rate of mitochondrial respiration, a subsequently increased mitochondrial superoxide production, and finally to an increased Nrf2 activity as cellular adaptive response to the AMPK-driven rise in intracellular redox stress (mitohormesis). We therefore checked whether different levels of basal and XN-induced ROS levels between WT and AMPK-/- cells could account for the observed distinct strength of Nrf2-mediated HO-1 induction. We examined total ROS (Fig. 4A) and mitochondrial superoxide (Fig. 4B) levels as well as the ratio between GSSG and GSH (Fig. 4C) under basal conditions and on exposure to XN. Acute administration of XN led to an increase in ROS levels, however, to the same extent in both investigated cell types. Moreover, WT and AMPK-/- did not differ in their basal ROS load and showed a comparable doubling time of about 15–16 h, largely excluding altered proliferation-related redox stress as explanation for the distinct Nrf2 response [36]. The observed AMPK-mediated boost of XN-induced Nrf2 signaling cannot be explained by an altered cellular redox balance.
Fig. 4.
WT and AMPK-/- MEF do not differ in their level of oxidative stress under basal and XN-exposed conditions. WT and AMPK-/- MEF were treated with vehicle (ctrl) or XN (5 µM) for 30 min before their mitochondrial (A) and total intracellular ROS (B) load as well as their GSH/GSSG ratio (C) were assessed by MitoSOX, H2DCF-DA staining, and a commercially available glutathione determination kit, respectively. Compiled data of three independent experiments are depicted (mean +SD).
The positive effect of activated AMPK on Nrf2 signaling does not involve inhibition of mTOR or GSK3β
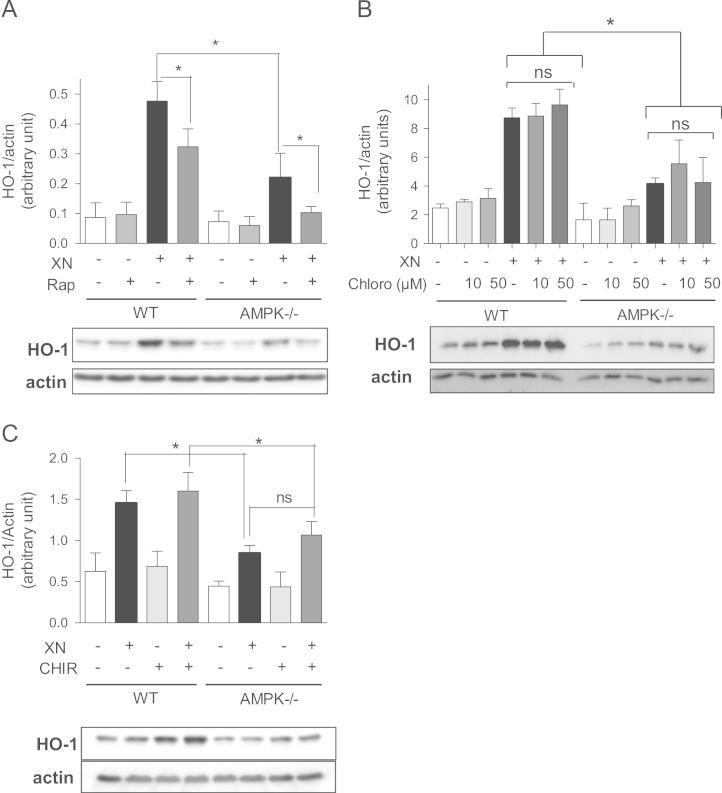
AMPK shifts cellular signal transduction toward a direction with potential positive impact on the Nrf2 pathway. Activated AMPK favors autophagy which, in turn, has been involved in augmented Nrf2 levels and signaling in some reports [37], [38]. We assessed whether autophagy constitutes the signaling interface on which the observed AMPK/Nrf2 cross talk takes place. Mammalian target of rapamycin (mTOR) is one of the major upstream inhibitors of autophagy and is inhibited by phosphorylation by AMPK [39]. If activated AMPK mediates its boost on Nrf2 signaling by mTOR inhibition and autophagy activation, rapamycin, an inhibitor of mTOR, should rescue the blunted XN response in AMPK-/- cells. However, treatment with rapamycin reduced rather than increased XN-mediated increased HO-1 protein levels in AMPK-/- cells. The same behavior was observed in WT cells, probably due to generally impaired protein synthesis on rapamycin exposure owing to interference with ribosomal protein S6 kinase 1 activity (Fig. 5A). Inhibition of autophagy by the lysosomal inhibitor chloroquine did not show any effect on the XN-induced HO-1 levels in WT or AMPK-/- cells either (Fig. 5B). Thus, based on these data with pharmacological activators and inhibitors altered mTOR activity or autophagy does not occur as an obvious explanation for the AMPK-mediated enhancement of Nrf2 signaling and HO-1 protein expression. However, thinking of the complex regulation of autophagy additional experiments are needed to unambiguously corroborate this conclusion. AMPK also inactivates GSK3β by phosphorylation, and GSK3β negatively influences Nrf2 signaling by increased Nrf2 degradation via the βTrcP pathway or increased nuclear exclusion [40], [41], [42], [43]. It is therefore conceivable that AMPK-mediated GSK3β inhibition accounts for the higher Nrf2 response in WT than in AMPK-/- cells. However, inhibition of GSK3β by CHIR9921 (Fig. 5C), lithium chloride, or siRNA-mediated knockdown (data not shown) did not significantly elevate HO-1 levels in AMPK-/- cells, largely excluding GSK3β as mediator between AMPK and Nrf2 in the used test system.
Fig. 5.
Inhibition of mTOR or of GSK3β does not play an obvious role in the AMPK-mediated boost of Nrf2-mediated HO-1 induction. WT and AMPK-/- MEF were pretreated with the mTOR inhibitor rapamycin (Rap; 100 nM) (A), the autophagy inhibitor chloroquine (chloro; 10 and 50 µM) (B), or the GSK3β inhibitor CHIR99201 (CHIR; 10 µM) (C) for 30 min before they were incubated with XN (5 µM) for 7 h. Total cell lysates were subjected to an immunoblot analysis for HO-1 and actin. Representative blots and compiled densitometric data of three independent experiments are depicted (mean +SD; ⁎P< 0.05, ANOVA).
WT and AMPK-/- cells do not differ in their total or nuclear level of Nrf2 on XN treatment
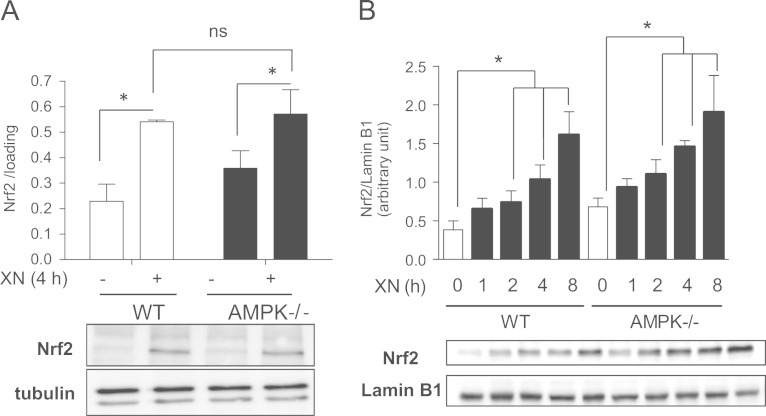
Transcriptional activity of Nrf2 is highly regulated via degradation and/or nuclear import [7], [44]. The blunted Nrf2 response in AMPK-/- cells could therefore still be due to reduced total or nuclear levels of Nrf2 in these cells, mediated by players other than GSK3β or mTOR. However, experiments assessing total levels and time-dependent nuclear translocation of Nrf2 in WT and AMPK-/- cells did not reveal significant differences in total level of the transcription factor (Fig. 6A) or in its nuclear accumulation on XN treatment (Fig. 6B). Overall, these data indicate that the positive effect of AMPK on Nrf2-mediated transcription in our system does not imply altered abundance or nuclear translocation of Nrf2.
Fig. 6.
WT and AMPK-/- MEF do not differ in their total and nuclear levels of Nrf2 on XN exposure. (A) WT and AMPK-/- MEF were treated with vehicle or XN (5 µM) for 4 h before total cell lysates were subjected to immunoblot analysis for Nrf2 and α⧸β tubulin. (B) WT and AMPK-/- MEF were treated with XN (5 µM) for the indicated periods of time before nuclear extracts were subjected to immunoblot analysis for Nrf2 and lamin B1. Representative blots from three independent experiments and compiled densitometric data are depicted (mean + SD; ⁎P<0.05; ANOVA).
AMPK-/- cells show an elevated ER stress that hampers Nrf2-mediated HO-1 induction
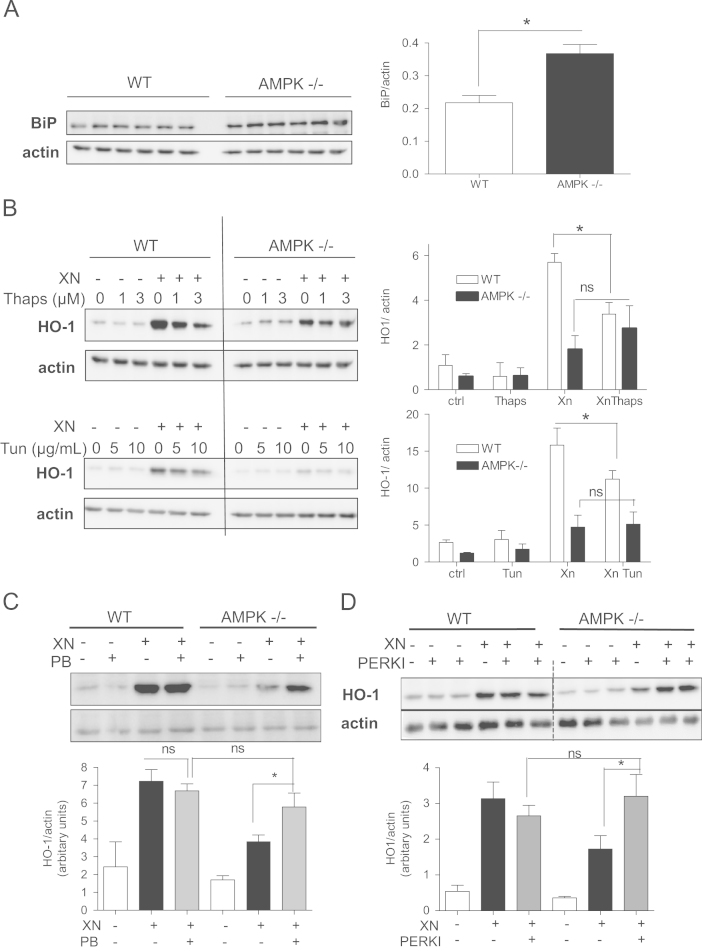
Nrf2 not only alleviates stress imposed by oxidative damage but also by xenobiotics, nutritional imbalance, growth factors, or accumulation of unfolded proteins in the endoplasmic reticulum (ER). The latter merges in a cellular defense program called unfolded protein response (UPR) counteracting ER stress primarily by inhibition of translation and activation of protein degradation. Recent studies suggest a vivid cross talk between the oxidative stress and the unfolded protein response, also with regard to Nrf2 signaling [45], [46], [47]. Of note, activated AMPK has been reported to alleviate ER stress [48], [49]. We therefore were prompted to test whether altered ER stress could possibly explain the AMPK-triggered boost of HO-1 induction. Fig. 7A shows that AMPK-/- display higher levels of GRP78/BiP, a protein accumulating on ER stress. Induction of ER stress by tunicamycin, a glycosylation inhibitor, or thapsigargin, an inhibitor of ER Ca2+-ATPase, reduced the XN-launched HO-1 expression in WT, but not AMPK-/- cells (Fig. 7B). Alleviation of ER stress by phenylbutyrate (Suppl. Fig. S3) was able to increase HO-1 expression in AMPK-/- but not WT cells (Fig. 7C). A consistent picture arose when cells were treated with GSK260641, an inhibitor of ER-resident protein kinase RNA-like endoplasmic reticulum kinase (PERK) controlling one of the three branches of the UPR [50] (Fig. 7D). These data demonstrate distinct levels of ER stress signaling between WT and AMPK-/- cells which relates to the different strength of Nrf2-mediated HO-1 expression and involves the PERK branch of the UPR.
Fig. 7.
Increased ER stress and PERK activity hamper Nrf2-mediated HO-1 induction in AMPK-/- cells. (A) Total cell lysates of WT and AMPK-/- cells were subjected to immunoblot analysis for GRP78/BiP and actin. The graph on the right depicts compiled data of six independently prepared protein extracts. (⁎P<0.05; Student´s t test (WT vs AMPK-/-).) (B) WT and AMPK-/-MEF were pretreated with the indicated concentrations of the ER stress inducers thapsigargin (Thaps) and tunicamycin (Tun) for 30 min and then exposed to vehicle or XN (5 µM) for 7 h. Total cell lysates were subjected to immunoblot analysis for HO-1 and actin. Representative blots are depicted and the graphs on the right show compiled densitometric data of three independent experiments (using Thaps at 3 µM and Tun at 5 µg/mL) (mean + SD; ⁎P<0.05; ANOVA). (C) WT and AMPK-/- MEF were treated with phenylbutyrate (PB, 10 mM), an ER stress inhibitor, and exposed to vehicle or XN (5 µM) for 7 h as indicated before total cell lysates were subjected to immunoblot analysis for HO-1 and actin. Representative blots are depicted and the graph on the right shows compiled densitometric data of three independent experiments (mean + SD; ⁎P<0.05; ANOVA). (D) WT and AMPK-/- MEF were treated with 1 µM PERK inhibitor GSK 2606414 (PERKI) for 30 min before they were exposed to XN (5 µM) for 7 h. Total cell lysates were subjected to immunoblot analysis for HO1 and actin. Representative blots and graphs showing the compiled densitometric analyses of three independent experiments are depicted (mean + SD; ⁎P<0.05; ANOVA). The dashed line in the blot picture symbolizes cutout of interjacent bands of no interest; all bands originate from the same membrane.
Discussion
In this work we made use of XN as small molecular probe activating both AMPK and Nrf2 signaling and analyzed the Nrf2/HO-1 response dependent on AMPK. Administration of XN to WT and AMPK-/- MEF showed a robust positive influence of AMPK on Nrf2-mediated HO-1 expression at the level of HO-1 mRNA and protein. AMPK deficiency accounted for elevated ER stress which was associated with increased PERK activity and subsequently reduced HO-1 expression. Total and nuclear levels of Nrf2 in WT and AMPK-/- cells were comparable. Moreover, AMPK activation by XN could be associated with mitochondrial dysfunction and for the first time with LKB1 as upstream AMPK kinase.
During the course of our work an AMPK-mediated enhancement of Nrf2 signaling has been reported by other investigators. Mo and coauthors observed a positive influence of AMPK on the berberine-induced Nrf2 response in septic mice [51]. Lee and Kim noted a reduced Nrf2 response on dehydrodiconiferyl alcohol (DHCA) exposure in macrophages when the AMPK inhibitor compound C is coadministered [52]. However, molecular details underlying the cross talk in those models remained largely elusive. For the model system used in this study, we could exclude increased intracellular redox stress and inhibition of mTOR or GSK3β to markedly contribute to the AMPK-mediated boost of Nrf2-mediated HO-1 induction. Regarding the role of GSK3β as a downstream target of AMPK, it must be stressed that our cellular system runs in 2–10% fetal calf serum and, thus, under conditions in which GSK3β is already significantly inhibited by the contained growth factors. Moreover, XN activates Nrf2 largely in a Keap1 rather than a GSK3β /βTrcP-dependent manner ([20] and Suppl. Fig. S4). Both system-inherent parameters override possible effects of the AMPK/GSK axis on Nrf2 which may apply in other systems based on quiescent cells or Keap1-independent Nrf2 activators and deserves further investigation.
The cellular UPR influences the oxidative stress response and vice versa on multiple levels. On the one hand, increased ER stress goes along with activation of PERK that is reported to phosphorylate and activate Nrf2 [53]. On the other hand, the ER stress-related ubiquitin ligase synoviolin/Hrd1 was found to trigger degradation and inactivation of Nrf2 [5]. In C. elegans several components of the UPR are target genes of SKN-1, the homologue of Nrf2, and signaling from the ER was shown to be necessary for the response of SKN-1 to oxidative stress [47]. In addition and of particular interest for our model system, activation of AMPK was found to activate the unfolded protein response [54] and to lower ER stress induced by various stressors [49]. In line with this emerging cross talk among AMPK, ER stress, and oxidative stress response we could link the three players for the first time. AMPK-/- MEF suffered from elevated ER stress which, in turn, contributed to the reduced Nrf2-mediated HO-1 expression compared to WT cells. Accordingly, induction of ER stress in WT or alleviation of ER stress in AMPK-/- cells approximated the extent of XN-triggered HO-1 induction between both cell types. It will be of interest to further investigate how AMPK signals to the individual branches of the UPR—the inositol-requiring protein 1 (IRE1), PERK, or activating transcription factor (ATF)6 branch [50], [55]—in order to control Nrf2 activity. Using pharmacological inhibition we could already show that elevated PERK activity contributes to the reduced strength of the Nrf2/HO-1 axis in AMPK-deficient cells. The effect of PERK on Nrf2 activity seems to be context dependent. In cells lacking an AMPK signal PERK dampens the Nrf2/HO-1 signaling axis whereas in cells with AMPK PERK activity generally exerts an activating influence on the Nrf2 response ([45], [53]; see also Fig. 7D: no significant but reproducible trend of reduced HO-1 induction in WT cells on inhibition of PERK).
Despite slightly higher levels of the ER stress-linked ubiquitin ligase Hrd1 (see Suppl. Fig. S5) AMPK-/- cells do not show reduced Nrf2 levels compared to WT MEF. Kinetics and quantity of total and nuclear Nrf2 accumulation on XN exposure showed no dependency on the presence of AMPK. Thus, activated AMPK and ER homeostasis seem to positively influence Nrf2-mediated HO-1 mRNA production in the nucleus. This could be mediated on the molecular level, e.g., by altered posttranslational modification of Nrf2, altered binding partners in the coactivator complex, or altered binding behavior at ARE-dependent promoters (duration, strength, preference for certain promoters). Xue et al. recently suggested that amplitude and frequency of constitutive Nrf2 oscillations between cytosol and nucleus are determinants for Nrf2-mediated gene transcription, independently from Nrf2 abundance [56]. Future studies are warranted to scrutinize in detail the role of AMPK and ER stress for those features in the Nrf2 signaling pathway. A competition between the UPR and the oxidative stress response for Nrf2´s transcriptional activity has been suggested for C. elegans [47]. It is tempting to speculate that such competition explains our findings. Current studies investigate whether Nrf2 of AMPK-deficient cells is preferentially targeted to promoters of genes connected with the unfolded protein response in disfavor of those genes mainly involved in the oxidative stress response such as HO-1.
Overall, using a chemical biological approach we showed the positive influence of AMPK on Nrf2/HO-1 signaling and revealed a hitherto unknown involvement of the PERK branch of the UPR in the signaling axis between AMPK and Nrf2/HO-1. These findings made in MEF may prompt further exploration of Nrf2 as a general sensor and executor of the cellular redox, energy, and protein homeostasis in specialized cell, organ, or whole animal systems. Furthermore, the demonstrated cooperation between the LKB1/AMPK and Nrf2/HO-1 axis may provide an important step in the etiology of metabolic disease or life span prolongation by caloric restriction.
Acknowledgments
The authors thank Thomas Kensler, Benoit Viollet, and Reuben Shaw for kindly providing WT MEF and isogenic Nrf2-, Keap1-, AMPKα-, or LKB1-deficient counterparts. The expert technical assistance from Hortenzia Beres and Daniel Schachner and the execution of pilot experiments by Barbara Arthofer are gratefully acknowledged. This work was financially supported by the FWF (P23317 to E.H.H.), the Herzfelder´sche Familienstiftung, and the graduate program IK BioProMoTion of the University of Vienna.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2015.03.030.
Appendix A. Supplementary materials
Supplementary data
References
- 1.Brandes R.P., Weissmann N., Schroder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 2.Heiss E.H., Dirsch V.M. Regulation of eNOS enzyme activity by posttranslational modification. Curr. Pharm. Des. 2014;20(22):3503–3513. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metallo C.M., Vander Heiden M.G. Metabolism strikes back: metabolic flux regulates cell signaling. Genes Dev. 2010;24(24):2717–2722. doi: 10.1101/gad.2010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Wu T. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28(7):708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copple I.M. The Keap1-Nrf2 cell defense pathway—a promising therapeutic target? Adv. Pharmacol. 63. 2012:43–79. doi: 10.1016/B978-0-12-398339-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 7.Bryan H.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013;85(6):705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Hardie D.G. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895-–08. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley S.A. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Shaw R.J. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero-Martin G. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28(6):677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirwany N.A., Zou M.H. AMPK: a cellular metabolic and redox sensor. A minireview. Front. Biosci. 2014;19:447–474. doi: 10.2741/4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill L.A., Hardie D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 15.Ludtmann M.H. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 2014;457(3):415–424. doi: 10.1042/BJ20130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heiss E.H. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J. Biol. Chem. 2009;284(46):31579–31586. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita K. Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc. Natl. Acad. Sci. USA. 2011;108(4):1427–1432. doi: 10.1073/pnas.1014156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X.L. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006;290(5):H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 19.Lee I.S. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via Nrf2-ARE signaling in microglial BV2 cells. Neurochem. Int. 2011;58(2):153–160. doi: 10.1016/j.neuint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y. Sites of alkylation of human Keap1 by natural chemoprevention agents. J. Am. Soc. Mass Spectrometry. 2007;18(12):2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doddapattar P. Xanthohumol ameliorates atherosclerotic plaque formation, hypercholesterolemia, and hepatic steatosis in ApoE-deficient mice. Mol. Nutr. Food Res. 2013;57(10):1718–1728. doi: 10.1002/mnfr.201200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paine A. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010;80(12):1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Farombi E.O. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 2008;46(4):1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 24.Guzman-Beltran S. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci. Lett. 2008;447(2-3):167–171. doi: 10.1016/j.neulet.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra D. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38(17):5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw R.J. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Laderoute K.R. 5’-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 2006;26(14):5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiss E.H. Identification of chromomoric acid C-I as an Nrf2 activator in Chromolaena odorata. J. Nat. Prod. 2014;77(3):503–508. doi: 10.1021/np400778m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motyl M., Kraus B., Heilmann J. Pitfalls in cell culture work with xanthohumol. Pharmazie. 2012;67(1):91–94. [PubMed] [Google Scholar]
- 30.Heiss E.H. Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biol. 1. 20132013:359–365. 359–365. doi: 10.1016/j.redox.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiss E.H. Glycolytic switch in response to betulinic acid in non-cancer cells. PloS One. 2014;9(12):e115683. doi: 10.1371/journal.pone.0115683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkwood J.S. A metabolomics-driven elucidation of the anti-obesity mechanisms of xanthohumol. J. Biol. Chem. 2013;288(26):19000–19013. doi: 10.1074/jbc.M112.445452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strathmann J. Xanthohumol-induced transient superoxide anion radical formation triggers cancer cells into apoptosis via a mitochondria-mediated mechanism. FASEB J. 2010;24(8):2938–2950. doi: 10.1096/fj.10-155846. [DOI] [PubMed] [Google Scholar]
- 34.Wu S.B. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim. Biophys. Acta. 2014;1840(4):1331–1344. doi: 10.1016/j.bbagen.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Onken B., Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PloS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burhans W.C., Heintz N.H. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic. Biol. Med. 2009;47(9):1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Kim J. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichimura Y. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell. 2013;51(5):618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Gwinn D.M. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horike N. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J. Biol. Chem. 2008;283(49):33902–33910. doi: 10.1074/jbc.M802537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rada P. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31(6):1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain A.K., Jaiswal A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282(22):16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 43.Choi S.H., Kim Y.W., Kim S.G. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem. Pharmacol. 2010;79(9):1352–1362. doi: 10.1016/j.bcp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Theodore M. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J. Biol. Chem. 2008;283(14):8984–8994. doi: 10.1074/jbc.M709040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cullinan S.B., Diehl J.A. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006;38(3):317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Afonyushkin T. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via Nrf2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler. Thromb. Vasc. Biol. 2010;30(5):1007–1013. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]
- 47.Glover-Cutter K.M., Lin S., Blackwell T.K. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet. 2013;9(9):e1003701. doi: 10.1371/journal.pgen.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Y. Arctigenin alleviates ER stress via activating AMPK. Acta Pharmacol. Sin. 2012;33(7):941–952. doi: 10.1038/aps.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am. J. Physiol. Renal Physiol. 2014 doi: 10.1152/ajprenal.00495.2014. p. ajprenal 00495 2014. [DOI] [PubMed] [Google Scholar]
- 50.Sozen E., Karademir B., Ozer N.K. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic. Biol. Med. 2015;78C:30–41. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Mo C. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014;20(4):574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J., Kim S. Upregulation of heme oxygenase-1 expression by dehydrodiconiferyl alcohol (DHCA) through the AMPK-Nrf2 dependent pathway. Toxicol. Appl. Pharmacol. 2014;281(1):87–100. doi: 10.1016/j.taap.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Cullinan S.B. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L. Phenformin activates the unfolded protein response in an AMP-activated protein kinase (AMPK)-dependent manner. J. Biol. Chem. 2013;288(19):13631–13638. doi: 10.1074/jbc.M113.462762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohno K. Stress-sensing mechanisms in the unfolded protein response: similarities and differences between yeast and mammals. J. Biochem. 2010;147(1):27–33. doi: 10.1093/jb/mvp196. [DOI] [PubMed] [Google Scholar]
- 56.Xue M. Frequency modulated translocational oscillations of Nrf2 mediate the antioxidant response element cytoprotective transcriptional response. Antioxid. Redox Signal. 2014 doi: 10.1089/ars.2014.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data