Abstract
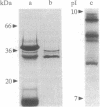
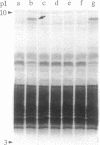
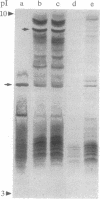
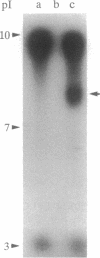
Methods are described for the removal of the sporophytic pollen grain coating of Brassica oleracea and for the isolation of coat polypeptides. The coat contains a small number of proteins ranging from 6 to 45 kDa. Many of the larger proteins are glycosylated, while all carry high positive charges resulting in pI values from 8.5 to 11. Polypeptides with pI values of 9.5, 9.0, and 8.5 possess strong esterase activity. No major differences could be detected in either pI values or molecular masses of pollen-coating polypeptides from grains carrying different sporophytically expressed S (self-incompatibility) alleles. Mixing pollen coat proteins with stigmatic extracts results in a conspicuous binding interaction involving female S-locus-specific and perhaps S-locus-related glycoproteins. This interaction, which is reversed by heating in the presence of SDS, results in an apparent charge shift of the female glycoprotein(s) of up to 2 pI units. The male participant in this interaction has been isolated by using a combination of fast protein liquid chromatography and reverse-phase HPLC and was shown to be a 7-kDa nonglycosylated peptide. Experiments with whole pollen cultured in vitro show challenge with stigmatic extracts to stimulate the release of gametophytic and sporophytic polypeptides and to result in the formation of a conspicuous interaction product, demonstrating the 7-kDa peptide to be freely available within the coating of pollen in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- East E. M., Mangelsdorf A. J. A New Interpretation of the Hereditary Behavior of Self-Sterile Plants. Proc Natl Acad Sci U S A. 1925 Feb;11(2):166–171. doi: 10.1073/pnas.11.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman C. J., Dickinson H. G. Pollen-stigma interactions in Brassica. IV. Structural reorganization in the pollen grains during hydration. J Cell Sci. 1986 Feb;80:141–157. doi: 10.1242/jcs.80.1.141. [DOI] [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem. 1985 Aug 15;149(1):218–224. doi: 10.1016/0003-2697(85)90498-1. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970 May;45(3):115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalonde B. A., Nasrallah M. E., Dwyer K. G., Chen C. H., Barlow B., Nasrallah J. B. A highly conserved Brassica gene with homology to the S-locus-specific glycoprotein structural gene. Plant Cell. 1989 Feb;1(2):249–258. doi: 10.1105/tpc.1.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure B. A., Haring V., Ebert P. R., Anderson M. A., Simpson R. J., Sakiyama F., Clarke A. E. Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature. 1989 Dec 21;342(6252):955–957. doi: 10.1038/342955a0. [DOI] [PubMed] [Google Scholar]
- Sarker R. H., Elleman C. J., Dickinson H. G. Control of pollen hydration in Brassica requires continued protein synthesis, and glycosylation in necessary for intraspecific incompatibility. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4340–4344. doi: 10.1073/pnas.85.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Thorsness M. K., Kandasamy M. K., Nishio T., Hirai M., Nasrallah J. B., Nasrallah M. E. Activity of an S Locus Gene Promoter in Pistils and Anthers of Transgenic Brassica. Plant Cell. 1991 Sep;3(9):867–876. doi: 10.1105/tpc.3.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Scutt C. P., Gates P. J., Gatehouse J. A., Boulter D., Croy R. R. A cDNA encoding an S-locus specific glycoprotein from Brassica oleracea plants containing the S5 self-incompatibility allele. Mol Gen Genet. 1990 Feb;220(3):409–413. doi: 10.1007/BF00391746. [DOI] [PubMed] [Google Scholar]
- Singh A., Ai Y., Kao T. H. Characterization of Ribonuclease Activity of Three S-Allele-Associated Proteins of Petunia inflata. Plant Physiol. 1991 May;96(1):61–68. doi: 10.1104/pp.96.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead A. D., Roberts I. N., Dickinson H. G. Pollen-stigma interaction in Brassica oleracea: the role of stigmatic proteins in pollen grain adhesion. J Cell Sci. 1980 Apr;42:417–423. doi: 10.1242/jcs.42.1.417. [DOI] [PubMed] [Google Scholar]
- Stein J. C., Howlett B., Boyes D. C., Nasrallah M. E., Nasrallah J. B. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsness M. K., Kandasamy M. K., Nasrallah M. E., Nasrallah J. B. A Brassica S-locus gene promoter targets toxic gene expression and cell death to the pistil and pollen of transgenic Nicotiana. Dev Biol. 1991 Jan;143(1):173–184. doi: 10.1016/0012-1606(91)90064-a. [DOI] [PubMed] [Google Scholar]
- Toriyama K., Thorsness M. K., Nasrallah J. B., Nasrallah M. E. A Brassica S locus gene promoter directs sporophytic expression in the anther tapetum of transgenic Arabidopsis. Dev Biol. 1991 Feb;143(2):427–431. doi: 10.1016/0012-1606(91)90094-j. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Zhang R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature. 1990 Jun 21;345(6277):743–746. doi: 10.1038/345743a0. [DOI] [PubMed] [Google Scholar]