Abstract
Aims
TOTAL (N = 10 732), a randomized trial of routine manual thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction, showed no difference in the primary efficacy outcome but a significant increase in stroke. We sought to understand these findings.
Methods and results
A detailed analysis of stroke timing, stroke severity, and stroke subtype was performed. Strokes were adjudicated by neurologists blinded to treatment assignment. Stroke within 30 days, the primary safety outcome, was increased [33 (0.7%) vs. 16 (0.3%), hazard ratio (HR) 2.06; 95% confidence interval (CI) 1.13–3.75]. The difference in stroke was apparent within 48 h [15 (0.3%) vs. 5 (0.1%), HR 3.00; 95% CI 1.09–8.25]. There was an increase in strokes within 180 days with minor or no disability (Rankin 0–2) [18 (0.4%) vs. 13 (0.3%) HR 1.38; 95% CI 0.68–2.82] and in strokes with major disability or fatal (Rankin 3–6) [35 (0.7%) vs. 13 (0.3%), HR 2.69; 95% CI 1.42–5.08]. Most of the absolute difference was due to an increase in ischaemic strokes within 180 days [37 (0.7%) vs. 21 (0.4%), HR 1.71; 95% CI 1.03–3.00], but there was also an increase in haemorrhagic strokes [10 (0.2%) vs. 2 (0.04%), HR 4.98; 95% CI 1.09–22.7]. Patients that had a stroke had a mortality of 30.8% within 180 days vs. 3.4% without a stroke (P < 0.001). A meta-analysis of randomized trials (N = 21 173) showed an increase in risk of stroke (odds ratio 1.59; 95% CI 1.11–2.27) but a trend towards reduction in mortality odds ratio (odds ratio 0.87; 95% CI 0.76–1.00).
Conclusion
Thrombectomy was associated with a significant increase in stroke. Based on these findings, future trials must carefully collect stroke to determine safety in addition to efficacy.
Keywords: STEMI, Thrombectomy, Stroke, Meta-analysis, Randomized trial
Introduction
Primary percutaneous coronary intervention (PCI) is the optimal method of achieving reperfusion in patients with ST segment elevation myocardial infarction (STEMI). However, one of the major limitations of primary PCI is distal embolization of thrombus after balloon inflation or stent deployment. Routine manual thrombectomy was considered to be a simple way of removing thrombus with the potential to reduce distal embolization during primary PCI.
A randomized trial of moderate size showed an apparent large benefit of routine manual thrombectomy, prompting a guideline recommendation for manual thrombectomy leading to incorporation into clinical practice.1–4 However, the confidence limits of the trial was wide and a significantly larger trial did not confirm a benefit.5,6
On the other hand, meta-analyses of thrombectomy have suggested the possibility that stroke may be increased but these were cautiously interpreted given small numbers of events.7,8
We conducted the Trial of Routine Aspiration Thrombectomy with PCI vs. PCI alone in patients with STEMI (TOTAL, N = 10 732) which showed no difference in cardiovascular (CV) death, recurrent myocardial infarction, cardiogenic shock, or class IV heart failure within 180 days but a significant increase in stroke.9 In this report, we set out to analyse in detail the occurrence of stroke in an attempt to understand the nature of this important finding as this may have important implications for the use of thrombectomy or other devices in similar circumstances.
Methods
Study design
The TOTAL trial, the design of which was previously published,10 was an international, investigator-initiated, multi-centre prospective randomized trial of routine manual thrombectomy with the Export catheter (Medtronic Inc., Santa Rosa, CA, USA) vs. PCI alone. Patients with STEMI who were referred for primary PCI within 12 h of symptom onset were eligible to participate. Although the trial design was open label, the adjudication of outcomes was blinded and neurologists specifically adjudicated all stroke events.
In the thrombectomy group, aspiration thrombectomy was to be the first procedure after wire crossing. The guide catheter was to be fully engaged with the coronary ostium during removal of the thrombectomy catheter to avoid embolizing thrombus to the systemic vasculature. Following each thrombectomy run, the guide catheter was to be aspirated to ensure removal of air or thrombus. It was recommended that a minimum of two syringes be aspirated during thrombectomy.
In the PCI alone group, thrombectomy was allowed as a bailout procedure if there was failure of the initial PCI alone strategy, defined as either thrombolysis in myocardial infarction (TIMI) 0 or 1 flow with large thrombus after balloon pre-dilation or large thrombus persisting after stent deployment.
The trial protocol recommended use of guideline recommended PCI pharmacotherapies but did not mandate a specific regimen.4
Outcomes
The primary efficacy outcome was the occurrence of CV death, recurrent MI, cardiogenic shock, or new or worsening New York Heart Association class IV heart failure within 180 days. The key safety outcome was stroke within 30 days. A central events committee, blinded to treatment allocation, adjudicated all of the primary outcome events, strokes, transient ischaemic attacks (TIAs), major bleeding, target vessel revascularization, and stent thromboses. Neurologists who were members of a central adjudication committee, blinded to treatment assignment, specifically adjudicated all stroke events.
Stroke was defined as the presence of a new focal neurologic deficit thought to be vascular in origin, with signs or symptoms lasting >24 h. It was strongly recommended that an imaging procedure such as computerized tomography (CT) or magnetic resonance imaging (MRI) be performed to confirm the diagnosis. Transient ischaemic attack was defined as a transient episode of a new focal neurologic deficit thought to be vascular in origin, with signs or symptoms lasting <24 h.
The modified Rankin score (Supplementary material online, Table S1) was used to assess disability from stroke over a range from 1 (full recovery) to 6 (fatal stroke).11 Stroke was categorized as stroke with minor or no disability if the modified Rankin score was ≤2 and as stroke associated with major disability or fatal if the Rankin score was 3–6 as previously published.12
Stroke subtype was categorized by neurologists as either ischaemic, or primary haemorrhagic (intra-cerebral or subarachnoid haemorrhage) based on CT or MRI, or as uncertain (no neuroimaging or autopsy). Ischaemic strokes that had had secondary haemorrhagic transformation were categorized as ischaemic in aetiology. The diagnosis of haemorrhagic stroke subtype was based on local interpretation of imaging and reviewed by a senior stroke neurologist adjudicator, with images obtained and reviewed by a neuroradiologist when local interpretation was equivocal. Images were not routinely reviewed by a central core laboratory for neurologic imaging. Neurologic events which include TIA were categorized as ischaemic (ischaemic, unknown stroke, or TIA) or primary haemorrhagic.
Statistical considerations
The primary analysis was conducted using a modified intention-to-treat approach that was pre-specified to include only randomized patients who had undergone primary PCI. Other pre-specified sensitivity analyses included full intention-to-treat, on-treatment, and per-protocol analyses as described previously.9
A two-sided, log-rank test was used to compare the two randomized groups with a P-value <0.05 considered significant. The hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using a Cox proportional hazards regression model with treatment group as the predictor variable.
To better understand the time course of stroke in relation to the procedure, a series of cut points were used for landmark analyses (0–48 h, >48 h to <7 days to <30 days, 30 to <90 days, 90 to 180 days). This was performed for overall stroke, ischaemic and haemorrhagic neurologic events.
The subgroup analyses for stroke examined procedural factors that may impact stroke as well as traditional risk factors for stroke and included: TIMI thrombus grade <4 vs. 4 or greater, initial TIMI flow (0–1 vs. 2–3), age (65 or younger vs. over 65 years), centres divided into tertiles of annual primary PCI volume, radial vs. femoral access, prior stroke, history of hypertension and diabetes.
A stepwise multivariable analysis using a Cox proportional hazards model was performed to determine independent predictors of stroke after primary PCI for STEMI. Known predictors of stroke after PCI were used including: age, sex, prior stroke, peripheral arterial disease, diabetes, intra-aortic balloon pump use, coronary bypass surgery during follow-up, prior hypertension, smoking, and additional PCI variables were tested including PCI procedure time, staged PCI, initial TIMI flow, radial access, GP IIb IIIa use, TIMI thrombus grade, and thrombectomy group.
We performed an updated meta-analysis of manual thrombectomy vs. PCI alone in patients with STEMI (see Supplementary material online, Appendix for detailed methods). A comprehensive systematic search strategy was performed of Medline, EMBASE, and Cochrane for all published studies up to April 12, 2015. Randomized trials of manual thrombectomy vs. PCI alone in patients with STEMI were included. Two reviewers independently reviewed abstracts and extracted data. The outcomes of CV mortality, myocardial infarction, and stroke were examined. When CV mortality was not available all-cause mortality was used. Odds ratios were calculated using the Mantel Haenszel method with a fixed effects model. Sensitivity analyses were performed using a random effects model.
Results
Of the 10 732 patients enrolled between August 2010 and July 2014, from 87 hospitals in 20 countries, 10 058 (93.7%) underwent PCI for index STEMI and were included in the primary analysis. Since our previous publication, one additional stroke has been reported within 180 days.9 In the PCI alone group, 18 patients withdrew consent and 39 were lost to follow-up and in the thrombectomy group, 16 withdrew consent, and 33 were lost to follow-up within 180 days.
Baseline risk factors for stroke and procedural aspects that may impact stroke were well balanced between the groups (Table 1). Percutaneous coronary intervention procedure time was longer with thrombectomy (39 vs. 35 min, P < 0.001). The use of five French diameter guide catheters was more frequent in the PCI alone group (0.8 vs. 2.5%) as was the use of upfront glycoprotein IIb/IIIa inhibitors (22.7 vs. 25.4% P = 0.001). There were no differences in dose of unfractionated heparin during PCI. There were no differences in the use of P2Y12 inhibitors or oral anti-coagulants at discharge or during follow-up.
Table 1.
Baseline risk factors for stroke and invasive procedures
| Thrombectomy (N = 5033) | PCI alone (N = 5030) | |
|---|---|---|
| Demographics | ||
| Mean age (years) (SD) | 61.1 (11.8) | 61.0 (11.9) |
| Age > 75 years (%) | 666 (13.2) | 630 (12.5) |
| Male (%) | 3864 (76.8) | 3933 (78.2) |
| Mean BMI (SD) | 27.5 (4.6) | 27.6 (4.6) |
| Mean systolic blood pressure at presentation (SD) | 135 (26) | 135 (27) |
| Location of MI | ||
| Anterior | 1961/5027 (39.0) | 2055/5026 (40.9) |
| Inferior | 2807/5027 (55.8) | 2710/5026 (53.9) |
| Lateral or other | 259/5027 (5.2) | 261/5026 (5.2) |
| History | ||
| Prior stroke | 158 (3.1) | 151 (3.0) |
| Hypertension | 2533 (50.3) | 2516 (50.0) |
| Peripheral arterial disease | 119 (2.4) | 110 (2.2) |
| Diabetes | 919 (18.3) | 936 (18.6) |
| Initial PCI procedure | ||
| Radial access | 3435 (68.3) | 3430 (68.2) |
| PCI procedure time (median) | 39.0 (29.0−53.0) | 35.0 (26.0−50.0) |
| ≤5 French guide catheters | 42 (0.8) | 124 (2.5) |
| 6 French guide catheters | 4857 (96.7) | 4793 (95.4) |
| ≥7 French guide catheters | 123 (2.4) | 105 (2.1) |
| ≥2 guide catheters | 650 (12.9) | 596 (11.9) |
| Unfractionated heparin | 4065 (80.8) | 4110 (81.7) |
| Median heparin dose for PCI (U) | 7500 | 7500 |
| Bivalirudin | 942 (18.7) | 872 (17.3) |
| Enoxaparin | 415 (8.2) | 425 (8.4) |
| Upfront glycoprotein IIb/IIIa inhibitora | 1140 (22.7) | 1276 (25.4) |
| Bailout glycoprotein IIb/IIIa inhibitor | 742 (14.7) | 808 (16.1) |
| Pre-procedure TIMI 0 flow | 3303 (66.3) | 3378 (67.8) |
| Coronary artery bypass grafting | 136 (2.7) | 140 (2.8) |
| Intra-aortic balloon pump | 96 (1.9) | 110 (2.2) |
| Therapies at 30 days | ||
| Aspirin | 4713 (98.4) | 4659 (98.1) |
| Clopidogrel | 3132 (65.4) | 3055 (64.3) |
| Prasugrel | 581 (12.1) | 581 (12.2) |
| Ticagrelor | 975 (20.4) | 985 (20.7) |
| β-Blockers | 4001 (83.5) | 3962 (83.4) |
| Oral anti-coagulants | 332 (6.9) | 318 (6.7) |
| ACE inhibitor | 3534 (73.8) | 3525 (74.2) |
| Statin | 4652 (97.1) | 4608 (97.0) |
aUpfront GP IIb IIIa inhibitor, P −0.0014.
Stroke within 30 days occurred in 33 (0.7%) patients in the thrombectomy group and 16 (0.3%) patients in the PCI alone group (HR 2.06; 95% CI 1.13–3.75; P = 0.015, Table 2). The composite of stroke or TIA within 30 days occurred in 42 (0.8%) patients in the thrombectomy group and 19 (0.4%) in the PCI alone group (HR 2.21; 95% CI 1.29–3.80, P = 0.003).
Table 2.
Stroke and transient ischaemic attack for thrombectomy vs. percutaneous coronary intervention alone at 30 and 180 days
| Thrombectomy (N = 5033) (%) | PCI alone (N = 5030) (%) | Hazard ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Stroke within 30 days | 33 (0.7%) | 16 (0.3%) | 2.06 | 1.13–3.75 | 0.015 |
| TIA within 30 days | 9 (0.2%) | 3 (0.1%) | 2.99 | 0.81–11.1 | 0.08 |
| Stroke or TIA within 30 days | 42 (0.8%) | 19 (0.4%) | 2.21 | 1.29–3.80 | 0.003 |
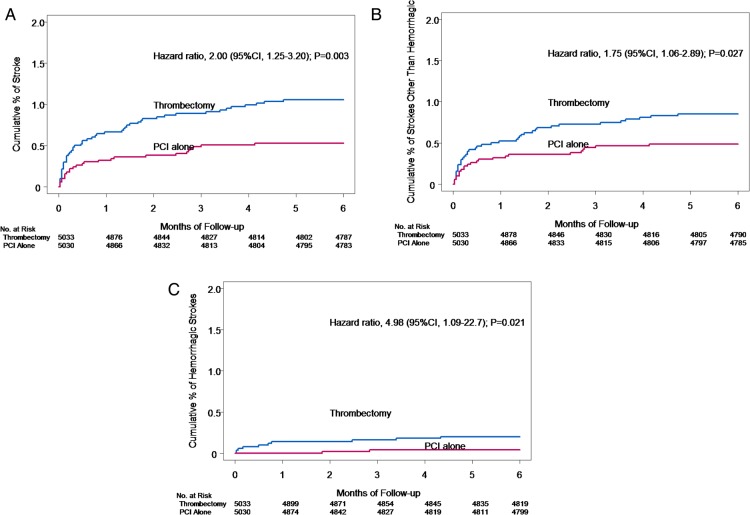
| Stroke within 180 days | 52 (1.0%) | 26 (0.5%) | 2.00 | 1.25–3.20 | 0.003 |
| TIA within 180 days | 11 (0.2%) | 5 (0.1%) | 2.19 | 0.76–6.31 | 0.14 |
| Stroke or TIA within 180 days | 62 (1.2%) | 31 (0.6%) | 2.00 | 1.30–3.08 | 0.001 |
The difference in stroke rates was apparent within 48 h [15 (0.3%) vs. 5 (0.1%), HR 3.00; 95% CI 1.09–8.25, Table 3) but not at 12 h [7 (0.14%) vs. 4 (0.08%), HR 1.75; 95% CI 0.51–5.98, P = 0.37]. A landmark analysis showed a similar risk of stroke in both groups during periods of >48 h to <7 days, 7 to <30 days, and 30 to <90 days, but a trend towards increased risk between 90 and 180 days [8 (0.2%) vs. 2 (0.04%), 3.99; 95% CI 0.85–18.8, Table 3]. The Kaplan–Meier curves for stroke and subtype of stroke are shown in Figure 1A–C. The full intent-to-treat analysis, as well as on treatment and per-protocol analyses showed similar patterns for stroke or TIA (Supplementary material online, Table S2).
Table 3.
Landmark analysis for stroke for thrombectomy vs. percutaneous coronary intervention alone
| Thrombectomy (N = 5033) (%) | PCI alone (N = 5030) (%) | Hazard ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| 0 to 48 h | 15 (0.30%) | 5 (0.10%) | 3.00 | 1.09–8.25 | 0.025 |
| >48 h to <7 days | 5 (0.10%) | 4 (0.08%) | 1.25 | 0.34–4.66 | 0.74 |
| 7 to <30 days | 13 (0.26%) | 7 (0.14%) | 1.85 | 0.74–4.65 | 0.18 |
| 30 to <90 days | 11 (0.23%) | 8 (0.16%) | 1.37 | 0.55–3.41 | 0.49 |
| 90 to 180 days | 8 (0.17%) | 2 (0.04%) | 3.99 | 0.85–18.8 | 0.06 |
Figure 1.
(A) Kaplan–Meier curves for stroke, (B) Kaplan–Meier curve for ischaemic or unknown stroke, (C) Kaplan–Meier curve for haemorrhagic stroke.
Atrial fibrillation during initial hospitalization was not recorded, but there was no difference in re-hospitalization for atrial fibrillation within 180 days [7 (0.1%) vs. 10 (0.2%)].
Strokes within 180 days with minor or no disability (Rankin 0–2) occurred in 18 (0.4%) of patients in the thrombectomy group and 13 (0.3%) in the PCI alone group (HR 1.38; 95% CI 0.68–2.82, P = 0.37) and strokes associated with major disability or that were fatal (Rankin 3–6) occurred in 35 (0.7%) of thrombectomy group vs. 13 (0.3%) of PCI alone group (HR 2.69; 95% CI 1.42–5.08, P = 0.002, Table 4).
Table 4.
Stroke severity for thrombectomy vs. percutaneous coronary intervention alone
| Modified Rankin score | Thrombectomy (N = 5033) (%) | PCI alone (N = 5030) (%) | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|---|
| Minor or no disability (0–2) | 18 (0.4%) | 13 (0.3%) | 1.38 | 0.68–2.82 | 0.37 |
| Major disability or fatal (3–6) | 35 (0.7%) | 13 (0.3%) | 2.69 | 1.42–5.08 | 0.002 |
| 1 | 13 (0.3%) | 9 (0.2%) | |||
| 2 | 5 (0.1%) | 4 (0.1%) | |||
| 3 | 9 (0.2%) | 1 (0.0%) | |||
| 4 | 9 (0.2%) | 5 (0.1%) | |||
| 5 | 5 (0.1%) | 1 (0.0%) | |||
| 6 | 13 (0.3%) | 6 (0.1%) |
There was an increase in both ischaemic strokes within 180 days [37 (0.7%) vs. 21 (0.4%), HR 1.76; 95% CI 1.03–3.00] and primary haemorrhagic strokes within 180 days [10 (0.2%) vs. 2 (0.04%), HR 4.98; 95% CI 1.09–22.7] but similar rates of strokes of uncertain aetiology within 180 days [5 (0.12%) vs. 3 (0.06%), HR 1.66; 95% CI 0.40–6.96].
The landmark analysis for ischaemic neurologic events show a similar pattern as overall stroke events (Table 5). The stroke severity using the modified Rankin score of both ischaemic and haemorrhagic events showed consistent findings with overall stroke group (Supplementary material online, Table S3)
Table 5.
Landmark analysis for different types of neurologic events including transient ischaemic attack
| Type of neurologic event | Thrombectomy (N = 5033) (%) | PCI alone (N = 5030) (%) | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|---|
| Ischemic/unknown | |||||
| 0 to 48 h | 15 (0.30%) | 6 (0.12%) | 2.50 | 0.97–6.44 | 0.050 |
| >48 h to <7 days | 5 (0.10%) | 5 (0.10%) | 1.00 | 0.29–3.45 | 1.00 |
| 7 to <30 days | 15 (0.30%) | 8 (0.16%) | 1.87 | 0.79–4.42 | 0.15 |
| 30 to <90 days | 11 (0.23%) | 8 (0.16%) | 1.37 | 0.55–3.41 | 0.49 |
| 90 to 180 days | 6 (0.12%) | 2 (0.04%) | 3.00 | 0.60–14.8 | 0.16 |
| Primary haemorrhagic | |||||
| 0 to 48 h | 3 (0.06%) | 0 | Not estimable | 0.08 | |
| >48 h to <7 days | 1 (0.02%) | 0 | Not estimable | 0.32 | |
| 7 to <30 days | 3 (0.06%) | 0 | Not estimable | 0.08 | |
| 30 to <90 days | 1 (0.02%) | 2 (0.04%) | 0.50 | 0.55–5.48 | 0.56 |
| 90 to 180 days | 2 (0.04%) | 0 | Not estimable | 0.16 | |
The findings of subgroup analyses for stroke within 180 days (Supplementary material online, Figure S1) were consistent with overall results and did not show any significant interactions by treatment.
The baseline characteristics of those patients who suffered a stroke vs. those that did not are shown in Supplementary material online, Table S4. Patients that suffered a stroke had a 30.8% mortality within 180 days vs. 3.4% for those patients without a stroke (HR 10.2; 95% CI 6.70–15.40, P < 0.001). The cause of death for patients that suffered a stroke (N = 24) was fatal stroke in 62.5% (15), cardiac death in 29.1% (7), haemorrhagic death in 4% (1), and other vascular cause in 4% (1).
Independent predictors for stroke were age, sex, peripheral arterial disease, prior stroke, diabetes, intra-aortic balloon pump, and randomization to thrombectomy (Figure 2). The C statistic for this model for stroke was 0.75.
Figure 2.
Predictors of stroke in multivariable analysis.
In the PCI alone group, no strokes occurred in the 354 patients (7%) who underwent protocol allowed bailout thrombectomy.
For the meta-analysis, 669 abstracts were identified by the search strategy; 20 randomized trials (N = 21 173) met the inclusion criteria. Stroke occurred in 0.8% patients undergoing thrombectomy vs. 0.5% with PCI alone, odds ratio 1.59; 95% CI 1.11–2.27, P = 0.01, I2 = 0% (Figure 3). Death occurred in 3.8% of patients undergoing thrombectomy vs. 4.3% with PCI alone, odds ratio 0.87; 95% CI 0.76–1.00, P = 0.05, I2 = 0%, Figure 4). There was no difference in recurrent myocardial infarction (2.1 vs. 2.3%, odds ratio 0.94; 95% CI 0.78–1.13, P = 0.51, I2 = 0%). The sensitivity analyses using the random effects model are shown in Supplementary material online, Table S4; the only outcome sensitive to the method of analysis was mortality (random effects model odds ratio 0.88; 95% CI 0.77–1.01, P = 0.07).
Figure 3.

Meta-analysis for stroke outcome. Ten of 20 thrombectomy trials published outcomes for stroke.
Figure 4.
Meta-analysis for mortality outcome.
Discussion
A strategy of routine thrombectomy compared to PCI alone was associated with an increased risk of stroke within 30 days that was apparent within 48 h. There was an increase primarily in ischaemic strokes but also in haemorrhagic strokes and in strokes of all degrees of disability. In addition to traditional risk factors for stroke, randomization to thrombectomy was an independent predictor of stroke.
A possible explanation of the increase in ischaemic stroke in the thrombectomy group would include embolization of thrombus from the coronary vasculature to the systemic vasculature. In addition, operators may have used more aggressive guide catheter manipulation to successfully cross lesions with the thrombectomy catheter and this could lead to dislodgement of atheroma from the aorta. As well, procedural times were longer in the routine thrombectomy arm. These mechanisms would explain strokes in the early period after PCI and those that were ischaemic.
The detailed landmark analysis showed that the greatest risk for stroke is within 48 h and then the risk appeared to be similar between 2 and 90 days. There is a trend for an increased risk of stroke again with thrombectomy between 90 and180 days. While the early increase is plausible, the very late (90–180 days) increase lacks a plausible explanation and may be due to chance.
While the increase in stroke associated with thrombectomy was not significant within 12 h, it was within 48 h. Caution should be used as the power to detect differences of the different time points is limited given the small numbers of events. In addition, it is important that events related to PCI may manifest between 12 and 48 h after the procedure which is particularly important in the era of early discharge after STEMI. We hypothesized that there might be greater efficacy and safety with thrombectomy performed in sites with high primary PCI volumes or in operators who perform more thrombectomy. However, stroke risk with thrombectomy did not vary with site primary PCI volume or operator thrombectomy volume. As well, stroke risk with thrombectomy did not vary between radial and femoral vascular access site.
The excess of haemorrhagic stroke in the thrombectomy arm is unexplained. There were no differences in the use of oral anti-coagulants and potent oral antiplatelet agents between the groups to explain these findings. There is no plausible mechanism to explain how thrombectomy would increase haemorrhagic stroke and given the small number of primary haemorrhagic stroke events (an excess of only eight events; n = 12), this may be due to chance.
A meta-analysis of thrombectomy trials from 2008 showed a trend for increase in stroke with thrombectomy and was the basis for specifying stroke as a key safety outcome in TOTAL.8 The more recent Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial (N = 7244) was a registry-based trial that showed findings for efficacy that were concordant with TOTAL, but there was no difference in stroke or other neurological complication during initial hospitalization.5 Why the two trials differed for this important outcome is a key question. A registry-based trial that relies on discharge diagnosis codes may underestimate non-fatal events, particularly less severe strokes. The larger sample size of TOTAL may have also resulted in increased power to detect differences in rare events.
In our meta-analysis of 20 randomized trials (N = 21 173), we found an increase in stroke with manual thrombectomy (0.8 vs. 0.5%; OR 1.59; 95% CI 1.11–2.27). Meta-analyses of mechanical thrombectomy (N = 1532) have shown similar trends for stroke (1.3 vs. 0.4%; RR 2.74; 95% CI 0.93–8.01) which provides external validation.13 Our updated meta-analysis also shows a lower mortality with thrombectomy of borderline significance but the CIs are wide (odds ratio 0.87; 95% CI 0.76–1.00, P = 0.05) and so these results are not robust. Furthermore, the effect on mortality is sensitive to the type of analysis model used and so the results should be interpreted cautiously. A planned individual patient meta-analysis of TOTAL, TAPAS, and TASTE will be able to assess whether manual thrombectomy has a preferentially beneficial or adverse effects in specific subgroups.
It is important to note that the stroke rates observed in the TOTAL trial (0.5 and 1.0%) are well within the expected stroke rates after primary PCI for STEMI (1.0–2.0%).14,15 Specifically the Assessment of Pexelizumab in Acute Myocardial Infarction study (APEX AMI, N = 5372) found a stroke rate of 1.3% stroke rate within 90 days for patients who have undergone primary PCI for STEMI.15
The strengths of TOTAL are that it is the largest randomized trial of thrombectomy to date, that stroke events were prospectively collected and adjudicated in a blinded fashion by neurologists and that stroke severity was documented. As a result, the TOTAL trial data on stroke is the most rigorous data available regarding thrombectomy and stroke. Nevertheless, stroke was an infrequent event and so the results at 30 days should be cautiously interpreted. Caution should be used to interpret stroke subtype given the difficulties with differentiating ischaemic strokes with haemorrhagic transformation vs. primary haemorrhagic. Given the small number of haemorrhagic strokes, only a few events reclassified to other subtypes could change the significance of effect on haemorrhagic stroke. An additional limitation is that CT and MRI scans were not reviewed routinely in a core laboratory. There are few events in each time period in landmark analysis and these analyses were not pre-specified and so the results should be interpreted cautiously. In addition, there was no adjustment for multiple statistical comparisons.
Furthermore, it is possible that delays in diagnosis of stroke occurred related to procedural sedation and procedures occurring in the middle of the night. Detailed data regarding presence of left ventricular aneurysms, occurrence of atrial fibrillation during index hospitalization, and relationship with stroke are not available in the TOTAL trial. Finally, the TOTAL was not designed to test the effectiveness of selective or bailout thrombectomy and so cannot assess the benefit of these strategies compared to no thrombectomy.
The mortality of patients who suffered a stroke in the TOTAL trial was 30%. These findings emphasize the importance of stroke as a clinical outcome.
A strategy of routine manual thrombectomy was associated with an increased risk of stroke compared with a strategy of PCI alone with thrombectomy reserved for only bailout. Future thrombectomy trials need to carefully collect stroke outcomes to determine their safety in addition to efficacy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Canadian Institutes of Health Research, Canadian Network and Centre for trials Internationally (CANNeCTIN), and Medtronic Inc. (ClinicalTrials.gov number, NCT01149044).
Conflict of interest: D.A. reports personal fees from AstraZeneca, personal fees from The Medicines, and personal fees from Boeringer Ingelheim outside the submitted work; V.D. reports grants from Astra Zeneca, grants and personal fees from Abbott Vascular, and grants from Medtronic Inc., outside the submitted work; S.S.J. reports grants from Medtronic during the conduct of the study; S.K. reports fees for enrolling subjects as a participating investigator; R.M. reports personal fees from Medtronic during the conduct of the study.
References
- 1.Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 2008;358:557–567. [DOI] [PubMed] [Google Scholar]
- 2.Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 2008;371:1915–1920. [DOI] [PubMed] [Google Scholar]
- 3.Kushner FG, Hand M, Smith SC, Jr, King SB, III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009;54:2205–2241. [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 5.Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 6.Lagerqvist B, Frobert O, Olivecrona GK, Gudnason T, Maeng M, Alstrom P, Andersson J, Calais F, Carlsson J, Collste O, Gotberg M, Hardhammar P, Ioanes D, Kallryd A, Linder R, Lundin A, Odenstedt J, Omerovic E, Puskar V, Todt T, Zelleroth E, Ostlund O, James SK. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med 2014;371:1111–1120. [DOI] [PubMed] [Google Scholar]
- 7.Tamhane UU, Chetcuti S, Hameed I, Grossman PM, Moscucci M, Gurm HS. Safety and efficacy of thrombectomy in patients undergoing primary percutaneous coronary intervention for acute ST elevation MI: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J 2008;29:2989–3001. [DOI] [PubMed] [Google Scholar]
- 9.Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, Kedev S, Thabane L, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemela K, Steg PG, Bernat I, Xu Y, Cantor WJ, Overgaard CB, Naber CK, Cheema AN, Welsh RC, Bertrand OF, Avezum A, Bhindi R, Pancholy S, Rao SV, Natarajan MK, Ten Berg JM, Shestakovska O, Gao P, Widimsky P, Dzavik V; for the TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolly SS, Cairns J, Yusuf S, Meeks B, Shestakovska O, Thabane L, Niemela K, Steg PG, Bertrand OF, Rao SV, Avezum A, Cantor WJ, Pancholy SB, Moreno R, Gershlick A, Bhindi R, Welsh RC, Cheema AN, Lavi S, Rokoss M, Dzavik V. Design and rationale of the TOTAL trial: a randomized trial of routine aspiration thrombectomy with percutaneous coronary intervention (PCI) versus PCI alone in patients with ST-elevation myocardial infarction undergoing primary PCI. Am Heart J 2014;167:315–321 e1. [DOI] [PubMed] [Google Scholar]
- 11.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538–1541. [DOI] [PubMed] [Google Scholar]
- 12.Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, Kappeler L, Mono ML, Brekenfeld C, Schroth G, Mattle HP. What is a minor stroke? Stroke 2010;41:661–666. [DOI] [PubMed] [Google Scholar]
- 13.Kumbhani DJ, Bavry AA, Desai MY, Bangalore S, Bhatt DL. Role of aspiration and mechanical thrombectomy in patients with acute myocardial infarction undergoing primary angioplasty: an updated meta-analysis of randomized trials. J Am Coll Cardiol 2013;62:1409–1418. [DOI] [PubMed] [Google Scholar]
- 14.Budaj A, Flasinska K, Gore JM, Anderson FA, Jr, Dabbous OH, Spencer FA, Goldberg RJ, Fox KA. Magnitude of and risk factors for in-hospital and postdischarge stroke in patients with acute coronary syndromes: findings from a Global Registry of Acute Coronary Events. Circulation 2005;111:3242–3247. [DOI] [PubMed] [Google Scholar]
- 15.Guptill JT, Mehta RH, Armstrong PW, Horton J, Laskowitz D, James S, Granger CB, Lopes RD. Stroke after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: timing, characteristics, and clinical outcomes. Circ Cardiovasc Interv 2013;6:176–183. [DOI] [PubMed] [Google Scholar]