Abstract
Today, there is an enormous amount of excitement in the field of stroke victim care due to the recent success of MR. CLEAN, SWIFT PRIME, ESCAPE, EXTEND-IA, and REVASCAT endovascular trials. Successful intravenous (IV) recombinant tissue plasminogen activation (rt-PA) clinical trials [i.e.: National Institutes of Neurodegenerative Disease and Stroke (NINDS) stroke trial; Third European Cooperative Acute Stroke Study (ECASSIII) and Third International Stroke study (IST-3)] also need to be emphasized. In the recent endovascular and thrombolytic trials, there is statistically significant improvement using both the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Score (mRS) scale, but neither approach promotes complete recovery in patients enrolled within any particular NIHSS or mRS score tier. Absolute improvement (mRS 0–2 at 90 days) with endovascular therapy is 13.5–31%, whereas thrombolytics alone also significantly improve patient functional independence, but to a lesser degree (NINDS rt-PA trial 13%).
This article has 3 main goals: (1) first to emphasize the utility and cost-effectiveness of rt-PA to treat stroke; (2) second to review the recent endovascular trials with respect to efficacy, safety and cost-effectiveness as a stroke treatment; and (3) to further consider and evaluate strategies to develop novel neuroprotective drugs. A thesis will be put forth so that future stroke trials and therapy development can optimally promote recovery so that stroke victims can return to “normal” life.
Keywords: MR. CLEAN, SWIFT PRIME, ESCAPE, EXTEND-IA, REVASCAT, rt-PA, NINDS, embolism
Introduction
Time is penumbra [1–6], an often use phrase to encompass the scientific observation that that at-risk neurons are recruited to die at an astounding rate after a stroke if the victim does not receive therapy. Penumbral expansion and death is a process that we can attenuate.
Even though attempts are being made to reduce the incidence of stroke, each year approximately 795,000 patients have a primary (76.7%) or secondary (23.3%) stroke [7]. Ischemic stroke is prevalent with 87% of patients having an ischemic stroke compared to 13% for hemorrhagic stroke (10% intracererbal hemorrhage; 3% subarachnoid hemorrhage). Ischemic strokes are categorized as embolic of thrombotic in nature: population percentages appear to vary with 50–65% of all strokes being thrombotic, with the remainder being embolic strokes.
For purposes of therapy development, a thrombotic stroke is usually related to the production of a blood clot which forms directly in a primary blood vessel supplying the brain such as the as the common carotid artery. Thrombotic strokes are the results of conditions such as atherosclerosis, narrowing of vessels due to cholesterol and fatty acid build-up. Thrombotic strokes can also be associated with blockage of smaller blood vessels deep within the brain (i.e.: lacunar infarction). An embolic stroke is the result of a blood clot that navigates its way into the brain from a distal part of the body, most often the heart due to pre-existing conditions such as atrial fibrillation (AFib) [7], an irregular heart rhythm as a primary example. The result of blood clot deposition in a blood vessel is reduced oxygen and nutrients reaching the one or more brain areas. The deposition of a blood clot in brain presents a unique pathophysiology that cannot be replicated by any other means [8]. In a recent article by Hossman [8], an intriguing and important argument is made for the use of embolization models to develop therapeutics because an embolization model would recapitulate many of the salient features of clinical stroke. This idea is now emphasized by a growing number of stroke groups in the USA and worldwide [7, 9–16].
The clinical presentation of stroke is usually the sudden onset of weakness or numbness in the face, arm of leg, paralysis, slurred speech, aphasia (sudden problem talking or expressing thoughts and words), dizziness, loss of coordination/balance, or other problems walking, problems with vision and other manifestations that correlate with interruption of blood flow to a particular area of the brain. The type of the pathophysiological deficit is dependent upon the extent of the ischemic area and brain regions involved in the stroke.
A) Embolic stroke therapy
There are now 2 therapeutic strategies that have been shown to be statistically and clinically effective in ischemic stroke patients. 1) Thrombolysis and 2) mechanical clot removal.
1) Thrombolysis (rt-PA)
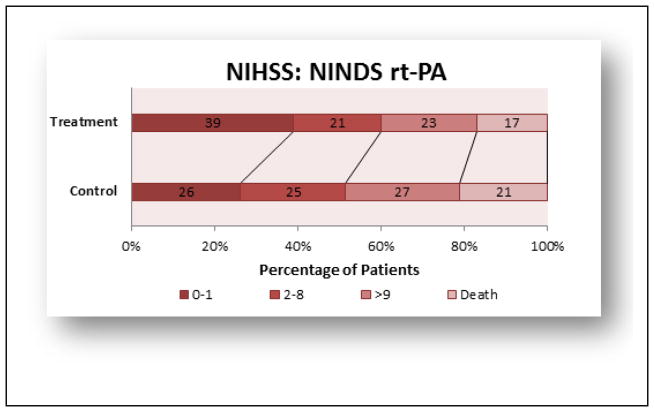
This section will focus on the advances made with the Food & Drug Administration (FDA)-approved thrombolytic, tissue plasminogen activator (rt-PA) since the original NINDS rt-PA study. Important clinical trials with rt-PA are summarized in below. rt-PA (Alteplase®, is a plasminogen activator that promotes thrombolysis by activating the endogenous fibrinolytic system [17]. The recommended Door-to-Needle (DTN) time for thrombolytic therapy administration is less than 60 minutes [18–22]. While rt-PA can be administered in under 60 minutes, and there is a movement to reduce DTN, it is not commonplace [23] and historical data shows that rt-PA is currently being administered well in excess of the 60 minute recommendation [24]. rt-PA is FDA approved for use within the first 3 hours of an embolic stroke, in patients presenting if brain hemorrhage can be excluded, and has shown to be effective when administered up to 4.5 hours after a stroke [25, 26] Figures 1 and 2 provide the original, gold standard efficacy result from the original NINDS rt-PA clinical trial [27]. In the trial, the rt-PA administration time was 0–180 minutes and then stratified between 0–90 minutes and 91–180 minutes. The study showed that there was a significant, modest, and absolute increase in the number of patients with minimal or no disability (12% Barthel index) and all 4 outcome measures (NIHSS and mRS shown), as well as Barthel Index and Glasgow Outcome Scale. The NINDS trial established efficacy within 3 hours, and subsequent clinical have enrolled patients outside of the 3 hour window, in an attempt to expand the therapeutic window for rt-PA [26, 25]. Since the 1995 NINDS rt-PA trial, the treatment is still underutilized in stroke victims [28, 29], but there has been constant improvement in recognizing the target patient population, which has reduced the DTN across the USA [30, 31] and promoted patient metrics such as reduced mortality, reduced hemorrhage, increased functional independence and increased time of discharge [32].
Figure 1.
NINDS rt-PA trial 3 months outcome (NIHSS scores)
Figure 2.
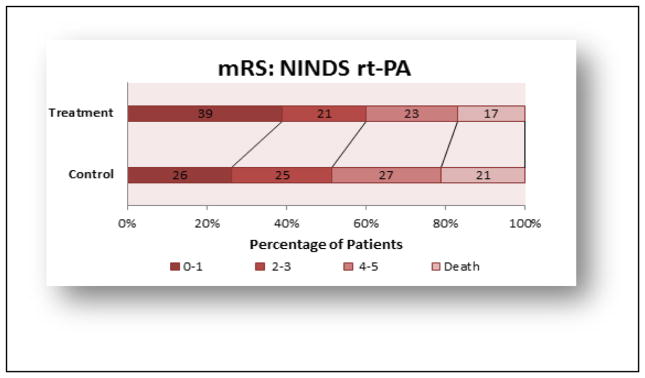
NINDS rt-PA trial 3 months outcome (mRS scores)
In the Third International Stroke study (IST-3) [33], 3035 patients were fully randomized to receive rt-A or vehicle, which included treating eligible patients within 6 hours of a stroke: 1007 were treated after 4.5 hours and 1617 patients were > 80 years at trial entry, an age where patients are normally excluded from thrombolysis clinical trials.
The Third European Cooperative Acute Stroke Study (ECASS III) trial [25, 34, 35] enrolled patients, with standard exclusion criteria (i.e.:age >80 years, diabetes, oral anticoagulants, and NIHSS>25) and investigated the use, safety and efficacy of rt-PA when administered up to 4.5 hours following an ischemic stroke. Most notably is the shift in the mRS 0–3 rt-PA-treated group (66.5% of patients) compared to 61.5% in the control group, an absolute change of 5%. Figure 3 provides the mRS data for Intention-to-Treat at 90 days.
Figure 3.
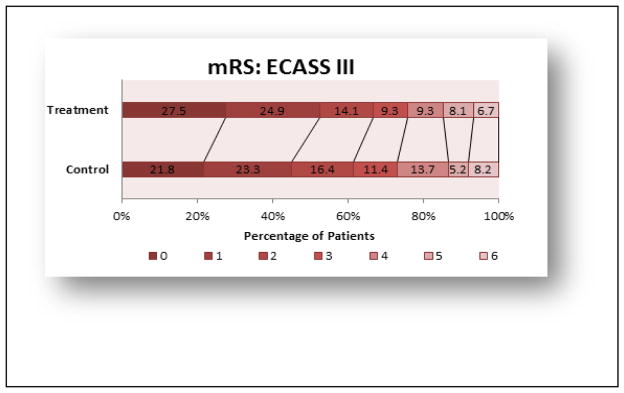
ECASS III trial 3 months outcome Intention-to Treat Population (mRS scores)
The Cochrane report by Wardlaw and colleagues[36, 37] emphasizes that rt-PA is most effective when administered within 3 hours of a stroke (p<0.0001 for 6 trials including 1779 patients), but results from 8 IV rt-PA clinical trials which included 6729 patients, also show significant benefit of thrombolysis (p=0.0006) up to 6 hours after a stroke.
1) Safety
Concerns about the administration of rt-PA abound, and have recently been questioned by Lyden [38]. For example, rt-PA efficacy, ease of administration and safety, has been questioned despite significant efficacy and a good safety profile in numerous trials in a wide (NIHSS) patient population. In the landmark NINDS rt-PA trial, in rt-PA-treated patients, symptomatic intracerebral hemorrhage (sICH) was significantly higher in rt-PA-treated patients (6.4%) than in placebo (0.6%)-treated controls [27].
In the ECASSIII trial [25, 34, 35], as a measure of safety, the incidence of intracerebral hemorrhage (ICH) and sICH was measured and shown to be higher in the rt-PA-treated group compared with placebo (ICH: 27.0% vs. 17.6%; P=0.001 and sICH: 2.4% vs. 0.2%; P=0.008), respectively. Despite higher ICH rates, rt-PA-induced mortality was not significantly between the rt-PA and placebo groups (7.7% and 8.4%, respectively; P=0.68. There was also no significant difference in the rate of other serious adverse events in the ECASSIII trial.
The Cochrane report by Wardlaw and colleagues [36, 37] also documents that rt-PA increases the risk of ICH and sICH, early death and death within 6 months of stroke and treatment; early treatment with rt-PA reduces death.
2) Cost-Effectiveness
There is little information regarding cost-effectiveness of rt-PA or the impact that rt-PA has on quality of life or quality-adjusted life-years (QALY) for stroke victims. As touched upon by Lyden [38], Center for Disease Control data reported by Taylor et al. [39] estimate that an ischemic stroke has a financial burden of $90,981 (unadjusted 1990 value) and the lifetime cost associated with all stroke occurring in 1990 (i.e.: estimated 392,344) was $29.0 billion.
Fagan et al. [40] have reported that treatment with rt-PA is cost-effective to society and the medical welfare infrastructure in the USA based upon analysis of the NINDS rt-PA clinical trial [27]. Using the Markov model and multiway sensitivity analysis, there was a greater than 90% probability of cost savings associated with rt-PA treatment and significantly increased QALY per 1000 patients when estimated over 30 years.
Boudreau et al. [41] estimate that the 2010 financial burden due to stroke was $74 billion in the USA. Cost analysis based upon treatment with rt-PA, within 3–4.5 hours of stroke onset (ECASSIII trial), shows incremental cost benefit, which is age-dependent ($6,255 per QALY <65 years old; $35,813 per QALY >65 years old), NIHSS score dependent (NIHSS 0 to 9, $16,322 per QALY; NIHSS 10 to 19, $37,462 per QALY; ≥ 20, $2,432 per QALY). In another report, Boudreau et al. [42] also estimate that rt-PA promotes a gain of 0.39 QALY per stroke patient and a lifetime cost-saving of $25,000 due to thrombolysis, compared with no r-rt-PA.
3) Patient population
Inclusion and exclusion criteria are routinely used in clinical trials and numerous guidelines have been published [43–46]. As recently emphasized by Lyden in Expert Reviews [38], too few patients are receiving rt-PA, even when they are eligible to receive thrombolysis. The original NINDS rt-PA trial [47] established that IV rt-PA improved neurological outcome when given within 3 hours of stroke onset, and subsequent trials have established efficacy within a 6 hour window [48–55, 17, 25] measured on various standardized scales 3 months after stroke, independent of the source and type of stroke. In the NINDS trial, patients with a variety of stroke types responded to rt-PA: small vessel lacunar stroke, rt-PA 59% vs. placebo 42%, cardio-embolic stroke, rt-PA 59 vs. placebo, large-vessel strokes rt-PA 65% vs. 42% with placebo. Thus, stroke subtype should not be considered as a limiting factor. Correlative analysis of rt-PA trials have shown reduced benefit in patients with an NIHSS score >19, and also pointed to decreased or no significant benefit in diabetic patients or patients with atrial fibrillation [56].
Summary
Taken together, IV rt-PA should be used in ischemic stroke patients within 3–6 hours of a stroke, and early treatment is most beneficial with fewer side effects. The use of rt-PA translates into increased QALY for the stroke victim.
B) Mechanical Embolectomy
Another effective treatment for stroke, albeit less frequently used, is mechanical thrombectomy in patients with documented large vessel occlusion [57–60]. A large vessel occlusion is defined as thromboembolic blockage of the distal internal carotid artery (ICA) which supplies the anterior part of the brain, the M1 or proximal M2 portions of the middle cerebral artery (MCA) which supplies blood to the cerebrum, the anterior temporal lobes and the insular cortices, or the proximal anterior cerebral artery (ACA), which supplies blood to the medial aspect of the cerebral hemispheres and parietal lobe.
In some of these trials presented herein, patients who were ineligible for IV rt-PA, were treated with thrombectomy and showed benefit, but they represented a small fraction of all enrolled patients. In 3 prior embolectomy trials, thrombectomy was performed late (> 6 hours after stroke onset) and did not appear to be successful [61–63], arguing that early treatment is prerequisite for reperfusion, efficacy and safety. The original trials used first generation devices whereas the 5 recent trials used newer (2nd or 3rd) generation devices, so the prior negative results and more recent positive results could reflect improvements made in the thrombectomy technology and methodology, but could also reflect intervention during a reasonable therapeutic window [4, 6, 22]. Previous studies with transcranial laser therapy attempted to achieve efficacy with a very long treatment delay (Time-to-treatment 16.3 + 5.4 hours), but the final trial ultimately ended in futility [64]. Regardless of the mechanism, it is clear that patients benefit when treatment was initiated with the newest devices within 6 hours of stroke onset. Use of mechanical thrombectomy, with or without rt-PA treatment, is considered standard of care in patients with documented large vessel occlusion [45].
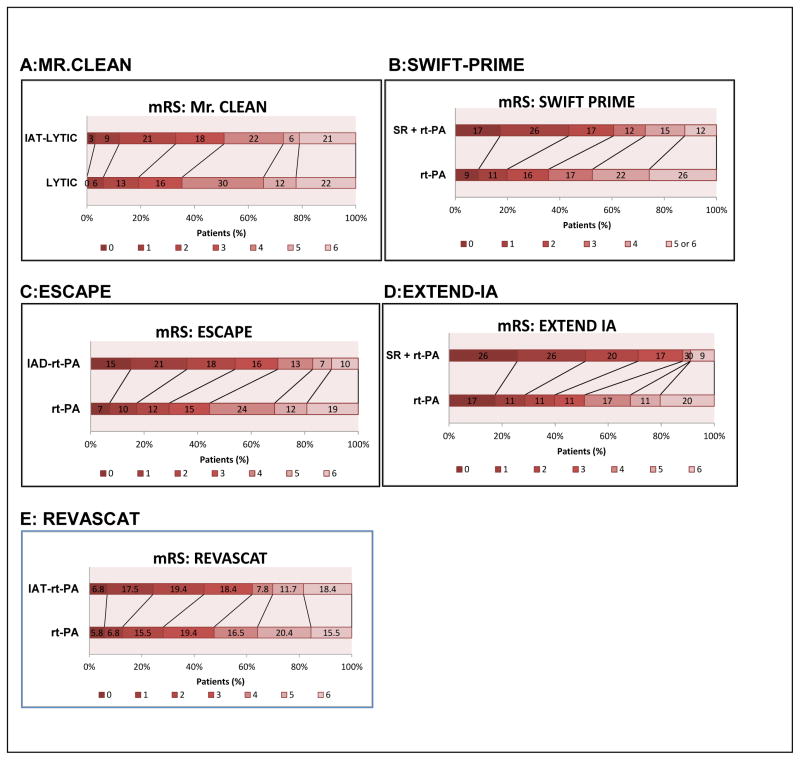
As shown in Figures 4 and 5, four randomized clinical trials showed benefit of thrombectomy when added to rt-PA treatment.
Figure 4.
Embolectomy-Thrombolytic Trials 3 month mRS outcome
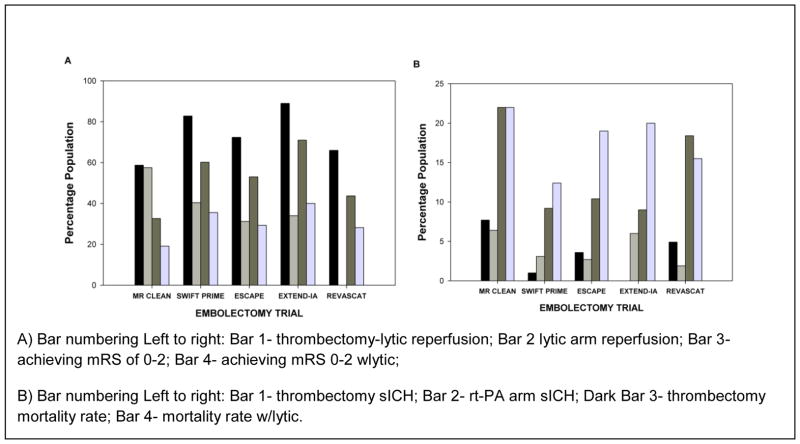
Figure 5. Summary of Mechanical Thrombectomy Study Outcomes.
A) Bar numbering Left to right: Bar 1- thrombectomy-lytic reperfusion; Bar 2 lytic arm reperfusion; Bar 3- achieving mRS of 0–2; Bar 4- achieving mRS 0–2 wlytic;
B) Bar numbering Left to right: Bar 1- thrombectomy sICH; Bar 2- rt-PA arm sICH; Dark Bar 3- thrombectomy mortality rate; Bar 4- mortality rate w/lytic.
Stroke clinicians who routinely manage stroke victims in an emergency room would dispute the fact that an embolic stroke results from the movement of a blood clot into the brain [9, 10, 7]. The recent positive intravascular embolectomy studies reported from MR. CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME, and REVASCAT [57, 60, 58, 59, 65] emphasize that the embolic stroke event can now be treated using the new generation of devices, in addition to rt-PA as described above. Restoration of perfusion by safe embolectomy is not novel, but the fact that surgeons have now managed to show substantial and significant benefit of the procedure is a major breakthrough in the stroke therapy field. Several intra-arterial approaches have been used to remove a clot from a large vessel; this is done by fragmenting the clot followed by suction aspiration or retrieved using a retriever as summarized below for each of the individual trials. Figure 4 shows a side-by-side comparison of the clinical function scoring using mRS for 90 day outcome, while Figure 5 shows efficacy and safety metrics. Table 1 presents cumulative clinical trial data for the treatment populations, time to treatment, age, initial NIHSS and the device used in each of the trials.
Table 1.
Embolectomy Efficacy Compared with rt-PA
| Trial | Embolectomy + rt-PA | rt-PA Control |
|---|---|---|
| A) MR CLEAN [57] N=500 | 233 patients | 267 patients |
| % Achieving Reperfusion | 58.7% (TICI score of 2b or 3) | 57.5 (mAOL score of 2 or 3) |
| mRS 0–2 | 32.6% | 19.1% |
| Age range | 54.5–76 (65.8) | 55.5–76.4 (65.7) |
| NIHSS range | 14–21 (17) | 14–22 (18) |
| Time-to-Treat [range (median)] min | Endo 210–313 (260) rt-PA 67–110 (85) |
rt-PA 65–116 (87) |
| Device: MERCI Retriever [68] | ||
| B) SWIFT PRIME [60] N=196 | 98 patients | 93 patients |
| % Achieving Reperfusion | 82.8% (reperfusion ≥90%) | 40.4% (reperfusion ≥90%) [p< 0.0001] |
| mRS 0–2 | 60.2% | 35.5% [p = 0.0008] |
| Age range | 65.0 ± 12.5 | 66.3 ± 11.3 |
| NIHSS range | 13–20 (17) | 13–22 (17) |
| Time-to-Treat range (median) min | Endo 190–300 (252) rt-PA 85–156 (110.5) |
rt-PA 80–155 (117) |
| Device: Solitaire Flow Restoration (FR) or Solitaire 2 | ||
| C) ESCAPE [58] N=238 | 164 patients | 147 patients |
| % Achieving Reperfusion | 72.4% (TICI score of 2b or 3) | 31.2% (mAOL score of 2 or 3) |
| mRS 0–2 | 53% | 29.3% [p<0.001] |
| Age range | 60–81 (71) | 60–81 (70) |
| NIHSS range | 13–20 (16) | 12–20 (17) |
| Time-to-Treat [range (median)] min | Endo 65–115 (84) rt-PA 120 |
rt-PA 118 |
| Device: Unidentified retrievable stent, suction via a balloon catheter guide Coviden | ||
| D) EXTEND-I [59] N=70 | 35 patients | 35 patients |
| % Achieving Reperfusion | 89% (reperfusion >90% without SICH) | 34% (reperfusion >90% without SICH) [p< 0.001] |
| mRS 0–2 | 71% | 40% [p=0.01] |
| Age range | 68.6 ±12.3 | 70.2 ±11.8 |
| NIHSS range | 13–20 (17) | 9–19 (13) |
| Time-to-Treat range (median) min | Endo 204–277 (248) rt-PA 127 (93–162) |
rt-PA 145 (105–180) |
| Device: Solitaire FR retrievable stent | ||
| E) REVASCAT [65] N=206 | 103 patients | 103 patients |
| % Achieving Reperfusion | 66% | NA% |
| mRS 0–2 | 43.7% | 28.2% |
| Age range | 65.7 ±11.3 | 67.2 ±9.5 |
| NIHSS range | 14–20 (17) | 12–19 (17) |
| Time-to-Treat range (median) min | Endo 201–340 (269) rt-PA 90–150 (117.5) |
rt-PA 86–137.5 (105) |
Device: Solitaire FR retrievable stent
MR. CLEAN [57]
Figure 4–5 and Table 1. MR CLEAN enrolled 500 patients (233 intra-arterial treatment and 267 thrombolytic treatment) with mean time-to-treatment of 260 minutes for the embolectomy arm and 87 minutes for the rt-PA arm. The comparison study used the MERCI stent retriever. There was no significant difference in patients achieving reperfusion between the 2 groups, but there was a difference of 13.5% in the rate of functional independence (mRS 0–2) in favor of combined intervention (32.6% vs. 19.1%).
SWIFT-PRIME [60]
Figure 4–5 and Table 1. SWIFT PRIME, which enrolled 196 patients (98 embolectomy plus IV rt-PA and 93 IV rt-PA) within 252 minutes for the embolectomy arm and 117 minutes for the rt-PA arm also demonstrated a significant improvement in functional independence (mRS 0–2) at 90 days, and greatly enhanced reperfusion in the majority of patients in the combined treatment group. Moreover, 60% of patients treated with embolectomy and intravenous thrombolysis had an mRS of 0–2 compared with standard care alone (35%).
ESCAPE [58]
Figure 4–5 and Table 1. ESCAPE enrolled 238 patients (164 embolectomy plus IV rt-PA and 147 IV rt-PA) within 84 minutes for the embolectomy arm and 118 minutes for the rt-PA arm. The trial used retrievable stents or balloon catheters for suction clot removal. Combination treatment achieved 53% inclusion rate of patients with mRS of 0–2 compared to only 29.3% in the rt-PA group.
EXTEND-IA [59]
Figure 4–5 and Table 1. EXTEND-IA was a small 70 patient Australian clinical trial, which was halted because of significant benefit in the endovascular therapy arm. Patients were treated with the Solitaire FR retrievable stent within a mean time of 248 minutes for endovascular therapy in combination with rt-PA (within 127 minutes mean) compared to a mean time of 145 minutes for rt-PA. 71% of patients in the endovascular therapy arm achieved mRS of 0–2 and >90% reperfusion) compared to 40% in the rt-PA group (same reperfusion).
REVASCAT [65]
Figure 5 and Table 1. REVASCAT enrolled 206 patients (103 embolectomy plus IV rt-PA and 103 IV rt-PA) within 269 minutes for the embolectomy arm and 105 minutes for the rt-PA arm. The thrombectomy arm used the Solitaire device. The rates of functional independence (mRS 0–2) at 90 days were 43.7% and 28.1% in the thrombectomy combined arm and standard care arms respectively, with an adjusted odds ratio for 1 point improvement of 1.7 (1.05–2.8) in favor of embolectomy.
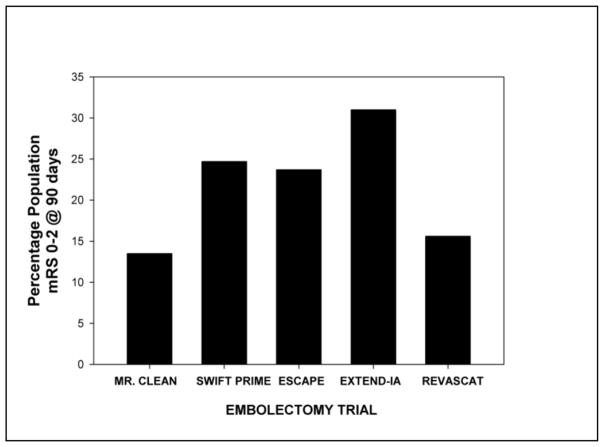
As shown in Figure 6, the difference in the rates of functional independence in the 5 embolectomy trials has an absolute range of 13.5 to 31 percentage points, when data was directly compared to the thrombolytic-treated group of patients. Clearly, embolectomy is beneficial in a select patient population presenting with a large vessel stroke within approximately 6 hours.
Figure 6.
Functional Independence After Embolectomy.
B1) Cost-Effectiveness
There is no doubt that embolectomy will be cost-effective in the treatment of ischemic stroke, especially when combined with thrombolysis. In 2012, Chen [66] provided interesting tiered QALY data which showed that incremental cost-effectiveness ratio (ICER) of thrombectomy was partially related to recanalization rate: the less reperfusion, the higher the ICER. Recently, Leppert et al. [67] considered the cost-effectiveness of IA treatment based upon the MR CLEAN trial, which as shown in Figure 6, was the least effective trial to promote functional independence (mRS 0–2 13.5%). Nevertheless, the concomitant use of IA thrombolysis with embolectomy within 6 hours of a stroke, at least using the MERCI retriever, was estimated to increase QALY by 0.7 years. The analysis also shows that the acute cost of IA plus thrombolytic is approximately $14,000 more than thrombolysis alone, but long term financial cost of the combined treatment is $4000 less than rt-PA alone. The cost-benefit to the patient is in functional independence and not in the financial burden associated with receiving the best current care.
B2) Patient population
Inclusion and exclusion criteria for embolectomy as an adjunct to thrombolysis are routinely used in clinical trials and the 2015 AHA embolectomy guidelines have been published [45]. Who should be treated with IA-rt-PA? Based upon 5 successful clinical trials, it appears that treatment IA treatment should be offered to patients with large artery occlusion up to 6 hours after a stroke. Either stent retrievers or aspiration devices may be used, as long as they are formally approved for the specific procedures. Patients should be included if less than 80 years of age, and having an NIHSS of <17. This may be expanded when the results of future trials are published. As shown in Figure 5, safety of embolectomy was not a significant concern; sICH, ICH and mortality in thrombectomy patients were not significantly different from standard thrombolytic care.
Summary
Taken together, IA therapy in combination with thrombolysis (i.e.: rt-PA) should be used in ischemic stroke patients within 6 hours of a stroke to promote independence and increase QALY) for stroke victim.
C) Neuroprotection: On the horizon?
With all data collected from endovascular and thrombolytic trials, treatments that are not mutually exclusive of each other, and given the fact that concomitant thrombolysis is used with embolectomy, it appears that future stroke therapy development studies should utilize embolic stroke models so that research studies can parallel the clinical situation. Currently, there are established rodent, rabbit and primate embolism models that can be used to develop novel neuroprotective strategies [11, 12, 69–72, 16, 15, 14]. Of importance to modeling stroke for stroke therapy research and development, there may be a need to include glycemic variability [73–75], age [76], gender/sex [77], and hypertension [76], but there are still no FDA-approved neuroprotectives to document the best path forward, nor is there critical data demonstrating that extensive studies in animals with comorbidities are required for success (see also [12]. With the discoveries described above, early intervention was best (within 6 hours) and recommendations are made to prevent any delay in treating the stroke victim [1–6, 36]. Even though endovascular approaches and thrombolysis are effective, and up to 30% of patients will be functionally independent, at least based upon current 90-day clinical outcome data, patient well-being can be further improved upon with safe, effective neuroprotective therapy. The stroke community must now take up the challenge of identifying and developing neuroprotective therapy.
Footnotes
Conflict of Interest Statement & Disclosure: PAL serves as Editor-in-Chief of the Journal of Neurology & Neurophysiology and Associate Editor of Translational Stroke Research. The scientific content of this work was not directly supported by the National Institutes of Health (NIH), National Institute of Neurodegenerative Disease and Stroke (NINDS) or any other funding source external to Cedars-Sinai Medical Center. PAL was supported in part by a U01 Translational research grant NS060685 for initial concepts of this article.
D) References
- 1.Campbell BC, Donnan GA, Davis SM. Vessel occlusion, penumbra, and reperfusion - translating theory to practice. Frontiers in neurology. 2014;5:194. doi: 10.3389/fneur.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis S, Donnan GA. Time is Penumbra: imaging, selection and outcome. The Johann jacob wepfer award 2014. Cerebrovasc Dis. 2014;38(1):59–72. doi: 10.1159/000365503. [DOI] [PubMed] [Google Scholar]
- 3.Phan TG, Wright PM, Markus R, Howells DW, Davis SM, Donnan GA. Salvaging the ischaemic penumbra: more than just reperfusion? Clin Exp Pharmacol Physiol. 2002;29(1–2):1–10. doi: 10.1046/j.1440-1681.2002.03609.x. [DOI] [PubMed] [Google Scholar]
- 4.Lapchak PA. Fast neuroprotection (fast-NPRX) for acute ischemic stroke victims: the time for treatment is now. Transl Stroke Res. 2013;4(6):704–9. doi: 10.1007/s12975-013-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hader R, Saver C, Steltzer T. No time to lose. Nursing management. 2006;37(7):23–6. 8–9, 48. [PubMed] [Google Scholar]
- 6.Saver JL. Time is brain--quantified. Stroke. 2006;37(1):263–6. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 8.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32(7):1310–6. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jickling GC, Sharp FR. Improving the translation of animal ischemic stroke studies to humans. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner RJ, Jickling GC, Sharp FR. Are Underlying Assumptions of Current Animal Models of Human Stroke Correct: from STAIRs to High Hurdles? Transl Stroke Res. 2011;2(2):138–43. doi: 10.1007/s12975-011-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapchak PA. Translational Stroke Research Using a Rabbit Embolic Stroke Model: A Correlative Analysis Hypothesis for Novel Therapy Development. Transl Stroke Res. 2010;1(2):96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapchak PA. A cost-effective rabbit embolic stroke bioassay: insight into the development of acute ischemic stroke therapy. Transl Stroke Res. 2015;6(2):99–103. doi: 10.1007/s12975-015-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culp BC, Culp WC. Rabbit subselective angiography stroke model. Stroke. 2008;39(11):e165. doi: 10.1161/STROKEAHA.108.526335. author reply e6. STROKEAHA.108.526335 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483(7388):213–7. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 15.Cook DJ, Teves L, Tymianski M. A translational paradigm for the preclinical evaluation of the stroke neuroprotectant Tat-NR2B9c in gyrencephalic nonhuman primates. Science translational medicine. 2012;4(154):154ra33. doi: 10.1126/scitranslmed.3003824. [DOI] [PubMed] [Google Scholar]
- 16.Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9(2):371–9. doi: 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapchak PA. Development of thrombolytic therapy for stroke: a perspective. Expert Opin Investig Drugs. 2002;11(11):1623–32. doi: 10.1517/13543784.11.11.1623. [DOI] [PubMed] [Google Scholar]
- 18.Desai JA, Smith EE. Prenotification and other factors involved in rapid tPA administration. Curr Atheroscler Rep. 2013;15(7):337. doi: 10.1007/s11883-013-0337-5. [DOI] [PubMed] [Google Scholar]
- 19.Olson DM, Constable M, Britz GW, Lin CB, Zimmer LO, Schwamm LH, et al. A qualitative assessment of practices associated with shorter door-to-needle time for thrombolytic therapy in acute ischemic stroke. J Neurosci Nurs. 2011;43(6):329–36. doi: 10.1097/JNN.0b013e318234e7fb. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association’s Target: Stroke initiative. Stroke. 2011;42(10):2983–9. doi: 10.1161/STROKEAHA.111.621342. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750–8. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41(7):1431–9. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrock JW, Lum M. Drill down analysis of door-to-needle time of acute ischemic stroke patients treated with intravenous tissue plasminogen activator. Am J Emerg Med. 2014;32(11):1330–3. doi: 10.1016/j.ajem.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw JM, Koumellis P, Liu M. Thrombolysis (different doses, routes of administration and agents) for acute ischaemic stroke. Cochrane Database Syst Rev. 2013;5:CD000514. doi: 10.1002/14651858.CD000514.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. 359/13/1317 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8(12):1095–102. doi: 10.1016/S1474-4422(09)70264-9. S1474-4422(09)70264-9 [pii] [DOI] [PubMed] [Google Scholar]
- 27.NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 28.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010;5(7):406–9. doi: 10.1002/jhm.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543–9. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 30.Ricci S, Cenciarelli S, Mazzoli T. Italian guidelines on thrombolysis indications in ischaemic stroke have been revised after IST 3 trial and Cochrane Review: PROS. Internal and emergency medicine. 2013 doi: 10.1007/s11739-013-0987-x. [DOI] [PubMed] [Google Scholar]
- 31.Costantino G, Podda GM, Bonzi M, Sbrojavacca R, Gruppo di Autoformazione M. Italian guidelines on thrombolysis indications in ischemic stroke have been revised after IST-3 trial and Cochrane revision: cons. Internal and emergency medicine. 2013 doi: 10.1007/s11739-013-0986-y. [DOI] [PubMed] [Google Scholar]
- 32.Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–8. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 33.Sandercock P, Lindley R, Wardlaw J, Dennis M, Innes K, Cohen G, et al. Update on the third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke and baseline features of the 3035 patients recruited. Trials. 2011;12:252. doi: 10.1186/1745-6215-12-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cronin CA, Smith EE. Response to letter regarding article, “Adherence to third European cooperative acute stroke study 3- to 4.5-hour exclusions and association with outcome: data from get with the guidelines-stroke”. Stroke. 2015;46(1):e16. doi: 10.1161/STROKEAHA.114.007906. [DOI] [PubMed] [Google Scholar]
- 35.Cronin CA, Sheth KN, Zhao X, Messe SR, Olson DM, Hernandez AF, et al. Adherence to Third European Cooperative Acute Stroke Study 3- to 4.5-hour exclusions and association with outcome: data from Get with the Guidelines-Stroke. Stroke. 2014;45(9):2745–9. doi: 10.1161/STROKEAHA.114.005443. [DOI] [PubMed] [Google Scholar]
- 36.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7:CD000213. doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyden P. Why don’t more patients receive intravenous rt-PA for acute stroke? Expert Rev Neurother. 2015;15(6):571–4. doi: 10.1586/14737175.2015.1041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27(9):1459–66. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 40.Fagan SC, Morgenstern LB, Petitta A, Ward RE, Tilley BC, Marler JR, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt-PA Stroke Study Group. Neurology. 1998;50(4):883–90. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 41.Boudreau DM, Guzauskas G, Villa KF, Fagan SC, Veenstra DL. A model of cost-effectiveness of tissue plasminogen activator in patient subgroups 3 to 4.5 hours after onset of acute ischemic stroke. Ann Emerg Med. 2013;61(1):46–55. doi: 10.1016/j.annemergmed.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boudreau DM, Guzauskas GF, Chen E, Lalla D, Tayama D, Fagan SC, et al. Cost-effectiveness of recombinant tissue-type plasminogen activator within 3 hours of acute ischemic stroke: current evidence. Stroke. 2014;45(10):3032–9. doi: 10.1161/STROKEAHA.114.005852. [DOI] [PubMed] [Google Scholar]
- 43.Paciaroni M. Italian guidelines on thrombolysis indications in ischemic stroke have been revised after the IST-3 trial and Cochrane review. Internal and emergency medicine. 2014;9(7):823–4. doi: 10.1007/s11739-014-1109-0. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Zhang Y, Wei H, Xu Y, Wang M, Cai Z, et al. A comparison of rt-PA thrombolysis guidelines between China and the USA: are changes needed? Neurol Res. 2015;37(1):57–63. doi: 10.1179/1743132814Y.0000000415. [DOI] [PubMed] [Google Scholar]
- 45.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 AHA/ASA Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015 doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 46.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 47.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 48.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. Jama. 2000;283(9):1145–50. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 49.Alberts MJ. tPA in acute ischemic stroke: United States experience and issues for the future. Neurology. 1998;51(3 Suppl 3):S53–5. doi: 10.1212/wnl.51.3_suppl_3.s53. [DOI] [PubMed] [Google Scholar]
- 50.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31(8):1812–6. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 51.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31(4):811–6. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 52.Grotta JC, Alexandrov AV. tPA-associated reperfusion after acute stroke demonstrated by SPECT. Stroke. 1998;29(2):429–32. doi: 10.1161/01.str.29.2.429. [DOI] [PubMed] [Google Scholar]
- 53.Grotta JC, Burgin WS, El-Mitwalli A, Long M, Campbell M, Morgenstern LB, et al. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001;58(12):2009–13. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- 54.Hacke W, Brott T, Caplan L, Meier D, Fieschi C, von Kummer R, et al. Thrombolysis in acute ischemic stroke: controlled trials and clinical experience. Neurology. 1999;53(7):S3–14. [PubMed] [Google Scholar]
- 55.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) Jama. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 56.Boudreau DM, Guzauskas G, Villa KF, Fagan SC, Veenstra DL. A Model of Cost-effectiveness of Tissue Plasminogen Activator in Patient Subgroups 3 to 4.5 Hours After Onset of Acute Ischemic Stroke. Ann Emerg Med. 2012 doi: 10.1016/j.annemergmed.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berkhemer OAFP, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 58.Goyal MDA, Menon BK, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 59.Campbell BCMP, Kleinig TJ, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 60.Saver J. SOLITAIRE FR with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. 2015 doi: 10.1161/STROKEAHA.115.010710. [DOI] [PubMed] [Google Scholar]
- 61.Kidwell CS, JR, Alger JR. Design and rationale of the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) Trial. Int J Stroke. 2014;9(1):110–6. doi: 10.1111/j.1747-4949.2012.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciccone AVL, Nichelatti M. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broderick JPPY, Demchuk AM. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hacke W, Schellinger PD, Albers GW, Bornstein NM, Dahlof BL, Fulton R, et al. Transcranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke. 2014;45(11):3187–93. doi: 10.1161/STROKEAHA.114.005795. [DOI] [PubMed] [Google Scholar]
- 65.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 66.Chen M. Cost-effectiveness of endovascular therapy for acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S16–21. doi: 10.1212/WNL.0b013e31826957df. [DOI] [PubMed] [Google Scholar]
- 67.Leppert MH, Campbell JD, Simpson JR, Burke JF. Cost-Effectiveness of Intra-Arterial Treatment as an Adjunct to Intravenous Tissue-Type Plasminogen Activator for Acute Ischemic Stroke. Stroke. 2015;46(7):1870–6. doi: 10.1161/STROKEAHA.115.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Lingsma H, van der Lugt A, et al. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials. 2014;15:343. doi: 10.1186/1745-6215-15-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Zhang RL, Jiang Q, Ding G, Chopp M, Zhang ZG. Focal embolic cerebral ischemia in the rat. Nat Protoc. 2015;10(4):539–47. doi: 10.1038/nprot.2015.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4(3):279–85. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapchak PA. Recommendations and practices to optimize stroke therapy: developing effective translational research programs. Stroke. 2013;44(3):841–3. doi: 10.1161/STROKEAHA.112.680439. [DOI] [PubMed] [Google Scholar]
- 72.Lyden P, Lapchak P. Sisyphus and translational stroke research. Science translational medicine. 2012;4(156):156ps20. doi: 10.1126/scitranslmed.3005083. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Moreno EI, Camara-Lemarroy CR, Gonzalez-Gonzalez JG, Gongora-Rivera F. Glycemic variability and acute ischemic stroke: the missing link? Transl Stroke Res. 2014;5(6):638–46. doi: 10.1007/s12975-014-0365-7. [DOI] [PubMed] [Google Scholar]
- 74.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5(4):442–53. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mandava P, Martini SR, Munoz M, Dalmeida W, Sarma AK, Anderson JA, et al. Hyperglycemia worsens outcome after rt-PA primarily in the large-vessel occlusive stroke subtype. Transl Stroke Res. 2014;5(4):519–25. doi: 10.1007/s12975-014-0338-x. [DOI] [PubMed] [Google Scholar]
- 76.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 77.Hoda MN, Bhatia K, Hafez SS, Johnson MH, Siddiqui S, Ergul A, et al. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res. 2014;5(4):484–90. doi: 10.1007/s12975-013-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]