Infectious and non-infectious inflammatory conditions are often associated with significant changes in systemic iron metabolism. One of the important consequences of this altered metabolic state is a decrease in plasma iron levels that is caused by reduced intestinal absorption and increased intracellular sequestration of the metal. The decrease in circulating iron can compromise erythropoiesis and lead ultimately to the development of an anemia that is usually referred to as the anemia of inflammation (AI). AI is usually a mild to moderate normocytic, normochromic anemia characterized by low plasma iron concentrations in the presence of normal or elevated serum ferritin levels (1). It typically occurs in the setting of chronic infections and autoinflammatory states but can also accompany malignancies and chronic renal disease. Even though hypoferremia is an important factor in the development of AI, other abnormalities that are associated with inflammation, such as decreased erythropoietin production, impaired erythropoiesis and increased erythrocyte destruction are additional contributors to the pathogenesis of the anemia (1-3). It is also worth remembering that persistently low circulating iron levels may have adverse effects on biological processes other than erythropoiesis, e.g. neurocognitive development, an issue of particular importance in infants and young children (4). This article will review our current understanding of the mechanisms that lead to hypoferremia in inflammatory diseases. Most of what we know about this problem is derived from studies in adult humans or from experiments in mice. It is likely that the basic pathophysiology will be very similar in infants and children but this is an issue that deserves further investigation. We will start by summarizing the mechanisms that regulate systemic iron metabolism under normal conditions, and then go on to describe how inflammation causes abnormalities in this process. We will conclude with a brief description of the management of inflammation-associated hypoferremia.
NORMAL IRON HOMEOSTASIS AND ITS REGULATION
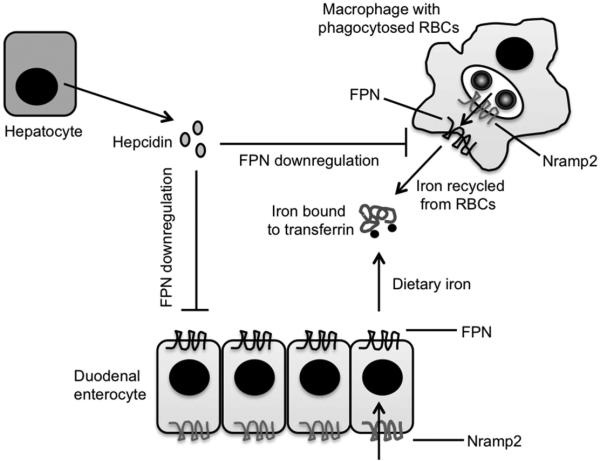
Over the last 10-15 years, there has been a tremendous increase in our understanding of how systemic iron homeostasis is regulated at the molecular level (5) (Figure 1). Most of the iron that is required for carrying out normal biological processes (about 25 mg/day) is recycled from the hemoglobin liberated during the destruction of aged red blood cells (RBCs), with only a minor fraction (about 2 mg/day) being obtained from the diet. Macrophages, chiefly in the spleen but also in other tissues, constitute the major cell type involved in iron recycling from RBCs, and duodenal enterocytes represent the main site of dietary iron absorption. Very similar sets of molecules handle iron in both types of cell. The transporter natural resistance-associated macrophage protein 2 (Nramp2, also known as Slc11a2 and DMT1) moves iron across the phagosomal membrane of macrophages as well as the apical membrane of enterocytes. Iron that reaches the cytosol by this means is then pumped out, either at the basolateral surface of the enterocyte or the plasma membrane of the macrophage, by ferroportin (FPN, also known as Slc40a1), the only known iron exporter in mammals. Iron that is in excess of that released into the plasma or utilized by the cell is stored in the cytoplasm complexed to ferritin. Plasma iron circulates bound to transferrin and can be taken up by cells via the ubiquitously expressed type 1 transferrin receptor. Because the entry of iron into the plasma from both macrophages and enterocytes is mediated by FPN, one of the major mechanisms that regulates circulating iron levels involves modulating the expression of this transporter. This function is carried out by the peptide hormone hepcidin (6). Hepcidin is secreted predominantly by the liver, and its expression is regulated, exclusively at the level of transcription, in response to changes in iron status. When plasma or tissue iron levels increase, hepcidin expression is up-regulated. Hepcidin binds to FPN, leading to internalization and degradation of the transporter (7), thus reducing the amount of iron entering the plasma. Conversely, when plasma or tissue iron levels decrease or when iron requirements increase (as in states of hypoxia), hepcidin expression is suppressed, allowing increased FPN levels and greater efflux of iron into the circulation. Thus, the hepcidin-FPN axis is a key component of a negative feedback loop that maintains plasma iron concentrations within a narrow physiologic range. However, it is important to note that in young animals – suckling rodents and 4-6 month old human infants – intestinal absorption of iron may be insensitive to regulation by hepcidin, possibly as a way of maximizing uptake of the metal from breast milk (8-11).
Figure 1. Mechanisms that regulate iron homeostasis under normal conditions.
Iron recycled from RBCs (Red Blood Cells) or absorbed from the diet is transported by Nramp2 (Natural resistance-associated macrophage protein-2) and FPN (Ferroportin) into the blood, where it circulates bound to transferrin. Circulating levels of iron are regulated by controlling the amount of FPN expressed on the plasma membrane, a function that is carried out by the peptide hormone hepcidin, which is secreted by the liver in response to changes in iron status and iron requirements.
DYSREGULATION OF IRON METABOLISM BY INFLAMMATION
The major factor responsible for altered iron metabolism in inflammatory states is increased expression of hepcidin (12). The abnormally elevated levels of hepcidin lead to intracellular sequestration and decreased absorption of iron as a result of down-regulation of FPN expression on macrophages and enterocytes, respectively. Plasma iron levels fall, usually within hours of the inflammatory stimulus (13,14), and if the hypoferremia persists, it can impair erythropoiesis and other processes that are dependent on iron.
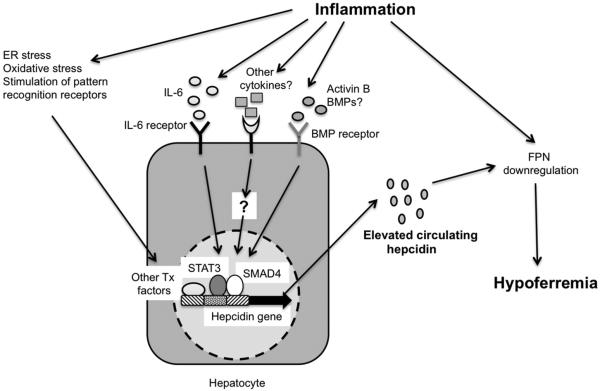
There has been a great deal of interest in clarifying the molecular pathways that connect inflammation to the increased expression of hepcidin (Figure 2). The cytokine interleukin (IL)-6 has emerged as one of the most important mediators of this up-regulation. Studies with cultured hepatocytes, wild-type and knockout mice and human volunteers have shown that IL-6 is sufficient to induce hepcidin expression, and is required for hepcidin up-regulation in response to inflammatory stimuli such as endotoxin or turpentine (13,15). Subsequent experiments have confirmed IL-6-dependent up-regulation of hepcidin in mouse models of inflammatory bowel disease (IBD) and systemic bacterial, viral and parasitic infection (16-18). The importance of IL-6 in the elevated hepcidin levels associated with human inflammatory diseases is highlighted by the good correlation between serum or urine hepcidin levels and serum IL-6 concentrations in IBD, and by the fact that anti-IL-6 treatment in rheumatoid arthritis is associated with a decline in circulating hepcidin (19-22). Mechanistic experiments have demonstrated that IL-6 acts by binding to its receptor gp130 on hepatocytes, thereby activating signals that phosphorylate the transcription factor signal transducer and activator of transcription 3 (STAT3), which binds to the promoter of the hepcidin gene and increases its expression (15,23-25). Cytokines other than IL-6, such as IL-1 and IL-22, have been shown to be involved in hepcidin up-regulation in cultured cells and in mice, but the relevance of these observations to the pathophysiology of inflammatory states is yet to be determined (26-28). Tumor necrosis factor α (TNFα) is another cytokine that is often elevated in inflammatory conditions, but its role in hepcidin expression is unclear. TNFα has been shown to inhibit hepcidin expression when added to cultured hepatocyte cell lines and has also been shown to have an inhibitory effect on hepcidin expression in two different mouse models of IBD (13,29). However, anti-TNFα therapy in human rheumatoid arthritis has been shown to be associated with decreases in hepcidin levels (21). Further work will be required to clarify this discrepancy. Besides cytokines, other inflammation-associated factors, such as oxidative and endoplasmic reticulum stress and direct stimulation of pattern recognition receptors, may also contribute to increased hepcidin expression, at least in mice (30-33). Recent experiments have suggested that the composition of the intestinal microbiota may influence hepcidin expression in mouse models of IBD, possibly by altering the cytokine milieu associated with intestinal inflammation (34).
Figure 2. The major factors contributing to hypoferremia during inflammation.
Inflammatory stimuli such as IL-6 (Interleukin-6), other cytokines, activin B, BMPs (Bone morphogenetic proteins), endoplasmic reticulum (ER) and oxidative stress, and pattern recognition receptor ligands activate STAT3 (Signal transducer and activator of transcription-3), SMAD4 (Small-mothers against decapentaplegic-4) and other transcription (Tx) factors to induce increased expression of hepcidin, leading to downregulation of FPN (Ferroportin) and consequent hypoferremia. Inflammatory mediators may also act directly to cause decreased expression of FPN. For clarity, inflammation-associated factors that inhibit hepcidin expression have not been shown.
In addition to IL-6 and the other inflammatory mediators described above, activators of the bone morphogenetic protein (BMP) receptors are also involved in the increased hepcidin expression associated with inflammatory conditions. Soluble forms of BMP co-receptors, as well as inhibitors of BMP signal transduction, have been shown to suppress hepcidin up-regulation in mouse models of IBD and bacterial infection (16,35). One of the BMP receptor ligands that appears to play a role in the inflammation-induced elevation of hepcidin is activin B. This member of the transforming growth factor β super-family is highly up-regulated in an IL-6-independent manner in the livers of mice treated with endotoxin, and is capable of inducing hepcidin expression when added to mouse hepatocytes and human hepatoma cells (36). Interestingly, the expression of activins has been shown to be increased in conditions such as IBD, rheumatoid arthritis and sepsis (37). BMP receptor stimulation by ligands such as activin B leads to the activation of the transcription factor small-mothers against decapentaplegic 4 (SMAD4), which cooperates with STAT3 and is required for IL-6-induced hepcidin up-regulation (38,39).
Not all inflammation-associated events induce increased hepcidin expression. Besides the possible inhibitory effect of TNFα mentioned above, the increased erythropoietic activity that can accompany inflammation can also decrease hepcidin levels, probably as a result of RBC precursors releasing factors that suppress hepcidin expression (34,40). Some components of the microbiota may also have an inhibitory effect on the production of hepcidin (34). Thus, the net level of hepcidin during inflammation is likely to reflect the combined influence of factors that alter expression either positively or negatively.
Mechanisms that do not involve up-regulation of hepcidin may also contribute to the hypoferremia of inflammation. Injection of mice with polyinosinic:polycytidylic acid, a ligand for the pattern recognition receptor Toll-like receptor 3, has been shown to cause a prompt decrease in serum iron concentration without a significant increase in hepcidin expression in the liver or spleen (41). It is not clear how the hypoferremia occurs under these circumstances but it may involve down-regulation of FPN mRNA levels.
Measurement of serum or urine levels of bioactive hepcidin can be a useful tool in the diagnosis of the hypoferremia and anemia associated with inflammation. Several methods are now available for this purpose, including enzyme-linked immunosorbent assays and mass spectrometry-based approaches (42). There is considerable variability in the hepcidin concentrations obtained with the different methods, even when using comparable samples, making further standardization essential before the tests can be implemented clinically (43). Nevertheless, serum or urine hepcidin levels have been shown to be significantly elevated in a number of human inflammatory conditions, including IBD, rheumatoid arthritis, malaria, human immunodeficiency virus infection and neonatal sepsis (19-22,44-47).
MANAGEMENT OF INFLAMMATION-ASSOCIATED HYPOFERREMIA
Treatment of the underlying inflammatory condition will restore normal iron homeostasis and is all that is required in situations where the hypoferremia and associated anemia are mild. However, more severe cases of AI reduce quality of life and benefit from specific treatment, as has been demonstrated in studies of rheumatoid arthritis, cancer and chronic renal failure (48-50). When intervention is called for, the general therapeutic strategies include iron supplementation, interference with the hepcidin-FPN axis and, in the case of anemia associated with chronic kidney disease, use of erythropoiesis stimulating agents (5,51). Oral iron supplements can be used but may be poorly effective because of the decreased intestinal absorption caused by elevated hepcidin levels. The impaired absorption can lead to elevation of iron concentrations in the intestinal lumen, which may alter the gut microbiota and exacerbate intestinal inflammation (52). Intravenous iron therapy is effective at correcting the functional iron deficiency associated with increased hepcidin expression, but supraphysiologic levels of iron can further up-regulate hepcidin and worsen the dysregulation of iron homeostasis by decreasing FPN expression (5). Because iron can promote the growth and virulence of some pathogens, it has been suggested that iron supplementation may increase the incidence or severity of infections, particularly in iron replete individuals. This is a concern that has been supported by some studies, but several others have indicated that iron supplementation does not increase infectious disease morbidity (53-56).
Interfering with the hepcidin-FPN axis constitutes a rational form of therapy that is directed at correcting the basic pathophysiology of the hypoferremia associated with inflammation. Several reagents targeting this axis are in various stages of development or evaluation (5,51) and include those that (a) inhibit hepcidin expression (antibodies that block IL-6 or the IL-6 receptor, inhibitors of IL-6 signaling, antibodies, soluble receptors and other molecules that block or sequester BMPs, inhibitors of BMP signaling, anti-sense oligonucleotides and short interfering RNAs directed against the hepcidin transcript, vitamin D), (b) block hepcidin (anti-hepcidin antibodies, proteins and aptamers that bind hepcidin), and (c) prevent hepcidin from down-regulating FPN (anti-FPN antibodies, thiol modifier compounds, cardiac glycosides).
CONCLUSION
Extensive basic research into the mechanisms that regulate iron metabolism has resulted in a solid understanding of the pathophysiology of the hypoferremia associated with inflammatory states, which in turn has allowed the development of rational therapeutic strategies. Application of these novel forms of treatment, and others that will undoubtedly emerge with further elucidation of the fine details of hepcidin and FPN function and regulation, will make it easier for the clinician to correct the abnormalities of iron homeostasis caused by inflammatory diseases.
Acknowledgments
B.C.’s laboratory is supported by National Institute of Allergy and Infectious Diseases (R01 AI089700).
ABBREVIATIONS
- AI
Anemia of inflammation
- BMP
Bone morphogenetic protein
- FPN
Ferroportin
- IBD
Inflammatory bowel disease
- IL
Interleukin
- Nramp
Natural resistance-associated macrophage protein
- RBC
Red blood cells
- SMAD
Small-mothers against decapentaplegic
- STAT
Signal transducer and activator of transcription
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
B.C. received an honorarium to serve as a member of the Mead Johnson Pediatric Institute Iron Expert Panel to write a manuscript; the sponsor had no involvement in preparing the manuscript.
REFERENCES
- 1.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–681. doi: 10.1016/j.hoc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freireich EJ, Ross JF, Bayles TB, Emerson CP, Finch SC. Radioactive iron metabolism and erythrocyte survival studies of the mechanism of anemia associated with rheumatoid arthritis. J Clin Invest. 1957;36:1043–1058. doi: 10.1172/JCI103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libregts SF, Gutierrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van Ijcken W, et al. Chronic IFNγ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118:2578–2588. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 4.Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:585. doi: 10.3389/fnhum.2013.00585. doi: 10.3389/fnhum.2013.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 8.Domellöf M, Lönnerdal B, Abrams SA, Hernell O. Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr. 2002;76:198–204. doi: 10.1093/ajcn/76.1.198. [DOI] [PubMed] [Google Scholar]
- 9.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr. 2001;138:679–87. doi: 10.1067/mpd.2001.112895. [DOI] [PubMed] [Google Scholar]
- 10.Leong WI, Bowlus CL, Tallkvist J, Lönnerdal B. DMT1 and FPN1 expression during infancy: developmental regulation of iron absorption. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1153–61. doi: 10.1152/ajpgi.00107.2003. [DOI] [PubMed] [Google Scholar]
- 11.Darshan D, Wilkins SJ, Frazer DM, Anderson GJ. Reduced expression of ferroportin-1 mediates hyporesponsiveness of suckling rats to stimuli that reduce iron absorption. Gastroenterology. 2011;141:300–9. doi: 10.1053/j.gastro.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–122. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 15.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Trebicka E, Fu Y, Ellenbogen S, Hong CC, Babitt JL, et al. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:112–119. doi: 10.1002/ibd.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez R, Jung CL, Gabayan V, Deng JC, Ganz T, Nemeth E, et al. Hepcidin induction by pathogens and pathogen-derived molecules is strongly dependent on interleukin-6. Infect Immun. 2014;82:745–752. doi: 10.1128/IAI.00983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HZ, He YX, Yang CJ, Zhou W, Zou CG. Hepcidin is regulated during blood-stage malaria and plays a protective role in malaria infection. J Immunol. 2011;187:6410–6416. doi: 10.4049/jimmunol.1101436. [DOI] [PubMed] [Google Scholar]
- 19.Semrin G, Fishman DS, Bousvaros A, Zholudev A, Saunders AC, Correia CE, et al. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–1106. doi: 10.1097/01.mib.0000235097.86360.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basseri RJ, Nemeth E, Vassilaki ME, Basseri B, Enayati P, Shaye O, et al. Hepcidin is a key mediator of anemia of inflammation in Crohn’s disease. J Crohns Colitis. 2013;7:e286–e291. doi: 10.1016/j.crohns.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNFα inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15:R141. doi: 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15:R204. doi: 10.1186/ar4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 24.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Sakamori R, Takehara T, Tatsumi T, Shigekawa M, Hikita H, Hiramatsu N, et al. STAT3 signaling within hepatocytes is required for anemia of inflammation in vivo. J Gastroenterol. 2010;45:244–248. doi: 10.1007/s00535-009-0159-y. [DOI] [PubMed] [Google Scholar]
- 26.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- 28.Smith CL, Arvedson TL, Cooke KS, Dickmann LJ, Forte C, Li H, et al. IL-22 regulates iron availability in vivo through the induction of hepcidin. J Immunol. 2013;191:1845–1855. doi: 10.4049/jimmunol.1202716. [DOI] [PubMed] [Google Scholar]
- 29.Shanmugam NK, Ellenbogen S, Trebicka E, Wang L, Mukhopadhyay S, Lacy-Hulbert A, et al. Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One. 2012;7:e38136. doi: 10.1371/journal.pone.0038136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira SJ, Pinto JP, Picarote G, Costa VM, Carvalho F, Rangel M, et al. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLoS One. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, et al. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig CL, Miller JC, Nelson JM, Ward DM, Kushner JP, Bockenstedt LK, et al. Toll-like receptors mediate induction of hepcidin in mice infected with Borrelia burgdorferi. Blood. 2009;114:1913–1918. doi: 10.1182/blood-2009-03-209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millonig G, Banzleben I, Peccerella T, Casanovas G, Brodziak-Jarosz L, Breitkopf-Heinlein K, et al. Sustained micromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3) J Biol Chem. 2012;287:37472–37482. doi: 10.1074/jbc.M112.358911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanmugam NK, Trebicka E, Fu LL, Shi HN, Cherayil BJ. Intestinal inflammation modulates expression of the iron-regulating hormone hepcidin depending on erythropoietic activity and the commensal microbiota. J Immunol. 2014;193:1398–1407. doi: 10.4049/jimmunol.1400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Harrington L, Trebicka E, Shi HN, Kagan JC, Hong CC, et al. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119:3322–3328. doi: 10.1172/JCI39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, et al. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood. 2012;120:431–439. doi: 10.1182/blood-2012-02-411470. [DOI] [PubMed] [Google Scholar]
- 37.Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci USA. 2007;104:16239–16244. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122:4635–4644. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layoun A, Huang H, Calve A, Santos MM. Toll-like receptor signal adaptor protein MyD88 is required for sustained endotoxin-induced acute hypoferremic response in mice. Am J Pathol. 2012;180:2340–2350. doi: 10.1016/j.ajpath.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 42.Konz T, Montes-Bayon M, Vaulont S. Hepcidin quantification: methods and utility in diagnosis. Metallomics. 2014;6:1583–1590. doi: 10.1039/c4mt00063c. [DOI] [PubMed] [Google Scholar]
- 43.Kroot JJ, van Herwaarden AE, Tjalsma H, Jansen RT, Hendriks JC, Swinkels DW. Second round robin for plasma hepcidin methods: first steps towards harmonization. Am J Hematol. 2012;87:977–983. doi: 10.1002/ajh.23289. [DOI] [PubMed] [Google Scholar]
- 44.Atkinson SH, Armitage AE, Khandwala S, Mwangi TW, Uyoga S, Bejon PA, et al. Combinatorial effects of malaria season, iron deficiency, and inflammation determine plasma hepcidin concentration in African children. Blood. 2014;123:3221–3229. doi: 10.1182/blood-2013-10-533000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisaksana R, de Mast Q, Alisjahbana B, Jusuf H, Sudjana P, Indrati AR, et al. Inverse relationship of serum hepcidin levels with CD4 cell counts in HIV-infected patients selected from an Indonesian prospective cohort study. PLoS One. 2013;8:e79904. doi: 10.1371/journal.pone.0079904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, et al. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci USA. 2014;111:12187–12192. doi: 10.1073/pnas.1402351111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu TW, Tabangin M, Kusano R, Ma Y, Ridsdale R, Akinbi H. The utility of serum hepcidin as a biomarker for late-onset neonatal sepsis. J Pediatr. 2013;162:67–71. doi: 10.1016/j.jpeds.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Moreno F, Sanz-Guajardo D, Lopez-Gomez JM, Jofre R, Valderrabano F. Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialyisis patients. Spanish cooperative renal patients quality of life study group of the Spanish Society of Nephrology. J Am Soc Nephrol. 2000;11:335–342. doi: 10.1681/ASN.V112335. [DOI] [PubMed] [Google Scholar]
- 49.Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 50.Swaak A. Anemia of chronic disease in patients with rheumatoid arthritis: aspects of prevalence, outcome, diagnosis, and the effect of treatment on disease activity. J Rheumatol. 2006;33:1467–1468. [PubMed] [Google Scholar]
- 51.Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol. 2012;87:392–400. doi: 10.1002/ajh.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherayil BJ, Ellenbogen S, Shanmugam NN. Iron and intestinal immunity. Curr Opin Gastroenterol. 2011;27:523–528. doi: 10.1097/MOG.0b013e32834a4cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–633S. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 54.Gera T, Sachdev HP. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ. 2002;325:1142–1152. doi: 10.1136/bmj.325.7373.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomized, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 56.Lemaire M, Islam QS, Shen H, Khan MA, Parveen M, Abedin F, et al. Iron-containing micronutrient powder provided to children with moderate-to-severe malnutrition increases hemoglobin concentrations but not the risk of infectious morbidity: a randomized, double-blind, placebo-controlled, noninferiority safety trial. Am J Clin Nutr. 2011;94:585–593. doi: 10.3945/ajcn.110.009316. [DOI] [PubMed] [Google Scholar]