Abstract
Iron oxide nanoparticles have been extensively used as T2 contrast agents for liver-specific magnetic resonance imaging (MRI). The applications, however, have been limited by their mediocre magnetism and r2 relaxivity. Recent studies show that Fe5C2 nanoparticles can be prepared by high temperature thermal decomposition. The resulting nanoparticles possess strong and air stable magnetism, suggesting their potential as a novel type of T2 contrast agent. To this end, we improve the synthetic and surface modification methods of Fe5C2 nanoparticles, and investigated the impact of size and coating on their performances for liver MRI. Specifically, we prepared 5, 14, and 22 nm Fe5C2 nanoparticles and engineered their surface by: 1) ligand addition with phospholipids, 2) ligand exchange with zwitterion-dopamine-sulfonate (ZDS), and 3) protein adsorption with casein. It was found that the size and surface coating have varied levels of impact on the particles' hydrodynamic size, viability, uptake by macrophages, and r2 relaxivity. Interestingly, while phospholipid- and ZDS-coated Fe5C2 nanoparticles showed comparable r2, the casein coating led to an r2 enhancement by more than 2 fold. In particular, casein coated 22 nm Fe5C2 nanoparticle show a striking r2 of 973 mM-1s-1, which is one of the highest among all of the T2 contrast agents reported to date. Small animal studies confirmed the advantage of Fe5C2 nanoparticles over iron oxide nanoparticles in inducing hypointensities on T2-weighted MR images, and the particles caused little toxicity to the host. The improvements are important for transforming Fe5C2 nanoparticles into a new class of MRI contrast agents. The observations also shed light on protein-based surface modification as a means to modulate contrast ability of magnetic nanoparticles.
Keywords: iron carbides, magnetic nanoparticles, magnetic resonance imaging, casein, surface modification, macrophages
Introduction
Liver cancer remains a major cause of mortality worldwide. In the United States, liver cancer is estimated to be diagnosed in more than 35,600 new patients in 2014 and cause 24,500 deaths 1. The five-year survival rate is 27% and 42% for regional and localized tumors, respectively, but the rate is dropped to only 18% for liver cancer that has metastasized 2. This status underscores the significance of early diagnosis of liver cancer. Liver-specific magnetic resonance imaging (MRI) is one of the most extensively used methods in detection of hepatocellular diseases and tumor metastasis from other organs 3-7. To improve detection accuracy, roughly 30-40% of the scans are performed with the assistance of either T1 (spin-lattice) or T2 (spin-spin) contrast agents 8-10. So far, the most commonly used T2 contrast agents are iron oxide nanoparticles (IONPs) 11, 12. IONPs are administered prior to a MRI scan and are taken up by the Küpffer cells in the liver. The particles cause signal decrease on a T2-weighted image, producing contrast against lesions (e.g. a tumor) that are less abundant of the resident macrophages thereby improving the diagnosis sensitivity and accuracy 13. However, clinically used IONP formulations, such as Feridex and Resovist, exhibit moderate contrast ability due to their mediocre magnetizations (~60-70 emu/g) 14-16. Over the years, researchers have endeavored to synthesize nanoparticles made of higher magnetization materials such as Co 17, Fe 18, and FePt 19. The resulting nanoparticles, however, have been often associated with issues including rapid oxidation 20, unstable magnetization in the air 21, harsh and often hazardous synthesis conditions 22, and high toxicity 23, and these drawbacks dim their perspectives of clinical translation.
Very recently, the Hou group and we reported the synthesis of iron carbide (Fe5C2) nanoparticles 15, 24. Fe5C2 nanoparticles exhibit a high magnetization (~140 emu/g), relatively low toxicity, air stability, and facile synthesis 24; more importantly, the high magnetization translates to r2 relaxivity that is 2-3 folds higher than IONPs 15. These findings suggest a great potential of Fe5C2 nanoparticles as an alternative T2 contrast agent. In our previous studies, only 20 nm Fe5C2 nanoparticles were investigated 15. It is postulated that the size of Fe5C2 nanoparticles may an impact on their cellular uptake, magnetization, r2 relaxivity, circulation half-lives 14,25, and therefore affecting their role as a liver contrast agent. These, however, have not been studied yet.
As-synthesized Fe5C2 particles inherit a thin shell of iron oxide from the synthesis 24. This means that the surface modification methods previously developed for IONPs can be borrowed to modify Fe5C2 nanoparticles. These include ligand addition with amphiphilic ligands such as PEGylated phospholipids 25 and surface exchange with iron-philic molecules such as 2,3-dimercaptosuccinic acid (DMSA) 26 and zwitterion-dopamine-sulfonate (ZDS) 27. In addition, protein based surface modification approaches that are more recently developed by us 28, 29 and others 30-32 are expected to be applicable to Fe5C2 nanoparticles. The surface coating may affect particles' interaction with the biological milieu 33, which has always been a topic of interest in nanoparticle developments, including the recent attention on protein corona of nanoparticles 31. The coating may also affect the r2 relaxivity of nanoparticles, but the topic has been much less studied, probably due to the relatively minor impact observed previously 34. However, recent studies by Huang et al. showed that the r2 relaxivity of IONPs can be increased by as much as ~2.5 fold when using casein as coating material 30, suggesting a bigger role surface coatings, and in particular protein-based surface coatings, can play in engineering T2 contrast agents. Whether the coating effect can be modulated to further enhance the r2 of Fe5C2 nanoparticles is worthy of investigations.
In the present study, we modified the previously reported synthetic approach and we prepared three sizes of Fe5C2 nanoparticles (5, 14, and 22 nm). These nanoparticles were then coated with PEGylated phospholipid, ZDS, or casein and the resulting conjugates were compared for their hydrodynamic size, macrophage uptake, toxicity, and r2 relaxivity. Significant size and surface effects were observed, especially to the r2 of the particles. In particular, the casein-coated 22 nm Fe5C2 nanoparticles show an extraordinary r2 of 973 mM-1s-1 (on the basis of Fe), making it one of the highest among all the reported T2 contrast agents to date 35. In small animal studies, we found that casein-coated 22 nm Fe5C2 nanoparticles can induce at least 2.5 fold greater hypointensity to the liver than IONPs. These observations confirm the promise of Fe5C2 nanoparticles as novel T2 contrast agents for liver imaging. In addition, the study sheds light on the great potential of modulating coatings for enhanced r2 of magnetic nanoparticles.
Methods
Preparation of Fe5C2 Nanoparticles
Fe5C2 NPs were synthesized using a previously published protocol with minor modifications 15. All of the chemical were from Sigma-Aldrich unless specified otherwise. For 22 nm Fe5C2 nanoparticle synthesis, 7.5 g of octadecylamine (ODA) and 56 mg of cetyltrimethylammonium bromide (CTAB) was added to a four-neck flask. The flask was purged with Ar gas and the temperature was increased to 393 K. 0.25 mL (3.6 mmol) of Fe(CO)5 was added to the reaction mixture and the temperature was raised to 453 K to induce oxidation of Fe(CO)5. After 10 min, the temperature was further raised to 693 K and maintained at the temperature for 10 min. The reaction system was then cooled to room temperature. The raw product was dissolved in a hexane/ethanol mixture and centrifuged for 10 min at 7500 rpm (6,174 g). This step was repeated for 6 times for the particles to be purified and the residual CTAB removed. For particles of larger and smaller sizes, the amount of Fe(CO)5 added was doubled (7.2 mmol) and halved (1.8 mmol), respectively. The nanoparticles synthesized above were characterized by TEM (FEI Tecnai 20), dynamic light scattering (DLS, Malvern Zetasizer Nano S90), and X-ray diffraction (XRD, Bruker D8 Advanced X-ray diffractometer, Cu source).
Surface Modification Using Phospholipid
The as-synthesized Fe5C2 nanoparticles (1 mg/mL) were dried and redissolved in 1 mL CHCl3. ~60 µL of DSPE-PEG-COOH (10 mg/mL, Avanti Polar Lipids, Inc.) was added dropwise to the solution during stirring. The solution was left to stir for 1 hr and the solvent was evaporated off. The dried product was dispersed in deionized water with sonication and purified by centrifugation (6,174 g for 5 min) 15.
Surface Modification Using Zwitterion Dopamine Sulfonate (ZDS)
In a similar fashion to the previous modification, 50 mg of ZDS in 2 mL DMSO was added to a 4 mL CHCl3 solution containing ~10 mg/mL Fe5C2 until the solution showed visible emulsion. The solution was then isolated by centrifugation (6,174 g for 10 min). The collected product was redispersed in water and centrifuged for 3 times to remove residual DMSO.
Surface Modification Using Casein
To prepare an aqueous solution of Fe5C2 particles, a solution of Fe5C2 in CHCl3 was mixed with a preheated solution of excess glucose in DMF and refluxed for 1 hr. The resulting solution was then precipitated by ethanol and centrifuged (6,174 g for 10 min) and the process was repeated for three times. The purified product was redispersed in water. To prepare the casein addition, 100 mg of casein was treated with 0.01 M NaOH to form a soluble base in water. At a molar ratio of particle:casein at 1:200, the two mixtures were added to a flask and mixed for 4 hrs at room temperature. After 4 hrs, a 0.4% solution of glutaraldehyde (glutaraldehyde:casein 1:2) was dropwise added and the mixture was stirred for 1 hr at room temperature. The final product was purified by centrifugation and re-dissolved in water.
MRI Phantom Study
To analyze the relaxivities of these nanoparticles, a MRI phantom study was run. Nanoparticles at different concentrations were dispersed in 1% agarose gel in 300 μL tubes. The tubes were scanned on a 7T Varian small animal MRI system. T2-weighted fast spin echo images were obtained using the following parameters: TR = 2,000 ms; TE = 20, 40, 60, 80, and 100 ms; ETL = 8; field-of-view (FOV) = 40 mm × 80 mm; slice thickness = 1 mm.
Cell Toxicity
1 × 104 RAW264.7 cells were placed in each well of a 96-well plate. After 24 hr incubation, Fe5C2 nanoparticles of varying concentrations (0 - 100 μg Fe/mL) were added and the incubation lasted for 4 hrs. The culture medium was removed after 4 hrs and replenished with fresh medium. Standard MTT assay was performed 24 hrs later to determine the cell viability.
Cell Uptake
1 × 106 RAW264.7 cells (murine macrophages) were placed in each of the three 2-well incubation chambers 24 hrs prior to the uptake study. Fe5C2 nanoparticles were added (0 - 100 μg Fe/mL) and incubated with the cells for 4 hrs. After incubation, the cells were washed using PBS. The cells were fixed with cold 95% ethanol for 15 min. Prussian blue staining was used to stain Fe while the nuclei of cells were stained with Nuclear Fast Red (Sigma-Aldrich). The slides were imaged on an optical microscope (Olympus X71). For quantitative analysis, the cells after incubation were collected and lysed by nitric acid (pH = 5.0, 72 hrs). The Fe content was determined by inductively coupled plasma mass spectrometry (ICP-MS) and the result was divided by cell count. Cells that had been incubated with casein-coated Fe5C2 nanoparticles were also collected for MRI phantom studies. The parameters were the same as those described above.
in vivo MRI
All the animal studies conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, USA, and a protocol approved by the Institutional Animal Care and Use Committee (IACUC), University of Georgia. Normal athymic nude mice were used for the in vivo imaging studies. The mice were anesthetized with isoflurane and tail-vein injected with casein-coated Fe5C2 or Fe3O4 nanoparticles at a dose of 2.5 mg Fe/kg (n = 3). Fe3O4 nanoparticles were Ocean Nanotech and were surface coated with casein. From T2-weighted fast spin echo images were obtained on a 7T Varian small animal system prior to as well as 1 hr and 4 hrs after the particle injection. The scan parameters were the following: TR = 2500 ms, TE = 40 ms, field-of-view (FOV) = 40 mm × 80 mm, matrix size = 2562 and, thickness = 2 mm. After the 4 hr scan, the mice were sacrificed and their liver, kidneys, and spleen were excised and frozen in OCT (optimal cutting temperature compound) gel at -80 °C. The tissue blocks were cryo-sectioned into 8 μm slices and fixed in 10% formalin solutions for 25 min. The slides were rinsed with PBS carefully and immersed in a mixture of 20% HCl solution and 10% K4[Fe(CN)6]·3H2O for 20 min. After washing by PBS, the slices were then counterstained with Fast Red solution for 5 min then washed again with PBS.
Results
Nanoparticle Synthesis and Characterization
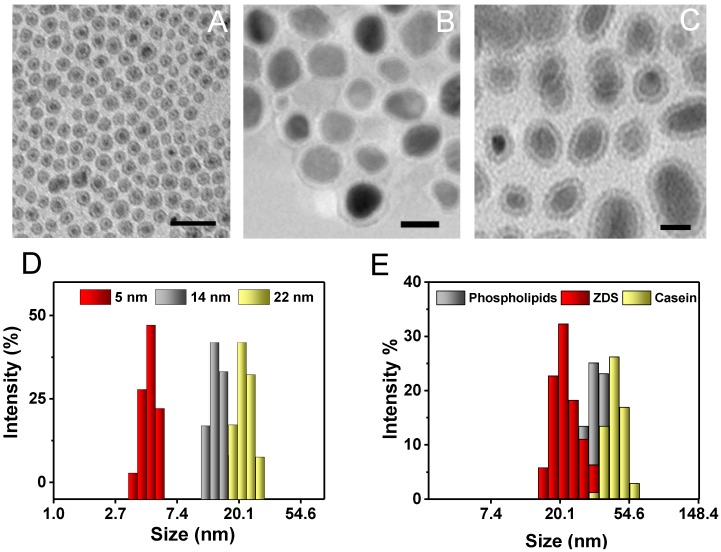
Fe5C2 nanoparticle synthesis was similar to a previously published protocol 24. Briefly, Fe(CO)5 was added to a Ar-purged mixture of octadecylamine and cetyltrimethylammonium bromide (CTAB) and the solution was heated up to the boiling point to induce Fe(CO)5 oxidation and carbonization 24. In order to tune the size of these nanoparticles, the amount of the Fe(CO)5 precursor was varied. More specifically, we doubled and halved the amount of Fe(CO)5 previously used to yield Fe5C2 nanoparticles of relatively large and small sizes (Table 1). The nanoparticle size was determined by transmission electron microscopy (TEM, Figure 1A-C). All of the particles display a core-shell structure, with the shell about 1 nm in depth. The overall particle sizes are 5, 14, and 22 nm, respectively. The dynamic light scattering (DLS) results overall agree with the TEM measurements albeit slightly larger (Figure 1D and Table 1); the difference was attributed to the organic coating on the particle surface that is invisible under TEM. X-ray diffraction (XRD) analysis confirmed that the majority of the particles were θ-Fe5C2 (JCPDS ID: 00-036-1248, Figure S1).
Table 1.
TEM and DLS analysis results of Fe5C2 nanoparticles.
| TEM results | DLS results | ||||
|---|---|---|---|---|---|
| Shell (nm) | Core (nm) | Total (nm) | PL (nm) | ZDS (nm) | Casein (nm) |
| ~1 | 2.7± 0.8 | 4.8 ± 0.9 | 12.1 ± 1.7 | 8.2 ± 0.6 | 16.8 ± 2.7 |
| ~1 | 11.4 ± 2.1 | 14.3 ± 2.3 | 23.2 ± 2.1 | 13.1 ± 0.2 | 32.4 ± 3.3 |
| ~1 | 19.5 ± 3.2 | 22.0 ± 3.4 | 35.3 ± 5.3 | 22.4 ± 1.4 | 44.9 ± 6.5 |
Figure 1.
Characterization of Fe5C2 nanoparticles. A-C) TEM images of 5 nm (A), 14 nm (B), and 22 nm (C) Fe5C2 nanoparticles. The particle sizes were tuned by varying the amount of Fe(CO)5 precursor used for synthesis. Scale bars: 20 nm. D) DLS analysis of as-synthesized Fe5C2 nanoparticles of three sizes. E) DLS analysis of 22 nm Fe5C2 nanoparticles coated with phospholipids, ZDS, or casein.
Surface modification
The as-synthesized Fe5C2 nanoparticles cannot be dispersed in water. To make them water soluble, we used three surface modification methods. These include 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N[carboxy(polyethylene glycol)-2000] (DSPE-PEG-COOH), which had used in our previous study 15. The other two are ZDS-based ligand exchange and casein-based protein adsorption, which were proven to be successful to modify IONPs 30, 36. The three strategies were all efficient to render Fe5C2 nanoparticles with good aqueous stability but the hydrodynamic sizes of the resulting formulations are varied. Taking 22 nm Fe5C2 nanoparticles for instance, ZDS, which is a small molecule, minimally affects the overall particle size (22.4 ± 1.4 nm, Figure 1E, Table 1, and Figure S2A&B). Phospholipid and casein coatings, on the other hand, significantly increased the hydrodynamic sizes to 35.3 ± 5.3 nm and 44.9 ± 6.5, respectively (Figure 1E, Table 1, and Figure S2C&D). The nanoparticles were also stable in PBS containing 10% fetal bovine serum (FBS), showing no aggregation for over 1 week (Figure S3).
Size and surface effects on r2 relaxivity
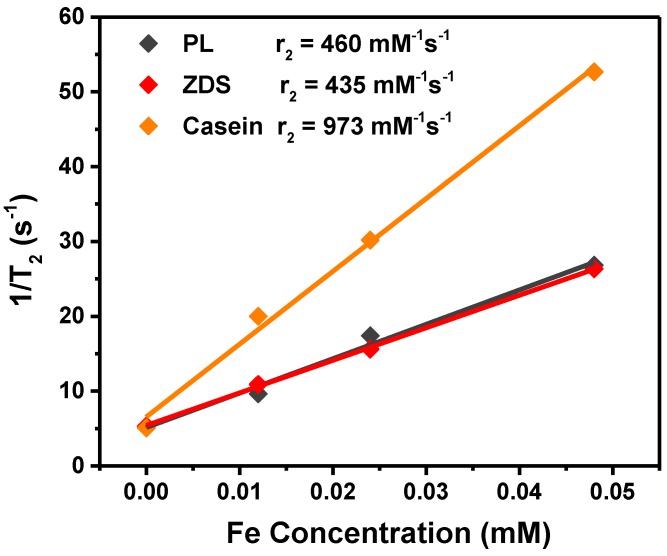
We next assessed the r2 relaxivities of all the nine Fe5C2 formulations. To do so, Fe5C2 nanoparticles of elevated Fe concentrations (0 - 0.05 mM Fe) were dispersed in 1% agarose gel and scanned on a 7T magnet. For particles of the same coating, there was a clear size effect on the T2 shortening effect, with larger nanoparticles more efficiently inducing hypointensities. For instance, for phospholipid coated Fe5C2 nanoparticles, r2 values were 342, 385, and 450 mM-1s-1 for 5, 14, and 22 nm particles, respectively (Figure 2A and Table 2). This size effect is attributed to surface canting caused magnetism drop, which is more severe for particles of smaller sizes 37.
Figure 2.
r2 relaxivity rates of Fe5C2 nanoparticles, measured with agarose gel samples containing different concentrations of particles. r2 values for 22 nm Fe5C2 coated with phospholipids, ZDS, and casein. While phospholipids and ZDS coated nanoparticles show comparable r2, casein coating increased the r2 by more than two fold to 973 mM-1s-1.
Table 2.
r2 relaxivities of Fe5C2 nanoparticles of different sizes and surface coatings.
| Size (nm) | PL (mM-1s-1) | ZDS (mM-1s-1) | Casein (mM-1s-1) |
|---|---|---|---|
| 5 | 342 | 338 | 836 |
| 14 | 385 | 389 | 879 |
| 22 | 460 | 435 | 973 |
For particles of the same size, we compared the r2 values to assess the impact from coatings. For ZDS coated Fe5C2 nanoparticles, we found that the r2 values were overall comparable to phospholipid-coated ones (Table 2). With the casein coating, however, we observed striking r2 increase (>200%) for particles of all the three sizes (Table 2). In particular, the casein-coated 22 nm Fe5C2 nanoparticles exhibited an exceptional r2 of 973 mM-1s-1 (per Fe basis), which is one of the highest for all T2 probes reported (Figure 2B).
Cell uptake and cytotoxicity
Cellular uptake and toxicity were studied with RAW264.7 cells (a murine macrophage cell line). Figure 3A shows representative Prussian blue staining images for cells incubated with casein-coated 22 nm Fe5C2 nanoparticles of different concentrations (incubation time was 4 h). Clearly, more particles were internalized when the initial particle concentration was increased. When the starting iron content was higher than 20 µg/mL, more than 80% cells in the scope were heavily laden with iron (Figure 3A).
Figure 3.
Cellular uptake and cytotoxicity studies. A) Representative Prussian blue staining images of RAW264.7 cells labeled with casein coated Fe5C2 nanoparticles. The starting particle concentration was increased from 0 to 100 µg Fe/mL and the incubation lasted for 4 h. Scale bars: 100 µm. B) Quantitative cell uptake data, measured by ICP-MS. The starting concentration was 50 µg/mL. The uptake was slightly higher for 22 nm nanoparticles. Meanwhile, little difference was observed among particles of the same core size but different coatings. C) MTT assays with phospholipid coated Fe5C2 nanoparticles using RAW264.7 macrophages. The cells retained over 85% viability at the tested concentrations (0 - 100 µg Fe/mL). D) MTT assays with 22 nm Fe5C2 nanoparticles of different coatings. Comparable viability was observed among the three coatings. All of the formulations showed over 85% viability in the tested concentrations.
Quantitative cell uptake analysis was performed using inductively coupled plasma-mass spectrometery (ICP-MS). The starting particle concentration was set as 10 µg Fe/mL, and the uptake in pg Fe per cell was compared among formulations of different sizes and coatings. For particles of the same size, no significant difference in uptake was found among the three coatings (Figure 3B). Meanwhile, the particle size has some but no dramatic impact on the cell uptake. Taking casein coated Fe5C2 nanoparticle for instance, 22 nm Fe5C2 nanoparticles exhibit an uptake of 13.86 pg Fe/cell, compared to that of 13.05 and 10.55 pg Fe/cell for the 14 nm and 5 nm ones, respectively (Figure 3B). This level of Fe loading is in general comparable to that observed with Fe3O4 nanoparticles 16. The nanoparticles induced comparable or even slightly enhanced contrast ability within cells (Figure S4).
Despite of the high iron loading, the cells remain overall healthy. Even at 100 µg Fe/mL, cells maintained ~ 90% viability, regardless of the size and coating (Figures 3B&3C). Notably, in surface modification, we took extra washing steps to remove residual CTAB from the synthesis (Experimental section). That, we believe, is responsible for the increase of cell tolerance relative to what was reported by us previously 15.
in vivo MRI
Based on the cell uptake and relaxivity results, it is determined that casein-coated Fe5C2 nanoparticles are the most promising contrast probes. We next set out to investigate these nanoparticles' in vivo performances in healthy nude mice. All three size (5, 14, and 22 nm) casein-coated Fe5C2 nanoparticles were intravenously (i.v.) injected (2.5 mg Fe/kg, n = 3). Sagittal T2-weighted MR images were acquired before and 1 and 4 h after the injection (Figure 4A). For comparison, casein coated 15 nm IONPs were injected as a control (Ocean Nanotech Inc, r2 was 268 mM-1s-1, Figure S5A&B). In all animal groups, a significant drop of signal intensity was observed in the liver at 1 h after injection. The hypointensities were maintained but less prominent at 4 h. To quantitatively analyze the signal change caused the nanoparticles. Specifically, we calculated the change in T2 (ΔT2%) using the equation ΔT2% = (T2pre - T2post/T2pre) * 100%. For Fe5C2 nanoparticles, there was a clear size effect on the hypointensity induced, with ΔT2% at 1 h being 43.9 ± 1.4%, 70.8 ± 1.7%, and 82.9 ± 1.8%, respectively, for 5, 14, and 22 nm Fe5C2 particles, and 37.9 ± 0.9%, 62.6 ± 1.1%, and 73.3 ± 1.0% at 4 h. All the three formulations outperformed Fe3O4 nanoparticles, which showed a ΔT2% of 48.8 ± 1.0% and 33.1 ± 0.7% for the 1 h and 4 h time points, respectively (Figure 4B). The enhanced contrast effect was mostly attributed to the high r2 of the Fe5C2 nanoparticles. After the 4 h imaging, we euthanized the animals and performed Prussian blue staining with tissue samples. There was a large amount of particles accumulated in the liver and spleen, which was attributed to the particle uptake by Küpffer cells and splenocytes. Interestingly, for 5 nm Fe5C2 nanoparticles, extensive positive staining was observed in the kidneys (Figure 4C). This is attributed to the relatively small size of the particles and indicates possible renal clearance of them. Meanwhile, no detectable damage to the tissues was observed.
Figure 4.
MR imaging and in vivo particle distribution. A) MR imaging results. Normal athymic nude mice were intravenously injected with casein-coated Fe5C2 (5 nm, 14 nm, 22 nm) or casein-coated 20 nm Fe3O4 nanoparticles. MRI scans were performed on a 7T magnet pre- and 1 hr, and 4 hrs after the injection. Darkening of livers appear prevalent in all the animals. The 22 nm Fe5C2 exhibited the most significant contrast among all of the formulations. B) Quantification of liver contrast changes. 14 and 22 nm Fe5C2 nanoparticles induced more significant signal drop in the liver than Fe3O4 nanoparticles did at 1 and 4 h time points (*P < 0.05). C) Prussian blue staining with tissue samples from the liver, kidney, and spleen. Positive staining was found across the liver and spleen. For 5 nm Fe5C2 nanoparticles, positive staining was also found in the kidneys, which was likely attributed to the small size of the particles. Scale bars, 10 µm.
Discussion and Conclusion
With high magnetization and good stability in ambient conditions, Fe5C2 nanoparticles hold great promise as a novel type of MRI contrast agent for liver imaging. To this end, it is important to optimize the size and surface features of the particles so as to achieve desired contrast ability, toxicity, relaxivity, and macrophage uptake. In the present study, we modified the synthetic approach to prepare Fe5C2 nanoparticles of different sizes. Based on the consideration that there is a layer of iron oxide shell on the Fe5C2 core, we adopted three surface modification methods used previously for IONPs to modify Fe5C2 nanoparticles. We showed that all the three methods are adequate to grant Fe5C2 nanoparticles with good aqueous stability. The size and coating have varied levels of impact on the particles' size, cellular uptake, in vivo contrast abilities, and in particular, their r2 relaxivity. These observations provide useful information for future engineering of Fe5C2 nanoparticles for either diagnosis or therapy purposes.
The in vivo MRI studies confirm the advantages of Fe5C2 nanoparticles over IONPs in the liver imaging. At the same Fe concentration, Fe5C2 nanoparticles can more efficiently induce hypointensities to the liver, which is mainly attributed to the high r2 relaxivity. An interesting observation is the accumulation of 5 nm Fe5C2 nanoparticles in the kidneys. This suggests possible renal clearance of Fe5C2 particles of small sizes, which may serve as an advantage in other imaging applications. For instance, for targeted cancer imaging, it is preferred that the unbound nanoparticles are rapidly cleared from the host 38. In this scenario, the 5 nm Fe5C2 nanoparticles, which provide comparable relaxivity but efficient renal clearance, may be a better option than the 22 nm formulation. This possibility will be assessed in our future studies.
The exceptionally high r2 relaxivity of Fe5C2 nanoparticles is intriguing. By definition, T2 contrast agents interact with nearby water molecules by inducing a local magnetic field in which the transverse relaxation (T2) of water is shortened. This manifests as a relatively “dark” area on T2 weighted images. The outer-sphere relaxivity, which describes the relaxation behavior of water molecules surrounding the contrast agents, is one of the most important contributors to MRI contrast. According to the outer-sphere model of transverse relaxation 39, the r2 relaxivity is proportional to particle magnetization (M) 40, 41. This explains the high r2 of Fe5C2 nanoparticles relative to IONPs. For Fe5C2 nanoparticles of the same coating, there was a clear size effect on r2. This is attributed to the surface canting effect which was previously observed with IONPs 37. A more interesting observation is a dramatic r2 increase found with casein coated nanoparticles. This is attributed to the impact of the casein coating on the water diffusion correlation time (τD), which is also proportional to r2 42. Casein is essentially a family of phosphoproteins consisting of four members, αS1, αS2, β, and κ caseins. κ-casein, which is the most soluble variant of the four, is believed to play a most contributing role to the enhanced r2. It is known that κ-casein has a unique, elongated “hair-like” structure 43; when coated onto nanoparticles, κ-casein may form a protein layer presenting long, hydrophilic channels, which enable water molecules to come in and interact with the inner water layer that is close to particle surface. The abundant surface hydroxyl and amides of casein may help promote fast proton exchange with water molecules. Meanwhile, the protein coating increases the overall particle size and restricts fast water diffusion. These factors all together lead to an enhanced τD, leading to increase of apparent r2. More investigations are under way to further elucidate the surface impact of casein, and hopefully, to guide the future design and engineering of magnetic probes with high r2 relaxivity.
Overall, we have prepared Fe5C2 nanoparticles of different sizes and surface coatings. We found that both size and surface have an impact on the particles performance as T2 contrast agents. In particular, with casein coated 22 nm Fe5C2 nanoparticles, we observed a striking r2 of 973 mM-1s-1, making it one of the highest reported T2 contrast agents to date. The particles showed low toxicity and they outperformed IONPs in inducing hypointensities to the liver. These observations confirm the great potential of Fe5C2 nanoparticles as a novel type of MRI contrast agents.
Supplementary Material
Figures S1-S5.
Acknowledgments
We thank Pradip Basnet from Dr. Yiping Zhao's group for his assistance in XRD analysis. The research was supported by an NCI/NIH R00 grant (R00CA153772, J.X.), an Elsa U. Pardee Foundation Award (J.X.), and a UGA-GRU seed grant (J.X.).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975-2011. Howlader NN, Krapcho M, Garshell J, et al; http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 3.Ba-Ssalamah A, Uffmann M, Saini S. et al. Clinical value of MRI liver-specific contrast agents: a tailored examination for a confident non-invasive diagnosis of focal liver lesions. Euro Radiol. 2009;19:342–57. doi: 10.1007/s00330-008-1172-x. [DOI] [PubMed] [Google Scholar]
- 4.Cheon J, Lee JH. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Accounts Chem Res. 2008;41:1630–40. doi: 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]
- 5.Perilongo G, Shafford EA. Liver tumours. Eur J Cancer. 1999;35:953–8. doi: 10.1016/s0959-8049(99)00049-0. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M. Surveillance for hepatocellular carcinoma. Best Pract Res Cl Ga. 2014;28:783–93. doi: 10.1016/j.bpg.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Thian YL, Riddell AM, Koh DM. Liver-specific agents for contrast-enhanced MRI: role in oncological imaging. Cancer imaging. 2013;13:567–79. doi: 10.1102/1470-7330.2013.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longmaid HE 3rd, Dupuy DE, Kane RA. et al. Noninvasive liver imaging: new techniques and practical strategies. Semin Ultrasound CT. 1992;13:377–98. [PubMed] [Google Scholar]
- 9.Van Beers BE, Daire JL, Garteiser P. New imaging techniques for liver diseases. J Hepatol. 2015;3:690–700. doi: 10.1016/j.jhep.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Issadore D, Min C, Liong M. et al. Miniature magnetic resonance system for point-of-care diagnostics. Lab Chip. 2011;11:2282–7. doi: 10.1039/c1lc20177h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouliquen D, Le Jeune JJ, Perdrisot R. et al. Iron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolism. Magn Reson Imaging. 1991;9:275–83. doi: 10.1016/0730-725x(91)90412-f. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Jiang W, Luo K. et al. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595–615. doi: 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HY, Lee SH, Xu C. et al. Synthesis and characterization of PVP-coated large core iron oxide nanoparticles as an MRI contrast agent. Nanotechnology. 2008;19:165101. doi: 10.1088/0957-4484/19/16/165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowger T, Xie J. Polyaspartic acid coated iron oxide nanoprobes for PET/MRI imaging. Methods Mol Bio. 2013;1025:225–35. doi: 10.1007/978-1-62703-462-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang W, Zhen Z, Yang C. et al. Fe5C2 nanoparticles with high MRI contrast enhancement for tumor imaging. Small. 2014;10:1245–9. doi: 10.1002/smll.201303263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie J, Liu G, Eden HS. et al. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Accounts Chem Res. 2011;44:883–92. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi HM, Lin YP, Aslam M. et al. Effects of shape and size of cobalt ferrite nanostructures on their MRI contrast and thermal activation. J Phys Chem C, Nanomaterials and interfaces. 2009;113:17761–7. doi: 10.1021/jp905776g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadjipanayis CG, Bonder MJ, Balakrishnan S. et al. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4:1925–9. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Yang K, Cheng L. et al. PEGylated FePt@Fe2O3 core-shell magnetic nanoparticles: potential theranostic applications and in vivo toxicity studies. Nanomed-Nanotechnol. 2013;9:1077–88. doi: 10.1016/j.nano.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Teng X, Yang H. Iron oxide shell as the oxidation-resistant layer in SmCo5 @ Fe2O3 core-shell magnetic nanoparticles. J Nanosci Nanotechno. 2007;7:356–61. [PubMed] [Google Scholar]
- 21.Nocera TM, Chen J, Murray CB. et al. Magnetic anisotropy considerations in magnetic force microscopy studies of single superparamagnetic nanoparticles. Nanotechnology. 2012;23:495704. doi: 10.1088/0957-4484/23/49/495704. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Wu J, Liu F. et al. Controlled synthesis of FePt-Au hybrid nanoparticles triggered by reaction atmosphere and FePt seeds. Nanoscale. 2013;5:9141–9. doi: 10.1039/c3nr02911e. [DOI] [PubMed] [Google Scholar]
- 23.Horev-Azaria L, Baldi G, Beno D. et al. Predictive toxicology of cobalt ferrite nanoparticles: comparative in-vitro study of different cellular models using methods of knowledge discovery from data. Part Fibre Toxicol. 2013;10:32. doi: 10.1186/1743-8977-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C, Zhao H, Hou Y. et al. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis. J Am Chem Soc. 2012;134:15814–21. doi: 10.1021/ja305048p. [DOI] [PubMed] [Google Scholar]
- 25.Tong S, Hou S, Ren B. et al. Self-assembly of phospholipid-PEG coating on nanoparticles through dual solvent exchange. Nano Lett. 2011;11:3720–6. doi: 10.1021/nl201978c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Wang J. Effects of DMSA-coated Fe3O4 nanoparticles on the transcription of genes related to iron and osmosis homeostasis. Toxicol Sci. 2013;131:521–36. doi: 10.1093/toxsci/kfs300. [DOI] [PubMed] [Google Scholar]
- 27.Wei H, Insin N, Lee J. et al. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett. 2012;12:22–5. doi: 10.1021/nl202721q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan Q, Xie J, Gao H. et al. HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy. Mol Pharm. 2011;8:1669–76. doi: 10.1021/mp200006f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Xie J, Zhou X. et al. Ferritin enhances SPIO tracking of C6 rat glioma cells by MRI. Mol Imaging Bio. 2011;13:87–93. doi: 10.1007/s11307-010-0338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Wang L, Lin R. et al. Casein-coated iron oxide nanoparticles for high MRI contrast enhancement and efficient cell targeting. ACS Appl Mater Interfaces. 2013;5:4632–9. doi: 10.1021/am400713j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakulkhu U, Mahmoudi M, Maurizi L. et al. Protein corona composition of superparamagnetic iron oxide nanoparticles with various physico-chemical properties and coatings. Sci Rep. 2014;4:5020. doi: 10.1038/srep05020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin MN, Allen AJ, MacCuspie RI. et al. Dissolution, agglomerate morphology, and stability limits of protein-coated silver nanoparticles. Langmuir. 2014;30:11442–52. doi: 10.1021/la502973z. [DOI] [PubMed] [Google Scholar]
- 33.Juneja R, Roy I. Surface modified PMMA nanoparticles with tunable drug release and cellular uptake. Rsc Adv. 2014;4:44472–9. [Google Scholar]
- 34.Tong S, Hou S, Zheng Z. et al. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010;10:4607–13. doi: 10.1021/nl102623x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Z, Zhou Z, Bao J. et al. Octapod iron oxide nanoparticles as high-performance T(2) contrast agents for magnetic resonance imaging. Nat Commun. 2013;4:2266. doi: 10.1038/ncomms3266. [DOI] [PubMed] [Google Scholar]
- 36.Wei H, Bruns OT, Chen O. et al. Compact zwitterion-coated iron oxide nanoparticles for in vitro and in vivo imaging. Integr Biol (Camb) 2013;5:108–14. doi: 10.1039/c2ib20142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baaziz W, Pichon BP, Fleutot S. et al. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J Phys Chem C. 2014;118:3795–810. [Google Scholar]
- 38.Choi HS, Liu W, Misra P. et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattoussi H, Cheon J. Inorganic nanoprobes for biological sensing and imaging. Boston: Artech House; 2009. [Google Scholar]
- 40.Gillis P, Koenig SH. Transverse relaxation of solvent protons induced by magnetized spheres: application to ferritin, erythrocytes, and magnetite. Magn Reson Med. 1987;5:323–45. doi: 10.1002/mrm.1910050404. [DOI] [PubMed] [Google Scholar]
- 41.Koenig SH, Gillis P. Transverse relaxation (1/T2) of solvent protons induced by magnetized spheres and its relevance to contrast enhancement in MRI. Invest Radiol. 1988;23(Suppl 1):S224–8. doi: 10.1097/00004424-198809001-00046. [DOI] [PubMed] [Google Scholar]
- 42.Koenig SH, Kellar KE. Theory of 1/T1 and 1/T2 NMRD profiles of solutions of magnetic nanoparticles. Magn Reson Med. 1995;34:227–33. doi: 10.1002/mrm.1910340214. [DOI] [PubMed] [Google Scholar]
- 43.Richardson BC, Creamer LK, Munford RE. Comparative micelle structure. I. The isolation and chemical characterization of caprine kappa-casein. Biochim Biophys Acta. 1973;310:111–7. doi: 10.1016/0005-2795(73)90013-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S5.