Abstract
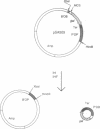
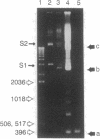
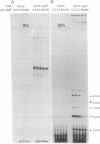
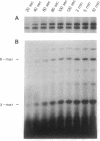
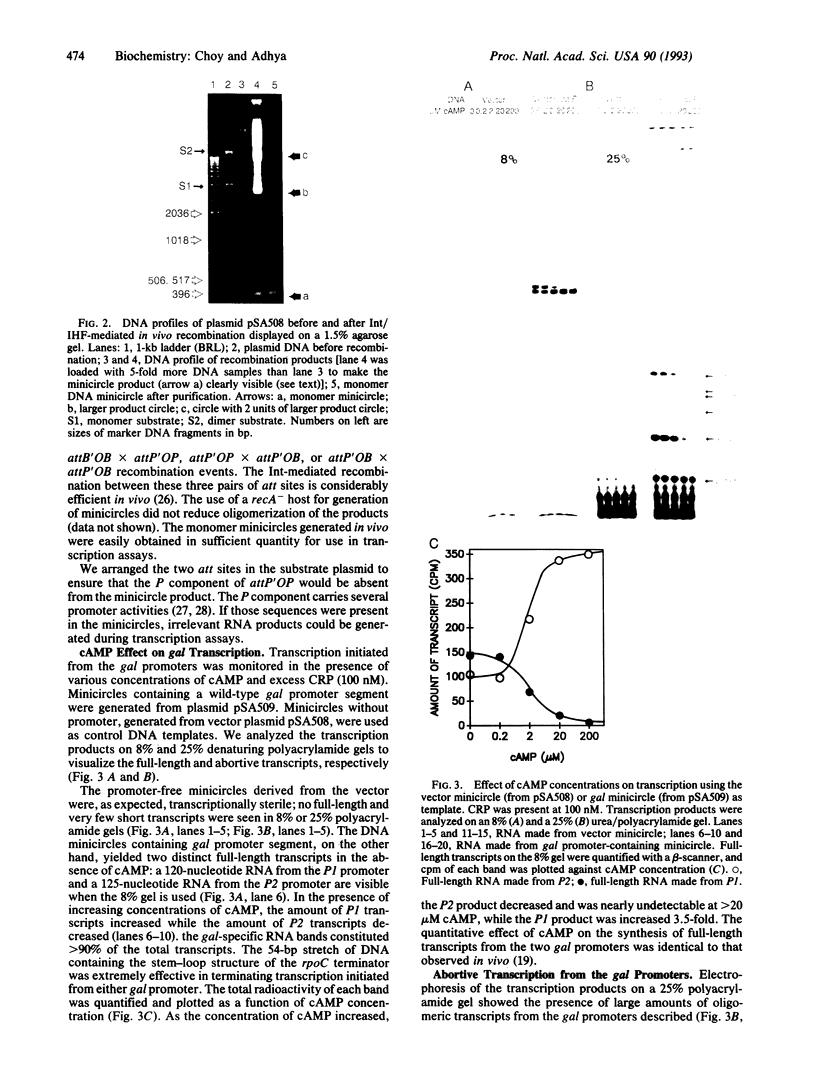
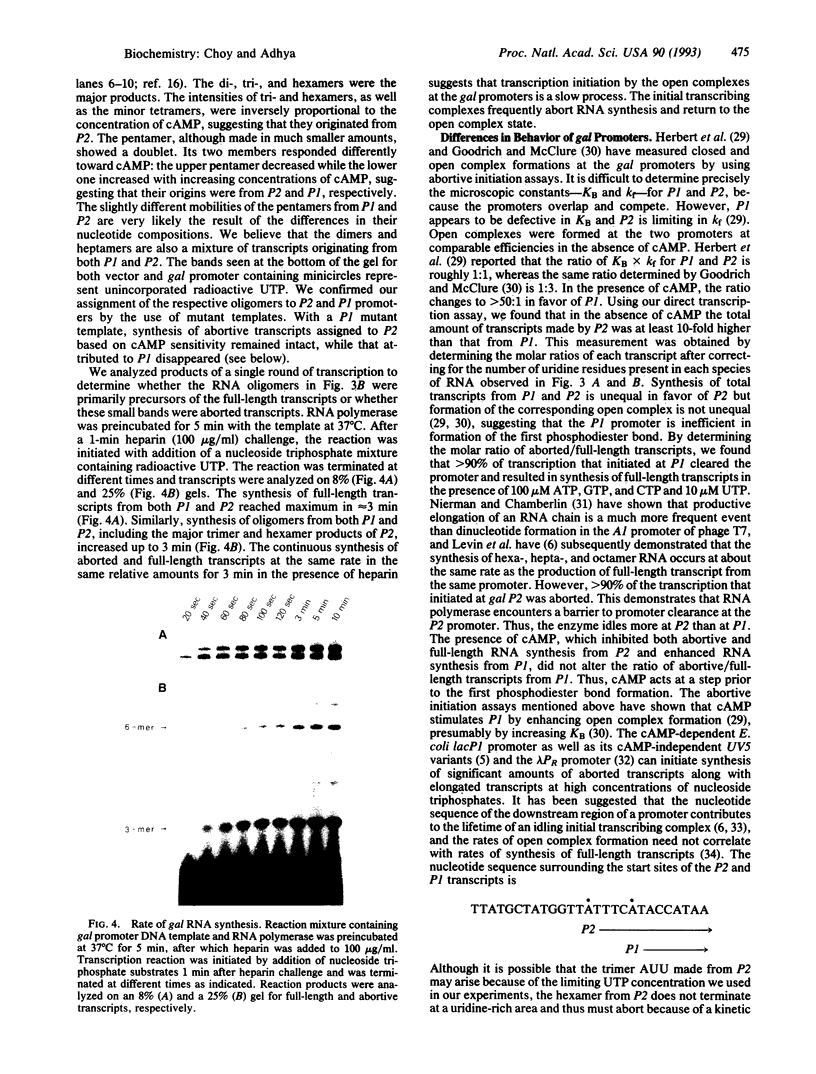
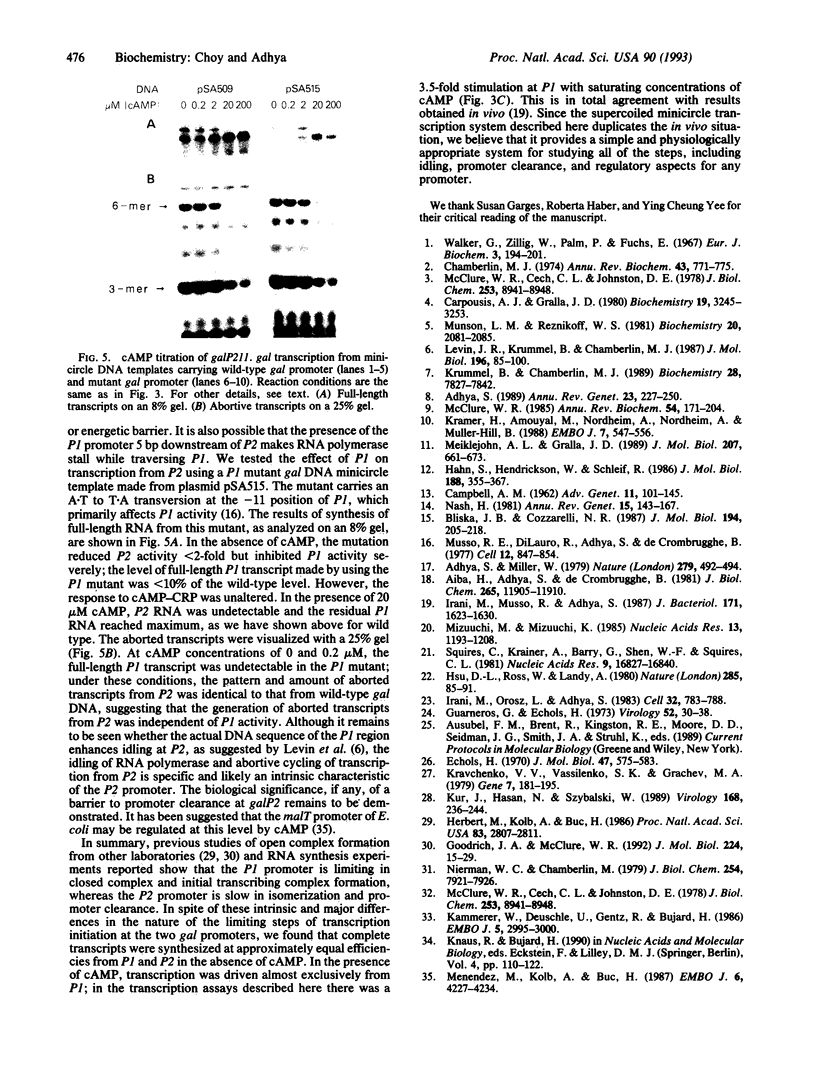
We have developed an in vivo system to engender supercoiled "minicircle" DNA containing a single promoter by using the integrative recombination system of bacteriophage lambda. The resulting minicircle templates allow quantitative analysis of the stages of transcription initiation from a promoter, including synthesis of both full-length and aborted transcripts in the same reactions under physiological conditions. We have used such minicircle DNA templates to study in vitro transcription of the Escherichia coli gal promoter. The full-length transcripts from gal P1 and P2 promoters responded to cAMP-cAMP receptor protein in a manner identical to that observed in vivo. There is a 3.5-fold stimulation of P1 and almost total inhibition of P2 in the presence of cAMP. Thus, the unitary promoter system described here duplicates the in vivo physiology. In spite of the synthesis in equimolar amounts of full-length transcripts from P1 and P2 in the absence of cAMP in vitro, as in vivo, RNA polymerase encountered different rate-limiting steps of transcription initiation at the two promoters.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Miller W. Modulation of the two promoters of the galactose operon of Escherichia coli. Nature. 1979 Jun 7;279(5713):492–494. doi: 10.1038/279492a0. [DOI] [PubMed] [Google Scholar]
- Adhya S. Multipartite genetic control elements: communication by DNA loop. Annu Rev Genet. 1989;23:227–250. doi: 10.1146/annurev.ge.23.120189.001303. [DOI] [PubMed] [Google Scholar]
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Echols H. Integrative and excisive recombination by bacteriophage lambda: evidence for an excision-specific recombination protein. J Mol Biol. 1970 Feb 14;47(3):575–583. doi: 10.1016/0022-2836(70)90324-4. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., McClure W. R. Regulation of open complex formation at the Escherichia coli galactose operon promoters. Simultaneous interaction of RNA polymerase, gal repressor and CAP/cAMP. J Mol Biol. 1992 Mar 5;224(1):15–29. doi: 10.1016/0022-2836(92)90573-3. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Echols H. Thermal asymmetry of site-specific recombination by bacteriophage lambda. Virology. 1973 Mar;52(1):30–38. doi: 10.1016/0042-6822(73)90395-4. [DOI] [PubMed] [Google Scholar]
- Hahn S., Hendrickson W., Schleif R. Transcription of Escherichia coli ara in vitro. The cyclic AMP receptor protein requirement for PBAD induction that depends on the presence and orientation of the araO2 site. J Mol Biol. 1986 Apr 5;188(3):355–367. doi: 10.1016/0022-2836(86)90160-9. [DOI] [PubMed] [Google Scholar]
- Herbert M., Kolb A., Buc H. Overlapping promoters and their control in Escherichia coli: the gal case. Proc Natl Acad Sci U S A. 1986 May;83(9):2807–2811. doi: 10.1073/pnas.83.9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. L., Ross W., Landy A. The lambda phage att site: functional limits and interaction with Int protein. Nature. 1980 May 8;285(5760):85–91. doi: 10.1038/285085a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Irani M., Musso R., Adhya S. Cyclic-AMP-dependent switch in initiation of transcription from the two promoters of the Escherichia coli gal operon: identification and assay of 5'-triphosphate ends of mRNA by GTP:RNA guanyltransferase. J Bacteriol. 1989 Mar;171(3):1623–1630. doi: 10.1128/jb.171.3.1623-1630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer W., Deuschle U., Gentz R., Bujard H. Functional dissection of Escherichia coli promoters: information in the transcribed region is involved in late steps of the overall process. EMBO J. 1986 Nov;5(11):2995–3000. doi: 10.1002/j.1460-2075.1986.tb04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko V. V., Vasilenko S. K., Grachev M. A. A rightward promoter to the left of the att site of lambda phage DNA: possible participant in site-specific recombination. Gene. 1979 Nov;7(3-4):181–195. doi: 10.1016/0378-1119(79)90045-3. [DOI] [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. RNA chain initiation by Escherichia coli RNA polymerase. Structural transitions of the enzyme in early ternary complexes. Biochemistry. 1989 Sep 19;28(19):7829–7842. doi: 10.1021/bi00445a045. [DOI] [PubMed] [Google Scholar]
- Krämer H., Amouyal M., Nordheim A., Müller-Hill B. DNA supercoiling changes the spacing requirement of two lac operators for DNA loop formation with lac repressor. EMBO J. 1988 Feb;7(2):547–556. doi: 10.1002/j.1460-2075.1988.tb02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kur J., Hasan N., Szybalski W. Repression of transcription from the b2-att region of coliphage lambda by integration host factor. Virology. 1989 Feb;168(2):236–244. doi: 10.1016/0042-6822(89)90263-8. [DOI] [PubMed] [Google Scholar]
- Levin J. R., Krummel B., Chamberlin M. J. Isolation and properties of transcribing ternary complexes of Escherichia coli RNA polymerase positioned at a single template base. J Mol Biol. 1987 Jul 5;196(1):85–100. doi: 10.1016/0022-2836(87)90512-2. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Cech C. L., Johnston D. E. A steady state assay for the RNA polymerase initiation reaction. J Biol Chem. 1978 Dec 25;253(24):8941–8948. [PubMed] [Google Scholar]
- McClure W. R., Cech C. L., Johnston D. E. A steady state assay for the RNA polymerase initiation reaction. J Biol Chem. 1978 Dec 25;253(24):8941–8948. [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Meiklejohn A. L., Gralla J. D. Activation of the lac promoter and its variants. Synergistic effects of catabolite activator protein and supercoiling in vitro. J Mol Biol. 1989 Jun 20;207(4):661–673. doi: 10.1016/0022-2836(89)90236-2. [DOI] [PubMed] [Google Scholar]
- Menendez M., Kolb A., Buc H. A new target for CRP action at the malT promoter. EMBO J. 1987 Dec 20;6(13):4227–4234. doi: 10.1002/j.1460-2075.1987.tb02771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi M., Mizuuchi K. The extent of DNA sequence required for a functional bacterial attachment site of phage lambda. Nucleic Acids Res. 1985 Feb 25;13(4):1193–1208. doi: 10.1093/nar/13.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson L. M., Reznikoff W. S. Abortive initiation and long ribonucleic acid synthesis. Biochemistry. 1981 Apr 14;20(8):2081–2085. doi: 10.1021/bi00511a003. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Nierman W. C., Chamberlin M. J. Studies of RNA chain initiation by Escherichia coli RNA polymerase bound to T7 DNA. Direct analysis of the kinetics and extent of RNA chain initiation at T7 promoter A1. J Biol Chem. 1979 Aug 25;254(16):7921–7926. [PubMed] [Google Scholar]
- Walter G., Zillig W., Palm P., Fuchs E. Initiation of DNA-dependent RNA synthesis and the effect of heparin on RNA polymerase. Eur J Biochem. 1967 Dec;3(2):194–201. doi: 10.1111/j.1432-1033.1967.tb19515.x. [DOI] [PubMed] [Google Scholar]