Big embryo 1 encodes a trans-Golgi localized MATE transporter required for regulation of lateral organ size and initiation rate in plants.
Abstract
Genetic networks that determine rates of organ initiation and organ size are key regulators of plant architecture. Whereas several genes that influence the timing of lateral organ initiation have been identified, the regulatory pathways in which these genes operate are poorly understood. Here, we identify a class of genes implicated in regulation of the lateral organ initiation rate. Loss-of-function mutations in the MATE transporter encoded by maize (Zea mays) Big embryo 1 (Bige1) cause accelerated leaf and root initiation as well as enlargement of the embryo scutellum. BIGE1 is localized to trans-Golgi, indicating a possible role in secretion of a signaling molecule. Interestingly, phenotypes of bige1 bear striking similarity to cyp78a mutants identified in diverse plant species. We show that a CYP78A gene is upregulated in bige1 mutant embryos, suggesting a role for BIGE1 in feedback regulation of a CYP78A pathway. We demonstrate that accelerated leaf formation and early flowering phenotypes conditioned by mutants of Arabidopsis thaliana BIGE1 orthologs are complemented by maize Bige1, showing that the BIGE1 transporter has a conserved function in regulation of lateral organ initiation in plants. We propose that BIGE1 is required for transport of an intermediate or product associated with the CYP78A pathway.
INTRODUCTION
Lateral organ size, number, and shape are key determinants of plant architecture. Formation of lateral organs is coordinated by multiple signaling networks that regulate meristem function. Over the past decade, key genes that are critical nodes of genetic networks regulating lateral organ formation have been identified (reviewed in Bar and Ori, 2014; Kalve et al., 2014; Vilches-Barro and Maizel, 2015). A remarkable characteristic of these networks is that maintenance and determination of cell fate is partly regulated by several distinct classes of mobile signals. Signaling molecules implicated in plant meristem function include classic hormones and peptides, as well as microRNAs that move between cell layers to regulate morphogenesis of lateral organs (Galinha et al., 2009; Murphy et al., 2012; Benkovics and Timmermans, 2014).
Leaves and other lateral organs are formed initially from groups of undifferentiated meristem cells. Classical hormones play key roles in this process. Auxin, mobilized by the PIN-FORMED1 (PIN1) transporter, promotes leaf initiation, whereas cytokinin and gibberellins are thought to fine tune organ formation by interacting with auxin signals (Galinha et al., 2009). Key downstream regulators include KNOTTED1-type transcription factors that repress leaf initiation and maintain meristem fate (Vollbrecht et al.,1991) and ABPHYL1 two-component regulators that mediate phyllotaxy (Giulini et al., 2004) in maize (Zea mays). These regulators in turn interact with ARF, IAA, and PIN1 genes involved in auxin signaling (Lee et al., 2009; Bolduc et al., 2012). Lateral root development is also mediated by auxin and cytokinin signals. Maize seedlings and adult plants form several types of lateral roots, including seminal roots, adventitious roots, and brace roots (Hochholdinger and Tuberosa, 2009), and genes that function in hormone biosynthesis and signaling have been implicated in lateral root formation (Hochholdinger et al., 2004).
Thus far, three classes of genes that regulate the rate and timing of leaf initiation have been identified in Arabidopsis thaliana, rice (Oryza sativa), and maize. The first class includes orthologs of maize terminal ear 1 (te1). The te1 mutant produces leaves more rapidly than the wild type, with an altered phyllotaxy (Matthews et al., 1974; Veit et al., 1998). The orthologous plastochron 2 (pla2) mutant of rice has a similar plastochron phenotype albeit with species-specific differences (Kawakatsu et al., 2006). Te1 and PLA2 each encode a protein with RNA recognition motifs (Veit et al., 1998; Kawakatsu et al., 2006). Because PLA2 is expressed almost exclusively at the site of leaf initiation, it is proposed that PLA2 interacts with an inhibitory field generated by existing leaf primordia in accordance with the Hofmeister Rule (Kawakatsu et al., 2006). The second class of organ initiation genes is identified from the Arabidopsis altered meristem program 1 (amp1), rice pla3, and maize viviparous 8 (vp8) mutants that show striking acceleration of leaf initiation rates (Chaudhury et al., 1993; Conway and Poethig, 1997; Evans and Poethig, 1997; Kawakatsu et al., 2009). The corresponding genes, which are orthologous and encode proteins related to mammalian membrane-localized glutamate carboxypeptidases, are proposed to function in biosynthesis or turnover of an unidentified signaling molecule involved in regulation of leaf initiation (Helliwell et al., 2001; Suzuki et al., 2008; Kawakatsu et al., 2009). Interestingly, a recent study has shown that AMP1 regulates microRNA-mediated translation inhibition (Li et al., 2013).
A third class of mutants that have rapid leaf initiation phenotypes includes CYP78A P450 genes. Leaf number of the rice pla1 mutant is increased 2-fold due to a shortened plastochron (Itoh et al., 1998). Arabidopsis kluh (klu), which was originally isolated as a mutant with small floral organ size (Anastasiou et al., 2007), causes a similar acceleration of leaf initiation (Wang et al., 2008). Mutations in a barley (Hordeum vulgare) CYP78A gene result in shortened plastochron (Mascher et al., 2014). Interestingly, effects of CYP78A mutants on organ size are typically dosage sensitive. Overexpression of KLU increases leaf size, while the loss-of-function mutants have smaller leaves (Anastasiou et al., 2007). In tomato (Solanum lycopersicum), quantitative trait locus analysis indicates that dosage of a CYP78A gene may determine fruit size (Chakrabarti et al., 2013). In addition to these phenotypes, non-cell-autonomous functions of KLU have been described in flower and seed (Anastasiou et al., 2007; Adamski et al., 2009; Eriksson et al., 2010). As originally proposed by Anastasiou et al. (2007), the CYP78A pathway likely generates a mobile signal that has not yet been identified.
CYP78A signaling is also implicated in seed development where the cotyledons and scutellum of dicot and monocot embryos, respectively, may be classified as lateral organs. Giant Embryo (GE) of rice is unique in specifically affecting scutellum size (Hong et al., 1996). GE encodes a CYP78A protein that is closely related to PLA1 and KLU (Nagasawa et al., 2013; Yang et al., 2013). In maize, quantitative trait locus analysis has implicated GE2 in determination of the embryo to endosperm ratio (Zhang et al., 2012). In Arabidopsis, a cyp78a5 cyp78a7 double mutant embryo has an increased number of cotyledons (Wang et al., 2008). Intriguingly, as in the case of the klu mutant, ge has non-cell-autonomous functions. Regulation of embryo and endosperm size involves an interaction between embryo and endosperm (Nagasawa et al., 2013). While the molecular basis of the nonautonomous signaling mediated by KLU and GE CYP78A proteins remains elusive, these phenotypes implicate production of a diffusible signaling molecule.
Here, we show that the maize big embryo 1 (bige1) mutant identifies a new class of genes that regulate the rate of lateral organ initiation, mutation of which results in increased leaf and lateral root number as well as large embryo size. In the bige1 mutant, increased embryo size is attributed to an accelerated transition to the cell expansion phase of scutellum development in the embryo without affecting overall kernel size. In addition to having increased lateral organ number, mutant plants flower earlier than the wild type. We show that Bige1 encodes a MATE-type transporter that is conserved in Arabidopsis. The broad similarity of the bige1 phenotype to the cyp78a mutants in Arabidopsis and rice suggests that bige1 may function in the CYP78A signaling pathway. Moreover, localization of BIGE1 to the proximal, trans-Golgi suggests that it may be specifically required for secretion of the as yet unidentified product of the CYP78A pathway. Consistent with that hypothesis, we show that a maize CYP78A gene, GE1, is upregulated in the bige1 mutant, implying that BIGE1 is required for feedback regulation of the CYP78A gene.
RESULTS
Seed Phenotypes of the bige1 Mutant
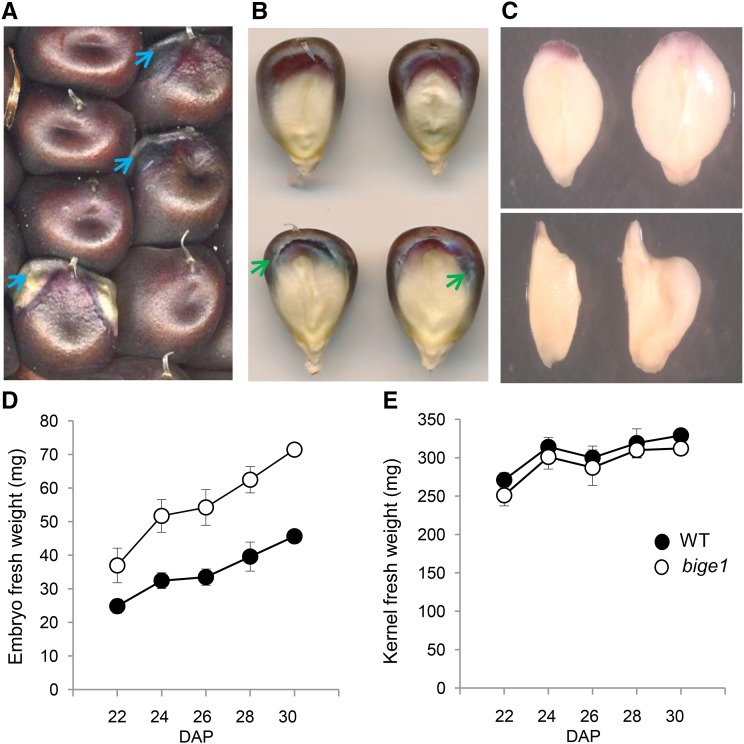
We initially isolated bige1 (bige1-umu1) as a recessive mutation in a screen of the UniformMu maize transposon population (McCarty et al., 2005) for embryo size mutants. The mutant kernels displayed an increase in the area of the embryo visible on germinal face of the kernel (Figure 1). On fully pollinated ears of wild-type W22 inbred, embryos are typically not visible unless adjacent kernels are removed. By contrast, embryos of bige1 mutant kernels could be readily discerned due to lateral outgrowth of the scutellum (Figure 1A). Mutant and wild-type kernels segregating on ears of self-pollinated heterozygotes were comparable in size at maturity. Inspection of the individual seeds confirmed that the bige1 mutant seeds have larger embryo surface area than the wild type (Figure 1B). The bige1 mutant seed also exhibited a distinctive pattern of intensified anthocyanin accumulation in aleurone cells that surround the embryo-endosperm interface. In extreme cases, outgrowth of the mutant embryo caused physical disruption of the normally smooth embryo-endosperm interface resulting in anthocyanin deficiency in endosperm near the embryo margin (Figure 1A, lower mutant kernel). Embryos excised from mature fresh mutant kernels were visibly larger than wild-type siblings (Figure 1C). Mutant embryos were both longer and wider than wild-type embryos. The size difference in bige1 embryos was attributed primarily to significant enlargement of the scutellum, the main lateral organ of the embryo. While mutant embryos could be distinguished from the wild type based on size as early as 12 and 15 d after pollination (DAP) in plants grown in spring and fall seasons, respectively, bige1 embryo size and fresh weight increased more rapidly than the wild type throughout development (Figures 1D and 1E). By contrast, fresh weights of wild-type and mutant kernels differed only slightly, indicating that the increase in embryo size was compensated for by a reduction in endosperm size in the mutant kernel. These results indicated that Bige1 function is required to coordinate growth of embryo and endosperm.
Figure 1.
The bige1 Mutant Has Increased Embryo Size Compensated for by a Proportional Decrease in Endosperm Size
(A) Mature wild-type and bige1 mutant seed segregating on an ear of a self-pollinated heterozygote. Blue arrows indicate margins of embryos visible on the mutant seeds.
(B) Germinal face views of wild-type (top) and bige1-umu1 mutant (bottom) kernels. Green arrows indicate regions of intense pigmentation at the interface between endosperm and embryo in bige1 seeds.
(C) Excised fresh mature embryos of the wild type (left) and bige1 (right) showing front (top) and side views (bottom).
(D) Fresh weight of developing embryos of the wild type and bige1 mutant excised from segregating ears at the indicated day after pollination.
(E) Fresh weight of wild-type and bige1 mutant whole seed recorded prior to dissection in (D). Error bars indicate se. All fresh weight differences between wild-type and bige1 embryos (D) and kernels (E) were statistically significant (P < 0.05; Student’s t test), except kernel fresh weight at 28 DAP (P > 0.09).
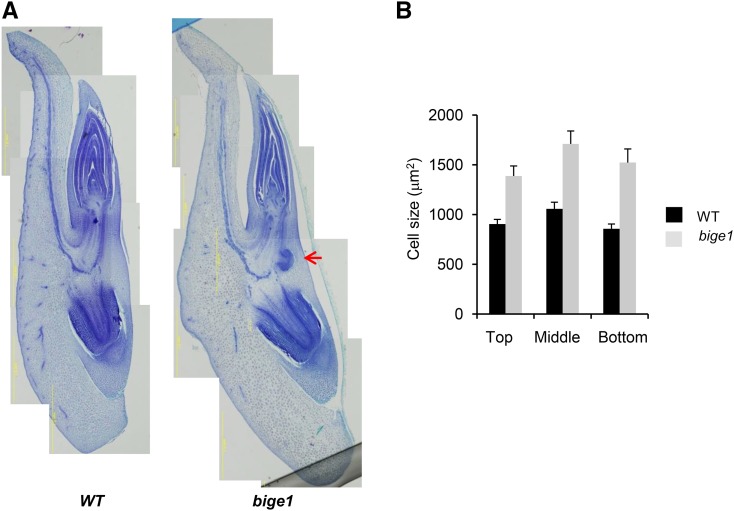
To gain insight into the cellular basis of the large-embryo phenotype, we conducted histological analysis of developing embryos (Figure 2). Analyses of maturation stage embryos (24 DAP) confirmed that the increased size and altered shape of the bige1 embryo (Figure 1C) was primarily attributable to enlargement of the scutellum (Figure 2A). Analysis of cell size in apical, middle, and basal regions of the scutellum (Supplemental Figure 1) indicated that the enlargement was due at least in part to increased cell size throughout the scutellum of bige1 embryos (Figure 2B). Flow cytometry analysis of nuclei isolated from developing scutellum at 22 DAP showed no difference in the ploidy profiles of wild-type and mutant embryos (Supplemental Figure 2), indicating that the increased cell size of the scutellum was not due to altered cell cycle regulation. In addition to increased scutellum cell size, sections of bige1 embryos revealed frequent precocious formation of lateral root primordia in the mesocotyl near the junction with the primary root.
Figure 2.
Morphology of Wild-Type and bige1 Mutant Embryos.
Developing embryos of wild-type and bige1-umu1 mutant seeds were dissected from ears of self-pollinated heterozygous plants at 24 DAP.
(A) Representative paraffin thin sections prepared from wild-type and mutant embryos. The red arrow indicates a precocious lateral root primordium.
(B) Cross-sectional area of cells (n = 52 for the wild type and n = 32 for bige1) located in the apical, middle, and basal regions of the scutellum, respectively, were measured from the digital images using ImageJ (Supplemental Figure 1). The difference in cell size between the wild type and bige1 mutant was significant by Student’s t test (P < 0.03).
Vegetative Phenotypes of bige1
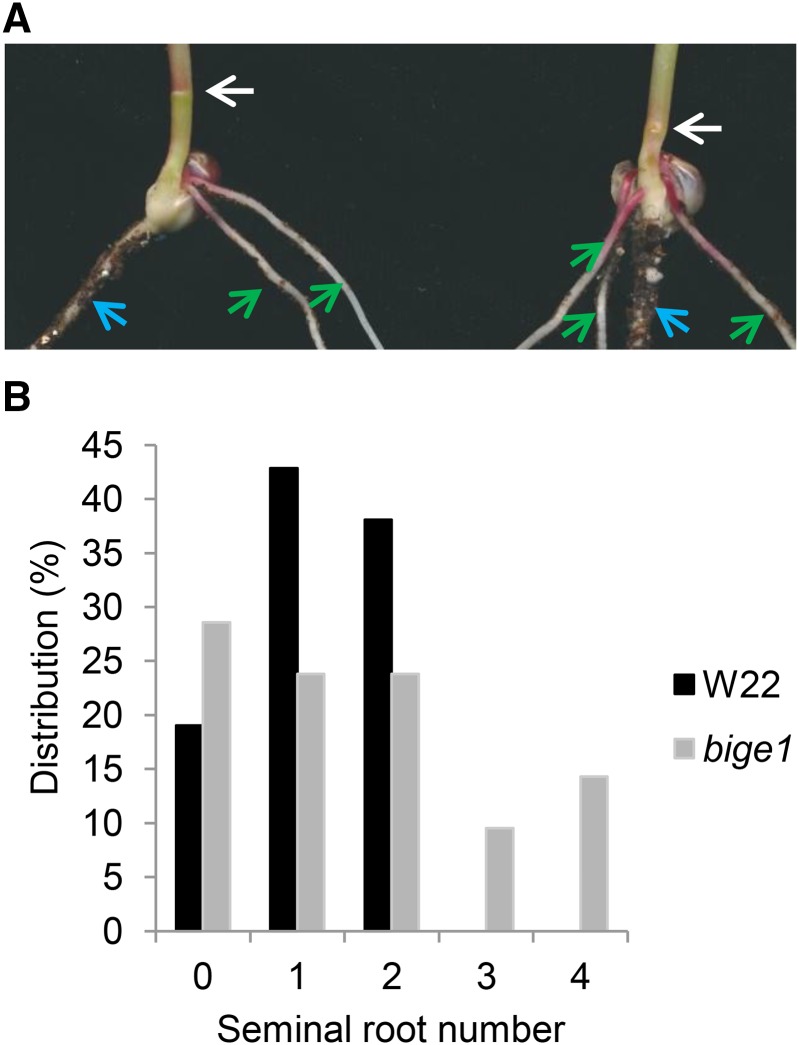
Homozygous bige1 kernels germinated to produce fully viable plants. Consistent with the precocious appearance of lateral root primordia in bige1 embryo sections noted above (Figure 2A), bige1 mutant seedlings exhibited increased numbers and accelerated production of seminal roots (Figure 3). At 5 d after sowing (DAS), mutant seedlings contained on average 1.6 roots and up to four seminal roots per seedling compared with an average of 1.2 roots and a maximum of two seminal roots per wild-type seedling. Furthermore, seminal roots formed earlier in development of mutant seedlings compared with the wild type, even though wild-type and mutant seeds germinated at similar rates (Supplemental Table 1). In addition to differences in root architecture, mesocotyl length differed in wild-type and bige1 mutant seedlings (Figure 3A). Wild-type seedlings at 5 DAS had a mean mesocotyl length of 10.6 mm (±0.37, n = 10) compared with 4.2 mm (±0.53, n = 10) in mutant seedlings. These phenotypes indicate that Bige1 acts during very early stages of postgermination development to suppress seminal root formation while promoting mesocotyl elongation.
Figure 3.
The bige1 Mutant Has Increased Seminal Root Number in Seedlings.
(A) Primary roots (blue arrow) and seminal adventitious roots (green arrow) of 5-d-old wild-type (left) and bige1-umu1 (right) seedlings (right). An emerging adventitious root initial is visible at the first node (white arrows) of the mutant seedling (right) but not in the wild-type seedling (left).
(B) Histogram showing the distribution of seminal root numbers observed in 5-d-old seedlings of the wild type and mutant (n = 21).
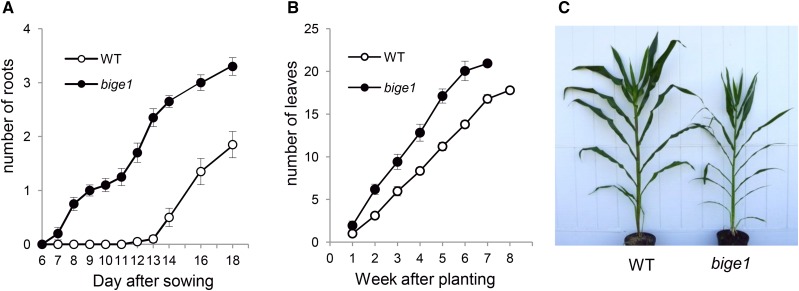
Excess formation of adventitious roots was also detected at later stages of seedling development. By 7 DAS, adventitious roots were visible at the first node of bige1 mutant seedlings but not in wild-type seedlings (Figure 4A). Wild-type seedlings eventually formed up to two adventitious roots from the first node at 18 DAS, whereas the mutants typically had comparable numbers of lateral roots by 12 DAS. This result indicated that timing of adventitious root emergence is accelerated in bige1 mutant plants. Increased numbers of lateral roots were not limited to young seedlings. At later stages of vegetative development, bige1 mutant plants produced brace roots at higher node positions on stem that typically did not produce brace roots in wild-type plants (Supplemental Figure 3). The rates of formation of other aerial lateral organs such as leaves were also increased in bige1 mutant plants (Figure 4B). Similar to adventitious root formation, the rate of leaf production in bige1 plants was accelerated compared with the wild type. Consequently, mutant plants produced up to four more leaves than wild-type plants by time of flowering, even though mutant plants flowered approximately 1 week earlier than wild-type plants. While adult mutant plants contained a greater number of lateral organs and internodes, the internodes were less elongated in the mutant (Figure 4C; Supplemental Figure 3), resulting in shorter stature overall. Taken together, these results indicate that Bige1 regulates the timing and rate of initiation of lateral organ formation in both seed and plant development.
Figure 4.
The bige1 Mutant Has Increased Lateral Organ Number during Vegetative Development.
(A) Number of adventitious roots formed at the first node of the wild type (white circles) and bige1-umu1 mutant (filled circles) seedlings. Error bars indicate se. The differences in root number between the wild type and bige1 mutant were statistically significant by Student’s t test (P < 0.05), except at 6 and 7 DAS.
(B) Number of emerged leaves produced by wild-type (white circles) and bige1 mutant (filled circles) plants. At 7 weeks after sowing, all mutant plants had flowered, whereas wild-type plants flowered 1 week later. The differences in leaf number between the wild type and bige1 mutant were statistically significant by Student’s t test at all stages (P < 0.05).
(C) Wild-type (left) and bige1 mutant (right) adult plants at the time of tassel emergence from the apex of the bige1 plant. The tassel was not yet visible in the apex of the wild-type plant.
Identification of the Bige1 Gene
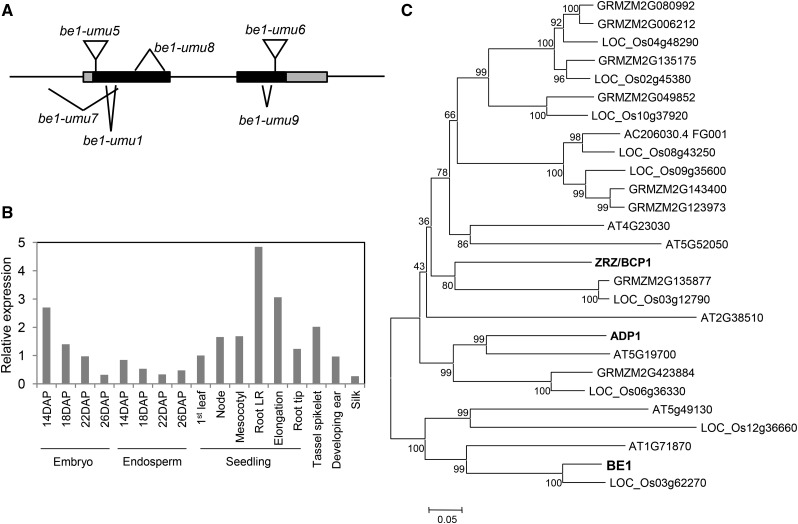
We identified the bige1 gene by positional cloning (see Methods) and confirmed identity of the locus by analyzing multiple independent bige1 alleles. Bige1 (GRMZM2G148937) encodes a putative membrane-localized protein that belongs to multidrug-and-toxin-extrusion (MATE) transporter family (Figure 5). The BIGE1 protein is predicted to have 12 membrane spanning regions. The bige1-umu1 mutation has a 12-bp in-frame deletion (Figure 5A) resulting in loss of four amino acids located in the second membrane spanning region as predicted by ConPred II (Arai et al., 2004). The bige1-umu9 allele contained a small deletion of 24 bp in the second coding exon that would truncate the 11th transmembrane domain of BIGE1. The bige1-umu7 and bige1-umu8 alleles each had deletions of more than 400 bp, causing significant disruptions of BIGE1 protein structure. The bige1-umu5 and bige1-umu6 alleles had Mutator (Mu) transposons inserted in the 5′ and 3′ regions of the coding sequence, respectively. The phenotypes caused by all alleles except bige1-umu5 were very similar to those of bige1-umu1. The bige1-umu5 insertion allele produced noticeably milder phenotypes in both seed and vegetative development (Supplemental Figure 4), indicating that it is a hypomorphic mutation. While the molecular basis of the hypomorphic phenotype has not been determined, we note that transcription initiation within the TIR of the Mu transposon (Barkan and Martienssen, 1991) would produce a transcript with a truncated open reading frame extending from the first in-frame translation initiation codon located downstream of the insertion site. The predicted mutant protein lacks the first 40 amino acids of the N terminus but retains all 12 MATE-related transmembrane domains intact. Alternatively, the Mu transposon could be spliced out, generating transcripts that encode a similarly truncated protein. Quantitative RT-PCR (RT-qPCR) analysis, together with expression profiles obtained from public transcriptome databases (Sekhon et al., 2011; Supplemental Figure 5), showed that Bige1 is broadly expressed with mRNA present in all tissues that we tested (Figure 5B).
Figure 5.
Structure and Expression of the Bige1 Gene.
(A) bige1 mutations including four deletion alleles and two Mu insertion alleles are shown in the context of Bige1 locus structure. Black and gray boxes represent protein coding exons and untranslated regions, respectively.
(B) RT-qPCR analysis of Bige1 expression in various tissues of maize. Seven-day-old seedlings were dissected into indicated tissues including three regions of the primary root: lateral root (LR) zone, elongation zone, and root tip. Values were normalized to 18S rRNA, and expression in the 1st leaf of the seedlings was set to 1. The values are means of technical duplicate assays.
(C) Unrooted tree of the subfamily of BIGE1-related MATE transporters from maize, rice, and Arabidopsis constructed using MEGA6 (Tamura et al., 2013) with a bootstrap value of 1000. The N-terminal variable regions of MATE proteins were removed for the phylogenic analysis (Supplemental Data Set 1). Characterized proteins including maize BIGE1 (GRMZM2G148937) and Arabidopsis ADP1 (AT4G29140) and ZRZ/BCP1 (At1G58340) are highlighted in bold.
MATE proteins are encoded by a large and diverse gene family in plants, and several MATE genes have been functionally characterized (Remy and Duque, 2014). Phylogenic analysis of MATE genes of multiple plant species, including maize, Arabidopsis, tomato, and rice, revealed that BIGE1-related MATE genes belong to a distinct subfamily; thus far, only a few members of which have genetically defined functions (Figure 5C; Supplemental Data Set 1). The BIGE1 group is related to a MATE subfamily that includes Arabidopsis ADP1 and related functionally redundant genes that have been implicated in regulation of overall plant growth (Li et al., 2014). In contrast to the maize genome, which contains a single Bige1 gene, genomes of Arabidopsis and rice each contain two closely related Bige1 paralogs. Hence, it is possible that in other model species, the bige1 phenotype has been obscured by genetic redundancy.
Subcellular Localization of the BIGE1 MATE Transporter
Thus far, most characterized MATE transporters in plants have been localized to the tonoplast membrane. However, recent studies have identified MATE transporters that are localized to plasma membrane, chloroplast, endomembrane systems (Li et al., 2002; Burko et al., 2011; Seo et al., 2012; Serrano et al., 2013; Li et al., 2014; Zhang et al., 2014). To determine to which membrane system the BIGE1 MATE transporter is localized, maize BIGE1 was fused with GFP at the C terminus and expressed under the control of the 35S promoter in stably transformed Arabidopsis. Root tissues of transgenic seedlings were then examined for localization of the GFP fusion protein using confocal microscopy (Figure 6). In contrast to the characteristic single-layered patterns reported for tonoplast- or plasma membrane-localized MATE-GFP fusion proteins, the BIGE1-GFP signal was detected as speckles in Arabidopsis root cells consistent with localization to the Golgi system. To resolve the location of the GFP fusion protein in the internal membrane system, we performed immunogold localization of BIGE1-GFP protein using an anti-GFP antibody (Figures 6A and 6B; Supplemental Figure 6). GFP-specific immunogold particles were detected exclusively on the trans side of Golgi stacks where secretory vesicles are produced.
Figure 6.
BIGE1-GFP Is Localized to the trans-Golgi.
Subcellular localization of BIGE1-GFP in roots of Arabidopsis plants transformed with Pro35S:BIGE1-GFP was determined using electron microscopy immunocytology.
(A) Confocal image of GFP fluorescence in root cells of a transgenic plant. Red line outlines a single root cell in the elongation zone.
(B) Localization of the BIGE1-GFP fusion protein detected by gold-labeled GFP polyclonal antibody.
(C) Schematic illustration of the image in (B) showing the position of the Golgi apparatus (blue). Trans and Cis indicate polarity of the Golgi apparatus. Immunolocalization images of two independent samples and a Col wild-type control sample are shown in Supplemental Figure 6.
Expression of CYP78A Genes
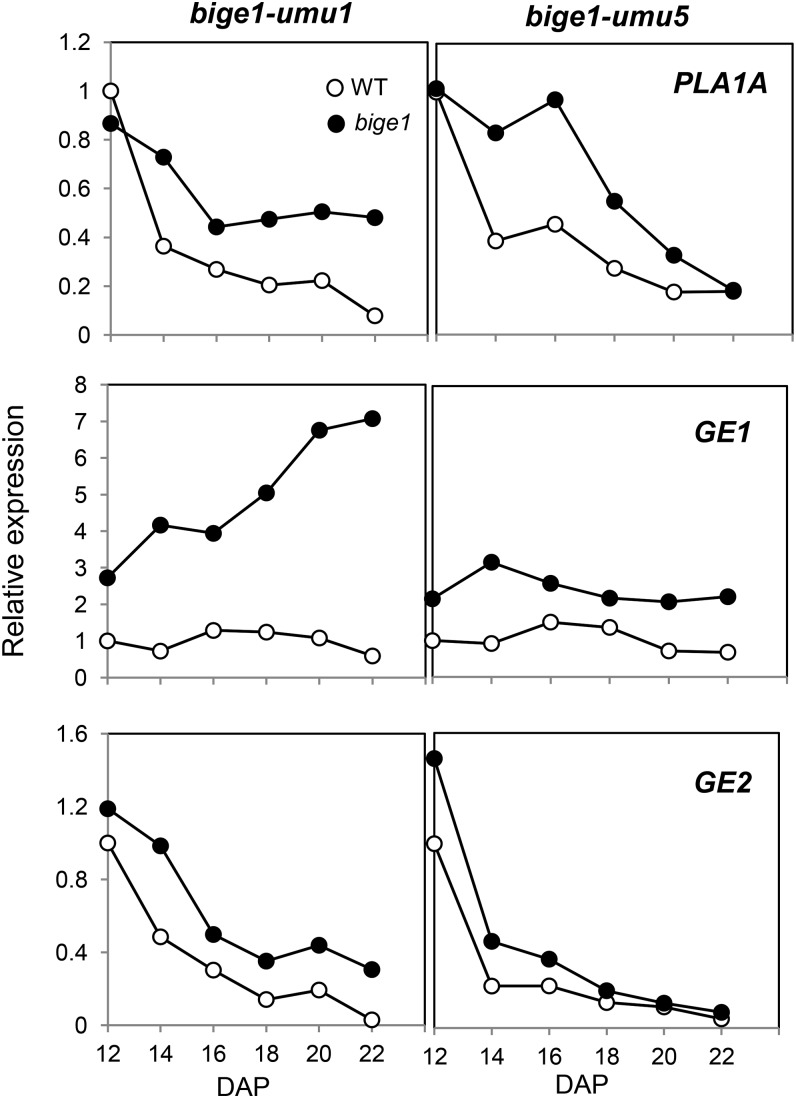
The bige1 phenotype has striking similarities to those of other known mutants affected in organ size and number. In Arabidopsis, mutations in KLU/CYP78A5 have been shown to accelerate initiation of leaf formation in association with early flowering (Wang et al., 2008). A CYP78A mutant of rice, pla1, has similar heterochronic effects on leaf initiation. In addition, a second rice CYP78A homolog identified from the ge mutant affects embryo size by altering cell size (Nagasawa et al., 2013; Yang et al., 2013). Notably, the GE transcript is upregulated in ge mutant seed, suggesting that GE is subject to feedback regulation by a product of the CYP78A pathway (Nagasawa et al., 2013). Hence, we reasoned that if BIGE1 functions in a CYP78A pathway, expression of a maize GE1 ortholog may be similarly upregulated in the bige1 mutant. To test this hypothesis, we quantified expression of a subset of maize CYP78A genes in the bige1 mutant by RT-qPCR. Based on phylogenetic analysis of nine maize CYP78A genes (Supplemental Figure 7 and Supplemental Data Set 2), we identified five maize CYP78A genes that belong to the same clade as the rice PLA1 and GE and Arabidopsis KLU/CYP78A5 genes. Among the five maize CYP78A genes, we detected significant expression of three genes, PLA1A, GE1, and GE2, in embryos of the wild type and bige1 mutant; thus, we focused on these three genes for expression analysis (Supplemental Figure 7). In developing embryos of wild-type maize, PLA1A, GE1, and GE2 genes were expressed throughout mid to late seed development (Figure 7; Supplemental Figure 8). Intriguingly, in both bige1-umu1 and bige1-umu5 mutant embryos, expression of GE1 was markedly upregulated during embryo development, whereas expression of GE2 differed little between the wild type and mutant. Expression of PLA1A showed greater variation among the alleles and seasons compared with consistently higher expression of GE1 (Figure 7; Supplemental Figure 8). These results showed that BIGE1 is required for feedback regulation of the GE1 CYP78A gene in developing maize embryos. Quantification of the bige1-umu1 mutant transcript showed no evidence of upregulation in the mutant compared with the wild type, suggesting that Bige1 itself is not feedback regulated (Supplemental Figure 9).
Figure 7.
RT-qPCR Analysis of CYP78A Genes in bige1 Mutant Embryos.
Expression of PLA1A, GE1, and GE2 genes in developing embryos of normal (WT) and the bige1-umu1 and bige1-umu5 mutants harvested from segregating ears grown in our 2013 spring field. Values were normalized to 18S rRNA, with expression in wild-type 12-DAP embryos set to 1. The values are means of technical duplicate assays. White circles represent the wild type, and black circles represent bige1 mutant. Independent replicate experiments are shown in Supplemental Figure 8.
Conserved Functions of Arabidopsis BIGE1 Orthologs, At-BIGE1A and At-BIGE1B
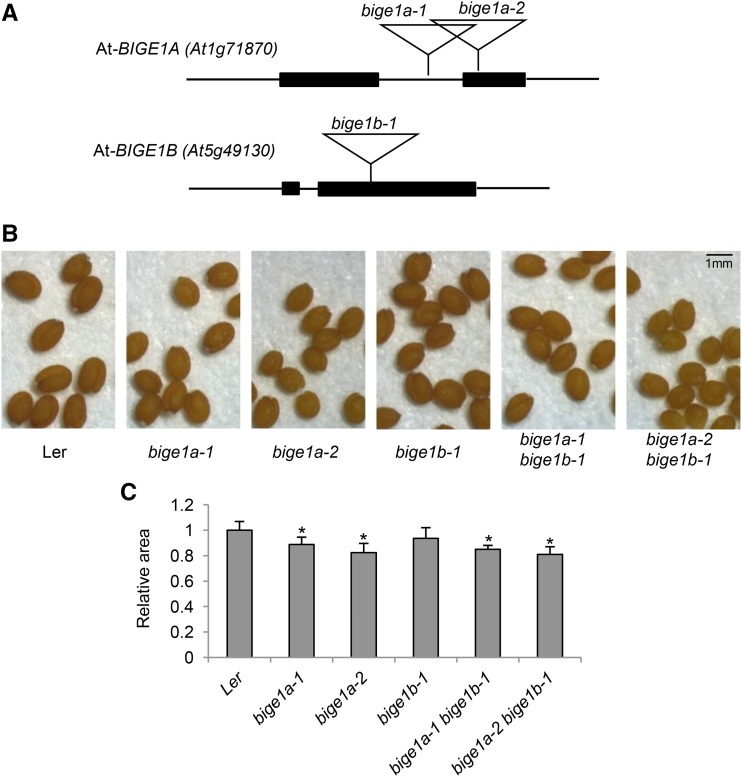
To determine whether function of the putative BIGE1 MATE transporter is conserved in other plant species, we analyzed mutants of two putative Arabidopsis Bige1 orthologs. We examined phenotypes of bige1a (At1g71870) and bige1b (At5g49130) transposon insertion mutants of Arabidopsis (Figure 8). Mature dry seed obtained from homozygous mutant plants were slightly smaller and rounder in shape compared with wild-type seed. This result suggested that At-BIGE1 genes regulate embryo size and shape. Intriguingly, the Arabidopsis klu (cyp78a5) mutant also produces small seeds (Adamski et al., 2009). Leaf number and flowering time phenotypes were quantified for the single mutants as well as the double mutant (Table 1; Supplemental Table 2). The bige1a single mutant plants showed an increase in total number of rosette leaves at the time of flowering compared with the wild type. The bige1a mutant plants also flowered earlier than the wild type. By contrast, the bige1b-1 mutant displayed variability in leaf number and flowering time in independent experiments with a trend toward slight elevation of leaf number and early flowering. The more pronounced effect of bige1a on leaf initiation and flowering time suggested that of the two paralogs, At-BIGE1A has a greater role in regulation of leaf initiation and flowering. The leaf number phenotype of the bige1a bige1b double mutant was enhanced slightly compared with the bige1a single mutant, consistent with an additive interaction of the single mutants, whereas there was little difference in flowering time of the double and single mutants. In contrast to maize bige1, overall statures of adult Arabidopsis wild-type and bige1 mutant plants differed only slightly (Supplemental Figure 10).
Figure 8.
Seed Phenotypes of Arabidopsis bige1a and bige1b Mutants.
(A) Structure of At-BIGE1 genes and Ds transposon insertion alleles used in this study. Filled boxes represent coding exons.
(B) Seeds were harvested from self-pollinated homozygous plants with indicated genotypes.
(C) Ten seeds for each genotype were used to measure the area of individual seeds in the images. Average values relative to wild-type Landsberg erecta (Ler) are shown. The error bars indicate sd. Seed sizes of bige1a and the double mutant were significantly less than the wild type (P < 0.001, Student’s t test; indicated by asterisks), whereas the difference between the wild type and bige1b was not significant (P = 0.08).
Table 1. Leaf Number and Flowering Time Phenotypes of Arabidopsis bige1 Mutants.
| Ler | bige1a-1 | bige1b-1 | bige1a-1 bige1b-1 | BIGE1:GFP#1 bige1a-1 | BIGE1:GFP#2 bige1a-1 | |
|---|---|---|---|---|---|---|
| Leaf number | 6.9 ± 0.3 | 8.1 ± 0.5* | 7.7 ± 0.5* | 8.3 ± 0.6* | 7.4 ± 0.5* | 6.8 ± 0.5 |
| Flowering days | 24.3 ± 0.8 | 22.8 ± 1.0* | 24.1 ± 1.1 | 23.3 ± 1.1* | 24.1 ± 1.2 | 23.8 ± 1.2 |
Statistically significant difference from the wild type (P < 0.05, Student’s t test).
To confirm that Arabidopsis BIGE1A is the functional ortholog of maize Bige1, we generated two independent transgenic Arabidopsis lines expressing a maize BIGE1-GFP fusion gene under the control of a 2.8-kb At-BIGE1A promoter fragment in the Arabidopsis bige1a-1 single mutant background. Both transgenic lines showed partial complementation of the leaf production and flowering time phenotypes of the bige1a-1 mutant. Collectively, these results show that Arabidopsis BIGE1A has a function orthologous to that of maize Bige1 in regulation of leaf initiation and flowering. It is noteworthy that, in contrast to ubiquitous expression of maize Bige1, GUS activity was exclusively observed in shoot and root meristem regions of plants transformed with the At-BIGE1A promoter:GUS transgene (Supplemental Figure 10). This apparent difference in developmental specificity of expression may reflect subfunctionalization of the partially redundant At-BIGE1A and At-BIGE1B paralogs. We cannot rule out the possibility that the apparent tissue-specific expression of the GUS reporter might be due to insufficient inclusion of regulatory sequences from At-BIGE1A or limited sensitivity of GUS staining. Alternatively, maize Bige1 may be regulated primarily by a posttranscriptional mechanism, in contrast to transcriptional regulation of At-BIGE1A.
DISCUSSION
Our results show that maize Bige1 encodes a trans-Golgi-associated MATE transporter implicated in regulation of organ initiation and size. Loss of Bige1 function causes increased embryo size and accelerated production of lateral organs, including leaves and roots, as well as early flowering. In the developing embryo, enlargement of the bige1 scutellum is due to increased cell size. Collectively, these phenotypes are reminiscent of mutants in a subset of CYP78A genes in plants. This association is reinforced by evidence that the CYP78A encoded by Zm-GE1 is upregulated in bige1 mutant embryos, suggesting that the BIGE1 transporter may operate in the CYP78A signaling pathway. We further show that functions of BIGE1-type MATEs are conserved in Arabidopsis; thus, the MATE-mediated regulation of organ initiation is likely to have a key role in determination of plant architecture.
Bige1 Regulates Timing of Organ Initiation
Based on vegetative phenotypes of the bige1 mutant, we conclude that Bige1 regulates the timing and rate of organ initiation rather than organ differentiation. The precocious seminal, adventitious, and brace roots formed by bige1 mutant plants have normal morphology, and the lateral roots form from pericycle cells as in the wild type (Figure 2). Although leaf blades of bige1 mutant plants are narrower than the wild type, leaf phyllotaxy is altered only slightly, with a small change in relative angle between each leaf (Figure 4). Accelerated leaf initiation in the mutant could entail recruitment of fewer cells into the primordium, leading to formation of a narrow leaf blade. Consistent with the hypothesis that bige1 leaves initiate from a smaller set of cells in the shoot apical meristem (SAM) with rapid timing, bige1 mutant plants produce narrow leaves occasionally with lobed margins at upper leaf positions (Supplemental Figure 11). In that case, if organ initiation is able to occur with fewer cells, then more leaves can be produced even if the overall cell production of the SAM is the same in mutant and the wild type.
Similarly, the more rapid expansion of bige1 embryo scutellum can be also interpreted as a heterochronic phenotype. Accelerated development of the bige1 mutant embryo is associated with an advance in the timing of the downregulation of maize LEC1 genes (Supplemental Figure 12) that mark the transition from embryogenesis to maturation in maize (Takacs et al., 2012). An early onset and lengthening of the period of cell expansion during the maturation phase in the bige1 mutant embryo would account for the overall increased cell size in the scutellum.
Coordination of Embryo and Endosperm Development by Bige1
Bige1 regulates both embryo and endosperm development. In developing seeds, the bige1 mutant embryo occupies relatively more space than the endosperm, while overall seed size is not changed. This phenotype indicates that Bige1 is essential to coordinate growth of the filial seed organs. Although Bige1 is expressed both in embryo and endosperm, it is not clear whether Bige1 functions independently in these organs. Our finding that the BIGE1 transporter is localized in the trans-Golgi suggests that secretion of a mobile signal may play a critical role in regulation of lateral organ initiation and relative growth of embryo and endosperm. Intriguingly, nonautonomous functions of CYP78A genes have been reported both in vegetative and reproductive organ formation. In Arabidopsis, KLU, which has been proposed to generate a mobile signal, regulates cell proliferation nonautonomously in leaves, flowers, and seeds (Anastasiou et al., 2007; Adamski et al., 2009). In rice, genetic mosaic analysis has shown that expression of GE is required in both embryo and endosperm to maintain the balance of size between these two organs (Nagasawa et al., 2013). Conceivably, Bige1 could mediate transport of a signal molecule that coordinates growth of embryo and endosperm nonautonomously. While we speculate that the signal is an intermediate and/or product associated with the CYP78A pathway, the identity of the signaling molecule remains to be determined.
BIGE1 MATE Transporter Subfamily
MATE transporter genes comprise a large gene family in plants. Several MATE transporters have been identified and functionally characterized in various plant species (Remy and Duque, 2014). Two major functional classes of the MATE transporters have been extensively studied. One is a class of tonoplast-localized MATE proteins that transport flavonoids and nicotines. The second class is plasma membrane-localized MATE transporters that mediate efflux of metals such as aluminum and iron with citrate. In addition, there are emerging lines of evidence that a subset of MATE transporters function in regulation of plant development and stress responses. Arabidopsis EDS5, which is a chloroplast-localized salicylic acid transporter, is essential for salicylic acid-dependent disease resistance responses (Nawrath et al., 2002; Serrano et al., 2013). Plasma membrane-localized At-DTX50 has been recently shown to mediate efflux of ABA in response to drought (Zhang et al., 2014). Arabidopsis ADP1 and ZRZ/BCD1, which are localized to the endomembrane system or possibly Golgi, respectively, cause highly branched phenotypes when overexpressed (Burko et al., 2011; Seo et al., 2012; Li et al., 2014). In addition, overexpression of ADP1 causes a reduction of auxin accumulation, indicating that MATE transporters are able to modulate hormone levels (Li et al., 2014). ADP1 and closely related MATE genes function redundantly to regulate overall plant growth including degree of branching (Li et al., 2014). Like members of the ADP1/ZRZ/BCD1 subfamily, BIGE1 is localized to a cellular compartment involved in secretion. ADP1 is localized to the non-Golgi endomembrane system. BCD1 is found in the Golgi, though its localization within the Golgi apparatus has not been resolved. The trans-Golgi localization of BIGE1 implies that it is likely involved in secretion of an unidentified small molecule.
The functions of MATE transporters in the secretory system are poorly understood. Because MATE transporters often have broad substrate specificities, the precise localization of BIGE1 and related MATEs within the apparatus may be a key to determining their functional specificity. By analogy to MATEs studied in other organisms, including plants, BIGE1 is likely to mediate transport of small molecules from cytosol into the Golgi lumen. Hence, substrates taken into lumen of the trans-Golgi would likely be loaded in transport vesicles that move to plasma membrane for secretion. Alternatively, BIGE1 may be “stored” at the trans-Golgi location in lieu of a final destination, such as the plasma membrane, a process regulated by vesicle cycling, as in the case of iron-regulated transporters (Barberon et al., 2011). In that case, it is possible that BIGE1-GFP localized elsewhere was below the limit of detection. We also cannot fully exclude the possibility that the trans-Golgi-specific BIGE1-GFP localization detected by immunolocalization in overexpressing lines is misleading due to overloading of the secretory pathway.
We have shown that BIGE1 function is conserved between maize and Arabidopsis. While we have not examined the role of BIGE1 genes in Arabidopsis seed development, we note that bige1a seeds that develop on mutant Arabidopsis plants are smaller than the wild type. Similarly, kernels that develop on homozygous mutant bige1 maize plants are also smaller than the wild type (Supplemental Figure 13), whereas bige1 kernels that develop on heterozygous plants are only slightly smaller than wild-type siblings (∼15% lower dry weight). Hence, in contrast to the big-embryo phenotype of maize bige1, the small seed size phenotype is evidently a maternal effect. Notably, klu/cyp78a5 homozygous mutant plants also generate smaller seeds due to reduced ovule size (Adamski et al., 2009), which is again consistent with the hypothesis that BIGE1 functions in a CYP78A pathway regulating organ size.
Relationship between BIGE1 and CYP78As
The upregulation of Zm-GE1 in the bige1 mutant suggests that BIGE1 function is at least indirectly related to the CYP78A signaling pathway. While upregulation of GE1 was more consistent across multiple seasons than effects on PLA1A expression, the responses of both genes showed substantial variation in field and greenhouse experiments, suggesting sensitivity to environment. With these limitations, we did not detect a consistent difference between effects of strong (bige1-umu1) and mild (bige1-umu5) alleles.
Despite extensive study, the product of the CYP78A pathway remains unknown, and thus far no substrates have been identified for any members of the BIGE1/ADP1/ZRZ/BCD1 subfamily of MATE transporters. Hence, it is plausible that BIGE1 transports a CYP78A-derived growth factor. As noted above, phenotypes of maize and Arabidopsis bige1 mutants are strikingly similar to those of cyp78a mutants in Arabidopsis, rice, and barley. The upregulation of Zm-GE1 in bige1 mutant embryo is reminiscent of the upregulation of GE in rice ge mutant embryos (Nagasawa et al., 2013), suggesting that rice GE and maize GE1 are feedback regulated by downstream signaling of the pathway that requires BIGE1. Overexpression of the ZRZ MATE causes a 2-fold increase in CYP78A5/KLU expression (Burko et al., 2011), although this could be an indirect effect due to morphological changes associated with proliferated meristems. Other circumstantial evidence conflicts with this idea. Rice GE/CYP78A13 and Arabidopsis KLUH/CYP78A5 are localized to the endoplasmic reticulum, whereas BIGE1 is localized at Golgi. Moreover, CYP78A genes are expressed in specific regions including SAM flanks and epithelial tissues of the embryo (Miyoshi et al., 2004; Anastasiou et al., 2007; Nagasawa et al., 2013; Yang et al., 2013), whereas Bige1 mRNA is expressed broadly in diverse tissues of maize. Nevertheless, it is intriguing that phenotypes of the bige1 mutant are markedly confined to developmental contexts associated with embryo formation and organ lateral initiation. Moreover, in Arabidopsis, expression of the At-BIGE1A promoter-GUS gene is restricted to the SAM and root tips. Expression of maize Bige1 driven by the At-BIGE1A promoter was sufficient to complement leaf and flowering phenotypes of the Arabidopsis bige1a mutant, suggesting that action of the BIGE1 MATE in meristem regions is sufficient for proper regulation of organ initiation. Therefore, the specificity of the maize bige1 phenotype may be determined primarily by the tissue-specific expression of CYP78A genes rather than regulation of Bige1 per se. Alternatively, posttranscriptional regulation could be the basis for the difference between Bige1 transcript levels in maize and AtBIGE1A:GUS expression in Arabidopsis. In any case, identification of substrates of the BIGE1 MATE transporter as well as the intermediate(s) and product(s) of the CYP78A pathway will be essential for understanding the mechanism of regulation of lateral organ initiation.
Separate Pathways Regulating Leaf Initiation
Our analysis of the BIGE1 MATE transporter identifies a new class of genes implicated in plastochron regulation and seed organ size in plants. The heterochronic mutants from diverse plant species that show accelerated leaf initiation can be classified into two groups based on the phenotypic characteristics and gene products. Mutants in the first group, which includes maize and Arabidopsis bige1 mutants as well as cyp78a mutants of rice and Arabidopsis, have relatively subtle effects on organ number and embryo size without affecting timing of the juvenile-to-adult phase transition that is typically marked by patterns of trichome formation and cuticular wax production in leaves. CYP78A and BIGE1 encode proteins implicated in biosynthesis and movement of small molecules, respectively. Mutants in the second group show strong heterochronic effects that include shifts in the juvenile-to-adult phase transition. Maize te1 and vp8 mutants and their rice orthologous mutants, pla2 and pla3, respectively, have dwarf phenotypes associated with delayed timing of the juvenile-to-adult phase transition. An Arabidopsis Vp8/PLA3 ortholog mutant, amp1, also exhibits excessive production of leaves and early flowering. AMP1, PLA3, and Vp8 encode proteins with similarity to glutamate carboxypeptidases (Helliwell et al., 2001; Suzuki et al., 2008; Kawakatsu et al., 2009). AMP1 has been shown to mediate microRNA-regulated translation inhibition (Li et al., 2013). Maize Te1 and rice PLA2 encode a protein with an RNA recognition domain (Veit et al., 1998; Kawakatsu et al., 2006). The highly pleiotropic dominant Cg mutant of maize promotes axial branching and ectopic brace root formation, in addition to excessive leaf production and delayed juvenile-to-adult phase change (Singleton, 1951; Chuck et al., 2007). The Cg mutation causes ectopic expression of microRNA, miR156 (Chuck et al., 2007). Together, these observations suggest that there are at least two distinct modes of regulation in the leaf initiation pathway: one mediated by a secreted metabolite and the other mediated by microRNA signaling. How these pathways interact at the molecular level remains elusive. In Arabidopsis, expression of CYP78A5 is elevated in the amp1 mutant (Helliwell et al., 2001), whereas Kawakatsu et al. (2009) concluded that the PLA1 and PLA2/PLA3 genes function more or less independently, suggesting that regulation of the pathways may differ among plant species. In any case, identification of substrates or small molecules interacting with CYP78A, BIGE1, AMP1/VP8/PLA3, and TE1/PLA3 functions is required for understanding developmental mechanisms of regulation of organ initiation possibly mediated by mobile factors.
METHODS
Maize Plant Growth Conditions
Maize (Zea mays) plants were grown either in the field during spring and fall seasons or in a greenhouse during the winter season. For counting of seminal roots, seeds were sown in soil and seedlings grown in the lab under ambient conditions (22°C). For counting of adventitious nodal roots, seedlings were grown in soil-containing pots in the greenhouse.
Isolation and Positional Cloning of the bige1 Mutant
The independent bige1-umu1, bige1-umu5, bige1-umu6, bige1-umu7, bige1-umu8, and bige1-umu9 alleles were isolated in a screen of the UniformMu population (McCarty et al., 2005) for embryo size mutants. Allelism tests were conducted by crossing bige1-umu1 as a reference allele to each candidate allele. The bige1-umu5 allele was used for fine mapping of the bige1 locus. For this purpose, the bige1-umu5 mutant was crossed to the B73 inbred, and the resulting F1 plants were self-pollinated to produce an F2 mapping population. Preliminary mapping experiments placed the bige1 locus in a region on the short arm of chromosome 5 between public mapping markers umc1416 and bnlg1006 (maizegdb.org). To refine the map location, a total of 795 individual F2 bige1-umu5 homozygous plants were genotyped by PCR using in-del markers (Supplemental Table 3). Based on F2 mapping, the bige1 mutant locus was narrowed down to a segment of ∼1 Mb spanned by 267unk1map2 (15/795 recombinants) and 321unk2map2 (1/795 recombinants) markers. Several candidate genes located in this region were then analyzed for polymorphisms by PCR and partial sequencing. Each of the bige1 mutant alleles contained polymorphisms in GRMZM2G148937. The polymorphisms associated with each mutation were subsequently confirmed by complete sequencing of the W22 wild-type and bige1 mutant alleles. For the sequencing, three overlapping segments of the GRMZM2G148937 locus were amplified using three different pairs of primers: 267oxy-mate F3 and 267mate scan-R4, 267mate scan-F2 and 267mate scan-R2, and 267mate scan-F7 and 267mate scan-R12. The three overlapping genomic regions were amplified with PrimeStar PCR enzyme (Takara/Clontech) and cloned into Invitrogen pCR-BluntII-TOPO vector (Thermo Fisher Scientific). The contiguous genomic regions were then assembled to obtain a 4193-bp Bige1 gene sequence including 1064 bp of 5′ untranslated region and 715 bp of 3′ untranslated region.
Phylogenetic Analysis
Predicted protein sequences of MATE transporters and CYP78A P450 were obtained from Phytozome (phytozome.jgi.doe.gov). The sequences were aligned using ClustalW embedded in MEGA6 (Tamura et al., 2013) with the default settings (pairwise alignment: gap opening penalty = 10, gap extension penalty = 0.1; multiple alignment: gap opening penalty = 10, gap extension penalty = 0.2; protein weight matrix = Gonnet; residue-specific penalties and hydrophilic penalties ON; gap separation distance = 4; end gap separation OFF; delay divergent cutoff = 30%). The phylogenic trees were constructed by the neighbor-joining method using Poisson model with Bootstrap value of 1000, implemented in the MEGA6 as the default setting. The N-terminal variable segments of the MATE and CYP78A were trimmed out for the phylogenetic analysis (Supplemental Data Sets 1 and 2).
Cloning of Full-Length cDNA and Plasmid Construction
To obtain the full-length protein coding region of the Bige1 gene, total RNA was prepared from 18-DAP W22 developing embryos and a full-length cDNA amplified and cloned using the Invitrogen SuperScriptIII One-Step RT-PCR system (Thermo Fisher Scientific) and RTXba F-1 and RTSal R-1 primers. The PCR products were cloned into pCR-BluntII-TOPO vector and sequenced to confirm 100% identity with the coding region of the W22 genomic sequence. The coding region of Bige1 gene was then excised from the pTOPO vector by XbaI and SalI restriction enzyme digest. The fragments were subcloned between 35S promoter and GFP of pCAM35SLGFP vector to construct pCAM35S267LGFP. The promoter region of At-BIGE1A was amplified by genomic PCR with Col-0 DNA as template and the primer pair of At1g71870proHind F4 and At1g71870proXba R2, and the products were subcloned into pCR-BluntII-TOPO vector. The promoter fragments were cut out from the vector by HindIII and XbaI digest and subsequently cloned into pBI121 replacing 35S promoter and pCAM35S267LGFP replacing 35S promoter, respectively, resulting in ProAtBIGE1A:GUS and ProAtBIGE1A:BIGE1-GFP transformation vectors.
Arabidopsis thaliana Mutants and Transformation
Seeds for Ds transposon insertion alleles of At-BIGE1A and At-BIGE1B were obtained from Cold Spring Harbor Laboratory (Sundaresan et al., 1995; Springer, 2000). Presence of the transposon insertions was confirmed by genomic PCR with the Ds border primers and At-BIGE1 gene-specific primers (Supplemental Table 3). The insertion sites for BIGE1A were further confirmed by sequencing the PCR products.
Transformation was performed by the floral dip method as described (Clough and Bent, 1998). Transformants were confirmed by selection on kanamycin plates for ProAtBIGE1:GUS construct and hygromycin plates for Pro35S:BIGE1-GFP and ProAtBIGE1A:BIGE1-GFP constructs, respectively.
RT-PCR Analysis
Total RNA was prepared from various tissues of maize and Arabidopsis plants using the RNeasy kit (Qiagen). Total RNA from maize endosperm was extracted as previously described (McCarty, 1986). The RNA samples were treated with DNaseI and purified by the DNA-free RNA kit (Zymo Research). RT-PCR reactions were performed with 100 ng total RNA in a total volume of 10 μL using the One-Step RT-PCR kit (Qiagen). For RT-qPCR analysis, 100 ng total RNA was used in a total volume of 20 μL using Power SYBR Green RNA-to-CT 1-step kit (Thermo Fisher Scientific), and gene expression was quantified by StepOnePlus (Applied Biosystems).
Microscopy
The subcellular localization analysis of BIGE1-GFP fusion protein was performed at UF ICBR core service. The immunogold labeling and electron microscopy imaging were performed as described by Kang (2010) with an anti-GFP antibody (Santa Cruz Biotechnology). Sections for the histological analyses were made as follows. Maize embryos were excised, fixed by formalin-acetic acid-alcohol, and dehydrated using an ethanol series. Samples were transferred to paraffin and embedded. Samples were section by microtome at 8 μm thick and stained by Toluidine blue O. The average size of cells in the sections of the wild type and bige1 was measured by counting the number of cells in 250 × 250 μm of square areas as shown in Supplemental Figure 2.
Flow Cytometry
The flow cytometry analysis was performed as described by Fouquet et al. (2011) at the UF ICBR flow cytometry service core facility.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: KT310084 for Bige1, At1g71870 for At-BIGE1A, and At5g49130 for At-BIGE1B.
Supplemental Data
Supplemental Figure 1. Analysis of cell size in scutellum tissues of wild-type and bige1 mutant embryos.
Supplemental Figure 2. Ploidy analysis of wild-type and bige1 mutant embryos.
Supplemental Figure 3. Brace root formation on wild-type and bige1 mutant plants.
Supplemental Figure 4. Phenotypes of independent bige1 alleles.
Supplemental Figure 5. Bige1 gene expression in various tissues.
Supplemental Figure 6. Subcellular localization of BIGE1-GFP fusion protein.
Supplemental Figure 7. CYP78A gene family in maize, rice, Arabidopsis, and tomato.
Supplemental Figure 8. RT-qPCR analysis of CYP78A gene expression.
Supplemental Figure 9. RT-qPCR analysis of Bige1 gene expression.
Supplemental Figure 10. Mature plant phenotypes of Arabidopsis bige1a and bige1b single and double mutants and GUS staining of a representative Arabidopsis plant transformed with an AtBIGE1Apro:GUS reporter gene.
Supplemental Figure 11. Leaf blades from upper nodes of wild-type and bige1 mutant maize plants.
Supplemental Figure 12. Effect of bige1 on expression of markers for onset of seed maturation.
Supplemental Figure 13. Seed size comparison between wild type and bige1.
Supplemental Table 1. Percentage of seeds with seminal roots.
Supplemental Table 2. Leaf number and flowering time phenotypes of Arabidopsis bige1 single and double mutants.
Supplemental Table 3. Primers used in this study.
Supplemental Data Set 1. Text file of the alignment used for the phylogenetic analysis shown in Figure 5C.
Supplemental Data Set 2. Text file of the alignment used for the phylogenetic analysis shown in Supplemental Figure 7.
Supplementary Material
Acknowledgments
We thank Funnce Liu for technical help. This material is based on work that is supported by the National Institute of Food and Agriculture, by the USDA, under Awards 2008-02561 and 2011-67013 (M.S. and D.R.M) and 2010-04228 (D.R.M.), and by the National Science Foundation (1116561 to D.R.M.).
AUTHOR CONTRIBUTIONS
M.S., Y.S., and D.R.M. designed the experiments. M.S., Y.S., S.W., and B.-H.K. performed the experiments. M.S., Y.S., and D.R.M. analyzed the data. M.S. and D.R.M. wrote the article.
Glossary
- DAP
days after pollination
- DAS
days after sowing
- RT-qPCR
quantitative RT-PCR
- SAM
shoot apical meristem
References
- Adamski N.M., Anastasiou E., Eriksson S., O’Neill C.M., Lenhard M. (2009). Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proc. Natl. Acad. Sci. USA 106: 20115–20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou E., Kenz S., Gerstung M., MacLean D., Timmer J., Fleck C., Lenhard M. (2007). Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev. Cell 13: 843–856. [DOI] [PubMed] [Google Scholar]
- Arai M., Mitsuke H., Ikeda M., Xia J.X., Kikuchi T., Satake M., Shimizu T. (2004). ConPred II: a consensus prediction method for obtaining transmembrane topology models with high reliability. Nucleic Acids Res. 32: W390–W393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Ori N. (2014). Leaf development and morphogenesis. Development 141: 4219–4230. [DOI] [PubMed] [Google Scholar]
- Barberon M., Zelazny E., Robert S., Conéjéro G., Curie C., Friml J., Vert G. (2011). Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 108: E450–E458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A., Martienssen R.A. (1991). Inactivation of maize transposon Mu suppresses a mutant phenotype by activating an outward-reading promoter near the end of Mu1. Proc. Natl. Acad. Sci. USA 88: 3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovics A.H., Timmermans M.C. (2014). Developmental patterning by gradients of mobile small RNAs. Curr. Opin. Genet. Dev. 27: 83–91. [DOI] [PubMed] [Google Scholar]
- Bolduc N., Yilmaz A., Mejia-Guerra M.K., Morohashi K., O’Connor D., Grotewold E., Hake S. (2012). Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 26: 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burko Y., Geva Y., Refael-Cohen A., Shleizer-Burko S., Shani E., Berger Y., Halon E., Chuck G., Moshelion M., Ori N. (2011). From organelle to organ: ZRIZI MATE-Type transporter is an organelle transporter that enhances organ initiation. Plant Cell Physiol. 52: 518–527. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M., Zhang N., Sauvage C., Muños S., Blanca J., Cañizares J., Diez M.J., Schneider R., Mazourek M., McClead J., Causse M., van der Knaap E. (2013). A cytochrome P450 regulates a domestication trait in cultivated tomato. Proc. Natl. Acad. Sci. USA 110: 17125–17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A.M., Letham S., Craig S., Dennis E.S. (1993). amp1-a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 4: 907–916. [Google Scholar]
- Chuck G., Cigan A.M., Saeteurn K., Hake S. (2007). The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 39: 544–549. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Conway L.J., Poethig R.S. (1997). Mutations of Arabidopsis thaliana that transform leaves into cotyledons. Proc. Natl. Acad. Sci. USA 94: 10209–10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S., Stransfeld L., Adamski N.M., Breuninger H., Lenhard M. (2010). KLUH/CYP78A5-dependent growth signaling coordinates floral organ growth in Arabidopsis. Curr. Biol. 20: 527–532. [DOI] [PubMed] [Google Scholar]
- Evans M.M., Poethig R.S. (1997). The viviparous8 mutation delays vegetative phase change and accelerates the rate of seedling growth in maize. Plant J. 12: 769–779. [Google Scholar]
- Fouquet R., Martin F., Fajardo D.S., Gault C.M., Gómez E., Tseung C.W., Policht T., Hueros G., Settles A.M. (2011). Maize rough endosperm3 encodes an RNA splicing factor required for endosperm cell differentiation and has a nonautonomous effect on embryo development. Plant Cell 23: 4280–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinha C., Bilsborough G., Tsiantis M. (2009). Hormonal input in plant meristems: A balancing act. Semin. Cell Dev. Biol. 20: 1149–1156. [DOI] [PubMed] [Google Scholar]
- Giulini A., Wang J., Jackson D. (2004). Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430: 1031–1034. [DOI] [PubMed] [Google Scholar]
- Helliwell C.A., Chin-Atkins A.N., Wilson I.W., Chapple R., Dennis E.S., Chaudhury A. (2001). The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell 13: 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F., Park W.J., Sauer M., Woll K. (2004). From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci. 9: 42–48. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F., Tuberosa R. (2009). Genetic and genomic dissection of maize root development and architecture. Curr. Opin. Plant Biol. 12: 172–177. [DOI] [PubMed] [Google Scholar]
- Hong S.K., Kitano H., Satoh H., Nagato Y. (1996). How is embryo size genetically regulated in rice? Development 122: 2051–2058. [DOI] [PubMed] [Google Scholar]
- Itoh J.I., Hasegawa A., Kitano H., Nagato Y. (1998). A recessive heterochronic mutation, plastochron1, shortens the plastochron and elongates the vegetative phase in rice. Plant Cell 10: 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalve S., De Vos D., Beemster G.T. (2014). Leaf development: a cellular perspective. Front. Plant Sci. 5: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H. (2010). Electron microscopy and high-pressure freezing of Arabidopsis. Methods Cell Biol. 96: 259–283. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T., Itoh J., Miyoshi K., Kurata N., Alvarez N., Veit B., Nagato Y. (2006). PLASTOCHRON2 regulates leaf initiation and maturation in rice. Plant Cell 18: 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu T., et al. (2009). PLASTOCHRON3/GOLIATH encodes a glutamate carboxypeptidase required for proper development in rice. Plant J. 58: 1028–1040. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Johnston R., Yang Y., Gallavotti A., Kojima M., Travençolo B.A., Costa Lda.F., Sakakibara H., Jackson D. (2009). Studies of aberrant phyllotaxy1 mutants of maize indicate complex interactions between auxin and cytokinin signaling in the shoot apical meristem. Plant Physiol. 150: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., He Z., Pandey G.K., Tsuchiya T., Luan S. (2002). Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 277: 5360–5368. [DOI] [PubMed] [Google Scholar]
- Li R., et al. (2014). ADP1 affects plant architecture by regulating local auxin biosynthesis. PLoS Genet. 10: e1003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., et al. (2013). MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell 153: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M., Jost M., Kuon J.E., Himmelbach A., Aßfalg A., Beier S., Scholz U., Graner A., Stein N. (2014). Mapping-by-sequencing accelerates forward genetics in barley. Genome Biol. 15: R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.L., Grogan C.O., Manchester C.E. (1974). Terminal ear mutant of maize (Zea mays). J. Agric. Sci. 82: 433–435. [Google Scholar]
- McCarty D.R. (1986). A simple method for extraction of RNA from maize tissues. Maize Genet. Coop. News Lett. 60: 61. [Google Scholar]
- McCarty D.R., et al. (2005). Steady-state transposon mutagenesis in inbred maize. Plant J. 44: 52–61. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Ahn B.O., Kawakatsu T., Ito Y., Itoh J., Nagato Y., Kurata N. (2004). PLASTOCHRON1, a timekeeper of leaf initiation in rice, encodes cytochrome P450. Proc. Natl. Acad. Sci. USA 101: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Smith S., De Smet I. (2012). Small signaling peptides in Arabidopsis development: how cells communicate over a short distance. Plant Cell 24: 3198–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa N., Hibara K., Heppard E.P., Vander Velden K.A., Luck S., Beatty M., Nagato Y., Sakai H. (2013). GIANT EMBRYO encodes CYP78A13, required for proper size balance between embryo and endosperm in rice. Plant J. 75: 592–605. [DOI] [PubMed] [Google Scholar]
- Nawrath C., Heck S., Parinthawong N., Métraux J.P. (2002). EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy E., Duque P. (2014). Beyond cellular detoxification: a plethora of physiological roles for MDR transporter homologs in plants. Front. Physiol. 5: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon R.S., Lin H., Childs K.L., Hansey C.N., Buell C.R., de Leon N., Kaeppler S.M. (2011). Genome-wide atlas of transcription during maize development. Plant J. 66: 553–563. [DOI] [PubMed] [Google Scholar]
- Seo P.J., Park J., Park M.J., Kim Y.S., Kim S.G., Jung J.H., Park C.M. (2012). A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochem. J. 442: 551–561. [DOI] [PubMed] [Google Scholar]
- Serrano M., Wang B., Aryal B., Garcion C., Abou-Mansour E., Heck S., Geisler M., Mauch F., Nawrath C., Métraux J.P. (2013). Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol. 162: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton W.R. (1951). Inheritance of corn grass a macromutation in maize, and its possible significance as an ancestral type. Am. Nat. 85: 81–96. [Google Scholar]
- Springer P.S. (2000). Gene traps: tools for plant development and genomics. Plant Cell 12: 1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V., Springer P., Volpe T., Haward S., Jones J.D., Dean C., Ma H., Martienssen R. (1995). Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9: 1797–1810. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Latshaw S., Sato Y., Settles A.M., Koch K.E., Hannah L.C., Kojima M., Sakakibara H., McCarty D.R. (2008). The Maize Viviparous8 locus, encoding a putative ALTERED MERISTEM PROGRAM1-like peptidase, regulates abscisic acid accumulation and coordinates embryo and endosperm development. Plant Physiol. 146: 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs E.M., Li J., Du C., Ponnala L., Janick-Buckner D., Yu J., Muehlbauer G.J., Schnable P.S., Timmermans M.C., Sun Q., Nettleton D., Scanlon M.J. (2012). Ontogeny of the maize shoot apical meristem. Plant Cell 24: 3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit B., Briggs S.P., Schmidt R.J., Yanofsky M.F., Hake S. (1998). Regulation of leaf initiation by the terminal ear 1 gene of maize. Nature 393: 166–168. [DOI] [PubMed] [Google Scholar]
- Vilches-Barro A., Maizel A. (2015). Talking through walls: mechanisms of lateral root emergence in Arabidopsis thaliana. Curr. Opin. Plant Biol. 23: 31–38. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E., Veit B., Sinha N., Hake S. (1991). The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350: 241–243. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Schwab R., Czech B., Mica E., Weigel D. (2008). Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell 20: 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Gao M., Yin X., Liu J., Xu Y., Zeng L., Li Q., Zhang S., Wang J., Zhang X., He Z. (2013). Control of rice embryo development, shoot apical meristem maintenance, and grain yield by a novel cytochrome p450. Mol. Plant 6: 1945–1960. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhu H., Pan Y., Yu Y., Luan S., Li L. (2014). A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 7: 1522–1532. [DOI] [PubMed] [Google Scholar]
- Zhang P., et al. (2012). A transposable element insertion within ZmGE2 gene is associated with increase in embryo to endosperm ratio in maize. Theor. Appl. Genet. 125: 1463–1471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.