Abstract
Esthesioneuroblastoma (ENB) is a rare malignant neoplasm arising from the olfactory neuroepithelium. ENB constitutes only 3% of all malignant intranasal neoplasm. Because of the rarity, the number of patients of ENB treated in individual departments is small. Most of these patients presents in locally advanced stages and require multimodality treatment in form of surgery, chemotherapy and radiotherapy. Multimodality approach with a risk-adapted strategy is required to achieve good control rates while minimizing treatment related toxicity.
Keywords: Surgery, Radiotherapy, Chemotherapy, Esthesioneuroblastoma
Core tip: This article is a comprehensive review of literature of a rare and aggressive neoplasm. This article outlines the various newer details of diagnosis, staging and treatment aspects of esthesioneuroblastoma (ENB). The importance of multimodality approach in management of ENB is reviewed in detail.
INTRODUCTION
Esthesioneuroblastoma (ENB) is a rare malignant neoplasm arising from the olfactory neuroepithelium, including the superior one third of the nasal septum, cribriform plate, and superior turbinates, extending to base of the skull and to the intracranial space[1]. ENB was first described by Berger et al[2] in 1924. Because of uncertainty regarding the precise histological origin of the tumour, it has been described using various names in the literature, but the commonly used terms in recent publications are ENB and olfactory neuroblastoma. It constitutes only 3% of all intranasal neoplasms and its etiology remains unclear[3]. ENB manifests across all ages, with peaks in the second and sixth decades of life[4].
CLINICAL PRESENTATION
ENB have varying biological behaviour, ranging from slow indolent growth to a highly aggressive neoplasm with rapid widespread metastasis resulting in poor survival[5]. The most common presentation is unilateral nasal obstruction followed by epistaxis. Other clinical features develops with local spread of tumor like proptosis (infiltration into orbital canal), cranial nerve palsies (infiltration into cranial foramens and brain), swelling in neck (neck nodes) and metastatic symptoms[6].
PATHOLOGY
Light microscopy features consists of homogeneous small cells having uniform round to oval nuclei. Rosette or pseudorosette formation, and eosinophilic fibrillary intercellular background material is seen. However, in undifferentiated tumors, with anaplastic hyperchromatic small cells showing many mitotic figures and scant cytoplasm, differentiation from other small-cell nasal neoplasms is difficult with light microscopy[7]. Hyams Grading system is routinely used for histopathological grading and it consists of four grades (I-IV)[8]. Hyams grading takes into account the architectural pattern, mitosis, nuclear pleomorphism, fibrillary matrix, rosettes and necrosis (Table 1). On immunohistochemistry (IHC), the tumour is positive for neuron specific enolase (NSE), synaptophysin, chromogranin and S-100 protein, but most cases are negative for cytokeratin, vimentin, desmin, actin, glial fibrillary acidic protein, UMB45, and leucocytic common antigen[7]. Electron microscopy is reliable in visualising uniform round nuclei, dense core neurosecretory granules, neuronal processes with microtubules and neurofilaments, and rare synapses[7]. The closest histopathological differential diagnosis is neuroblastoma. The neuroblastoma stains positive for NSE, synaptophysin, Leu7, and neurofilament protein. Elevated serum catecholamines are also suggestive of neuroblastoma.
Table 1.
Hyams histopathological grading
| Grade | Lobular architecture preservation | Mitotic index | Nuclear polymorphism | Fibrillary matrix | Rosettes | Necrosis |
| I | + | Zero | None | Prominent | HW rosettes | None |
| II | + | Low | Low | Present | HW rosettes | None |
| III | +/- | Moderate | Moderate | Low | FW rosettes | Rare |
| IV | +/- | High | High | Absent | None | Frequent |
INVESTIGATIVE WORK-UP
The gold standard of diagnosis is biopsy of the lesion showing characteristic histopathological features along with IHC. Fine needle aspiration cytology of suspected lymph nodes in neck is recommended to rule out nodal spread. Local extension of the tumor is evaluated using contrast enhanced computed tomography (CECT) of the face and neck (Figure 1). Magnetic resonance imaging (MRI) is better in evaluating sinonasal, intraorbital or intracerebral extension (Figure 2). As computed tomography (CT) can better demonstrate bony erosions, both studies are usually required in most of the patients[9]. Chest X ray, Ultrasound abdomen and CECT chest and abdomen is helpful in detecting systemic metastasis. Baseline hemogram, renal function tests and liver function tests are done for further treatment planning. Positron emission tomography is not routinely recommended for staging evaluation but can be used as an adjunct to CT and MRI.
Figure 1.
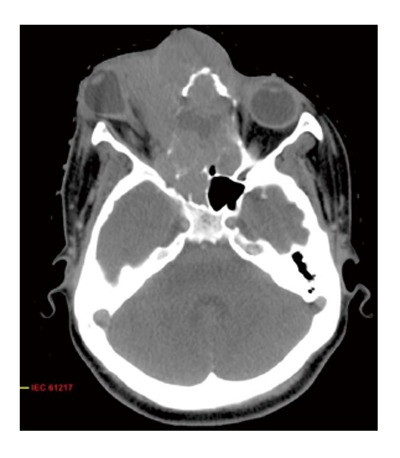
Axial computed tomography scan showing locally advanced esthesioneuroblastoma with extension to paranasal sinus and orbit.
Figure 2.
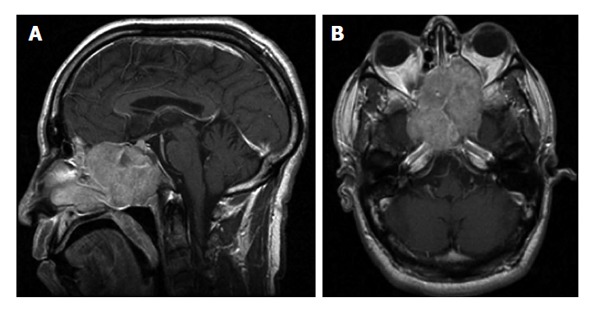
T1 W sagital section (A) and axial section (B) magnetic resonance imaging showing locally advanced esthesioneuroblastoma. Reprinted with permission from medscape drugs and diseases (http://emedicine.medscape.com/), 2015, Available from: URL: http://emedicine.medscape.com/article/250237-overview.
STAGING
Tumor staging is an important guide for prognosis and therapy. Several staging systems, including Hymans, Kadish, and tumor, node, metastasis (TNM) systems, have been proposed as a guide to choosing treatment modalities. Staging for ENB was first proposed by Kadish et al[10] and the tumors were staged as three group categories. Group A is limited to tumors of the nasal cavity; group B is extension to the paranasal sinuses; and group C is extension beyond the paranasal sinuses and nasal cavity. Morita et al[11] in 1993 published a revised Kadish system that redefined stage C (consisting of local disease spreading beyond the paranasal sinuses) and included a stage D (distant metastasis) (Table 2). In 1992, Dulguerov et al[6] proposed TNM staging system based on the TNM system, determined on pre-treatment CT and MRI findings (Table 3). However, modified Kadish staging system is the most widely used staging system.
Table 2.
Modified kadish staging
| Stage A: Tumour limited to the nasal fossa |
| Stage B: Tumour extension into the paranasal sinuses |
| Stage C: Tumour extension beyond the paranasal sinuses and nasal cavity |
| Stage D: Distant metastasis |
Table 3.
Tumor, node, metastasis staging system
| T1 | Tumour involving the nasal cavity and/or paranasal sinuses (excluding sphenoid), sparing the most superior ethmoidal cells |
| T2 | Tumour involving the nasal cavity and/or paranasal sinuses (including the sphenoid) with extension to or erosion of the cribriform plate |
| T3 | Tumour extending into the orbit or protruding into the anterior cranial fossa, without dural invasion |
| T4 | Tumour involving the brain |
| N0 | No cervical lymph-node metastasis |
| N1 | Any form of cervical lymph-node metastasis |
| M0 | No metastases |
| M1 | Distant metastasis |
TREATMENT MODALITIES
The various treatment modalities used in the management of ENB are surgery, chemotherapy, radiation therapy (RT) and palliative care. Nowadays, the multimodal approach is recommended for improved survival and quality of life of the patients.
Surgery
The mainstay of the treatment is surgery. The advantage of surgery is tumor removal, immediate improvement in compressive symptoms, proper tissue for histopathological and prognostic evaluation. Intracranial extension and close proximity to the cribriform plate and ethmoidal roof requires a combined transfacial and neurosurgical approach[12]. For T1 tumors, craniotomy is not justified when there is clear radiological evidence of a normal cribriform plate and upper ethmoid cells. All other patients should be managed by combined craniofacial approach. Dulguerov et al[5] showed 100% local control with craniofacial resection as compared to 40% local control with other surgical resections. Similarly, Spaulding et al[13] at the University of Virginia showed reduction of 20% local recurrence rate with the craniofacial resection as compared to non-craniofacial approach. An international collaborative study of 17 centres reported the role of craniofacial resection in ENB in 2012. Five-year overall survival was 78% and 5-year recurrence-free survival was 64%[14]. Craniofacial resection allows en bloc resection of the tumour with better assessment of intracranial extension and protection of the brain and optic nerves. The current accepted practice is open or endoscopic craniofacial surgical resection.
RT
Specimens from the nasal cavity and paranasal sinuses, even en bloc, are difficult to orient, and surgical margins are difficult to analyze properly. Due to locally infiltrative nature of the disease, surgically clear margins are difficult to achieve. Thus there is a role of adjuvant RT to minimize the risk of local recurrence[12,15]. Adjuvant RT is indicated for Kadish stage B and C, whereas Kadish A disease can be managed with surgery alone. RT is delivered to the tumor bed and local extension with nodal irradiation reserved for involved nodes. Elective nodal irradiation is not practiced routinely. The RT doses have varied from 50 to 60 Gy in the literature[16,17]. With higher RT doses, there is always a risk of long term neural toxicity. But with advancement in technologies in delivery of RT with Intensity modulated RT (IMRT), Image guided RT and proton therapy, the long term neural toxicities can be minimized. RT is also used in neoadjuvant settings in locally advanced tumors and in palliation in metastatic settings. In small local recurrences, stereotactic radiosurgery and stereotactic radiotherapy can be used even for re-radiation[18].
Chemotherapy
The role of chemotherapy is not very clear in adjuvant settings in early tumors, but in locally advanced and metastatic tumors it has a definitive role. It decreases the chances of systemic failure by acting on systemic micro-metastasis[19]. In neoadjuvant settings, it decreases the size of tumor, decreases compressive symptoms and helps in further complete removal of the tumour. It can be combined with RT in both neoadjuvant and adjuvant settings for better results[20]. The common drugs used are cisplatin, etoposide, adriamycin, vincristine and cyclophosphamide. Initial literature in support of neoadjuvant CT (NACT) comes from the University of Virginia, where Kadish C stage patients received 2 cycles of NACT with vincristine and cyclophosphamide with or without doxorubicin, followed by RT dose of 50 Gy, followed by craniofacial resection[21]. The 5-year and 10-year actuarial survival was 72% and 60% repectively. Subsequently, Cisplatin-based regimens became the preferred CT regimen in ENB at the Mayo Clinic, Gustave-Roussy Institute and Harvard Medical Institute[22-24]. Presently, the preferred chemotherapy regimen is cisplatin (33 mg/m2 daily) and etoposide (100 mg/m2 daily) for 3 Da.
Management of neck
Neck metastases at presentation in seen in 5% of patients[25]. In such patients, neck should be addressed by neck dissection or radiotherapy. Delayed neck metastases has been reported in 16% of patients in older series[25]. But with newer investigative, surgical, CT and RT techniques, these incidences are better managed. Patients with advanced local disease should be evaluated radiologically for neck metastases and regional treatment in form of neck dissection or neck RT should be offered in case of positive neck nodes.
STAGE WISE TREATMENT
Kadish A
Surgery alone with clear margins is sufficient in Kadish A staged tumors. Adjuvant RT is indicated in close and positive margins or with residual disease. No role of adjuvant chemotherapy.
Kadish B
Surgery followed by adjuvant RT is the treatment of choice. Role of adjuvant CT is controversial. Recent reports shows use of adjuvant CT. Neoadjuvant CT or RT can be used in inoperable cases.
Kadish C
Kadish C staged tumors requires all the three modalities. Neoadjuvant approach (CT/RT/concurrent CT-RT) is preferred. Definitive role of adjuvant CT in these settings.
Kadish D
Systemic chemotherapy and palliative RT to local site and metastatic sites are advised. Palliative care should be incorporated for improving the quality of life.
RECURRENCE
Local recurrence and/or distant metastases remain the main problem in the management of ENB. Salvage treatment consists of surgery, surgery and postoperative RT, RT alone, palliative chemotherapy (CCT), or supportive care. The management options depend upon the type of relapse and initial treatment received by the patient. Bachar et al[26] reported local recurrence was documented in 30.7%, regional recurrence in 17.9%, and distant metastasis in 7.7% of patients. In a metaanalysis by Dulguerov et al[6], local regional, and distant recurrence rates were reported in 29%, 16%, and 17%, respectively.
PROGNOSIS
The most important prognostic factors influencing the outcome reported in ENB are Hyams grade, positive lymph nodes, Kadish stage, extent of resection and postoperative RT with atleast 54 Gy[27-29].
ENB IN CHILDREN
In pediatric population upto 15 years of age, the incidence of ENB is 0.1/100000[30]. In younger patients, the tumors have a more aggressive presentation and advanced disease. Bisogno et al[31] reported 9 young patients of ENB managed with combined modality approach. The 5-year progression-free survival and overall survival were 77.8% and 88.9% respectively[31]. El Kababri et al[32] reported 11 children and adolescents treated with combined modality approach between 1982 and 2002. The 5-year actuarial disease-free survival and overall survival rate was 91% with ten patients being long term survivors[32]. Thus, pediatric ENB has an aggressive presentation but has good clinical results with combined modality approach.
CONCLUSION
Most of the patients of ENB present in locally advanced stage and the optimal management depends on the cooperation between clinicians, surgeons, radiologists and pathologists from establishing diagnosis to organizing the therapeutic strategy. Novel strategies including combined CCT with RT and/or dose escalation with advanced RT techniques such as IMRT and proton therapy should be prospectively investigated to improve the survival results in ENB.
Footnotes
P- Reviewer: Kupeli S, Noussios G, Unal M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: The authors declare no potential conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 22, 2015
First decision: May 19, 2015
Article in press: July 27, 2015
References
- 1.Levine PA, McLean WC, Cantrell RW. Esthesioneuroblastoma: the University of Virginia experience 1960-1985. Laryngoscope. 1986;96:742–746. [PubMed] [Google Scholar]
- 2.Berger L, Luc R, Richard D. L’esthesioneuroepitheliome olfactif. Bull Assoc Fr Etude Cancer. 1924;13:410–421. [Google Scholar]
- 3.Kumar M, Fallon RJ, Hill JS, Davis MM. Esthesioneuroblastoma in children. J Pediatr Hematol Oncol. 2002;24:482–487. doi: 10.1097/00043426-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Elkon D, Hightower SI, Lim ML, Cantrell RW, Constable WC. Esthesioneuroblastoma. Cancer. 1979;44:1087–1094. doi: 10.1002/1097-0142(197909)44:3<1087::aid-cncr2820440343>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102:843–849. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2:683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 7.Hirose T, Scheithauer BW, Lopes MB, Gerber HA, Altermatt HJ, Harner SG, VandenBerg SR. Olfactory neuroblastoma. An immunohistochemical, ultrastructural, and flow cytometric study. Cancer. 1995;76:4–19. doi: 10.1002/1097-0142(19950701)76:1<4::aid-cncr2820760103>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Hyams VJ. Olfactory neuroblastoma. In: Hyams VJ, Baksakis JG, Michaels L, editors. Tumors of the upper respiratory tract and ear. Washington DC: Armed Forces Institute of Pathology; 1988. pp. 240–248. [Google Scholar]
- 9.Pickuth D, Heywang-Köbrunner SH, Spielmann RP. Computed tomography and magnetic resonance imaging features of olfactory neuroblastoma: an analysis of 22 cases. Clin Otolaryngol Allied Sci. 1999;24:457–461. doi: 10.1046/j.1365-2273.1999.00295.x. [DOI] [PubMed] [Google Scholar]
- 10.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37:1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32:706–714; discussion 714-715. doi: 10.1227/00006123-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Diaz EM, Johnigan RH, Pero C, El-Naggar AK, Roberts DB, Barker JL, DeMonte F. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck. 2005;27:138–149. doi: 10.1002/hed.20127. [DOI] [PubMed] [Google Scholar]
- 13.Spaulding CA, Kranyak MS, Constable WC, Stewart FM. Esthesioneuroblastoma: a comparison of two treatment eras. Int J Radiat Oncol Biol Phys. 1988;15:581–590. doi: 10.1016/0360-3016(88)90298-2. [DOI] [PubMed] [Google Scholar]
- 14.Patel SG, Singh B, Stambuk HE, Carlson D, Bridger PG, Cantu G, Cheesman AD, Donald P, Fliss D, Gullane P, et al. Craniofacial surgery for esthesioneuroblastoma: report of an international collaborative study. J Neurol Surg B Skull Base. 2012;73:208–220. doi: 10.1055/s-0032-1311754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber G, Laedrach K, Baumert B, Caversaccio M, Raveh J, Greiner R. Esthesioneuroblastoma: irradiation alone and surgery alone are not enough. Int J Radiat Oncol Biol Phys. 2002;54:486–491. doi: 10.1016/s0360-3016(02)02941-3. [DOI] [PubMed] [Google Scholar]
- 16.Slevin NJ, Irwin CJ, Banerjee SS, Gupta NK, Farrington WT. Olfactory neural tumours--the role of external beam radiotherapy. J Laryngol Otol. 1996;110:1012–1016. [PubMed] [Google Scholar]
- 17.Platek ME, Merzianu M, Mashtare TL, Popat SR, Rigual NR, Warren GW, Singh AK. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol. 2011;6:41. doi: 10.1186/1748-717X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Gompel JJ, Carlson ML, Pollock BE, Moore EJ, Foote RL, Link MJ. Stereotactic radiosurgical salvage treatment for locally recurrent esthesioneuroblastoma. Neurosurgery. 2013;72:332–339; discussion 339-340. doi: 10.1227/NEU.0b013e31827fcdc2. [DOI] [PubMed] [Google Scholar]
- 19.Porter AB, Bernold DM, Giannini C, Foote RL, Link MJ, Olsen KD, Moynihan TJ, Buckner JC. Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J Neurooncol. 2008;90:201–204. doi: 10.1007/s11060-008-9645-y. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Ghoshal S, Khosla D, Bharti S, Das A, Kumar N, Kapoor R, Sharma SC. Survival and failure outcomes in locally advanced esthesioneuroblastoma: a single centre experience of 15 patients. Eur Arch Otorhinolaryngol. 2013;270:1897–1901. doi: 10.1007/s00405-012-2280-4. [DOI] [PubMed] [Google Scholar]
- 21.Eden BV, Debo RF, Larner JM, Kelly MD, Levine PA, Stewart FM, Cantrell RW, Constable WC. Esthesioneuroblastoma. Long-term outcome and patterns of failure--the University of Virginia experience. Cancer. 1994;73:2556–2562. doi: 10.1002/1097-0142(19940515)73:10<2556::aid-cncr2820731017>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Koka VN, Julieron M, Bourhis J, Janot F, Le Ridant AM, Marandas P, Luboinski B, Schwaab G. Aesthesioneuroblastoma. J Laryngol Otol. 1998;112:628–633. doi: 10.1017/s0022215100141295. [DOI] [PubMed] [Google Scholar]
- 23.McElroy EA, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998;42:1023–1027; discussion 1027-1028. doi: 10.1097/00006123-199805000-00040. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya N, Thornton AF, Joseph MP, Goodman ML, Amrein PC. Successful treatment of esthesioneuroblastoma and neuroendocrine carcinoma with combined chemotherapy and proton radiation. Results in 9 cases. Arch Otolaryngol Head Neck Surg. 1997;123:34–40. doi: 10.1001/archotol.1997.01900010038005. [DOI] [PubMed] [Google Scholar]
- 25.Davis RE, Weissler MC. Esthesioneuroblastoma and neck metastasis. Head Neck. 1992;14:477–482. doi: 10.1002/hed.2880140610. [DOI] [PubMed] [Google Scholar]
- 26.Bachar G, Goldstein DP, Shah M, Tandon A, Ringash J, Pond G, Gullane PJ, Perez-Ordonez B, Gilbert RW, Brown DH, et al. Esthesioneuroblastoma: The Princess Margaret Hospital experience. Head Neck. 2008;30:1607–1614. doi: 10.1002/hed.20920. [DOI] [PubMed] [Google Scholar]
- 27.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133:276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 28.Ozsahin M, Gruber G, Olszyk O, Karakoyun-Celik O, Pehlivan B, Azria D, Roelandts M, Kaanders JH, Cengiz M, Krengli M, et al. Outcome and prognostic factors in olfactory neuroblastoma: a rare cancer network study. Int J Radiat Oncol Biol Phys. 2010;78:992–997. doi: 10.1016/j.ijrobp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto RC, Gleich LL, Biddinger PW, Gluckman JL. Esthesioneuroblastoma and sinonasal undifferentiated carcinoma: impact of histological grading and clinical staging on survival and prognosis. Laryngoscope. 2000;110:1262–1265. doi: 10.1097/00005537-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Benoit MM, Bhattacharyya N, Faquin W, Cunningham M. Cancer of the nasal cavity in the pediatric population. Pediatrics. 2008;121:e141–e145. doi: 10.1542/peds.2007-1319. [DOI] [PubMed] [Google Scholar]
- 31.Bisogno G, Soloni P, Conte M, Podda M, Ferrari A, Garaventa A, Luksch R, Cecchetto G. Esthesioneuroblastoma in pediatric and adolescent age. A report from the TREP project in cooperation with the Italian Neuroblastoma and Soft Tissue Sarcoma Committees. BMC Cancer. 2012;12:117. doi: 10.1186/1471-2407-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Kababri M, Habrand JL, Valteau-Couanet D, Gaspar N, Dufour C, Oberlin O. Esthesioneuroblastoma in children and adolescent: experience on 11 cases with literature review. J Pediatr Hematol Oncol. 2014;36:91–95. doi: 10.1097/MPH.0000000000000095. [DOI] [PubMed] [Google Scholar]


