Abstract
Spectinamides are new semi-synthetic spectinomycin derivatives with potent anti-tubercular activity. The reported synergism of the precursor spectinomycin with other antibiotics prompted us to examine whether spectinamides sensitize M. tuberculosis to other antibiotics not traditionally used in the treatment of tuberculosis to potentially expand therapeutic options for MDR/XDR Tuberculosis. Whole cell synergy checkerboard screens were performed using the laboratory strain M. tuberculosis H37Rv, lead spectinamide 1599, and a broad panel of 27 antibiotics. In vitro, 1599 synergized with 11 drugs from 6 antibiotic classes. The observed synergy was tested against clinical isolates confirming synergy with Clarithromycin, Doxycycline and Clindamycin, combinations of which were taken forward for in vivo efficacy determination. Co-administration of 1599 and clarithromycin provided additional bacterial killing in a mouse model of acute tuberculosis infection, but not in a chronic infection model. Further studies indicated that mismatched drug exposure profiles likely permitted induction of phenotypic clarithromycin resistance and subsequent loss of synergism. These studies highlight the importance of validating in vitro synergism and the challenge of matching drug exposures to obtain a synergistic outcome in vivo. Results from this study indicate that a 1599 clarithromycin combination is potentially viable, providing the drug exposures can be carefully monitored.
More than 130 years since after its identification as the causative agent of tuberculosis in 1882, Mycobacterium tuberculosis remains a severe global health threat, has infected more than one third of the world’s population, and is responsible for almost 2 million deaths annually1. Although most common in the developing world, more than 10,000 cases of tuberculosis and >500 associated deaths occur in the United States annually2. Adept at evasion of the human host’s immune system, M. tuberculosis establishes persistent infections and forms granulomas in the lung3. There the bacteria can remain in a latent state for decades before reactivating and disseminating to cause an active disease state4. The complexity and challenge of killing granuloma-resident bacilli is increasingly recognized as the result of multiple subpopulations, even within a single granuloma, each with distinct drug-susceptibility and resistance profiles5,6.
Treatment of Mycobacterium tuberculosis infections is lengthy and hindered by the emergence of drug resistance7. Standard treatment of drug-susceptible infections requires a 2 month initial phase with daily administration of frontline drugs rifampin, isoniazid, pyrazinamide, and ethambutol followed by a 4 month continuation phase composed of isoniazid and rifampin. Patient adherence to this lengthy regime is challenged by antibiotic-associated toxicity and inadequate access to individual regime components, particularly isoniazid for which supply shortages are not uncommon8. Underexposure of drug is proposed to permit selection of genetic mutants resistant to frontline drugs and further exacerbates the clinical challenge of managing tuberculosis by fueling the development of acquired drug resistance9. Drug resistant tuberculosis isolates can be spread from person to person, often with low fitness cost for the bacteria, and radically increases the length and cost of treatment for the disease10,11.
In response to the increasing prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis infections, numerous efforts are being undertaken to thwart the spread of this global killer. Several novel scaffolds with unique mechanisms of action (and thus potential to inhibit drug-resistant infections) are in various stages of pre-clinical and clinical development. This includes nitroaromatic prodrugs (PA-824, TBA-354, and delamanid), cell wall active compounds such as SQ109, the structurally unique aminoglycoside apramycin, and the recently approved bedaquiline (TMC207)12,13,14,15,16,17,18. Efforts to re-sensitize drug-resistant infections to frontline drugs by adjuvant therapy with non-tubercular agents including efflux inhibitors verapamil and thioridazine have also shown promise and are being pursued19,20. Synthetic modification of natural products has led to the successful development of treatments for Gram-positive bacterial infections and we have recently applied this approach in the discovery and preclinical development of anti-tuberculars known as spectinamides21.
Spectinamides are semi-synthetic derivatives of spectinomycin, an aminocyclitol that binds to a site within the bacterial 30S ribosome (helix 34 of 16S rRNA) distinct from that of other protein synthesis inhibitors, including aminoglycosides and macrolides22. Unlike aminoglycosides, spectinomycin does not inhibit human mitochondrial translation, a side effect of aminoglycosides that leads to ototoxicity23,24,25. Despite its potent activity against bacterial ribosomes, spectinomycin is only weakly antibacterial owing to limited intracellular accumulation and resultant access to the ribosomal target. Efflux pump Rv1258c is upregulated in MDR/XDR isolates in vitro, implicated in drug tolerance, upregulated in human sputum samples during the course of tuberculosis treatment, and provides M. tuberculosis with intrinsic resistance to spectinomycin5,19,21,26,27. Semi-synthetic spectinamide analogs avoid efflux by Rv1258c, thereby gaining potency against M. tuberculosis both in vitro and in in vivo models of tuberculosis infection21.
Spectinomycin was previously shown to synergize with several classes of drugs in vitro28. While the poor anti-tuberculosis activity of spectinomycin may restrict this synergism in vivo, spectinamides are efficacious in vivo and, so, afford the opportunity to further investigate the prospects for sensitization and synergism between spectinomycins and antibiotics lacking intrinsic anti-tuberculosis activity. Therefore, we investigated the interaction of spectinamides with a library of antibiotics not currently used in standard TB therapy.
Hits were confirmed in clinical isolates and mechanisms underlying synergy were explored using both genetic and biochemical approaches. These studies indicated that lead spectinamides display favorable interactions with some FDA approved antibiotics including macrolides in vitro. Combination treatment with 1599 and clarithromycin provided additive reduction in bacterial loads in an acute model of tuberculosis infection but this interaction was not seen in a lengthier model of chronic tuberculosis infection, apparently likely due to mismatched exposure profiles as we show here. These studies highlight the challenges of matching in vitro synergism in vivo due to the impact of differing pharmacokinetic profiles and dosing schedules on in vivo success of drug combinations identified in vitro.
Results
Chemically diverse antibiotics synergize with anti-tuberculosis spectinamide 1599 in vitro
The interaction of lead spectinamide 1599 with a library of 27 antibiotics not normally used to treat tuberculosis was examined in vitro using checkerboard synergy assays (Table 1). In this well-established technique, the reduction in the minimum treatment concentration required to inhibit growth (MIC) is established for 2 compounds alone and in combination. The mutual reductions in MICs are used to calculate the fractional inhibitory concentration index (FICI), where a FICI ≤ 0.5 indicates synergism. Preliminary screening performed with Mycobacterium tuberculosis laboratory strain H37Rv indicated synergy (FICI ≤ 0.5) with 13 partner drugs, including trimethoprim, bacitracin, vancomycin, tetracyclines, macrolides, and closely related lincosamides. Encouragingly, the finding of synergism between clarithromycin and 1599 is similar to previously reported synergism between clarithromycin and the parent drug spectinomycin28. Despite synergism with tetracyclines, 1599 did not synergize with the structurally-similar glycylcycline tigecycline (FICI = 0.6)29. Indifference (no antagonism) was seen between 1599 and aminoglycosides, beta-lactams, nitroimidazoles, and parent molecule spectinomycin. 1599 neither synergized with nor sensitized cells to spectinomycin, suggesting that it’s avoidance of efflux by pump Rv1258c is not due to inhibition of this pump, as the later would be expected to sensitize TB to spectinomycin.
Table 1. In vitro synergism in Mycobacterium tuberculosis laboratory strain H37Rv.
| Drug Class | Antibiotic | MIC alone | MIC in presence of 1599 | 1599 MIC in combination | FICI with 1599 (H37Rv) | Interaction | Number of Replicates |
|---|---|---|---|---|---|---|---|
| Acyl-depsipeptide | ADEP4 | >20 | 20 | 0.6 | ≤1.0 | Indifference | 1 |
| Aminocycitol | Spectinomycin | 100–200 | 50–100 | 0.8 | 1.0 | Indifference | 2 |
| Aminoglycoside | Gentamycin | 3.1 | 0.8 | 0.6 | 0.75 | Indifference | 1 |
| Aminoglycoside | Streptomycin | 3.1 | 3.1–6.3 | 0.8–1.6 | 2.0–4.0 | Indifference | 2 |
| Aminoglycoside | Tobramycin | 6.3 | 6.3 | 1.3 | 2.0 | Indifference | 1 |
| Antifolate | Methotrexate (DHFR) | 200–>200 | 100–200 | 1.3 | 0.75–≤2.0 | Indifference | 2 |
| Antifolate | Sulfamethoxazole (DHPS) | 200 | 50 | 0.8 | 1.25 | Indifference | 1 |
| Antifolate | Trimethoprim (DHFR) | >200 | 12.5–25 | 0.2–0.3 | ≤0.13–≤0.19 | Synergism | 3 |
| Antiprotozoal | Chloroquine | >200 | >200 | 2.5 | ≤3.0 | Indifference | 1 |
| Cephalosporin | Cefotaxime | >200 | >200 | 1.3 | ≤2.0 | Indifference | 1 |
| Carbapenem | Meropenem | 6.3 | 6.3 | 1.3 | ≤2.0 | Indifference | 1 |
| Cyclic polypeptide | Bacitracin | >200 | 25–50 | 0.6 | ≤0.31–≤0.38 | Synergism | 2 |
| Fluoroquinolone | Gatifloxacin | 0.2 | 0.1 | 0.6 | 1.0 | Indifference | 1 |
| Glycopeptide | Vancomycin | 50 | 3.1 | 0.3–0.6 | 0.19–0.31 | Synergism | 2 |
| Glycycline | Tigecycline | 6.3 | 0.8 | 0.6 | 0.6 | Indifference | 1 |
| Lincosamide | Clindamycin | >200–>400 | 0.1–6.3 | 0.02–0.2 | ≤0.02–≤0.16 | Synergism | 3 |
| Lincosamide | Lincomycin | >200 | 1.6–12.5 | 0.04–0.2 | ≤0.07–≤0.16 | Synergism | 2 |
| Lincosamide | Pirlimycin | >200 | 25–50 | 0.3 | ≤0.31–≤0.38 | Synergism | 2 |
| Macrolide | Azithromycin | >400–>800 | 6.3–12.5 | 0.3–0.4 | ≤0.13–≤0.28 | Synergism | 3 |
| Macrolide | Clarithromycin | 12.5–48 | 0.1–5.6 | 0.04–0.05 | 0.07–0.35 | Synergism | 3 |
| Macrolide | Erythromycin | >200–800 | 12.5–50 | 0.2–0.4 | 0.16–≤0.28 | Synergism | 3 |
| Nitroimidazole | Metronidazole | >200 | 200 | 0.6 | ≤1.0 | Indifference | 1 |
| Nitroimidazole | Nitrofurantoin | 50 | 25 | 0.6 | 1.0 | Indifference | 1 |
| Oxazolidinone | Linezolid | 3.1 | 3.1 | 0.1 | 1.5 | Indifference | 1 |
| Tetracycline | Doxycycline | 6.3–50 | 0.4–0.8 | 0.1–0.3 | 0.08–0.31 | Synergism | 5 |
| Tetracycline | Minocycline | 25 | 1.6 | 0.1 | 0.13 | Synergism | 1 |
| Tetracycline | Tetracycline | 25–50 | 0.8–1.6 | 0.1–0.2 | 0.08–0.13 | Synergism | 2 |
Whole cell, checkerboard assays were used to characterize interaction of 1599 with indicated antibiotics. Assays were performed with Mycobacterium tuberculosis strain H37Rv. Fractional Inhibitory Concentration Index (FICI) scores were interpreted as follows: synergy (≤0.5), indifference (>0.5–4.0) or antagonism (>4.0) and MICs are in μg/mL. Results are presented as the range from the indicated number of biologically independent experimental replicates. A less than or equal to symbol (≤) preceding a FICI score indicates that an MIC was higher than the greatest concentration tested, which was used in FICI calculation.
Spectinamides synergize with tetracyclines, macrolides, and lincosamides to enhance potency against clinical isolates
Representative hits identified in initial screens were verified in checkerboard synergy assays against the laboratory M. tuberculosis Erdman strain and two clinical isolates (Table 2). 1599 synergism with trimethoprim and the cyclic polypeptide bacitracin was restricted to laboratory strain H37Rv, as FICI scores determined for clinical isolates ranged from 0.8 to 2.0, indicating indifference. Synergism of 1599 and vancomycin was not tested in clinical isolates since this drug did not synergize with structurally related spectinamides (below), possibly indicating a non-specific interaction. The synergism of 1599 with clindamycin, clarithromycin, and doxycycline was maintained in clinical isolates. Synergism between clindamycin and 1599 was particularly notable, as clindamycin itself was inactive against all M. tuberculosis strains investigated (MIC ≥ 200 μg/mL). Drugs synergistic with spectinamide 1599 were also tested for synergy with structurally related spectinamide compounds 1329 and 1445 and their precursor, spectinomycin (Table 2). Synergy with clarithromycin, clindamycin, doxycycline, and tetracycline was equivalent for all three spectinamides and spectinomycin, indicating the structural requirement for synergy was not introduced by the spectinamide modification to the parent molecule. Synergy with trimethoprim and vancomycin was restricted to compound 1599, however. This observation was not studied further since 1599 interaction with these drugs was not seen in clinical isolates and, therefore, is inappropriate for further advancement.
Table 2. Validation of Preliminary Hits.
| Antibiotic |
FICI Score with 1599 |
FICI Score with other Spectinamides and Spectinamides (strain H37Rv) |
Consensus Interaction | |||||
|---|---|---|---|---|---|---|---|---|
| H37Rv | Erdman | TN14149 | TN043 | 1329 | 1445 | Spectinomycin | ||
| Bacitracin | ≤0.31–≤0.38 | ≤1.06* | ≤1.0* | ≤0.75* | nd | nd | nd | Indifference |
| Clarithromycin | 0.12–0.34 | 0.06–0.07 | 0.09–0.15 | 0.02* | 0.003–0.02 | 0.03* | 0.04–0.37 | Synergism |
| Clindamycin | ≤0.02–≤0.07 | ≤0.03* | ≤0.05* | ≤0.05* | ≤0.03–≤0.06 | ≤0.03–≤0.06 | ≤0.13–≤0.5 | Synergism |
| Doxycycline | 0.08–0.31 | 0.09 | 0.5* | 0.25* | 0.02* | 0.02* | 0.03–0.31 | Synergism |
| Tetracycline | nd | nd | nd | nd | 0.01* | 0.02* | 0.02 | Synergism |
| Trimethoprim | ≤0.13–0.19 | ≤0.13* | ≤2.0* | ≤0.5* | ≤0.75–≤2.0 | ≤0.75–≤2.0 | nd | Indifference |
| Vancomycin | nd | nd | nd | nd | 1.0–1.5 | 1.0 | 1.0 | Indifference |
Whole cell, checkerboard assays were used to characterize interaction of spectinamides (1599, 1329, 1445) or parent spectinomycin with indicated antibiotics. Assays were performed with Mycobacterium tuberculosis laboratory strains (H37Rv, Erdman) or clinical isolates (TN14149, TN043), as indicated.
Fractional Inhibitory Concentration Index (FICI) scores were interpreted as follows: synergy (≤0.5), indifference (>0.5–4.0) or antagonism (>4.0) and are representative of 2–3 independent experiments, except where *indicates results are from a single experiment or “nd” indicates not determined.
Synergism of 1599 and clarithromycin is achieved at relevant concentrations
An important consideration for the clinical potential of re-purposed antibiotics is if the concentration required to elicit the desired response, in this case synergy, is therapeutically achievable. To begin answering this question for the 1599 combinations identified, we examined the MIC of partner antibiotics in the presence and absence of 1599 (Table 3). The presence of sub-inhibitory 1599 (0.6 μg/mL) reduced the MIC of the partner drugs examined by over 85%. For example, 1599 decreased the MIC of clarithromycin from 25 to 0.1 μg/mL. This is well below the maximum plasma concentration (Cmax) of clarithromycin achieved in humans (2.3 μg/mL) receiving a single 200 mg oral dose of this antibiotic30. Sub-inhibitory concentrations of 1599 also reduced the MIC of other antibiotics to equal or slightly above the Cmax parameters achieved in humans with a single oral dose of clindamycin (600 mg dose, 3.5 μg/mL Cmax)31, Doxycycline (100 mg dose, 1.7 μg/mL Cmax)32, and Tetracycline (300 mg dose, 2.5 μg/mL Cmax)33. These data suggest that amongst the 1599 synergism partners identified, clarithromycin may have the greatest potential for synergism with spectinamides in vivo.
Table 3. Reduction in MIC produced by sub-inhibitory concentrations of compound 1599.
| Antibiotic |
MIC (μg/mL) |
% MIC | |
|---|---|---|---|
| (−) 1599 | (+) 1599 | ||
| Clarithromycin | 25–50 | 0.1 | 0.2–0.4% |
| Clindamycin | >200 | 6–25 | <1.5–6.3% |
| Doxycycline | 6.3–50 | 0.4–1.6 | 3.2–6.5% |
| Tetracycline | 50 | 6.3 | 12.6% |
MICs of macrolides and tetracyclines in the absence (−) or presence (+) of 0.6 μg/mL of spectinamide 1599. The % of original MIC values were calculated by dividing the MIC in the presence of 1599 by the MIC in the absence of 1599. Results presented are from two biologically independent experiments with Mycobacterium tuberculosis strain H37Rv.
Co-administration of 1599 and clarithromycin provides a statistically significant improvement in clearance of pulmonary infection loads in an acute but not chronic mouse model of tuberculosis infection
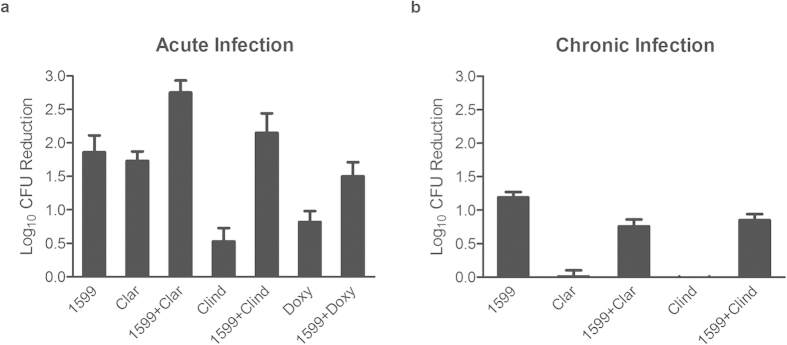
Combinations were tested in vivo in a murine model of acute tuberculosis infection. Gamma-interferon knock-out (GKO) mice were infected by low dose aerosol and treatments were initiated 13 days post infection (p.i.) twice per day (BID) for 9 days (Fig. 1a). Monotherapy with 1599 alone reduced bacterial loads in the lungs by 1.9 logs, similar to previous findings21. Clarithromycin alone reduced pulmonary loads by 1.7 logs despite having weak activity in vitro, which is in agreement with previous findings34 and may reflect this antibiotic’s high accumulation within lungs. Co-administration of 1599 with clarithromycin, however, yielded 2.8 logs of killing (Fig. 1a). This improvement in activity compared to monotherapy was statistically significant (p = 0.008 when compared to 1599 group) (Supplementary Table S1). Co-administration of 1599 with clindamycin or doxycyline did not yield a statistically significant difference in pulmonary loads in comparison with 1599 monotherapy (Supplementary Table S1)21.
Figure 1. Efficacy of 1599 combinations in acute and chronic infection models.
Log10 reduction in bacteria in lungs was determined by calculating the difference between bacillary loads in organs from the untreated group and treated groups. Mean log10 CFU reductions per lung ± the standard error of mean (SEM) are presented. (a) Murine model of acute tuberculosis infection where low-dose aerosol infected gamma interferon knock-out mice were treated twice a day for 9 consecutive days beginning 14 days post-infection. (b) Murine model of chronic tuberculosis infection where low-dose aerosol infected BALB/c mice received treatments once daily for 5/7 days for 28 days beginning 41 days post-infection. Abbreviations: Clar, clarithromycin; Clind, clindamycin; Doxy, doxycycline.
The activity of 1599 (150 mg/kg), clarithromycin (250 mg/kg), and clindamycin (100 mg/kg) were then tested alone and in combination in a mouse model of chronic tuberculosis infection (Fig. 1b). Owing to the protracted nature of the chronic infection trial, it was impractical to dose animals more than once per day. Hence, BALB/c mice were infected by low dose aerosol and treatments were initiated 41 days p.i., once per day (QD), using a 5 of 7 (5/7) day dosing schedule for 30 days35,36. Compared to carrier treated controls, 1599 reduced bacterial loads by ~1.2 logs (p < 0.001 vs. carrier) in lungs, as we have reported previously (Fig. 1b, Supplementary Table S2). Monotherapy with clarithromycin or clindamycin, however, was completely ineffective at reducing bacterial load in the lungs. Co-administration of 1599 with either clarithromycin or clindamycin failed to increase bacterial clearance as compared to 1599 monotherapy and, in fact, slightly but significantly reduced the activity of 1599 in the lungs.
Inducible macrolide resistance may restrict efficacy in vivo with 5/7 day dosing
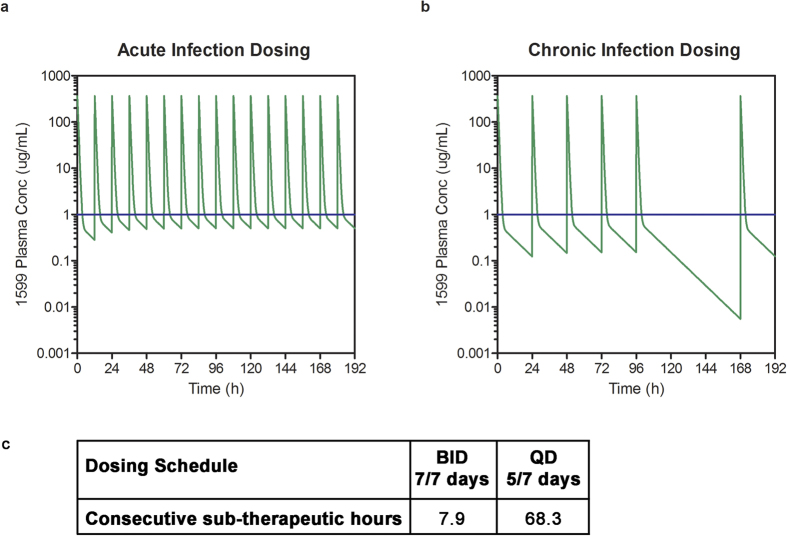
In light of the differing results for the combination of 1599 and clarithromycin in these two distinct infection models, mathematical modeling with the pharmacokinetic parameters of 1599 was performed retrospectively using plasma concentration-time data previously determined in mice after subcutaneous administration of 159921 (Fig. 2). Pharmacokinetic modeling was used to estimate plasma concentrations of 1599 when administered BID or QD to assess if the dosing schedule used in vivo may have negatively impacted the efficacy of this combination. The dosing schedule used in the acute infection model (BID dosing for 9 consecutive days) was predicted to result in free plasma concentrations above the MIC of 1599 (defined here as >1 μg/mL) for 7.9 h, representing 34% of the dosing interval. The remaining 66% of the time between doses (7.9 h), free plasma concentrations were predicted to be below MIC. In contrast the dosing schedule used in the chronic infection trial (QD dosing 5/7 days) was predicted to result in periods below MIC of 68.3 h. Additionally, the total daily dose of 1599 and clarithromycin received was decreased by 50% compared to BID dosing when QD dosing was used. This also likely contributed to the lack of efficacy seen in chronic infection trial.
Figure 2. Pharmacokinetic modeling.
Free predicted 1599 plasma concentrations during efficacy trials. The MIC for 1599, 1 μg/mL was used to defined the in vivo therapeutic concentration (blue line). Predicted plasma concentrations are shown in (a) for BID and (b) for QD dosing. (c) shows the maximum consecutive time of free 1599 concentrations below the MIC for each dosing regimen.
Exposure of mycobacteria to sub-inhibitory concentrations of macrolides (including clarithromycin) induces the bacterium’s erm methyltransferase activity that modifies the macrolide binding site to provide antibacterial resistance to this class of drugs. Since 5/7 dosing and lower dosing was predicted to cause periods of monotherapy in which clarithromycin may have induced its own resistance, we next determined if pre-exposure to clarithromycin prevented synergism with 1599. M. tuberculosis strains H37Rv and CDC1551 were pre-incubated for 24 hours with sub-inhibitory concentrations of clarithromycin or vehicle prior to performing checkerboard assays (Supplementary Table S3). The combination of 1599 and clarithromycin synergized against untreated H37Rv (FICIs ranged from 0.06 to 0.13), while synergism was greatly ablated in bacteria pre-treated with 0.1 μg/mL clarithromycin (FICIs ranged from 0.65–1.0). Similar results were seen in separate experiments performed with strain CDC1551 (Supplementary Table S4). In the absence of clarithromycin pre-treatment, the lowest concentration of clarithromycin required to reduce the MIC of 1599 ranged from 0.1 to 0.8 μg/mL, which is lower than clarithromycin’s maximum plasma concentration (3 μg/mL) predicted in mice dosed orally at 200 mg/kg clarithromycin37. Once bacteria were exposed to monotreatment with clarithromycin (meant to simulate the 2 days off in a 5/7 dosing schedule), the concentration of clarithromycin required to reduce the MIC of 1599 was >50 μg/mL in all experiments. This grossly exceeds the 3 μg/mL peak plasma concentration of this antibiotic that is achievable in vivo. These data, combined with pharmacokinetic modeling, suggest that periods of clarithromycin monotherapy resulting from mismatched exposure profiles restricted the synergism in vivo by induction of macrolide resistance.
1599 potentiates the potency of clarithromycin protein synthesis inhibition
To determine if the strong synergism seen between 1599 and clarithromycin in whole cell assays stemmed from interactions at the ribosomal target, in vitro protein translation assays were performed using purified mycobacterial ribosomes. As comparators we included clindamycin and tetracyline, the two other ribosomal inhibitors shown to exhibit synergistic interaction with 1599 in whole cell assays. Titrations of 1599 and partner drugs were prepared (6 concentrations per compound) and the percent inhibition resulting from each of the 36 unique drug concentration reactions measured (Supplementary Fig. S1). A mutual reduction on ribosomal IC50 was seen for the combination of 1599 and clarithromycin, where the IC50 of clarithromycin was reduced from 1 to 0.2 μg/mL in the presence of sub-IC50 1599. Although titration translation assays did indicate potentiation of protein translation inhibition, the magnitude of this interaction did not match the strong synergism seen in whole cell growth assays. However, among the three drug classes tested and shown to exhibit synergistic activity in whole cell assays, the interaction between 1599 and clarithromycin was the most potent in the ribosomal target assay. Further studies may clarify if strong whole-cell synergism between 1599 and clarithromycin arises from an interaction independent of their known ribosomal binding sites as has been reported for streptogramin ribosome inhibitors38.
Discussion
In the present study, we identified several non-conventional anti-tuberculosis antibiotics that synergized with the anti-tubercular spectinamide 1599 to inhibit growth of M. tuberculosis. Hits for whole cell (MIC-based) synergism were validated in clinical isolates, confirming in vitro synergism between spectinamides, tetracyclines, macrolides, and closely related lincosamides. The high attrition noted when validating H37Rv hits strongly emphasizes the need to confirm screening hits obtained using a laboratory strain by re-testing with clinical isolates. While in vitro synergism occurred at concentrations reportedly achievable in vivo, synergism was not strictly recapitulated when combinations were tested in mouse models of acute and chronic tuberculosis infection. Of the three drugs tested for synergism with 1599 in vivo, only clarithromycin produced significant additive killing when combined with 1599 in the treatment of an acute tuberculosis infection. This activity was restricted to the 9 day acute infection model, however, as the 1599-clarithromycin combination yielded weaker killing compared to monotherapy with 1599 when tested in the model of chronic tuberculosis infection. This result was unanticipated, and underlines the need for further investigation to better understand the impact of inadequate clarithromycin drug exposure in future combination dosing regimens.
Sufficient concentrations of antibiotic in the bloodstream and affected tissues following dose administration are important for achieving synergism in vivo. Indeed, the importance of appropriately matched pharmacokinetic profiles – and the impact of mismatched exposure profiles - has been recently emphasized by the work of Drusano and others39. Mismatched exposure profiles can lead to periods of monotherapy, where bacteria are exposed to only the drug with the longer half-life. This can lead to periods of sub-inhibitory concentrations that may permit induction of transient resistance mechanisms and the selection of genetic mutants, as has been reported for the combination of rifampicin and moxifloxacin in M. tuberculosis39. Indeed we demonstrate here that exposure of M. tuberculosis to subinhibitory concentrations of clarithromycin induces clarithromycin resistance and may lead to subsequent loss of synergism with 1599 in vivo. As the duration of treatment was extended from dosing BID for 9 consecutive days in the acute infection model to 28 days QD 5 days per week in the chronic infection model employed, it is therefore not surprising to see induction of phenotypic clarithromycin-resistance. The consequence of this was likely induction of transient macrolide resistance, associated loss of clarithromycin efficacy, and subsequent loss of synergism with 1599. Modeling based upon known in vivo exposure profiles for the doses used also predicted that increasing dose frequency from once to twice daily and providing treatment 7 days per week should reduce periods in which macrolide resistance is induced in bacilli exposed only to clarithromycin thus enabling synergism in vivo. The lack of in vivo efficacy produced by these combinations when using a 5/7 day dosing regimen, however, may limit this combination to specific clinical situations where dosing and drug exposures are carefully monitored. Despite unclear efficacy, clarithromycin is included in salvage therapy for MDR tuberculosis when first and second line drugs fail40,41. Clarithromycin is well tolerated and has an acceptable toxicity profile, making it distinct from most second-line and salvage therapy agents42. While sub-inhibitory concentrations of clarithromycin may induce phenotypic macrolide resistance, clarithromycin may offer additional benefits including its anti-inflammatory properties and positive influence on pharmacokinetics of orally co-administered antibiotics, including linezolid for which serum exposure in MDR TB patients increases with clarithromycin43, likely through increased oral bioavailability due to inhibition of P-gp mediated efflux. Therefore, future combination trials with clarithromycin should not be necessarily ruled out, but must involve carefully chosen and monitored dosing regimen to ensure optimal exposure. Alternatively, macrolide derivatives that do not induce macrolide resistance may improve prospects for combination treatment with anti-tuberculars, including spectinamides44.
The challenge of treating tuberculosis infections is complicated by numerous factors, including M. tuberculosis’s impermeable cell wall structure, and its ability to remain latent for decades walled off within granuloma. The complex tissue matrix surrounding granulomas represents a further obstacle that successful anti-tuberculosis must permeate to reach resident bacteria45. This challenge is further exacerbated by findings indicating that a single granuloma is composed of several microenvironments and harbors a heterogeneous population of bacilli with distinct metabolic states and drug resistance profiles5. Careful design of drug combinations that do not necessarily synergize in the act of killing individual bacilli but rather reach and kill distinct bacterial subpopulations within the infected host, then, may lead to more complete sterilization and successful treatment. Therefore in vivo combination trials of 1599 with frontline and developing tuberculosis drugs are of particular interest and ongoing in our laboratories.
Methods
Checkerboard synergy assays
Whole cell in vitro synergy assays were performed using M. tuberculosis strain H37Rv, which was cultured as described previously21. In a 96-well assay plate, two-fold serial dilutions of Drug A were prepared in 100 μl of Middlebrook 7H9 media (highest and lowest concentrations in rows A and G, respectively, with no drug in row H). Using a single dip with a 200ss pintool, 0.2 μl of drug B (1599) was transferred to the assay plate columns 1 to 11 of the assay plate, with drug-free DMSO transferred to column 12. To each well of the assay plate 100 μl of mid-log phase bacteria (diluted to OD600 of 0.01) was added, and plates incubated for 7 days prior to reading MICs by visual inspection. Fractional inhibitory concentration index (FICI) scores were calculated using the formula [MIC drug B in presence of Drug A]/[MIC of drug B) + [MIC of drug A in the presence of drug B]/[MIC of drug A]. FICI scores were interpreted as follows: synergy (≤0.5), indifference (>0.5–4.0), or antagonism (>4.0)28. For each drug combination, FICI ranges were reported from two biologically independent experiments.
Ethics Statement
In vivo efficacy trials were performed at Colorado State University according to Protocol number 13-4263A, approved by the Colorado State University Institutional Animal Care and Use Committee (IACUC).
In vivo dosing selection for combination efficacy trails
Based upon promising in vitro synergism, clarithromycin, clindamycin, and doxycycline were chosen for 1599 combination testing in an in vivo model of acute M. tuberculosis infection46. Dosing regimens were selected based on their tolerability and ability to generate therapeutically similar systemic exposures in vivo in mice to those reported in the literature for humans. For clindamycin, an oral dose of 100 mg/kg BID was chosen based on mouse PK and plasma protein binding data that indicted free peak plasma concentrations of approximately 4 μg/mL for this dose47,48. For clarithromycin, an oral dose of 250 mg/kg BID was used, as this dosage was expected to result in free peak plasma concentrations of approximately 1 μg/mL49,50. For doxycycline, oral administration of 150 mg/kg BID was used, with an expected peak of 0.3 μg/mL for free concentrations51,52.
In vivo efficacy model of acute tuberculosis infection
Efficacy of antibiotics alone and in combinations was tested as essentially as described previously35,46,53. Briefly, 8 week female GKO mice (C57BL/6-IFNγ knockout from Jackson Laboratories) were infected with a low dose aerosol (LDA; ~100 CFU’s per mouse) of M. tuberculosis Erdman, transformed with pFCA-LuxAB. Beginning 13 days post-infection mice were dosed with antibiotics twice daily (BID). Indicated groups of 5 mice per group received monotherapy with 1599 (subcutaneous injection, 150 mg/kg of body weight BID), clarithromycin (oral delivery, 250 mg/kg BID), clindamycin (oral, 100 mg/kg BID), doxycycline (oral delivery, 150 mg/kg BID), or 1599 dosing in combination with clarithromycin, clindamycin, or doxycycline at doses indicated for monotherapy. Mice were dosed for 9 consecutive days. 10 days post-initiation of treatment, lungs were harvested and bacterial loads determined by enumeration of CFU.
In vivo efficacy model of chronic tuberculosis infection
The combination of 1599 with clarithromycin or with clindamycin was evaluated in a chronic infection, using female Balb/c mice (Charles River Labs, Wilmington, MA) infected with a LDA35,54,55,56. At 21 days post-infection, mice were treated once daily (QD) for 5 days a week (drug “holiday dosing”). Indicated groups of 6 mice per group received monotherapy with 1599 (subcutaneous injection, 150 mg/kg of body weight QD), clarithromycin (oral delivery, 250 mg/kg QD), clindamycin (oral, 100 mg/kg QD), of 1599 dosing in combination with clarithromycin, or clindamycin, or doxycycline at doses indicated for monotherapy infection model. Lungs were harvested after 28 days of treatment and bacterial loads determined by enumeration of CFU.
Pharmacokinetic modeling
Plasma concentration-time profiles for 1599 were simulated using a 2-compartment pharmacokinetic model based on pharmacokinetic data after subcutaneous administration in mice21. Simulations were performed for 5/7 and 7/7 day dosing with either QD and BID dosing using the software package Phoenix WinNonlin 6.3 (Icon Development Solutions, Hanover, MD).
Macrolide resistance induction
Checkerboard assays were performed in M. tuberculosis strains H37Rv and CDC1551 with and without pre-treatment with 0.1 μg/mL of clarithromycin, a sub-inhibitory concentration that increases the resistance of mycobacteria to clarithromycin.
Ribosomal inhibition assays
Protein translation assays using mycobacterial ribosomes were performed as described previously modified to measure the inhibition produced by combinations of two compounds57. For each drug combination, two-fold dilutions of compound 1599 were combined with two-fold dilutions of partner antibiotic to achieve 35 unique reaction concentration combinations where the concentration of each compound ranged from 1 to 1/16th the previously established ribosomal IC50.
Additional Information
How to cite this article: Bruhn, D. F. et al. In vitro and in vivo Evaluation of Synergism between Anti-Tubercular Spectinamides and Non-Classical Tuberculosis Antibiotics. Sci. Rep. 5, 13985; doi: 10.1038/srep13985 (2015).
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (grant AI090810) and the American Lebanese Syrian Associated Charities. We are grateful to Gregory Robertson (Colorado State University), Armand Guiguemde (St. Jude Children’s Research Hospital) and Robin E. Lee (St. Jude Children’s Research Hospital) for critical evaluation of this manuscript.
Footnotes
RL, BM and JL disclose intellectual property rights associated with the spectinamide series.
Author Contributions D.F.B., R.E.L., E.C.B. and A.J.L. designed the experiments. D.F.B., M.S.S. and D.S. performed the biological experiments. D.F.B. and B.M. performed pharmacokinetic modeling. J.L. synthesized compound 1599. D.F.B., B.M. and R.E.L. wrote the paper. All authors critically reviewed the paper.
References
- Zumla A., Raviglione M., Hafner R. & von Reyn C. F. Tuberculosis. N Engl J Med 368, 745–755, 10.1056/NEJMra1200894 (2013). [DOI] [PubMed] [Google Scholar]
- CDC. Trends in Tuberculosis, 2013. (U.S. Department of Health and Human Services Centers for Disease Control and Prevention, 2013).
- Mattila J. T. et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol 191, 773–784, 10.4049/jimmunol.1300113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Kaul A., Tsolaki A. G., Kishore U. & Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology 217, 363–374, 10.1016/j.imbio.2011.07.008 (2012). [DOI] [PubMed] [Google Scholar]
- Adams K. N. et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145, 39–53, 10.1016/j.cell.2011.02.022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12, 352–366, 10.1038/nri3211 (2012). [DOI] [PubMed] [Google Scholar]
- Gunther G. Multidrug-resistant and extensively drug-resistant tuberculosis: a review of current concepts and future challenges. Clin Med 14, 279–285, 10.7861/clinmedicine.14-3-279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Impact of a Shortage of First-Line Antituberculosis Medication on Tuberculosis Control — United States, 2012–2013. (U.S. Department of Health and Human Services Centers for Disease Control and Prevention, 2013).
- Pasipanodya J. G. et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208, 1464–1473, 10.1093/infdis/jit352 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottger E. C., Springer B., Pletschette M. & Sander P. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nat Med 4, 1343–1344, 10.1038/3906 (1998). [DOI] [PubMed] [Google Scholar]
- Sander P. et al. Fitness cost of chromosomal drug resistance-conferring mutations. Antimicrob Agents Chemother 46, 1204–1211 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. et al. In vivo efficacy of apramycin in murine infection models. Antimicrob Agents Chemother 58, 6938–6941, 10.1128/AAC.03239-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt T. et al. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc Natl Acad Sci USA 109, 10984–10989, 10.1073/pnas.1204073109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K. et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405, 962–966, 10.1038/35016103 (2000). [DOI] [PubMed] [Google Scholar]
- Matsumoto M. et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3, e466, 10.1371/journal.pmed.0030466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A. H. et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 56, 3271–3276, 10.1128/AAC.06126-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopova M. et al. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J Antimicrob Chemother 56, 968–974, 10.1093/jac/dki319 (2005). [DOI] [PubMed] [Google Scholar]
- Upton A. M. et al. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 59, 136–144, 10.1128/AAC.03823-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw G. E. et al. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med 184, 269–276, 10.1164/rccm.201011-1924OC (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. et al. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med 188, 600–607, 10.1164/rccm.201304-0650OC (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. E. et al. Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med, 10.1038/nm.3458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. P. et al. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407, 340–348, 10.1038/35030019 (2000). [DOI] [PubMed] [Google Scholar]
- Duscha S. et al. Identification and evaluation of improved 4′-O-(alkyl) 4,5-disubstituted 2-deoxystreptamines as next-generation aminoglycoside antibiotics. mBio 5, e01827–01814, 10.1128/mBio.01827-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie S. N. et al. Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proc Natl Acad Sci USA 105, 20888–20893, 10.1073/pnas.0811258106 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fernandez D. et al. 4′-O-substitutions determine selectivity of aminoglycoside antibiotics. Nat Commun 5, 3112, 10.1038/ncomms4112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N. D. et al. Transcriptional adaptation of drug-tolerant Mycobacterium tuberculosis during treatment of human tuberculosis. J Infect Dis, 10.1093/infdis/jiv149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balganesh M. et al. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob Agents Chemother 56, 2643–2651, 10.1128/AAC.06003-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Garcia S. et al. Synergistic drug combinations for tuberculosis therapy identified by a novel high-throughput screen. Antimicrob Agents Chemother 55, 3861–3869, 10.1128/AAC.00474-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Berens C., Projan S. J. & Hillen W. Comparison of tetracycline and tigecycline binding to ribosomes mapped by dimethylsulphate and drug-directed Fe2+ cleavage of 16S rRNA. J Antimicrob Chemother 53, 592–599, 10.1093/jac/dkh125 (2004). [DOI] [PubMed] [Google Scholar]
- Chu S. Y. et al. Pharmacokinetics of clarithromycin, a new macrolide, after single ascending oral doses. Antimicrob Agents Chemother 36, 2447–2453 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur D. et al. Bioavailability and selected pharmacokinetic parameters of clindamycin hydrochloride after administration of a new 600 mg tablet formulation. Int J Clin Pharmacol Ther 37, 386–392 (1999). [PubMed] [Google Scholar]
- Malmborg A. S. Bioavailability of doxycycline monohydrate. A comparison with equivalent doses of doxycycline hydrochloride. Chemotherapy 30, 76–80 (1984). [DOI] [PubMed] [Google Scholar]
- Sjolin-Forsberg G. & Hermansson J. Comparative bioavailability of tetracycline and lymecycline. Br J Clin Pharmacol 18, 529–533 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Herrera J., Reddy V. M., Daneluzzi D. & Gangadharam P. R. Antituberculosis activity of clarithromycin. Antimicrob Agents Chemother 39, 2692–2695 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts A. J. et al. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob Agents Chemother 49, 2294–2301, 10.1128/AAC.49.6.2294-2301.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts A. J., Degroote M. A. & Orme I. M. Preclinical testing of new drugs for tuberculosis: current challenges. Trends Microbiol 16, 48–54, 10.1016/j.tim.2007.12.002 (2008). [DOI] [PubMed] [Google Scholar]
- Hoffman H. L. et al. Influence of macrolide susceptibility on efficacies of clarithromycin and azithromycin against Streptococcus pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 47, 739–746 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canu A. & Leclercq R. Overcoming bacterial resistance by dual target inhibition: the case of streptogramins. Curr Drug Target Infect Disord 1, 215–225 (2001). [DOI] [PubMed] [Google Scholar]
- Drusano G. L. et al. The combination of rifampin plus moxifloxacin is synergistic for suppression of resistance but antagonistic for cell kill of Mycobacterium tuberculosis as determined in a hollow-fiber infection model. mBio 1, 10.1128/mBio.00139-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung K. J. et al. Salvage therapy for multidrug-resistant tuberculosis. Clin Microbiol Infect 20, 441–446, 10.1111/1469-0691.12335 (2014). [DOI] [PubMed] [Google Scholar]
- Dooley K. E. et al. World Health Organization group 5 drugs for the treatment of drug-resistant tuberculosis: unclear efficacy or untapped potential? J Infect Dis 207, 1352–1358, 10.1093/infdis/jis460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminero J. A., Sotgiu G., Zumla A. & Migliori G. B. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 10, 621–629, 10.1016/S1473-3099(10)70139-0 (2010). [DOI] [PubMed] [Google Scholar]
- Bolhuis M. S. et al. Clarithromycin increases linezolid exposure in multidrug-resistant tuberculosis patients. Eur Respir J 42, 1614–1621, 10.1183/09031936.00001913 (2013). [DOI] [PubMed] [Google Scholar]
- Falzari K. et al. In vitro and in vivo activities of macrolide derivatives against Mycobacterium tuberculosis. Antimicrob Agents Chemother 49, 1447–1454, 10.1128/AAC.49.4.1447-1454.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartois V. & Barry C. E. 3rd. A medicinal chemists’ guide to the unique difficulties of lead optimization for tuberculosis. Bioorg Med Chem Lett 23, 4741–4750, 10.1016/j.bmcl.2013.07.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts A. J., Gruppo V., Brooks J. V. & Orme I. M. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob Agents Chemother 47, 783–785 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeterp J. J., Mattie H. & van Furth R. Activity of erythromycin and clindamycin in an experimental Staphylococcus aureus infection in normal and granulocytopenic mice. A comparative in vivo and in vitro study. Scand J Infect Dis 25, 123–132 (1993). [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Lowe B. R., Dzink J. L. & Bartlett J. G. Antibiotic levels in infected and sterile subcutaneous abscesses in mice. J Infect Dis 143, 487–494 (1981). [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W. & Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother 33, 1531–1534 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee E., Azoulay-Dupuis E., Swanson R., Bergogne-Berezin E. & Pocidalo J. J. Individual and combined activities of clarithromycin and its 14-hydroxy metabolite in a murine model of Haemophilus influenzae infection. J Antimicrob Chemother 27 Suppl A, 31–41 (1991). [DOI] [PubMed] [Google Scholar]
- Zeidner N. S. et al. Sustained-release formulation of doxycycline hyclate for prophylaxis of tick bite infection in a murine model of Lyme borreliosis. Antimicrob Agents Chemother 48, 2697–2699, 10.1128/AAC.48.7.2697-2699.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. & Wormser G. P. Pharmacodynamics of doxycycline for chemoprophylaxis of Lyme disease: preliminary findings and possible implications for other antimicrobials. Int J Antimicrob Agents 31, 235–239, 10.1016/j.ijantimicag.2007.11.011 (2008). [DOI] [PubMed] [Google Scholar]
- Vicente E. et al. Efficacy of quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives in experimental tuberculosis. Antimicrob Agents Chemother 52, 3321–3326, 10.1128/AAC.00379-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts A. J., Degroote M. A. & Orme I. M. Preclinical testing of new drugs for tuberculosis: current challenges. Trends Microbiol 16, 48–54, 10.1016/j.tim.2007.12.002 (2008). [DOI] [PubMed] [Google Scholar]
- De Groote M. A. et al. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrobial Agents Chemother 55, 1237–1247, 10.1128/AAC.00595-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote M. A. et al. Importance of confirming data on the in vivo efficacy of novel antibacterial drug regimens against various strains of Mycobacterium tuberculosis. Antimicrobial Agents Chemother 56, 731–738, 10.1128/AAC.05701-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian S. et al. Structure-activity relationships among the kanamycin aminoglycosides: role of ring I hydroxyl and amino groups. Antimicrob Agents Chemother 56, 6104–6108, 10.1128/AAC.01326-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.