Abstract
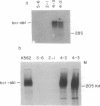
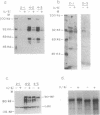
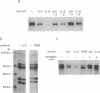
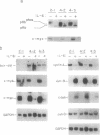
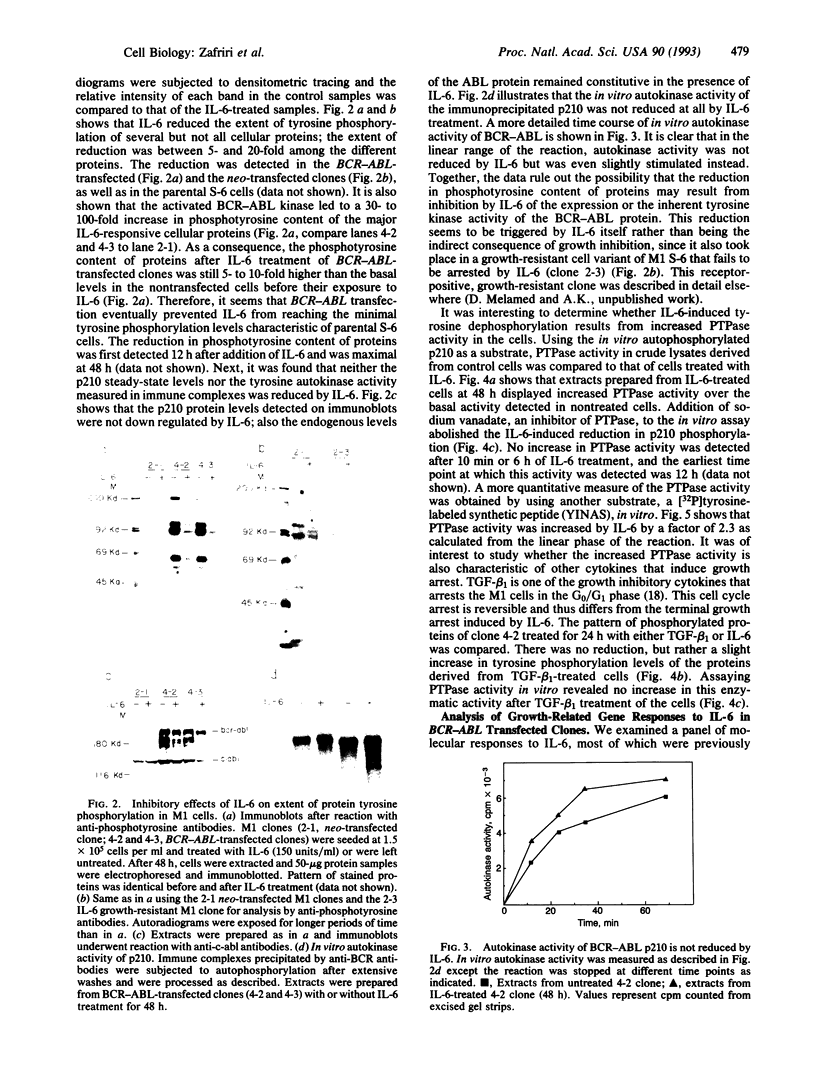
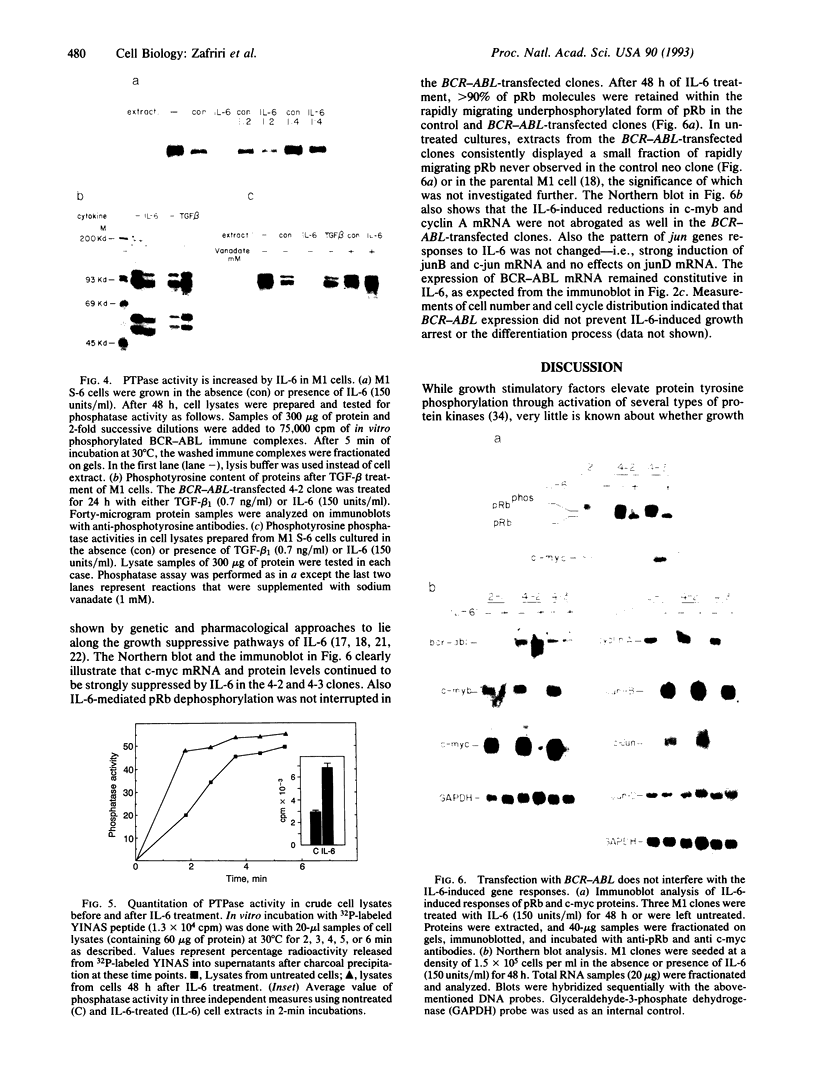
Interleukin 6 (IL-6) induces in M1 myeloblastic cells growth arrest and terminal differentiation toward monocytes. It is reported here that IL-6 reduced by 5- to 20-fold the tyrosine phosphorylation of cellular proteins in these cells. The same-fold reduction was also observed in M1 cells that were transfected with the BCR-ABL deregulated protein kinase. In these stable clones, the levels of tyrosine phosphorylation of cellular proteins were 30- to 100-fold higher than in the parental cells. IL-6 did not reduce the expression levels or the inherent tyrosine kinase activity of BCR-ABL p210. By measuring the protein-tyrosine-phosphatase (PTPase; protein-tyrosine-phosphate phosphohydrolase, EC 3.1.3.48) activity in crude cell lysates, we found that protein dephosphorylation resulted, at least partially, from induction of PTPase activity by IL-6. The induction of PTPase in the BCR-ABL-transfected clones was not sufficient to confer the minimal protein phosphorylation levels characteristic of IL-6-treated cells. Yet, the transfected M1 clones showed normal growth and differentiation responses to IL-6. None of the gene responses to IL-6 including suppression in the levels of c-myc, c-myb, and cyclin A mRNA; junB and c-jun mRNA induction; and dephosphorylation of retinoblastoma protein were rescued by the BCR-ABL oncogene. The functional relevance of PTPase induction by IL-6 is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollahi A., Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991 Aug;2(8):401–407. [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Fainstein E., Einat M., Gokkel E., Marcelle C., Croce C. M., Gale R. P., Canaani E. Nucleotide sequence analysis of human abl and bcr-abl cDNAs. Oncogene. 1989 Dec;4(12):1477–1481. [PubMed] [Google Scholar]
- Fainstein E., Marcelle C., Rosner A., Canaani E., Gale R. P., Dreazen O., Smith S. D., Croce C. M. A new fused transcript in Philadelphia chromosome positive acute lymphocytic leukaemia. 1987 Nov 26-Dec 2Nature. 330(6146):386–388. doi: 10.1038/330386a0. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. A., Sartorelli A. C. Regulation of protein phosphotyrosine content by changes in tyrosine kinase and protein phosphotyrosine phosphatase activities during induced granulocytic and monocytic differentiation of HL-60 leukemia cells. Biochem Biophys Res Commun. 1986 Oct 15;140(1):440–447. doi: 10.1016/0006-291x(86)91110-1. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Gough N. M., Dunn A. R., de Blaquiere J. Nucleotide sequence of cDNA clones of the murine myb proto-oncogene. EMBO J. 1985 Aug;4(8):2003–2008. doi: 10.1002/j.1460-2075.1985.tb03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A. Cytokine triggered molecular pathways that control cell cycle arrest. J Cell Biochem. 1992 Sep;50(1):1–9. doi: 10.1002/jcb.240500102. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Resnitzky D., Ber R., Gat G. Recessive genetic deregulation abrogates c-myc suppression by interferon and is implicated in oncogenesis. Mol Cell Biol. 1988 Jul;8(7):2828–2836. doi: 10.1128/mcb.8.7.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Activation of the abl oncogene in murine and human leukemias. Biochim Biophys Acta. 1985 Nov 12;823(1):1–17. doi: 10.1016/0304-419x(85)90012-5. [DOI] [PubMed] [Google Scholar]
- Laneuville P., Heisterkamp N., Groffen J. Expression of the chronic myelogenous leukemia-associated p210bcr/abl oncoprotein in a murine IL-3 dependent myeloid cell line. Oncogene. 1991 Feb;6(2):275–282. [PubMed] [Google Scholar]
- Lord K. A., Abdollahi A., Thomas S. M., DeMarco M., Brugge J. S., Hoffman-Liebermann B., Liebermann D. A. Leukemia inhibitory factor and interleukin-6 trigger the same immediate early response, including tyrosine phosphorylation, upon induction of myeloid leukemia differentiation. Mol Cell Biol. 1991 Sep;11(9):4371–4379. doi: 10.1128/mcb.11.9.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Pendergast A. M., Muller A. J., Witte O. N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990 Mar 2;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. A thousand and two protein tyrosine phosphatases. Biochim Biophys Acta. 1992 Jul 22;1136(1):35–43. doi: 10.1016/0167-4889(92)90082-m. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Kimchi A. Deregulated c-myc expression abrogates the interferon- and interleukin 6-mediated G0/G1 cell cycle arrest but not other inhibitory responses in M1 myeloblastic cells. Cell Growth Differ. 1991 Jan;2(1):33–41. [PubMed] [Google Scholar]
- Resnitzky D., Tiefenbrun N., Berissi H., Kimchi A. Interferons and interleukin 6 suppress phosphorylation of the retinoblastoma protein in growth-sensitive hematopoietic cells. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):402–406. doi: 10.1073/pnas.89.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Streuli M. Molecular characterization of protein tyrosine phosphatases. Cell Growth Differ. 1991 Jan;2(1):59–65. [PubMed] [Google Scholar]
- Shabo Y., Lotem J., Rubinstein M., Revel M., Clark S. C., Wolf S. F., Kamen R., Sachs L. The myeloid blood cell differentiation-inducing protein MGI-2A is interleukin-6. Blood. 1988 Dec;72(6):2070–2073. [PubMed] [Google Scholar]
- Shibata K., Nishimura J., Takahira H., Nawata H. Phosphotyrosine phosphatase activity prevents the detection of P210bcr/abl protein in mature cells in chronic myelogenous leukemia even by an immunoblotting technique. Leukemia. 1989 Sep;3(9):615–619. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden A., Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986 Dec 12;234(4782):1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]