Abstract
Purpose:
To evaluate retinal sensitivity over hard exudates in correlation with the spectral domain optical coherence tomography (SD-OCT) findings in eyes with diabetic retinopathy.
Methods:
Twelve eyes of 10 patients with hard exudates associated with diabetic retinopathy were enrolled in this study. All subjects underwent a complete ophthalmic examination including SD-OCT (Copernicus, Zawiercie, Poland) and microperimetry (MP1; Nidek Technologies, Padova, Italy). Retinal sensitivity was measured, over the areas with hard exudates and compared to corresponding locations devoid of hard exudates, using a semi-automatic program. The size of the hard exudate plaque was measured using the measurement software in the microperimeter. Retinal thickness in the area of the hard exudates and foveal thickness were measured using SD-OCT.
Results:
Mean retinal sensitivity over hard exudates was 4.97 ± 4.17 dB which was significantly (P = 0.0001) reduced as compared to locations devoid of hard exudates. No significant correlation (r=-0.23, P = 0.45) was found between the size of the hard exudates and retinal sensitivity. A significant negative correlation was found between retinal sensitivity and retinal thickness at the area of the hard exudates (r=-0.65, P = 0.05), and between retinal sensitivity and foveal thickness (r=-0.91, P = 0.001).
Conclusion:
In eyes with diabetic retinopathy, retinal sensitivity was reduced due to the presence of hard exudates in the outer retinal layers and retinal thickening but this was not correlated with the size of the hard exudates.
Keywords: Diabetic Retinopathy, Hard Exudates, Microperimeter, Retinal Sensitivity, Spectral Domain Optical Coherence Tomography
INTRODUCTION
Diabetic retinopathy is a common complication of diabetes mellitus, and remains one of the leading causes of visual impairment and blindness.[1,2,3] The deposition of hard macular exudates is a sight threatening consequence of macular edema in patients with diabetes mellitus.[4,5] Visual impairment has been correlated with the deposition of hard foveal exudates[6,7] which are one of the most common and early clinical signs of diabetic retinopathy. Hard exudates are lipid and lipoprotein deposits and appear as white, yellowish or waxy lesions situated mainly in the outer plexiform layer of the retina.[6,8] Large deposits of hard exudates increase the risk of developing subretinal fibrosis, which is one of the most devastating sequelae of diabetic maculopathy.[7] Previous investigations have shown that hard exudates may be associated with degeneration of both photoreceptors and neuronal elements in the outer plexiform layer.[9,10,11,12] Therefore, hard exudates may be an additional indicator for loss of retinal function. Disturbance of central vision, decreased fixation stability over hard exudates and retinal thickening in the fovea, have been reported in patients with diabetic maculopathy.[5,13]
The microperimeter (MP1, Nidek Technology, Italy) is an instrument used to assess retinal sensitivity at the area of interest, the presence of scotoma and fixation characteristics.[14,15,16] Spectral domain optical coherence tomography (SD-OCT, Copernicus, Poland) scans the posterior pole of the retina with high resolution (as high as 6 microns resolution) and provides a 3-dimensional image of the retina.[17]
Structural and functional integrity over the areas with hard exudates and locations devoid of hard exudates, has not been previously studied. The present study aimed to correlate retinal sensitivity with SD-OCT findings in areas with hard exudate deposition in eyes with diabetic retinopathy.
METHODS
Twelve eyes of ten patients with hard exudates due to diabetic macular edema, were studied in this prospective case-control study. Informed consent was obtained from the patients prior their participation. All patients underwent a comprehensive ophthalmic examination, including determination of best corrected visual acuity (BCVA) using an ETDRS chart, fundus evaluation, which included slit lamp biomicroscopy, indirect ophthalmoscopy, and digital fundus photography. Subjects with history of any ocular surgery or laser treatment and patients with hereditary or acquired macular disorders, other than hard exudates due to diabetic macular edema, were excluded. Patients with a significant ocular media opacity and pupil size less than 4 mm (due to poor quality SD-OCT scans) were excluded.
Different areas in the same eye were used as cases and controls. Retinal sensitivity, measured over the area with hard exudates, was recorded and compared with retinal sensitivity measured in areas devoid of hard exudates in the same eye. Retinal sensitivity was measured using MP1, and retinal thickness in areas with hard exudates and foveal thickness were measured using SD-OCT.
MP1 was performed [Figures 1 and 2] after mydriasis using Goldmann III size stimuli of 120 millisecond duration, a 4-2 threshold strategy and a white background with 4 apostilbs luminance. A semi-automatic program was used to manually select the area on the retina in which the stimuli were projected. Initially, the color photograph of the retina being examined was taken using MP1; the borders of the hard exudates were marked manually and stimulations were automatically projected inside the marked area of interest on the retina. An equal amount of area was stimulated in the area devoid of hard exudates in the same retina, considered as control points. The stimuli were projected, one at a time, and the patient was asked to respond to every stimulus seen by pressing a hand-held button. A well-defined reference mark in the retina was chosen by the examiner to track saccadic eye movements. A pre-test training was given before starting the test. False negative responses were tested once in a minute during the test. Fixation characteristics, like fixation stability and location, were measured during the test. Fixation patterns were analyzed based on data obtained during the retinal sensitivity testing. Fixation characteristics were classified according to the literature, explained elsewhere.[18]
Figure 1.
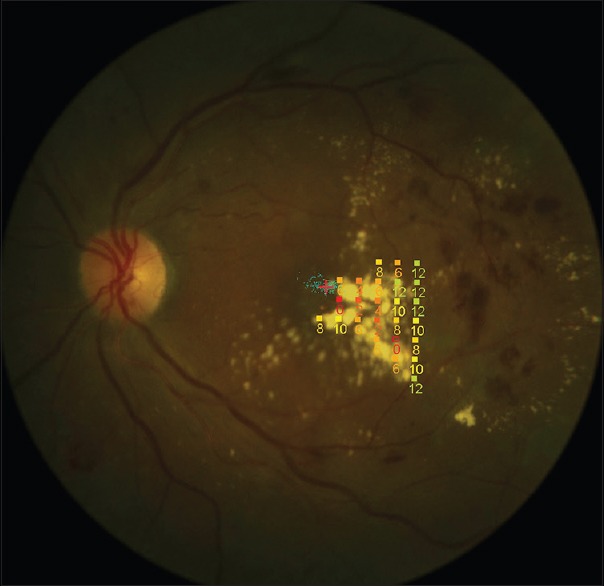
Stimulus pattern of retinal sensitivity measurements using microperimetry over the hard exudate area.
Figure 2.
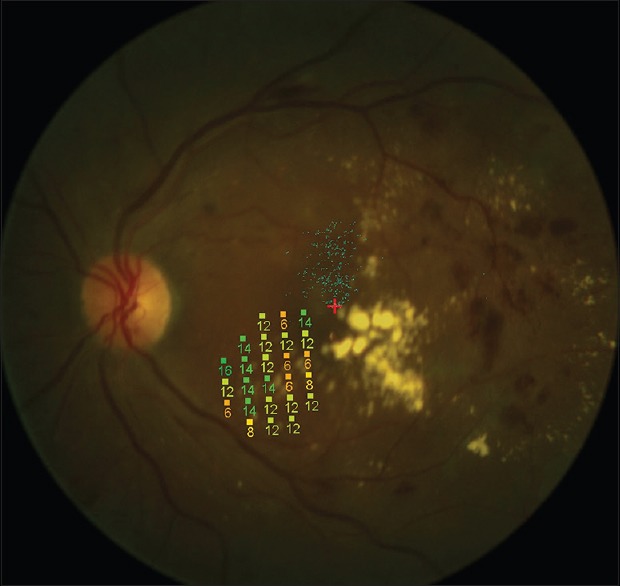
Stimulus pattern of retinal sensitivity measurements using microperimetry over the non-hard exudate area.
SD-OCT imaging [Figure 3] was performed after pupil dilatation using a 7 mm asterix scan (15 B-scans, 2,473 A-scans per B-scan) passing through the foveola and over the area of the hard exudates. The scan length was increased according to the location of the hard exudates. Retinal thickness at the area of the hard exudates and foveal thickness were measured manually, using the measurement software present in the instrument. Statistical analysis was performed using Statistical Package for Social Sciences, version 12.0, (SPSS, Chicago, Illinois) for windows. All measurements were recorded by the same observer.
Figure 3.
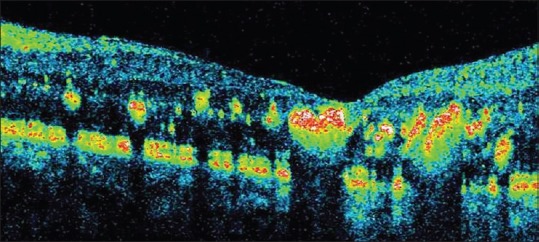
Spectral domain optical coherence tomography showing retinal thickening in areas with hard exudates and their location in outer plexiform layer.
RESULTS
A total of 10 patients with mean age of 56.33 ± 7.3 years were studied. Visual acuity of subjects ranged from 0.20-1.50 logMAR. Three hundred and twenty-nine points were stimulated over hard exudates in twelve eyes; one hundred and forty eight points were stimulated over non-hard exudates area of five eyes of the same patients. Areas without diabetic retinopathy lesions and without any increase in retinal thickness were used as control points. Retinal sensitivity measured over the hard exudates was compared to retinal sensitivity measured over the normal retina.
Retinal sensitivity over hard exudates was significantly (P < 0.001) lower as compared to retinal sensitivity over areas devoid of hard exudates. Retinal sensitivity ranged from 0 dB to 18 dB (mean: 4.97 ± 4.17 dB) in areas with hard exudates, and 2 dB to 20 dB (mean: 13.13 ± 3.7 dB) in control areas. Retinal thickness, measured at the area of hard exudates (r-0.65; P < 0.05), and foveal thickness (r-0.93; P < 0.001) were significantly and negatively correlated with retinal sensitivity. Retinal thickness at the areas of hard exudates was 401 ± 114 (range, 251-609) microns. The size of hard exudate plaques ranged from 1,381-6,652 microns and there was no significant correlation (r-0.22; P = 0.45) between the size of hard exudate plaques and retinal sensitivity over the hard exudates.
All eyes had central fixation. Three eyes (25%) showed stable fixation, four (33.3%) showed relatively unstable fixation and five showed (41.6%) unstable fixation.
DISCUSSION
Microperimetric evaluation of retinal sensitivity has been used for several macular disorders.[19,20,21] Previous studies have shown that microperimetry provides exact measurement of retinal sensitivity and directly correlates fundus details with retinal function.[16] In this study, a semiautomatic program was used to simulate the retina. This program provided measurement of retinal sensitivity over the retinal lesions. In patients with diabetic retinopathy, the correlation between retinal sensitivity using microperimetry, visual acuity and retinal thickness determined by OCT has been reported.[14,15]
Retinal sensitivity, measured using the microperimeter, has been reported in cases of diabetic retinopathy[14,15] and diabetic maculopathy.[16] In this study, an attempt was made to evaluate localized retinal sensitivity in areas affected with hard exudates and compare it with retinal sensitivity over areas devoid of hard exudates in the same eyes. Retinal sensitivity was decreased over the hard exudates when compared to the non-hard exudates area. The reduction of retinal sensitivity observed in this study was more than that reported in a previous study (13.3 ± 6.7 dB) using a scanning laser ophthalmoscope microperimeter.[22] This difference may be due to the use of a different type of stimulation program. Our study showed that loss of retinal sensitivity over hard exudates could cause visual impairment and scotoma, which is in line with previous reports.[5,6,23]
Studies have reported a direct association between the severity of hard exudates and visual disturbances.[5,24] In contrast, the current study found no significant association between the size of hard exudate plaques and retinal sensitivity or visual acuity. Fixation instability has been reported in diabetic macular edema;[22] according to our data, most patients with hard exudates showed unstable fixation. Foveal hard exudates may be associated with dense scotoma and deteriorated fixation.[5,16]
In the current series, the retina showed spongiform thickening at the area of hard exudates and retinal thickening in the area of hard exudates was negatively correlated with retinal sensitivity. As previously reported, hard exudates represent leakage of lipoprotein at the outer plexiform layer and lead to neurological and photoreceptor damage.[9,10,11,12] Reduced retinal sensitivity is due to the deposition of hard exudates in the macula.[25] In the current study, retinal sensitivity was not correlated with the size of the hard exudates plaque. Thus, it seems that probably the biochemical composition but not size of the hard exudates, determine retinal sensitivity as well as retinal thickness. Also, it may be that the vertical extent of the hard exudate rather than the horizontal area of the hard exudate, may affect retinal sensitivity.
Parisi et al[26] evaluated psychophysical and electrophysiological responses in eyes with early age-related macular degeneration and reported a significant correlation between multifocal electroretinogram (mfERG) and microperimetry responses, suggesting that the reduction in macular sensitivity using MP1 could be ascribed to retinal factors leading the abnormal mfERG responses such as a dysfunction in preganglionic macular elements, i.e. photoreceptors and bipolar cells.
To the best of the authors’ knowledge, no study has assessed the sensitivity and specificity of microperimetry in assessing localized retinal sensitivity. In the study done by Oztürk et al[27] who evaluated the macula in primary open angle glaucoma, the area under the curve was reported to be 0.65. However, this was based on mean retinal sensitivity and not localized retinal sensitivity which was used in this study.
In summary, this study evaluated localized retinal sensitivity in areas containing hard exudates in patients with diabetic retinopathy. Retinal sensitivity was correlated with retinal thickness, foveal thickness and the location of the hard exudates but not with the size of the hard exudates.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Kalesnykiene V, Sorri I, Voutilainen R, Uusitupa M, Niskanen L, Uusitalo H. The effect of glycaemic control on the quantitative characteristics of retinopathy lesions in patients with type 2 diabetes mellitus: 10-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2009;247:335–341. doi: 10.1007/s00417-008-1001-6. [DOI] [PubMed] [Google Scholar]
- 2.Porta M, Bandello F. Diabetic retinopathy. A clinical update. Diabetologia. 2002;45:1617–1634. doi: 10.1007/s00125-002-0990-7. [DOI] [PubMed] [Google Scholar]
- 3.Ong GL, Ripley LG, Newsom RS, Cooper M, Casswell AG. Screening for sight-threatening diabetic retinopathy: Comparison of fundus photography with automated color contrast threshold test. Am J Ophthalmol. 2004;137:445–452. doi: 10.1016/j.ajo.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo T. Disappearance of diabetic macular hard exudates after hemodialysis introduction. Acta Med Okayama. 2006;60:201–205. doi: 10.18926/AMO/30746. [DOI] [PubMed] [Google Scholar]
- 5.Møller F, Bek T. The relation between visual acuity, fixation stability, and the size and location of foveal hard exudates after photocoagulation for diabetic maculopathy: A 1-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2003;241:458–462. doi: 10.1007/s00417-003-0661-5. [DOI] [PubMed] [Google Scholar]
- 6.Otani T, Kishi S. Tomographic findings of foveal hard exudates in diabetic macular edema. Am J Ophthalmol. 2001;131:50–54. doi: 10.1016/s0002-9394(00)00661-9. [DOI] [PubMed] [Google Scholar]
- 7.Larsson J, Kifley A, Zhu M, Wang JJ, Mitchell P, Sutter FK, et al. Rapid reduction of hard exudates in eyes with diabetic retinopathy after intravitreal triamcinolone: Data from a randomized, placebo-controlled, clinical trial. Acta Ophthalmol. 2009;87:275–280. doi: 10.1111/j.1755-3768.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia M, Hornero R, Sánchez CI, López MI, Diez A. Feature extraction and selection for the automatic detection of hard exudates in retinal images. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4969–4972. doi: 10.1109/IEMBS.2007.4353456. [DOI] [PubMed] [Google Scholar]
- 9.Fong DS, Segal PP, Myers F, Ferris FL, Hubbard LD, Davis MD. Subretinal fibrosis in diabetic macular edema. ETDRS report 23. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1997;115:873–877. doi: 10.1001/archopht.1997.01100160043006. [DOI] [PubMed] [Google Scholar]
- 10.Bek T, Lund-Andersen H. Localised blood-retinal barrier leakage and retinal light sensitivity in diabetic retinopathy. Br J Ophthalmol. 1990;74:388–392. doi: 10.1136/bjo.74.7.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloodworth JM., Jr Diabetic retinopathy. Diabetes. 1962;11:1–22. [PubMed] [Google Scholar]
- 12.Bresnick GH. Nonproliferative diabetic retinopathy. In: Ryan SJ, editor. Retina. 2nd ed. St Louis: Mosby; 1994. pp. 1277–1318. [Google Scholar]
- 13.Møller F, Bek T. The relation between visual acuity and the size of fixational eye movements in patients with diabetic and non-diabetic macular disease. Acta Ophthalmol Scand. 1998;76:38–42. doi: 10.1034/j.1600-0420.1998.760107.x. [DOI] [PubMed] [Google Scholar]
- 14.Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F. Diabetic macular edema: Correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci. 2006;47:3044–3051. doi: 10.1167/iovs.05-1141. [DOI] [PubMed] [Google Scholar]
- 15.Okada K, Yamamoto S, Mizunoya S, Hoshino A, Arai M, Takatsuna Y. Correlation of retinal sensitivity measured with fundus-related microperimetry to visual acuity and retinal thickness in eyes with diabetic macular edema. Eye (Lond) 2006;20:805–809. doi: 10.1038/sj.eye.6702014. [DOI] [PubMed] [Google Scholar]
- 16.Vujosevic S, Pilotto E, Bottega E, Benetti E, Cavarzeran F, Midena E. Retinal fixation impairment in diabetic macular edema. Retina. 2008;28:1443–1450. doi: 10.1097/IAE.0b013e318183571e. [DOI] [PubMed] [Google Scholar]
- 17.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 18.Carpineto P, Ciancaglini M, Di Antonio L, Gavalas C, Mastropasqua L. Fundus microperimetry patterns of fixation in type 2 diabetic patients with diffuse macular edema. Retina. 2007;27:21–29. doi: 10.1097/01.iae.0000256658.71864.ca. [DOI] [PubMed] [Google Scholar]
- 19.Charbel Issa P, Helb HM, Rohrschneider K, Holz FG, Scholl HP. Microperimetric assessment of patients with type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2007;48:3788–3795. doi: 10.1167/iovs.06-1272. [DOI] [PubMed] [Google Scholar]
- 20.Midena E, Vujosevic S, Convento E, Manfre’ A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–1503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nittala MG, Laxmi G, Raman R, Rani PK, Bhargava A, Pal SS, et al. Spectral-domain OCT and microperimeter characterization of morphological and functional changes in X-linked retinoschisis. Ophthalmic Surg Lasers Imaging. 2009;40:71–74. doi: 10.3928/15428877-20090101-16. [DOI] [PubMed] [Google Scholar]
- 22.Kube T, Schmidt S, Toonen F, Kirchhof B, Wolf S. Fixation stability and macular light sensitivity in patients with diabetic maculopathy: A microperimetric study with a scanning laser ophthalmoscope. Ophthalmologica. 2005;219:16–20. doi: 10.1159/000081777. [DOI] [PubMed] [Google Scholar]
- 23.Ozer PA, Unlu N, Demir MN, Hazirolan DO, Acar MA, Duman S. Serum lipid profile in diabetic macular edema. J Diabetes Complications. 2009;23:244–248. doi: 10.1016/j.jdiacomp.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Chew EY, Klein ML, Ferris FL, 3rd, Remaley NA, Murphy RP, Chantry K, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 25.Cekiç O, Bardak Y, Tig US, Yildizoglu U, Bardak H. Quantitative evaluation of reduction of plaque-like hard exudates in diabetic macular edema after intravitreal triamcinolone injection. Int Ophthalmol. 2008;28:95–99. doi: 10.1007/s10792-007-9120-3. [DOI] [PubMed] [Google Scholar]
- 26.Parisi V, Perillo L, Tedeschi M, Scassa C, Gallinaro G, Capaldo N, et al. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina. 2007;27:879–890. doi: 10.1097/IAE.0b013e318042d6aa. [DOI] [PubMed] [Google Scholar]
- 27.Oztürk F, Yavas GF, Küsbeci T, Ermis SS. A comparison among Humphrey field analyzer, Microperimetry, and Heidelberg Retina Tomograph in the evaluation of macula in primary open angle glaucoma. J Glaucoma. 2008;17:118–121. doi: 10.1097/IJG.0b013e31814b97fd. [DOI] [PubMed] [Google Scholar]


