Abstract
The continued need to develop minimally invasive alternatives to surgical and radiologic interventions has driven the development of endoscopic ultrasound (EUS)-guided treatments. EUS has now stepped into the therapeutic arena. EUS provides the unique advantage of both real-time imaging and access to structures within and adjacent to the gastrointestinal (GI) tract. Hence, EUS-guided therapeutic techniques continue to evolve in several directions enabling a variety of minimally invasive therapies for pancreatic and biliary pathologies. Furthermore, the close proximity of the GI tract to vascular structures in the mediastinum and abdomen permits EUS-guided vascular access and therapy. Studies have demonstrated several EUS-guided vascular interventions by using standard endoscopic accessories and available tools from the interventional radiology armamentarium. This article provides an overview of the literature including clinical and nonclinical studies for the management of nonvariceal and variceal GI bleeding, formation of intrahepatic portosystemic shunts (IPSS), and EUS-guided cardiac access and therapy.
Keywords: EUS, EUS-guided coil, EUS-guided cyanoacrylate injection, EUS-guided sclerosant, EUS guided vascular therapy, guided vascular access, injection, non-variceal gastrointestinal bleeding, varices, vascular abnormality
INTRODUCTION
Since its inception in the 1980s, endoscopic ultrasound (EUS) has evolved from a mere diagnostic tool to an interventional platform employing the skills learnt from interventional radiology (IR) and minimally invasive surgery techniques. EUS provides the perfect combination of real-time imaging with the opportunity to guide anatomically specific therapy. EUS imaging provides vital detail, such as the appearance, size, and precise location of bleeding lesions, with the associated vascular anatomy. Precise therapy can be delivered to vascular sites that are inaccessible to usual hemostatic techniques that solely use endoscopic visualization.
Through the development of interventional EUS, pathologies such as pancreatic pseudocyst can be successfully managed endoscopically rather than traditional surgical methods. The evolution of EUS-guided vascular access is made possible by the close proximity of the gastrointestinal (GI) tract to vascular structures in the mediastinum and abdomen. EUS-guidance provides unique access to vessels which have been the target for therapy by IR techniques for many decades, such as selective angiographic embolization for refractory GI bleeding and transjugular intrahepatic portosystemic shunt (TIPSS) for refractory gastroesophageal variceal bleeding.[1] Major vessels such as the aorta, coeliac axis, portal vein (PV), hepatic vein (HV), mesenteric vessels, aberrant vascular shunts, and even smaller vessels such as the gastroduodenal artery (GDA) and splenic vessels can all be confidently traced and identified. Visceral pseudoaneurysms [Figure 1 and Video 1] are a rare but serious complication of pancreatitis or abdominal surgery. Pseudoaneurysmal rupture and bleeding is traditionally managed by IR or surgery, and is associated with considerable morbidity and mortality.[2,3] However, EUS-guidance offers an attractive, minimally-invasive, alternate access route and opportunity for therapeutic intervention. Agents such as sclerosants, cyanoacrylate (CYA), thrombin, and coils can all be delivered through a standard EUS fine needle aspirate (FNA) into the lesion.
Figure 1.
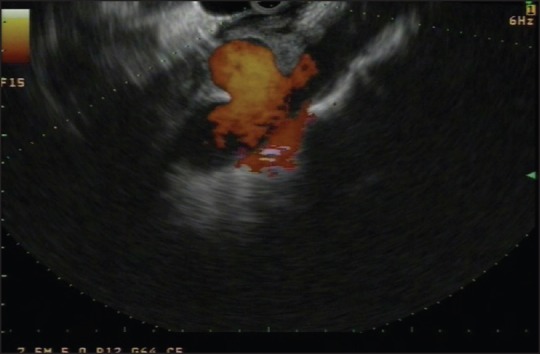
Endoscopic ultrasound image of a celiac artery aneurysm (white arrow) is obtained using a linear echoendoscopic and color Doppler
NONVARICEAL GI BLEEDING
Endoscopic techniques for treatment of nonvariceal GI bleeding include injection of adrenaline,[4,5] thermal contact therapy,[6,7] mechanical hemostasis with endoscopic clips,[8] and band ligation.[9] These well-established modalities are effective in majority of cases. However, unsuccessful treatment or refractory bleeding is reported in up to 15% of patients.[10,11,12]
EUS-guided vascular access and therapy was first described in 1996[13] when Fockens and colleagues treated four patients with Dieulafoy's lesion under direct guidance of a rotating sector scanner EUS with adrenaline/polidocanol injection using a standard 23-gauge sclerotherapy needle. More recently, Levy et al., reported a case series of five patients with refractory bleeding due to hemosuccus pancreaticus, Dieulafoy's lesion, duodenal ulcer, and GI stromal tumor (GIST).[14] The patients in their study presented with an average of three bleeding episodes requiring multiple units of packed red blood cell transfusions and had undergone prior heater probe therapy, adrenaline, CYA, or fibrin injection and coil/gel foam injection at angiography; all of which were unsuccessful. EUS was able to demarcate tortuous vessels and/or pseudoaneurysm feeding the bleeding lesions. Targeted EUS-guided therapy was performed using a linear echoendoscope in all cases. CYA or alcohol (99%) was injected into the feeding vessels using a 22-gauge FNA needle. Doppler ultrasound confirmed absence of visible flow post-injection, indicating control of the bleeding source. There were no rebleeding episodes or adverse events at mean 12-month (range 0.4-23 months) follow-up. Gonzalez and colleagues also reported a series of five patients with arterial GI bleeding (Dieulafoy's lesion, pancreatic tumor, pseudoaneurysm secondary to acute pancreatitis, and arterial anomaly post pancreaticoduodenectomy) refractory to endoscopic hemostasis.[15] EUS-guided polidocanol or CYA injection achieved immediate hemostasis which was confirmed on Doppler ultrasound. One patient required repeat EUS-guided therapy for rebleeding. There was no further rebleeding or adverse events at 9-month follow-up. The same group reported a patient with intracystic hemorrhage of a splenic pseudoaneurysm during EUS-guided pseudocyst drainage. Immediate EUS-guided CYA injection into the distal arm of the splenic artery was performed to achieve hemostasis.[16] Recently, Kumbhari et al., demonstrated successful EUS-guided CYA injection of a feeding vessel of a gastric GIST causing recurrent GI bleeding refractory to endoscopic over-the-scope-clip placement in a 94-year-old man[17] [Figure 2 and Video 2].
Figure 2.
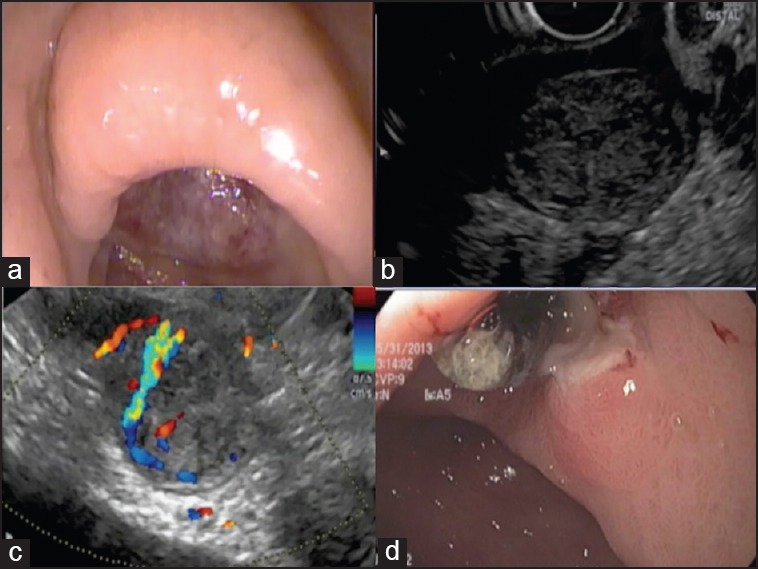
(a) Endoscopic image of a large ulcer in the antrum of the stomach. (b) Endoscopic ultrasound demonstrates a hypoechoic mass arising from the muscularis propria at the level of the antral ulcer, suggestive of a gastrointestinal stromal tumor (GIST). (c) Color Doppler of the GIST reveals a vessel feeding into the tumor, the likely cause of recurrent bleeding. (d) Endoscopic appearance of ulcer post EUS-guided injection of cyanoacrylate into the feeding vessel. The ulcer has reduced considerably in size
The current evaluation of EUS-guided interventions for the management of nonvariceal refractory GI bleeding is limited to small case series and there are no comparative studies. However, the feasibility and safety demonstrated in these studies is encouraging. There is a clear advantage of direct EUS and Doppler visualization of the “culprit” vessel responsible for the recurrent bleeding. EUS allows precise treatment delivery with the prospect of higher successful treatment rates in patients with bleeding refractory to standard endoscopic hemostasis techniques.
ESOPHAGEAL VARICEAL BLEEDING
Endoscopic sclerotherapy[18] was considered the standard endoscopic treatment for esophageal varices until endoscopic band ligation was introduced in 1986.[19] Band ligation is the standard method for primary and secondary treatment of esophageal varices.[20] However, recurrence rates of 15-65%[21,22] have been reported and are thought to be due to failure to treat the perforating veins and collateral vessels feeding the esophageal varices.[23,24]
In a small pilot study (n = 5), Lahoti et al., treated bleeding esophageal varices with EUS-guided sclerotherapy.[25] EUS permitted the endoscopist to target the perforating veins with sodium morrhuate. A mean of 2.2 sessions were required to achieve complete eradication of esophageal varices. Obliteration or thrombosis of vessels was confirmed using Doppler ultrasound. There were no rebleeding or adverse events at 15-month follow-up. Subsequently, a randomized controlled trial compared endoscopic sclerotherapy with EUS-guided sclerotherapy, of esophageal collateral veins in 50 patients with bleeding esophageal varices.[25] Sclerotherapy was repeated at 2-week intervals until complete eradication was achieved. EUS was performed in all patients at the end of treatment to assess for the presence of collateral veins. There was no difference in mean number of sessions to eradication (4.3 vs. 4.1, P = 0.52) and recurrence rates were similar (16.7% vs. 8.3%, P = 0.32) in the endoscopic versus EUS group, respectively. However, recurrence was significantly associated with the presence of collateral vessels (P = 0.003) which was higher in the endoscopic group (33.3% vs. 0%, P = 0.004) at 22-month follow-up.
Although EUS-guided therapy of esophageal varices carry the advantage of identifying collateral veins, larger randomized controlled trials are needed to determine the clinical benefit in terms of preventing recurrence and rebleeding rates or reducing the number of sessions needed to eradicate varices.
GASTRIC VARICES
Gastric varices (GV) are less common than esophageal varices, but may occur in up to 20% of patients with portal hypertension. Majority (up to 65%) will bleed over a 2-year period.[26] GV can be classified into two subgroups: Gastroesophageal varices (GOV) and isolated GV (IGV). GOV exist in connection with esophageal varices and are found along the lesser curvature (GOV1) or at the cardia (GOV2). IGV are located in the fundus (IGV1) or sporadically (IGV2) around the antrum and pylorus. Endoscopic band ligation is not preferred in treating GV, due to the larger size of GV (compared to esophageal varices) and higher risk of rebleed after band ligation.[27] Sclerosant injection into GV is avoided due to associated excessive adverse events including gastric ulceration, perforation, and high recurrent bleeding rates of 37-53%.[26,28]
The initial description of endoscopic CYA injection into GV by Soehendra in 1986 represented a milestone in its management and is now widely considered as a first-line therapy.[29] Hemostasis rates are very high (89-100%),[30,31,32] although a major adverse event related to CYA therapy is systemic embolization, which is thought to be related to the volume of CYA injection.[33,34] Several reports of cardiac embolism, pulmonary embolism, splenic vein thrombosis, splenic artery embolism, renal vein thrombosis, and cerebral infarct have been documented. Entrapment of the needle in the varix by CYA and damage to the endoscope has also been reported.[35,36,37] However, incomplete eradication of GV leads to the risk of recurrent and catastrophic bleeding.
The most notable role of EUS in the management of GV is its diagnostic capability [Video 3]. Bearing in mind that GV are located in the deep submucosal layer, they can have a similar appearance to prominent mucosal gastric folds. A prospective study showed EUS increased the detection of fundal varices sixfold.[38] Lee et al., demonstrated EUS reduced the risk of bleeding when performed to monitor GV obliteration after CYA injection.[39] Repeat injections were performed in the same procedure if EUS confirmed persistent flow on Doppler ultrasound. Iwase and colleagues showed similar results in their study.[40] Residual patency of treated varices correlated with risk of rebleeding after glue injection. Varix obliteration was also confirmed by the absence of blood flow on color Doppler.
EUS-guided CYA injection
There are several advantages of direct EUS-guided therapy in the management of GV. The variceal lumen can be precisely targeted, thereby reducing paravariceal injection which can occur during “blind” endoscopic injection; the variceal lumen can be “missed” in up to 60% of injections.[27] Furthermore, EUS enables visualization (and injection) of the deeper, feeding veins which can aid in minimization of CYA volume used to obliterate the resultant large varix.
A small pilot study showed that an average of only 1.6 mL of CYA was required to eradicate gastric varies in a mean of 1.6 sessions. There was no rebleeding at 10.6-month follow-up.[33] However, it is important to note that identification of the feeder vessel can be challenging, and direction of efferent or afferent vessel cannot always be determined. Injection of a small amount of contrast prior to CYA injection may help delineate the direction of blood flow.
EUS-guided coiling
Coil embolization is a hemostatic technique derived from our IR colleagues. To avoid the adverse events related to the use of CYA, commercially available embolization coils have been used to successfully obliterate varices. The coil is made of a metal alloy and contains radially extending synthetic fibers, which help induce clot formation and subsequent hemostasis. The fibers also act as a scaffold to retain CYA, if injected subsequently in the same session. The coil length ranges from 2 to 15 cm and coil diameter ranges from 2 to 20 mm. Coil selection is based on the size/diameter of the varix and is approximated to match the varix size. Most coils used in IR can be loaded into a 19 gauge (0.035 inch coil) or a 22 gauge (0.018 inch coil) EUS-FNA needle [Video 4]. The needle stylet is used as a pusher to deploy the coil into the varix. The coils used by the authors (MReye, Cook Medical, Bloomington IN, USA) are made of Inconel, a nickel-based superalloy which are magnetic resonance imaging (MRI) conditional up to 3 Tesla.
Levy et al., reported the first case of EUS-guided coil embolization of refractory, ectopic choledochojejunal variceal bleeding in a 50-year-old woman after total pancreatectomy.[41] Another small study demonstrated obliteration of GV in three out of four patients (75%) with mean 8.5 coils (range 2-22). The authors modified their technique to target the feeding vein, thereby reducing the number of coils used per patient.
A recent retrospective trial compared EUS-guided coil injection (n = 11) to EUS-guided CYA injection (n = 19) for the treatment of GV.[42] All patients underwent CT post treatment. There was no difference in the obliteration rate (91% vs. 100%, P = nonsignificant (NS), mean number of sessions (1.3% vs. 1.5%, P = NS), or recurrence (0% vs. 0%, P = NS) in the coil vs. CYA group, respectively, at 17-month follow-up. Notably, adverse events were significantly higher in the CYA group (58% vs. 9%, P = 0.01), although nine of the 11 adverse events in the CYA group were asymptomatic pulmonary CYA embolism found on post-procedure CT, which led to prolonged hospital length of stay. Thus, systemic glue embolization appears to be more common than being appreciated, although seldom symptomatic.
EUS-guided combined CYA injection and coiling
An ex vivo experiment revealed that CYA immediately adheres to the coil fibers when a coil is immersed into heparinized blood.[43] Since the coil fibers act as a scaffold for glue, combining coil and glue injection for treatment of large GV is appealing as it could potentially decrease the volume of CYA needed to achieve variceal obliteration. The combined technique is described in Table 1 and demonstrated in Video 5.
Table 1.
Technique of EUS-guided combined coil and cyanoacrylate injection for treatment of gastric varices
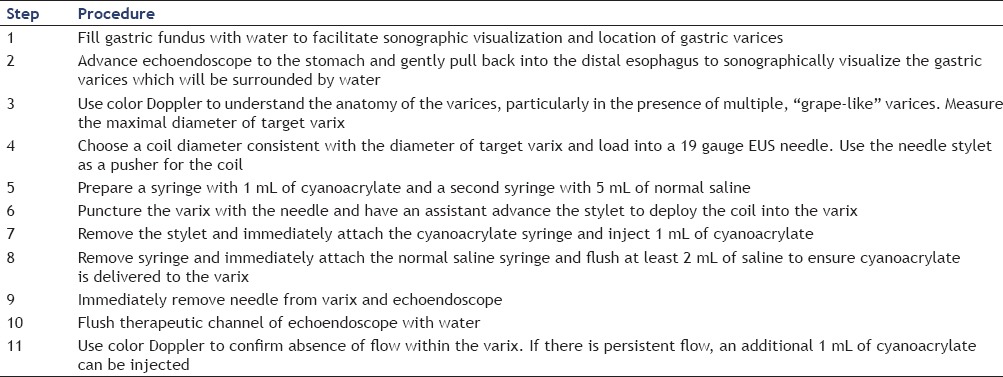
A recent retrospective study evaluated treatment of patients with active recent bleeding secondary to large GV (>1 cm diameter) who were poor candidates for TIPSS placement.[43] A total of 30 patients underwent combined treatment with EUS-guided coiling and CYA injection via a transesophageal route, avoiding direct puncture of the mucosal side of the gastric varix. Only one coil was used in majority (93%) of patients and mean volume of CYA injected was 1.4 mL. Two patients developed esophageal bleeding from the needle injection site which was treated with band ligation. Rebleeding occurred in 16.6% of patients at 6.6-month follow-up. There were no other adverse events or damage to the echoendoscope.
There are three major advantages of EUS-guided combined coil-glue injection. Firstly, direct puncture of the gastric mucosa overlying the varix can be avoided by adopting a transesophageal route, hence avoiding the risk of “back-bleeding” which may occur after varix puncture. Secondly, successful hemostasis is not hindered by the presence of blood or food which can accumulate in the fundus in cases of acute gastric variceal bleeding. Thirdly, the technique most likely has a beneficial role to play in the treatment of larger GV (>1 cm). Volume of CYA injection (and therefore the risk of systemic embolization) can be limited. Obliteration of large varices at high risk of bleeding can be immediately confirmed with Doppler ultrasound and repeat coil/glue injections can be performed at the same session. Nevertheless, comparative prospective studies are needed to determine the benefit of combined CYA and coil treatment of GV over CYA alone (EUS or endoscopy-guided).
Rectal varices
Rectal varices occur in 44-89%[44,45,46] of cases of cirrhosis, and are a significant cause of lower GI bleeding (LGIB) in portal hypertension. Bleeding can occur from varices which are visible endoscopically, as well as endoscopically inevident rectal varices (EIRV).[47] Hemodynamic evaluation of rectal varices is routinely done at some centers by utilizing EUS to assess site, size, velocity, or direction of flow.[48,49] Sharma et al., reported a series of five patients in which 40% required EUS to identify EIRV. In the presence of LGIB, EUS prevented potentially hazardous application of clips or coagulation methods to control a bleeding point. Furthermore, the authors were able to visualize perforator veins supplying the pararectal varices which could be targeted for therapy with band ligation.
Extrapolating from the experience of EUS-guided therapy of GV, several authors have reported EUS-guided coiling and/or CYA injection of rectal varices.[50,51,52] EUS affords the following benefits:
Ability to visualize EIRV,
Identification of perforator veins,
Precise delivery of treatment directly into the varix, and
Ability to target therapy unhindered by luminal contents.
Furthermore, we can confirm the absence of flow post-therapy using color Doppler.
PV ANGIOGRAPHY
PV angiography provides valuable clinical information which can assist in the management of patients with portal hypertension and other hepatobiliary diseases.[53] However, portal venography and direct PV pressure measurements are rarely performed due to the procedure's invasiveness and high rate of complications.[54] Although the PV is one of the most inaccessible vessels in the body, it can be easily identified by EUS. Also EUS permits transgastric access into PV, contrast injection, and pressure monitoring, using a standard FNA needle. Lai and colleagues demonstrated PV access and pressure measurements using a 22-gauge needle in a porcine model.[55] Subsequently, Magno and colleagues reported on EUS-guided PV angiography in a porcine model using a 25-gauge needle.[56] The smaller size needle was thought to reduce the risk of damage to the blood vessel and subsequent bleeding. However, large volume injection of viscous contrast media was technically challenging through the narrow caliber needle lumen and small volume of contrast media hindered high quality venography. Thereafter, Giday and colleagues demonstrated safety using carbon dioxide (CO2 ) for portal venography in a porcine model through a 25-gauge needle, which obviated the need for injection of any contrast media.[57] Using these techniques, the same authors performed EUS-guided transhepatic PV catheterization with a modified endoscopic retrograde cholangiography (ERCP) catheter. The group performed portal angiography and obtained continuous portal pressure readings over a 1 hour period in a porcine model (n = 3).[58] The position of the catheter was not affected by animal respiration or endoscope movement during the study. Consistent results and minimal variability were noted within each animal. The investigators used the transhepatic puncture of the PV with the FNA needle followed by transhepatic route for catheter placement into the PV to prevent the risk of bleeding. Hepatic parenchyma surrounding the catheter provided the tamponade of the track after catheter removal, thus preventing post-procedural bleeding. Necropsy examination did not show any signs of bleeding, hepatic, or intra-abdominal organ or vascular damage.
INTRAHEPATIC PORTOSYSTEMIC SHUNT (IPSS)
Decompression of the portal system by placement of a TIPSS is a procedure performed by interventional radiologists. The effectiveness of TIPSS has been well-documented in the treatment and prevention of acute or recurrent variceal bleeding and refractory ascites.[1,59,60,61]
Buscaglia et al., described the first EUS-guided creation of an IPSS in a survival porcine model of 10 animals.[62] The authors identified a sonographic plane which enabled visualization of the intrahepatic branches of both the hepatic and PVs. The PVs are distinct from the HVs owing to their thickened, hyperechoic walls. Under EUS guidance, the HV was punctured with a 19-gauge FNA needle, which was passed through hepatic parenchyma. The needle was directed towards the PV and a guide wire was advanced through the needle into the PV. A self-expandable uncovered metal biliary stent (6-10 mm diameter × 40-80 mm length) was deployed between the HV and PV, accordingly forming an IPSS. There were no adverse events in the survival period (2 weeks) or at necropsy in any of the animals. Binmoeller et al. used a similar technique to create an IPSS using a lumen-apposing stent in a porcine model (n = 5).[63] The Axios Stent (Xlumena, Mountain View, CA, USA) is a fully covered, dual-flanged metal stent. The flange and body diameters measure 10 and 4 mm, respectively. The stent length is 8 mm. Necropsy confirmed successful stent placement between PV and HV with no evidence of tissue injury or hematomas.
ACCESS TO THE HEART
The heart and pulmonary trunk are located next to the esophagus, and are therefore directly accessible via endosonography. The relationship is routinely used in cardiology for transesophageal echocardiography (TEE). Real-time imaging of the heart, cardiac valves, and measurement of blood flow is regularly performed.[64,65] Fritscher-Ravens et al., performed successful EUS-guided puncture of the heart in a survival porcine study followed by three clinical cases.[66] In the animal studies (n = 8), the authors accessed the left atrium, left ventricle, coronary arteries, and aortic valve using 22- and 19-gauge FNA needles. Also radiofrequency ablation of the aortic valve, insertion of pacing wires, and injection of contrast agents were performed. The animals underwent cardiac monitoring during the procedures and survived for 2 weeks post-procedure. There were no episodes of arrhythmias. Repeat electrocardiography, pulse oximetry, and endosonography were performed to assess for morphological cardiac abnormalities prior to euthanasia. At necropsy, penetration sites were identifiable, although unremarkable in appearance. There was no bleeding or hematoma in any of the animals.
The authors went on to perform EUS-guided cardiac access in three patients (age 56-75 years). Pericardial fluid aspiration was performed in two patients using a 22-gauge needle, and FNA of a 5 cm left atrial mass was performed using a 19-gauge needle in the third patient. There were no adverse events after the procedures. However, in current times, the potential risk of mediastinal infection needs to be addressed before universal acceptance of such an approach.
SUMMARY
The GI tract provides unique access to vascular structures in the mediastinum and abdomen. The maturation of EUS technology has led to the emergence of many EUS-guided therapeutic interventions, although clinical data is currently limited. While the novel list of indications for therapeutic EUS continues to grow, the currently available endoscopic devices and accessories limit interventional EUS techniques. The development of new tools designed for EUS-guided intervention is eagerly awaited.
Videos Available on: www.eusjournal.com
Footnotes
Source of Support: Nil.
Conflicts of Interest: Payal Saxena has received consulting fees from Olympus Australia, Pentax Medical, and Cook Medical. She is a consultant for Boston Scientific. She has received research support from Cook Medical and Boston Scientific. Sundeep Lakhtakia has no conflicts of interest.
REFERENCES
- 1.Boyer TD, Haskal ZJ. American Association for the Study of Liver D. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 2.Kalva SP, Yeddula K, Wicky S, et al. Angiographic intervention in patients with a suspected visceral artery pseudoaneurysm complicating pancreatitis and pancreatic surgery. Arch Surg. 2011;146:647–52. doi: 10.1001/archsurg.2011.11. [DOI] [PubMed] [Google Scholar]
- 3.Fankhauser GT, Stone WM, Naidu SG, et al. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2011;53:966–70. doi: 10.1016/j.jvs.2010.10.071. [DOI] [PubMed] [Google Scholar]
- 4.Lin HJ, Hsieh YH, Tseng GY, et al. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615–9. doi: 10.1067/mge.2002.123271. [DOI] [PubMed] [Google Scholar]
- 5.Song SY, Chung JB, Moon YM, et al. Comparison of the hemostatic effect of endoscopic injection with fibrin glue and hypertonic saline-epinephrine for peptic ulcer bleeding: A prospective randomized trial. Endoscopy. 1997;29:827–33. doi: 10.1055/s-2007-1004316. [DOI] [PubMed] [Google Scholar]
- 6.Kanai M, Hamada A, Endo Y, et al. Efficacy of argon plasma coagulation in nonvariceal upper gastrointestinal bleeding. Endoscopy. 2004;36:1085–8. doi: 10.1055/s-2004-826033. [DOI] [PubMed] [Google Scholar]
- 7.Chau CH, Siu WT, Law BK, et al. Randomized controlled trial comparing epinephrine injection plus heat probe coagulation versus epinephrine injection plus argon plasma coagulation for bleeding peptic ulcers. Gastrointest Endosc. 2003;57:455–61. doi: 10.1016/s0016-5107(03)80008-1. [DOI] [PubMed] [Google Scholar]
- 8.Chou YC, Hsu PI, Lai KH, et al. A prospective, randomized trial of endoscopic hemoclip placement and distilled water injection for treatment of high-risk bleeding ulcers. Gastrointest Endosc. 2003;57:324–8. doi: 10.1067/mge.2003.103. [DOI] [PubMed] [Google Scholar]
- 9.Mumtaz R, Shaukat M, Ramirez FC. Outcomes of endoscopic treatment of gastroduodenal Dieulafoy's lesion with rubber band ligation and thermal/injection therapy. J Clin Gastroenterol. 2003;36:310–4. doi: 10.1097/00004836-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: A population-based study. Am J Gastroenterol. 1997;92:419–24. [PubMed] [Google Scholar]
- 11.Targownik LE, Nabalamba A. Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993-2003. Clin Gastroenterol Hepatol. 2006;4:1459–66. doi: 10.1016/j.cgh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Barkun AN, Bardou M, Kuipers EJ, et al. International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–13. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 13.Fockens P, Meenan J, van Dullemen HM, et al. Dieulafoy's disease: Endosonographic detection and endosonography-guided treatment. Gastrointest Endosc. 1996;44:437–42. doi: 10.1016/s0016-5107(96)70096-2. [DOI] [PubMed] [Google Scholar]
- 14.Levy MJ, Wong Kee Song LM, Farnell MB, et al. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008;103:352–9. doi: 10.1111/j.1572-0241.2007.01616.x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez JM, Giacino C, Pioche M, et al. Endoscopic ultrasound-guided vascular therapy: Is it safe and effective? Endoscopy. 2012;44:539–42. doi: 10.1055/s-0031-1291609. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez JM, Ezzedine S, Vitton V, et al. Endoscopic ultrasound treatment of vascular complications in acute pancreatitis. Endoscopy. 2009;41:721–4. doi: 10.1055/s-0029-1214874. [DOI] [PubMed] [Google Scholar]
- 17.Kumbhari V, Gondal B, Okolo Iii PI, et al. Endoscopic ultrasound-guided angiotherapy of a large bleeding gastrointestinal stromal tumor. Endoscopy. 2013;45:E326–7. doi: 10.1055/s-0033-1344875. [DOI] [PubMed] [Google Scholar]
- 18.Eisen GM, Baron TH, Dominitz JA, et al. Standards Practice Committe, American Society for Gastrointestinal Endoscopy The role of endoscopic therapy in the management of variceal hemorrhage. Gastrointest Endosc. 2002;56:618–20. doi: 10.1016/s0016-5107(02)70105-3. [DOI] [PubMed] [Google Scholar]
- 19.Van Stiegmann G, Cambre T, Sun JH. A new endoscopic elastic band ligating device. Gastrointest Endosc. 1986;32:230–3. doi: 10.1016/s0016-5107(86)71815-4. [DOI] [PubMed] [Google Scholar]
- 20.Helmy A, Hayes PC. Review article: Current endoscopic therapeutic options in the management of variceal bleeding. Aliment Pharmacol Ther. 2001;15:575–94. doi: 10.1046/j.1365-2036.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- 21.Hou MC, Lin HC, Lee FY, et al. Recurrence of esophageal varices following endoscopic treatment and its impact on rebleeding: Comparison of sclerotherapy and ligation. J Hepatol. 2000;32:202–8. doi: 10.1016/s0168-8278(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 22.Krige JE, Bornman PC, Goldberg PA, et al. Variceal rebleeding and recurrence after endoscopic injection sclerotherapy: A prospective evaluation in 204 patients. Arch Surg. 2000;135:1315–22. doi: 10.1001/archsurg.135.11.1315. [DOI] [PubMed] [Google Scholar]
- 23.Irisawa A, Obara K, Bhutani MS, et al. Role of para-esophageal collateral veins in patients with portal hypertension based on the results of endoscopic ultrasonography and liver scintigraphy analysis. J Gastroenterol Hepatol. 2003;18:309–14. doi: 10.1046/j.1440-1746.2003.02956.x. [DOI] [PubMed] [Google Scholar]
- 24.Irisawa A, Saito A, Obara K, et al. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: Severe periesophageal collateral veins and large perforating veins. Gastrointest Endosc. 2001;53:77–84. doi: 10.1067/mge.2001.108479. [DOI] [PubMed] [Google Scholar]
- 25.Lahoti S, Catalano MF, Alcocer E, et al. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc. 2000;51:331–3. doi: 10.1016/s0016-5107(00)70363-4. [DOI] [PubMed] [Google Scholar]
- 26.Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–9. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 27.Sarin SK, Kumar A. Sclerosants for variceal sclerotherapy: A critical appraisal. Am J Gastroenterol. 1990;85:641–9. [PubMed] [Google Scholar]
- 28.Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointestinal endoscopy. 1986;32:264–8. doi: 10.1016/s0016-5107(86)71843-9. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 30.Oho K, Iwao T, Sumino M, et al. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: A nonrandomized study. Endoscopy. 1995;27:349–54. doi: 10.1055/s-2007-1005712. [DOI] [PubMed] [Google Scholar]
- 31.Sarin SK, Jain AK, Jain M, et al. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol. 2002;97:1010–5. doi: 10.1111/j.1572-0241.2002.05622.x. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa K, Ishikawa S, Naritaka Y, et al. Clinical evaluation of endoscopic injection sclerotherapy using n-butyl-2-cyanoacrylate for gastric variceal bleeding. J Gastroenterol Hepatol. 1999;14:245–50. doi: 10.1046/j.1440-1746.1999.01842.x. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: Results in 5 cases. Gastrointest Endosc. 2007;66:402–7. doi: 10.1016/j.gie.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SS, Kim HH, Park SH, et al. N-butyl-2-cyanoacrylate pulmonary embolism after endoscopic injection sclerotherapy for gastric variceal bleeding. J Comput Assist Tomogr. 2001;25:16–22. doi: 10.1097/00004728-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Soehendra N, Nam VC, Grimm H, et al. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy. 1986;18:25–6. doi: 10.1055/s-2007-1013014. [DOI] [PubMed] [Google Scholar]
- 36.Seewald S, Ang TL, Imazu H, et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos) Gastrointest Endosc. 2008;68:447–54. doi: 10.1016/j.gie.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 37.Soehendra N, Grimm H, Nam VC, et al. 10 years’ experience with endoscopic sclerotherapy of esophagogastric varices. Chirurg. 1989;60:594–8. [PubMed] [Google Scholar]
- 38.Boustiere C, Dumas O, Jouffre C, et al. Endoscopic ultrasonography classification of gastric varices in patients with cirrhosis. Comparison with endoscopic findings. J Hepatol. 1993;19:268–72. doi: 10.1016/s0168-8278(05)80581-1. [DOI] [PubMed] [Google Scholar]
- 39.Lee YT, Chan FK, Ng EK, et al. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168–74. doi: 10.1067/mge.2000.107911. [DOI] [PubMed] [Google Scholar]
- 40.Iwase H, Suga S, Morise K, et al. Color Doppler endoscopic ultrasonography for the evaluation of gastric varices and endoscopic obliteration with cyanoacrylate glue. Gastrointest Endosc. 1995;41:150–4. doi: 10.1016/s0016-5107(05)80599-1. [DOI] [PubMed] [Google Scholar]
- 41.Levy MJ, Wong Kee Song LM, Kendrick ML, et al. EUS-guided coil embolization for refractory ectopic variceal bleeding (with videos) Gastrointest Endosc. 2008;67:572–4. doi: 10.1016/j.gie.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: A multicenter study (with videos) Gastrointest Endosc. 2013;78:711–21. doi: 10.1016/j.gie.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Binmoeller KF, Weilert F, Shah JN, et al. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos) Gastrointest Endosc. 2011;74:1019–25. doi: 10.1016/j.gie.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 44.Hosking SW, Smart HL, Johnson AG, et al. Anorectal varices, haemorrhoids, and portal hypertension. Lancet. 1989;1:349–52. doi: 10.1016/s0140-6736(89)91724-8. [DOI] [PubMed] [Google Scholar]
- 45.Chawla Y, Dilawari JB. Anorectal varices – Their frequency in cirrhotic and non-cirrhotic portal hypertension. Gut. 1991;32:309–11. doi: 10.1136/gut.32.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goenka MK, Kochhar R, Nagi B, et al. Rectosigmoid varices and other mucosal changes in patients with portal hypertension. Am J Gastroenterol. 1991;86:1185–9. [PubMed] [Google Scholar]
- 47.Sharma M, Rai P, Bansal R. EUS-assisted evaluation of rectal varices before banding. Gastroenterol Res Pract 2013. 2013:619187. doi: 10.1155/2013/619187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T, Yamazaki K, Toyota J, et al. Diagnosis of rectal varices via color Doppler ultrasonography. Am J Gastroenterol. 2007;102:2253–8. doi: 10.1111/j.1572-0241.2007.01340.x. [DOI] [PubMed] [Google Scholar]
- 49.Sato T, Yamazaki K, Toyota J, et al. Evaluation of therapeutic effects on rectal varices using percutaneous color Doppler ultrasonography. Hepatol Res. 2009;39:694–9. doi: 10.1111/j.1872-034X.2009.00505.x. [DOI] [PubMed] [Google Scholar]
- 50.Weilert F, Shah JN, Marson FP, et al. EUS-guided coil and glue for bleeding rectal varix. Gastrointest Endosc. 2012;76:915–6. doi: 10.1016/j.gie.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 51.Connor EK, Duran-Castro OL, Attam R. Therapy for recurrent bleeding from rectal varices by EUS-guided sclerosis. Gastrointest Endosc. 2015;81:1280–1. doi: 10.1016/j.gie.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 52.Storm AC, Kumbhari V, Saxena P, et al. EUS-guided angiotherapy. Gastrointest Endosc. 2014;80:164–5. doi: 10.1016/j.gie.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Abraldes JG, Tarantino I, Turnes J, et al. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–8. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 54.Armonis A, Patch D, Burroughs A. Hepatic venous pressure measurement: An old test as a new prognostic marker in cirrhosis? Hepatology. 1997;25:245–8. doi: 10.1053/jhep.1997.v25.ajhep0250245. [DOI] [PubMed] [Google Scholar]
- 55.Lai L, Poneros J, Santilli J, et al. EUS-guided portal vein catheterization and pressure measurement in an animal model: A pilot study of feasibility. Gastrointest Endosc. 2004;59:280–3. doi: 10.1016/s0016-5107(03)02544-6. [DOI] [PubMed] [Google Scholar]
- 56.Magno P, Ko CW, Buscaglia JM, et al. EUS-guided angiography: A novel approach to diagnostic and therapeutic interventions in the vascular system. Gastrointest Endosc. 2007;66:587–91. doi: 10.1016/j.gie.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Giday SA, Ko CW, Clarke JO, et al. EUS-guided portal vein carbon dioxide angiography: A pilot study in a porcine model. Gastrointest Endosc. 2007;66:814–9. doi: 10.1016/j.gie.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 58.Giday SA, Clarke JO, Buscaglia JM, et al. EUS-guided portal vein catheterization: A promising novel approach for portal angiography and portal vein pressure measurements. Gastrointest Endosc. 2008;67:338–42. doi: 10.1016/j.gie.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 59.Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41:S344–51. doi: 10.1097/MCG.0b013e318157e500. [DOI] [PubMed] [Google Scholar]
- 60.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: A meta-analytic review. Hepatology. 1995;22:332–54. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 61.Gines P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–47. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 62.Buscaglia JM, Dray X, Shin EJ, et al. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video) Gastrointest Endosc. 2009;69:941–7. doi: 10.1016/j.gie.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 63.Binmoeller K, Shah J. EUS-guided transgastric intrahepatic portosystemic shunt using the axios stent. Gastointest Endosc. 2011;73:A–167. [Google Scholar]
- 64.Kuhl HP, Hanrath P. The impact of transesophageal echocardiography on daily clinical practice. Eur J Echocardiogr. 2004;5:455–68. doi: 10.1016/j.euje.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Orta R, Moreno E, Vidal M, et al. Three-dimensional versus two-dimensional transesophageal echocardiography in mitral valve repair. J Am Soc Echocardiogr. 2007;20:4–12. doi: 10.1016/j.echo.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Fritscher-Ravens A, Ganbari A, Mosse CA, et al. Transesophageal endoscopic ultrasound-guided access to the heart. Endoscopy. 2007;39:385–9. doi: 10.1055/s-2007-966440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


