Abstract
Different imaging techniques can bring different information which will contribute to the final diagnosis and further management of the patients. Even from the time of Hippocrates, palpation has been used in order to detect and characterize a body mass. The so-called virtual palpation has now become a reality due to elastography, which is a recently developed technique. Elastography has already been proving its added value as a complementary imaging method, helpful to better characterize and differentiate between benign and malignant masses. The current applications of elastography in lymph nodes (LNs) assessment by endoscopic ultrasonography will be further discussed in this paper, with a review of the literature and future perspectives.
Keywords: Elastography, endoscopic ultrasound, endoscopic-ultrasound elastography, lymph node, ultrasound
INTRODUCTION
Ultrasound (US), as a widely available imaging technique, has proved its value in diverse clinical applications and many studies and reviews have been published over the years, including its role in the assessment of rare gastrointestinal (GI) diseases or hardly accessible organs.[1,2,3,4] One of the best diagnostic tools to assess the digestive tract and surrounding organs is endoscopic ultrasound (EUS), but one of its major limitations is the limited capacity to determine the exact nature of a lesion.[5,6,7] Differential diagnosis between benign and malignant lymph nodes (LNs) based on the EUS appearance is difficult and frequently requires EUS-guided fine needle aspiration biopsy (EUS-FNAB) for confirmation of malignancy.[8,9,10,11,12] Recently, the European Society of Gastrointestinal Endoscopy (ESGE) published recommendations on EUS-guided sampling made for various settings, including LNs.[13,14] Although the specificity of EUS-FNAB is close to 100%,[9,15,16,17,18,19,20] it potentially misses microinvasion of malignancy into LNs.[21] Also, it requires experience and it is associated with a risk of complications which, even if it is low, is not negligible.[6,22] According to the guidelines of the ESGE published by Dumonceau et al., contrast-enhanced EUS and EUS-elastography are new techniques developed to increase the negative predictive value (NPV) of EUS-FNAB.[13] Recent researches and detailed technical explanations published indicate that these techniques may potentially be useful to select diagnostically significant LNs and also the most suspicious area of a LN to be targeted for FNAB.[14,21,23,24,25,26,27]
As a noninvasive technique, EUS elastography complements conventional EUS with minimal prolongation of the examination time, minimum cost, and no added complication or death.[28,29] Till now, EUS-elastography imaging has been proved to offer complementary information added to conventional EUS imaging, representing a promising method that allows the differential diagnosis of benign and malignant LNs.[5,30,31] It is easy to be included in clinical staging and, particularly with computer-aided pixel analysis, significantly improves the specificity of LN staging. The most significant advantage of EUS-elastography is that it can be performed in real-time during a diagnostic examination and can immediately give important information that can impact patient management.[32]
WHAT IS ELASTOGRAPHY?
Palpation has been used in medicine for more than 1,000 years, in order to assess a mass located in the body.[33] Elastography is the imaging equivalent of the ancient palpation, being able even to exceed the subjectivity of the physical exam and to quantify the stiffness of a lesion. It is based on the general principle that softer parts of tissues deform easier under compression than harder parts do, allowing an objective determination of tissue consistency and showing differences in hardness between normal and diseased (tissues).[34,35,36,37] A grossly division in two types of elastography can be made, a qualitative one based on tissue's response to an external or internal generated force, called strain elastography (SE); and a quantitative one, based on measurements of the shear waves generated by a ‘push-pulse’ of low frequency, called shear wave elastography (SWE).[38,39,40,41] A way to semiquantify information from strain images is strain ratio. This can be calculated by measuring tissue stiffness in the targeted area as well as outside it, in a region representing normal tissue. Then, the strain ratio value can be calculated as the quotient between the two assessed regions.[42] Another possible way to quantify strain is by mean of hue histograms.[6,43] Elastography has already proved its valuable clinical utility in applications such as breast cancer diagnosis[44,45,46,47,48,49] or assessment of liver fibrosis,[43,50,51,52,53,54,55,56,57,58] adding complementary information to conventional US. It has also shown promising results in applications such as thyroid nodules,[59,60] pancreatic masses,[19,21,28,61,62] chronic pancreatitis,[42,63,64] autoimmune pancreatitis,[65] prostate cancer,[66,67,68] or LNs assessment.[69] Recently, elastography has been also introduced during EUS examination.[70,71,72,73,74] SE is currently the only method available for EUS, and all studies used the Hitachi US systems, with the exception of a paper published in 2014 which used the new Olympus processor.[75]
EFSUMB GUIDELINES FOR THE USE OF US ELASTOGRAPHY
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) has recently published guidelines and recommendations on the clinical use of US elastography. While in the first paper they have been dealing with the basic principles and technology of US elastography,[76] the second paper assessed the clinically used applications.[77] Transcutaneous elastography of superficial LNs was discussed as being a promising application that may prove its clinical value if more experience is gained. An increase in stiffness is expected in malignancies, but might also occur in inflamed LNs.[77,78,79] On the other hand, the assessment of LNs by EUS-elastography has been presented as a useful tool in differentiating benign and malignant LNs, with a sensitivity of 88% and a specificity of 85% according to the meta-analysis of Xu and colleagues.[29] Benign (physiological and reactive) LNs are characterized by a homogeneous or scattered soft pattern (predominantly green or mixed red-yellow-green). Typically, the soft vascular structures of the LN hilum may be delineated [Figure 1]. The LN cortex may appear slightly harder than the LN medulla [Figure 2]. Malignant LNs (in particular those with diffuse metastatic infiltration) most often display a homogeneous hard elastographic pattern [Figure 3]. Focal necrosis or incomplete malignant infiltration may result in inhomogeneous (mixed), but predominantly hard patterns.[80,81] The accurate elastographic discrimination between benign (reactive) and malignant (metastatic) LNs is of great importance for prognosis and selection of appropriate therapy for many cancers (esophageal, gastric, rectal, pancreatobiliary, and lung cancer)[82] [Figures 4 and 5]. It may be also used for identification of the harder, and then most suspicious LN, or of the hardest regions within LNs to be targeted for EUS-FNAB.[24,77] Thus, EUS-elastography may be helpful by reducing the number of false-negative results and repeated procedures.[32]
Figure 1.
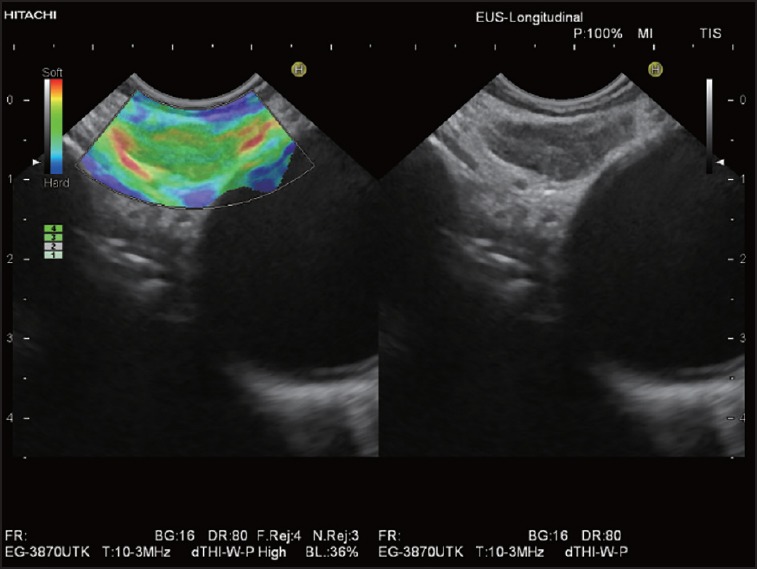
Elastographic image of a typical benign mediastinal LN: Homogeneous soft pattern (green) with delineation of soft vascular structures of the LN hilum (red and yellow). LN: Lymph node
Figure 2.

Elastographic image of a large reactive mediastinal LN: Heterogeneous soft pattern. The soft structures of the LN hilum and medulla are displayed in red, yellow, and green. The LN cortex is considerably harder (blue)
Figure 3.
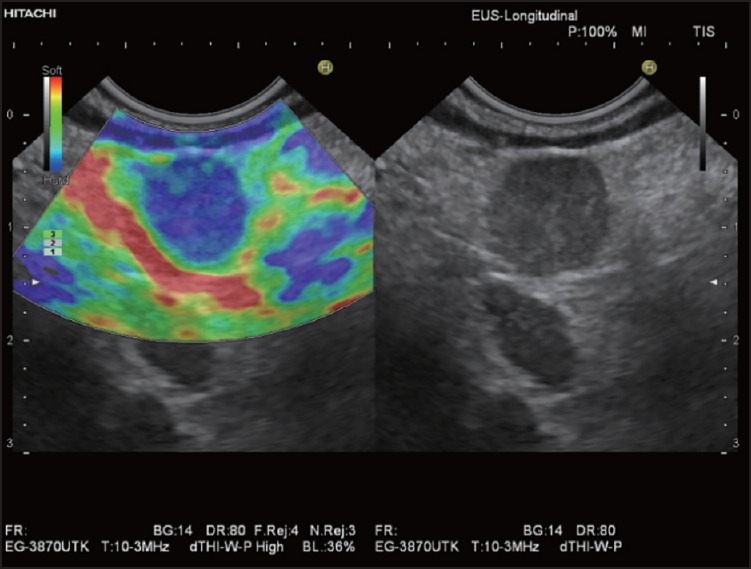
Elastographic image of a typical malignant LN: Homogeneous hard pattern (predominantly blue) of a round LN metastasis in a patient with gastric cancer
Figure 4.
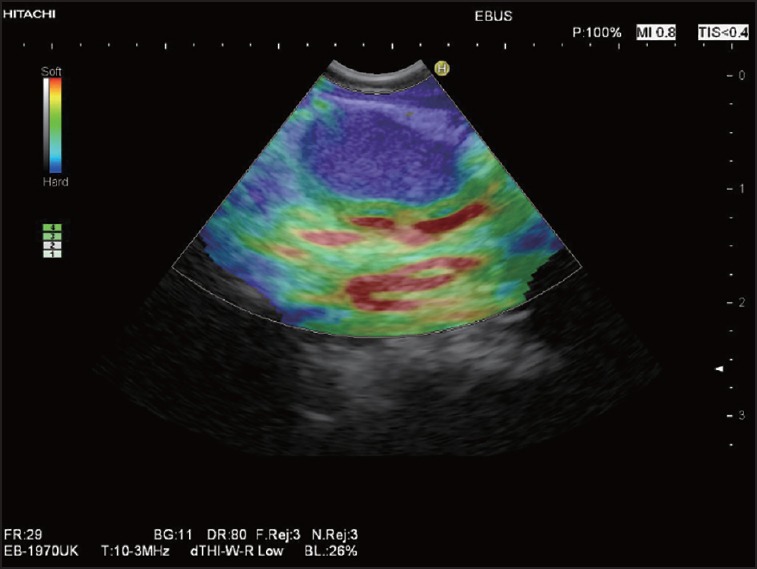
Elastographic evaluation of lower periesophageal LNs using an EBUS-scope in a patient with stenosing squamous cell cancer of the upper esophagus: Homogeneous hard pattern (blue) of the oval LN. EBUS: Endobronchial ultrasound
Figure 5.
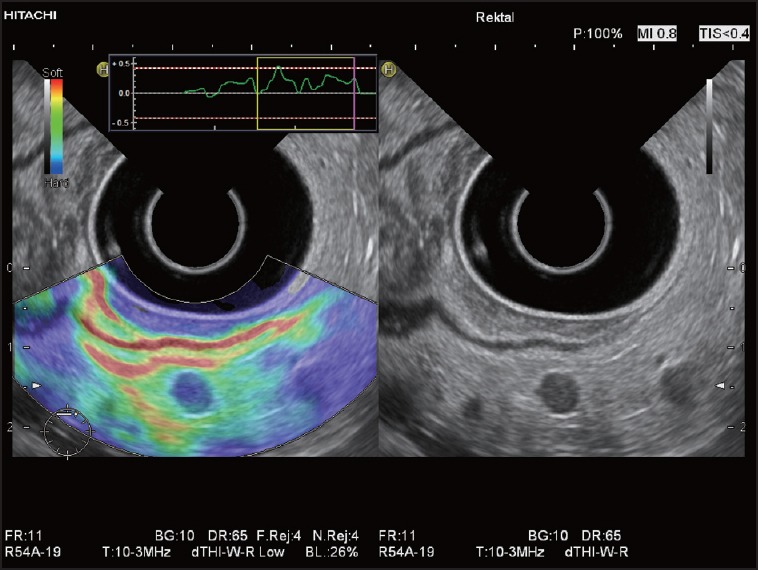
Elastographic image of a very small LN metastasis in a patient with rectal cancer: The round LN is depicted homogeneously blue and is significantly harder in comparison with surrounding perirectal connective tissue and fat (green, yellow, and red)
In summary, endoscopic elastography is real-time elastography (RTE) performed with an endoscopic probe (flexible upper GI echoendoscope, rectal US probe, and flexible endobronchial US (EBUS) probe), which has led to further improvement in B-mode imaging results for classification of benign and malignant LNs, particularly by targeting LNs for needle sampling. For clinical cases and high quality images, we recommend the EFSUMB Atlas (http://www.efsumb-atlas.org/v2/index.asp?xref=f1-info-elasto.asp ).[83]
NODAL STAGING IN CARCINOMA PATIENTS
Importance and current status
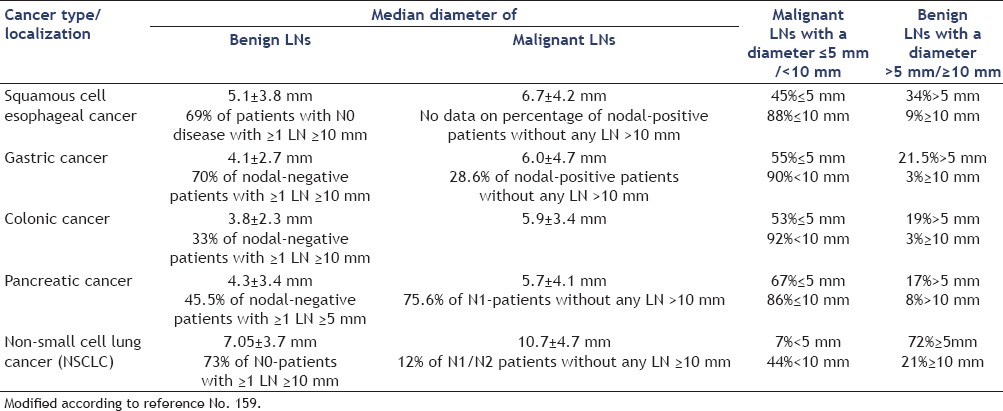
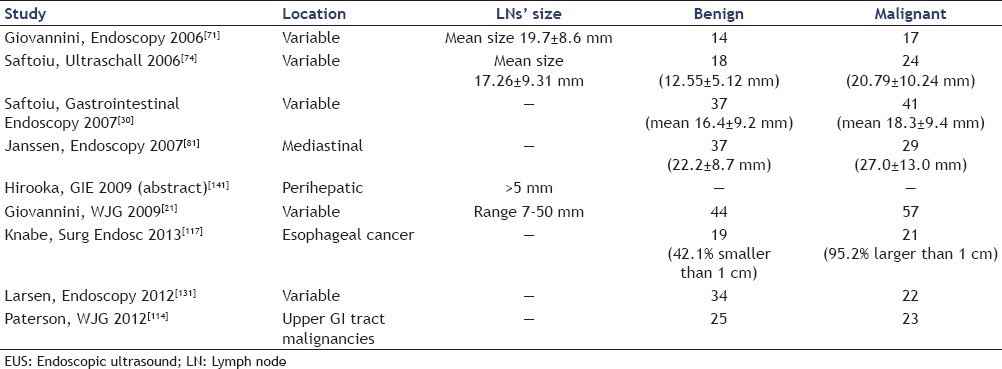
LNs characterization by cross-sectional imaging techniques is limited in accuracy, since they evaluate insensitive criteria, such as LN's size or gross morphology. It is well-known that LN metastases can be present in nonenlarged LNs and that not all enlarged LNs are malignant.[84,85,159] For example, in GI and pancreatic cancer approximately 50% of metastatic infiltrations are found in LNs with the largest diameter being ≤5 mm, which are almost undetectable for the standard imaging techniques.[86,87,88,89,90,91] In gastric cancer, the percentage of LNs with a diameter of ≥10 mm is the same in patients with LNs metastases as in patients without metastatic nodal involvement. Also in pulmonary cancer, LN size is not a reliable indicator of LN metastases. In one surgical study, 44% of mediastinal LN metastases proved to be <10 mm in largest diameter, and in 12% of all patients with N1 or N2 disease the pathologist did not find any LN with a diameter of ≥10 mm[87,88,92] [Table 1]. Therefore, sensitivity of LN size measurements cannot be expected to be higher than 70%.[93] Positron emission tomography (PET) has overcome some of these limitations, but is still constrained by current resolution limits for small nodal metastases.[84]
Table 1.
Diameter of benign and malignant lymph nodes (LNs) in surgical specimen of nodal-positive and nodal-negative patients with gastrointestinal, pancreatic, and non-small cell lung cancer.[86,87,88,91,92]
The number of LNs infiltrated with malignant cells and also LN's location have an impact in predicting patient's survival outcome. For example, in esophageal carcinoma, patients with three to six involved LNs on both sides of the diaphragm had a worse survival than those without nodal involvement, or with involved LNs located only on one side of the diaphragm.[94] The study of Twine et al., on a number of 267 patients with esophageal cancer, showed that survival was related to EUS tumor (T) stage (P < 0.0001), EUS node (N) stage (P < 0.0001), EUS tumor length (P < 0.0001), and to the EUS defined LNs metastasis count (P < 0.0001), with a median survival of 44, 36, 24, and 17 months, respectively, for patients with 0, 1, 2-4, and >4 LN metastases, respectively.[95]
Endosonographic LN characterization
Catalano et al., in a cohort of 100 patients with esophageal cancer defined endosonographic features predictive of LN metastasis: Hypoechoic structure, distinct margin, roundness, and a diameter <10 mm. Probability of malignancy increased with the number of malignancy criteria. Malignancy could be predicted with 100% accuracy when all four features were present.[96] Further studies using EUS and EBUS confirmed these criteria and added further malignancy criteria for LNs: Absence of hyperechoic LN hilum and lack of a central nodal vessel, occurrence of hyperechoic coagulation necrosis, and heterogeneous echo pattern.[97,98,99,100,101,102,103] The probability of malignancy of a particular LN is very low, if none of the malignant LN criteria is observed.[97,104,105] However, some of the features predictive for malignancy, in particular coagulation necrosis and heterogeneous echotexture are also observed in tuberculous LNs.[106] Using a scoring system based on the traditional Catalano criteria allowed definitive classification of a particular LN as either malignant or benign only in approximately 25% of mediastinal LNs.[107] Moreover, using a grey-scale texture analysis in EBUS did not improve differentiation of benign from malignant mediastinal LNs.[108] EUS-FNAB has a significantly higher accuracy for differentiation of benign vs. malignant LNs than echo features alone.[109,110] The incremental value of EUS-FNAB over EUS alone for the evaluation of mediastinal lymphadenopathy has also been shown in a meta-analysis. FNAB improved the sensitivity of EUS from 84.7% to 88.0% and its specificity from 84.6% to 96.4%.[111]
The added value of EUS-elastography
In the past few years, new techniques for nodal staging have been developed, providing staging information before the initiation of therapy. One of these techniques is sonographic elastography, which can be recommended as a complementary method to B-mode US, for the characterization and differentiation of benign and malignant LNs in real time.[35,112,113] The gold standard for determination of malignant cells within a LN at the time of preoperative staging remains FNAB, with a specificity and a positive predictive value (PPV) for cancer diagnosis of up till 100%.[82,114,115,116] EUS-elastography, brings additional information on LNs stiffness, being clinically useful in selecting the most suspicious LNs for tissue sampling, especially in patients presenting multiple LNs, with a high PPV[21,74,81] [Figure 6a and b]. Due to a high NPV, it might reduce the number of unnecessary biopsies.[21] The smallest LN metastases may escape both B-mode diagnosis and EUS-FNAB. However, elastography can detect circumscribed metastasis-related changes in tissue hardness, being helpful in delineating the very early circumscribed malignant infiltration for improved target EUS-guided FNAB [Figures 7 and 8].[82] Additionally, normal elastographic architecture of enlarged inflammatory LNs can be helpful to prove a benign inflammatory disease, for example, sarcoidosis[39] [Figure 9]. It can also help in guiding the puncture in a non-necrotic part of the suspicious LN when necrotic tissue is present, as in advanced cancer.[21,117] In cases of negative EUS-FNAB, or in situations where this procedure is not considered useful or it is not possible (technical problems, peritumoral LNs, interposed malignant tissue, or interposed vascular structures), EUS-elastography may offer an alternative for the differential diagnosis.[21,78,112,118]
Figure 6.
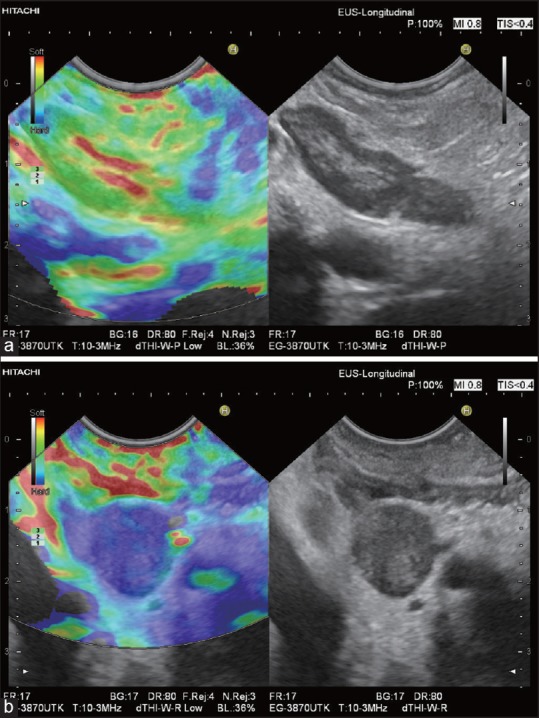
Two periduodenal LNs in one patient with pancreatic cancer: An oblong reactive LN (a) has an almost identical elasticity compared with the surrounding connective tissue (predominantly green). The soft hilar structures are clearly delimited (red and yellow). A round LN metastasis (b) in the same patient is homogenously hard (blue)
Figure 7.
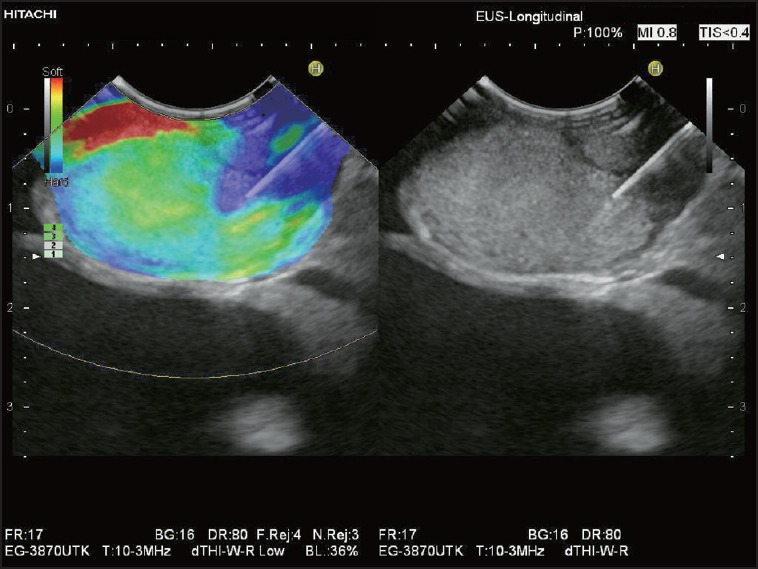
Elastography-guided EUS-FNAB in a patient with known hepatocellular carcinoma (HCC): Very large mediastinal LN with predominantly soft pattern (green) and a small area in the LN periphery, which is definitely much stiffer (blue): The 22-gauge aspiration needle is targeted to this “hard” LN region. Cytology and histology proved metastatic infiltration by HCC. FNAB: Fine needle aspiration biopsy
Figure 8.
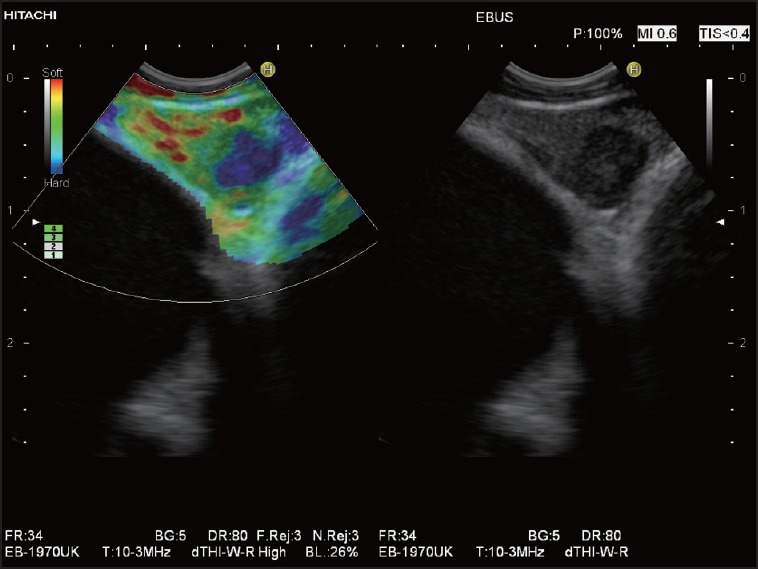
EBUS elastography in a patient with right-sided peripheral non-small cell lung cancer: A triangular LN is shown in the left mediastinal area 4 (4L). Elastography shows a scattered pattern with a circumscribed hard (blue) infiltration which was proven to be a metastasis by transbronchial EBUS-FNAB. Surgery was omitted, and palliative chemotherapy started
Figure 9.
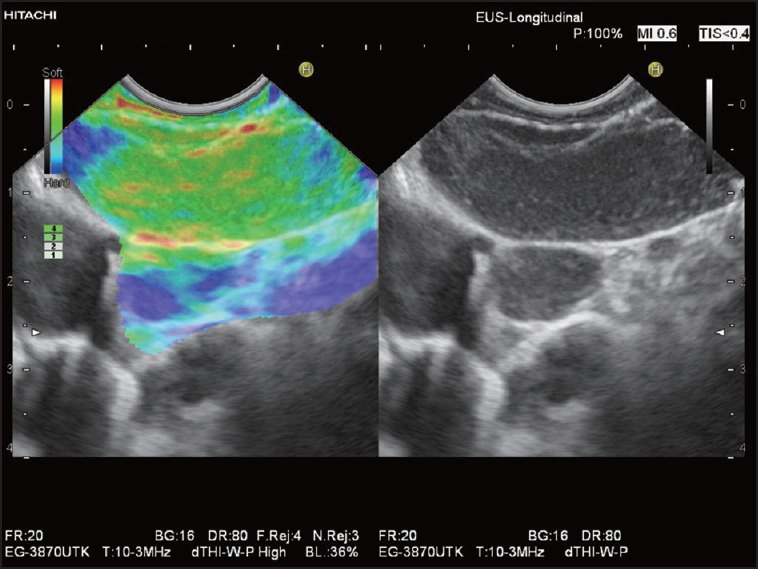
Elastographic image of a large oblong and hypoechoic mediastinal LN in a patient with sarcoidosis: The LN is predominantly green
EUS-ELASTOGRAPHY, DIFFERENTIATION OF BENIGN AND MALIGNANT LNS: REVIEW OF THE LITERATURE
The ability of EUS-elastography to differentiate benign and malignant LNs for the first time was evaluated by Giovannini et al. in 2006, on a number of 31 LNs with different locations. The final diagnosis was based on FNAB samples or surgical specimens. They found a sensitivity of 100% and a specificity of 50% using a color-coded scale, with blue for malignant tissue, green for fibrosis, yellow for normal tissue, and red for fat. They pointed out the need for specificity improvement, highlighting the promising result of EUS-elastography for guiding the biopsy.[71] In 2009, Giovannini et al., conducted a multicenter study which came in addition to the previous one. A total number of 101 LNs in 101 patients known with different malignancies were evaluated based on the same real-time elastographic pattern, and the result was compared with the classification based on the B-mode EUS images, using EUS-FNAB and/or surgical pathology as reference standard (44 benign LNs and 57 malignant LNs). They found improved specificity of 82.5%, as compared to 50% in the first study. Also, sensitivity, specificity, PPV, NPV, and global accuracy for EUS-elastography were significantly better than the respective parameters obtained for B-mode EUS images (91.8%, 82.5%, 88.8%, 86.8%, and 88.1% respectively, as compared to 78.6%, 50.0%, 70.5%, 60.6%, and 67.3%, respectively).[21]
Saftoiu et al., analyzed whether EUS-elastography may differentiate between benign and malignant LNs by using qualitative pattern and quantitative histogram analysis. Thirty-one patients diagnosed by EUS with cervical, mediastinal, or abdominal LNs were prospectively included, with a total number of 42 LNs examined. The gold standard used was fine needle aspiration cytology (FNAC) with further verification either by surgery or by follow-up. The qualitative pattern analysis showed a high sensitivity (91.7%), specificity (94.4%), and accuracy (92.86%). Even better results were obtained by defining an elasticity ratio based on separate red-green-blue channel histogram values (sensitivity, specificity, and accuracy of 95.8, 94.4, and 95.2%, respectively).[74] In a second study, by using a dynamic hue histogram analysis in order to reduce possible selection bias or artifacts, they obtained a sensitivity, specificity, and accuracy of 85.4, 91.9, and 88.5%, respectively, after the evaluation of total number of 78 LNs in 54 patients diagnosed by EUS with cervical, mediastinal, or abdominal LNs.[30]
Xu et al., assessed the accuracy of EUS-elastography by pooling data of existing trials. Seven studies involving 368 patients with 431 LNs were included, and a meta-analysis was performed. Pooling was conducted in a fixed-effect model or a random-effect model. The pooled sensitivity of EUS-elastography for the differential diagnosis of benign and malignant LNs was 88% (95% confidence interval (CI) 0.83-0.92), and the specificity was 85% (95% CI, 0.79-0.89). The subgroup analysis by excluding the outliers provided a sensitivity of 85% (95% CI, 0.79-0.90) and a specificity of 91% (95% CI, 0.85-0.95).[29] The most important data of studies evaluating the role of EUS elastography for characterization of LNs are detailed in Tables 2 and 3.
Table 2.
EUS-elastography — the ability to differentiate between benign and malignant LNs
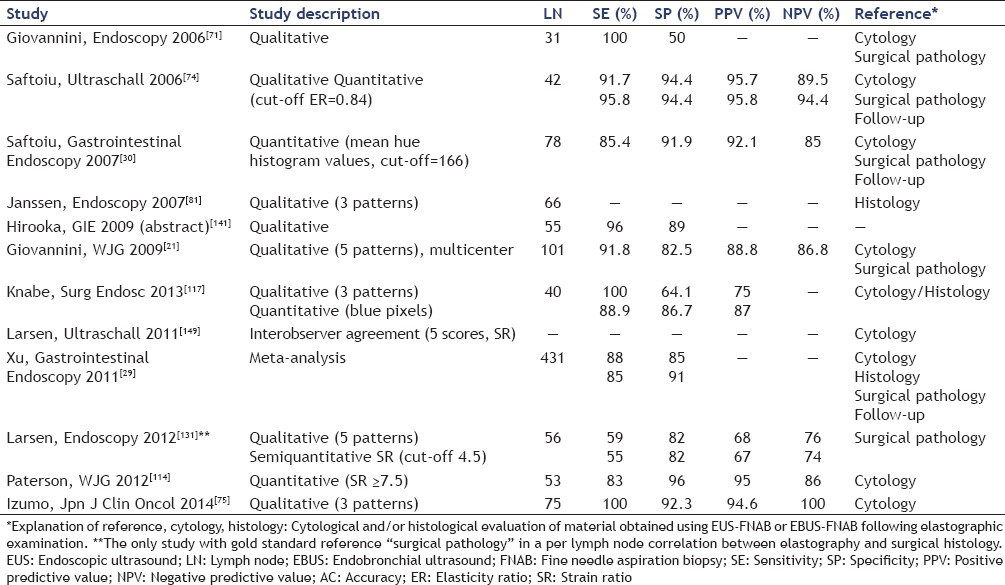
Table 3.
EUS-elastography studies and LNs' dimensions
Staging of carcinoma — the added value of EUS-elastography
Once detected, most primary tumors are staged using the American Joint Committee on Cancer classification[119] to assess the local extent and size of the primary tumor (T), regional LN involvement (N), or distant metastasis (M). Assessment of pathological LNs is now incorporated in revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline. LNs with a short axis ≥15 mm are considered measurable and assessable as target lesions. The short axis measurement is the one which should be included in the sum of lesions in calculation of tumor response. Nodes that shrink to <10 mm short axis are considered normal.[120] The presence of LN metastases can significantly alter patient management, and therefore accurate diagnosis of the presence and extent of nodal disease can help in optimizing patient management.[121] The accurate identification and characterization of LNs by imaging has important therapeutic and prognostic significance in patients with newly diagnosed cancers. The presence of nodal metastases limits the therapeutic options and also generally indicates worse prognosis in patients. Thus, it becomes crucial to have this information before commencing therapy. Current cross-sectional imaging modalities rely on insensitive size and morphologic criteria and, thus, lack the desired accuracy for characterizing LNs. This is mainly because metastases can be present in nonenlarged LNs and not all enlarged LNs are malignant. PET has overcome some of these limitations, but is still constrained by current resolution limits for small nodal metastases. This has fuelled the development of targeted techniques for nodal imaging and characterization as outlined in this article. In the past few years, studies have shown that these newer imaging techniques can bridge some of the limitations of existing imaging for nodal characterization, and thereby provide the much-needed staging information before the initiation of therapy.[84]
Esophagogastric cancer staging
In esophageal cancer, confirmation of metastasis to regional and nonregional LNs or to distant sites may affect patient management.[122] EUS-FNAB demonstration of distant LNs metastases have been found to change the management strategy in 7 and 12% in one prospective and one retrospective study, respectively, involving a total of 307 patients.[123,124] It is also helpful in selecting the best surgical approach of patients with resectable distal esophageal carcinoma and mediastinal LNs visualized on EUS. If the LNs are proved to be involved, a transthoracic esophagectomy is the best approach, while a negative result for mediastinal LNs involvement may allow a transhiatal resection.[125] In his study, Saltzman[126] stated that EUS was the most accurate technique for the locoregional (T and N) staging of esophageal cancer, and optimal staging strategies for esophageal cancer should use EUS-FNAB with either computed tomography (CT) or PET.
In upper GI cancer, EUS-FNAB of regional (e. g., periesophageal or perigastric) LNs is not advisable due to a high false-positive rate of up to 15%, which is caused by inadvertent needle-crossing of the primary tumor or by tumor-cell contamination of luminal fluid.[127,128] Therefore, nodal staging relies on EUS features alone and has limited diagnostic reliability (in gastric cancer; sensitivity 83% and specificity 67%).[129]
In a recent study by Hassan et al., in 234 patients with gastric cancer, EUS detected 99 lesions suspicious to be distant metastases, among them 85 were suspicious LNs, mostly located in the mediastinum. EUS-FNAB confirmed distant LN metastases in 58% of targeted LNs and changed the management in 34 of the 234 patients (15%) undergoing EUS for staging, avoiding unnecessary surgery.[130] Preliminary studies suggest that an elastography-based approach may increase the reliability of endosonographic nodal staging and the efficacy of EUS-FNAB for cytopathological characterization of distant LN metastases in upper GI malignancies [Table 4]. EUS elastography has been shown to be superior to standard EUS assessment in the differentiation between benign and malignant LNs in esophagogastric cancer.[114] Knabe et al., aimed to assess whether EUS-elastography was able to improve LN staging in patients with esophageal cancer. A total number of 40 patients with known esophageal cancer were prospectively enrolled. Using histological/cytological results, out of the 40 LNs examined, 21 were proved to be malignant. The proportions of color pixels were assessed using computer analysis of the elastography images. Sensitivity, specificity, and PPVs of EUS-elastography alone were of 100%, 64.1%, and 75%, respectively, as compared to the values of 91.3%, 64.7%, and 74%, respectively, obtained for B-mode criteria. When computer analysis of the elastographic images was added, with a cut-off value randomly set to 50% blue pixels, the specificity improved significantly from 64.1% to 86.7%, with a slight decrease in sensitivity, from 100% to 88.9%. PPV improved from 75% to 86%.[117]
Table 4.
Added value of EUS-elastography for esophagogastric cancer staging, as compared to EUS for benign-malignant LNs differentiation
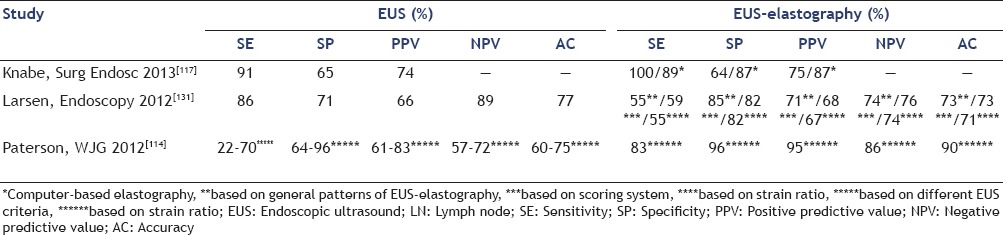
Larsen et al., evaluated the use of EUS, EUS-elastography, quantitative EUS-elastography (by calculating the strain ratio), and EUS-FNAB in the LN staging of upper GI cancer, using histology as the gold standard. A number of 56 LNs (34 benign and 22 malignant) in 56 patients were examined. Authors obtained values for sensitivity, specificity, accuracy, PPV, and NPV of 86%, 71%, 77%, 66%, and 89%, respectively, for EUS; 55%, 85%, 73%, 71%, and 74%, respectively for EUS-elastography; 59%, 82%, 73%, 68%, and 76%, respectively, for EUS-elastography using a 5 pattern system scoring; 55%, 82%, 71%, 67%, and 74%, respectively, for strain ratio evaluation with a cut-off value 4.5. The sensitivity, specificity, and accuracy of EUS-FNAB were 64%, 96%, and 85%, respectively.[131]
Paterson et al., assessed quantitative EUS-elastography in the nodal staging of esophagogastric cancers. Using EUS-FNAB cytology as the reference standard, out of the 50 examined LNs, 23 were proved to be malignant. With a strain ratio cut-off value of ≥7.5 for malignancy; sensitivity, specificity, PPV, NPV, and accuracy were 83%, 96%, 95%, 86%, and 90%, respectively, as compared to the values of 22-70%, 64-96%, 61-83%, 57-72%, and 60-75%, respectively, obtained for different B-mode EUS criteria.[114]
Lung cancer staging
Correct staging of non-small cell lung cancer (NSCLC) is important for further decisions regarding case management, by allocating the patient to surgery, neoadjuvant, or palliation therapy.[132,133]
According to the guidelines published by De Leyn et al., regarding the preoperative LN staging of NSCLC, CT is still the basic imaging modality in mediastinal LN staging, both primary and in restaging after induction therapy. However, according to the cited guidelines, CT chest is not accurate enough for mediastinal LN staging. When only CT is available, invasive staging is advised in every patient; except for T1 squamous cell cancer with LNs <1 cm on CT. EUS-FNAB is presented as a new minimally invasive technique that provides cytohistological diagnosis and may be complementary to the surgical invasive staging technique.[134] EUS-FNAB has a high sensitivity and specificity in the diagnosis of LNs metastases, being able even to detect small LNs metastases (less than 1 cm) often miss by CT.[135] This has a huge impact for staging of NSCLC, reducing futile thoracotomies in patients with suspected or proven lung cancer and enlarged (>1 cm) mediastinal LNs on chest CT.[136,137] EUS-FNAB is also able to detect advance disease in 11-25% of the cases with negative CT results.[85] However, EUS-FNAB cannot investigate anterior mediastinum.[138]
Janssen et al., tested the feasibility of EUS-elastography in the dorsal mediastinum, and compared the elastographic patterns of LNs with results from EUS-FNAB. A total number of 66 LNs (37 benign and 29 malignant) were examined in 50 consecutive patients. For EUS, accuracy was 71.2%, if lesions were stated as malignant when at least three out of four B-mode criteria (round, hypoechoic, >1 cm, and sharp border) were positive. For elastographic criteria, the assumption that a relatively homogeneous pattern, color-coded in green/yellow (intermediate tissue stiffness) is characteristic for benign LNs gave an accuracy of 87.9%. Using the dominance of blue color (relatively hard tissue) as marker for malignancy, regardless of pattern, the accuracy was 84.8%. Interobserver variability among the three examiners was also evaluated in this study. LNs were classified into three different categories (benign, malignant, and not classifiable). A final kappa score of 0.84 was obtained in this analysis, highlighting a very good correlation.[81] Preliminary reports have proved the feasibility of elastography for endobronchial longitudinal US[139,140] [Figure 10]. A recent study of Izumo et al., reported on the ability of EBUS elastography to differentiate mediastinal and hilar LNs using qualitative elastographic pattern analysis (n = 33 benign and 42 malignant LNs). Results were compared with final cytopathological results of EBUS-guided FNAB. Sensitivity, specificity, PPV, NPV, and diagnostic accuracy for elastographic diagnosis of a malignant mediastinal LN were 100%, 92.3%, 94.6%, 100%, and 96.7%, respectively.[75]
Figure 10.
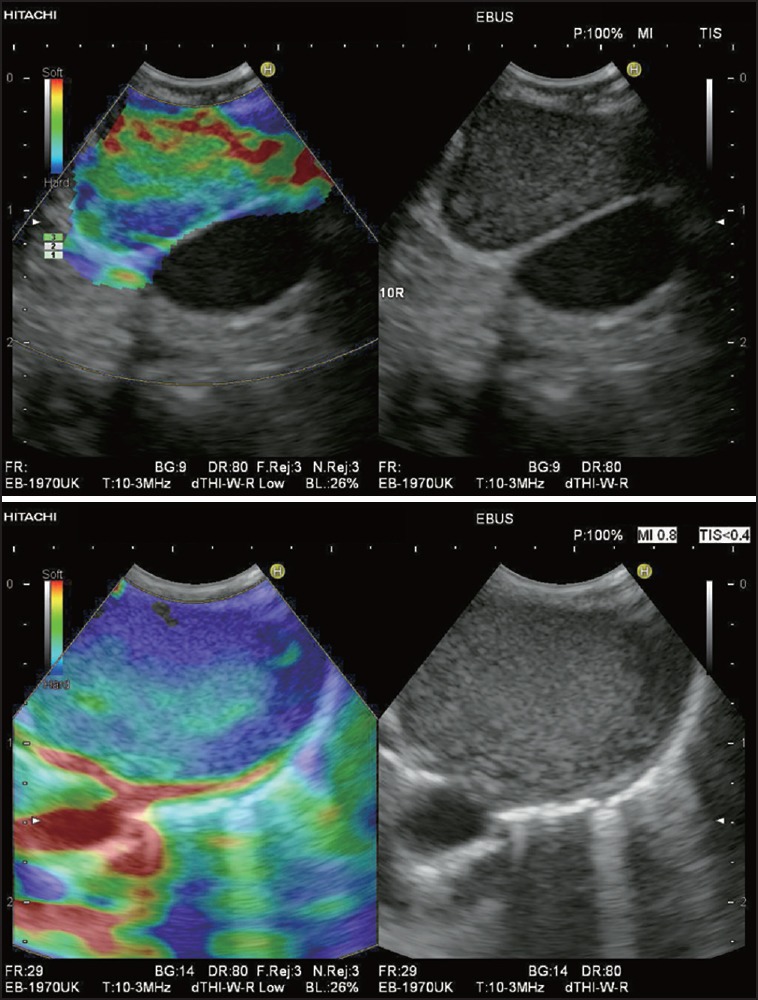
Elastography in EBUS showing an enlarged LN at the right hilum (10R) in two different patients with mediastinal lymphadenopathy of unknown origin. In both cases transbronchial EBUS-FNAB was performed. In one patient with an elastographic heterogeneous LN with clearly visible soft (red and yellow) hilar structures and a small hard (blue) peripheral zone of the cortex (a) cytology and histology showed lymphoid tissue with reactive changes. In the other patient with a predominantly hard LN (green and blue) without any obvious hilar structures (b) cytology and histology demonstrated metastasis of a renal cell cancer, which had been treated by surgery 7 years ago
Bile duct tumors staging
EUS-elastography may add new information and may be a useful method in the diagnosis of the involvement of hepatoduodenal ligament LNs in biliary malignancies.[141]
In patients with hilar tumors, EUS-FNAB of regional LNs has a tremendous impact in the selection of patients suitable for a multidisciplinary approach, including chemo- and radiation therapy in association with the liver transplantation, being able to avoid unnecessary transplantation in about 20% of the cases.[142,143]
Hirooka et al., assessed the diagnostic capability of EUS-elastography to diagnose the involvement of LNs in biliary malignancies. Fifty-five patients with enlarged LNs were included. Involved LN images were defined as a mass harder than the surrounding connective tissue or as a mass with inhomogeneous hardness. They obtained results for sensitivity and specificity of EUS-elastography in diagnosing LN involvement of 96% and 89%, respectively.[141]
Other possible applications of EUS-elastography for cancer staging
In pancreatic cancer, studies aiming to directly assess the clinical impact of EUS-FNAB by demonstrating the metastatic disease with impact on patients management are lacking.[144] In the study by Mortensen et al.,[123] EUS-FNAB disclosed metastatic LNs that affected treatment decisions in six of the 99 evaluated patients (6.06%).
In rectal cancer, two studies reported that EUS-FNAB evaluation of LNs for staging did not alter significantly the management of patients as compared to EUS alone.[145,146] This may be due to the fact that in rectal cancer there is a close correlation between T and N stages, and also to the fact that most perirectal LNs detected at EUS are malignant.[144] By contrast, Gagliardi et al.,[147] found that in rectal cancer magnetic resonance imaging (MRI) has 67% sensitivity, 71% specificity, and 69% accuracy in detecting malignant LNs, values which can be considered as low.
In prostate cancer patients with nodal metastases are excluded from radical prostatectomy as a curative option and, instead, receive adjuvant therapy to achieve disease control.[148]
All these types of cancers may benefit from the ability of EUS-elastography to improve nodal staging, but this should be assessed in further studies.
Are the results of EUS-elastography reproducible?
One of the main criticisms of EUS-elastography is the variability of the elastographic images and the difficulty of interpretation.[70] The main pitfall of EUS elastography is the endosonographer's inability to control tissue compression. Usually, none or minimal pressure is required to obtain a good compression from the pulsatility of abdominal vessels.[32] Studies have proved that elastography and elastographical strain ratio evaluation of LNs are feasible and may be reproduced with good interobserver agreement in a blinded clinical setup.[149]
The study of Giovannini et al., showed a satisfying interobserver concordance. Authors performed an interobserver study on a statistically representative and blinded selection of 15 videos of LNs. The videos were each evaluated by five endoscopists experienced in EUS and elastography. The agreement between two different examiners was measured by an adapted kappa coefficient. The interobserver study also evaluated the agreement between two examiners to classify the examined tissue as benign or malignant. This agreement was measured by the Cohen kappa coefficient. The kappa coefficient of the sonoelastography score was 0.519. The kappa coefficient for the differentiation between benign and malignant LNs was 0.657.[21]
Larsen et al., evaluated the intra- and interobserver agreement of EUS and EUS-elastography during the evaluation of a specific LN in patients with upper GI malignancies. The EUS-elastography strain ratio was used to differentiate between benign and malignant LNs and the interobserver agreement was evaluated. A total number of 62 LNs in 62 patients were included, and EUS-FNAB cytology was used as the reference standard. Values for kappa coefficient (95%-CI) and for interobserver agreement were 0.80 (0.55-1.00) and 90.2% for EUS; 0.58 (0.34-0.82) and 81.9% for EUS-elastography, 0.35 (0.2-0.50) and 59.0% for EUS-elastography using a 5 patterns scoring system, 0.59 (0.36-0.84) and 82.0% for highest EUS-elastography strain ratio, respectively using a cut-off value of 3.81.[149]
Janssen et al., aimed to test the feasibility of EUS-elastography in EUS of the dorsal mediastinum and to explore the elastographic patterns of benign and malignant lesions based on results from EUS-guided FNAB of these LNs. On a total number of 66 LNs, 37 benign and 29 malignant, by applying an elastographic pattern, they obtained an excellent interobserver agreement (kappa = 0.84).[81]
Perspectives — what's next?
EUS elastography has a potential capability of defining characteristics of benign and malignant lesions, but needs improvement of the specificity and clear definitions of the criteria required for an accurate elastographic evaluation. An improved grading system might help.[71] Elastographic strain ratio is promising, but it seems to overcome some of difficulties in classifying the LNs in the scoring system. For transcutaneous US-elastography, studies have demonstrated better sensitivity when using a strain ratio,[150,151] as compared to the values obtained by using visual scoring systems, which showed better results for specificity.[151] This was also reflected in a meta-analysis including nine studies using qualitative elastography score and five studies using strain ratio.[152] As far as EUS-elastography studies, the results on the utility of using a strain ratio over a visual scoring system were discordant.[153,154] Thus, the utility of the ‘elasticity ratio’ should be tested in larger prospective studies,[74] with a focus on cut-off points.[149] The presence of central necrosis may also impair the differentiation of lesions by a subjective interpretation of the elastography score according to the strain ratio.[155]
A correlation between the elasticity imaging results and a histologic study would also be highly desirable (’virtual biopsy’ for noninvasive characterization; real-time guidance of biopsies in stiffer areas of the lesions).[30] The goal is not to replace tissue confirmation. Instead, the information obtained by EUS elastography should be considered as complementary to the conventional EUS imaging. It may increase the yield of FNAB and reduce the number of unnecessary biopsies when assessing LNs. The second generation of elastography software providing quantitative analysis of tissue elasticity might be able to increase the accuracy of this technique.[21] A histogram-based evaluation has already been integrated into the next software generation, but this also needs to be tested in future investigations, with the need to define correct cut-off values.[117] Also further studies are necessary to prove if a computer-based analysis is superior to an examiner-based approach.[81]
There is a lack of studies on the intra- and interobserver variability of EUS-elastography. Image selection should be part of the intra- and interobserver evaluation.
An exact value of tissue elasticity would be best, but this requires the knowledge of the compression force applied to the tissue.[131] At the moment, this is only feasible with SWE.[76] Preliminary studies have shown high diagnostic accuracy of percutaneous SWE for discrimination of benign and malignant LNs in patients with head and neck malignancies and breast cancer.[156,157,158] Therefore, implementation of SWE into EUS systems is highly desirable.
CONCLUSION
Elastography can provide additional information about the structure and pathology of mediastinal and abdominal LNs. Whereas, the differential diagnosis of malignant and benign LNs cannot be solved for certain, it seems to be an excellent method for targeting different areas of the LN to avoid unnecessary needle passes in EUS-FNAB. The disadvantage of the missing controlled pressure of the probe in EUS might be counteracted by more advanced software solutions allowing increasing the reliability and reproducibility of the measurements and by implementation of SWE into EUS systems.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Barreiros AP, Chiorean L, Braden B, et al. Ultrasound in rare diffuse liver disease. Z Gastroenterol. 2014;52:1247–56. doi: 10.1055/s-0034-1384996. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich CF, Lembcke B, Jenssen C, et al. Intestinal ultrasound in rare gastrointestinal diseases, update, part 1. Ultraschall Med. 2014;35:400–21. doi: 10.1055/s-0034-1385154. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Mathis G, Cui XW, et al. Ultrasound of the pleurae and lungs. Ultrasound Med Biol. 2015;41:351–65. doi: 10.1016/j.ultrasmedbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Hirche TO, Hirche H, Cui XW, et al. Ultrasound evaluation of mediastinal lymphadenopathy in patients with sarcoidosis. Med Ultrason. 2014;16:194–200. doi: 10.11152/mu.2013.2066.163.2hh. [DOI] [PubMed] [Google Scholar]
- 5.Popescu A, Saftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound. 2014;3:109–17. doi: 10.4103/2303-9027.123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iglesias-Garcia J, Lindkvist B, Larino-Noia J, et al. Endoscopic ultrasound elastography. Endosc Ultrasound. 2012;1:8–16. doi: 10.7178/eus.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert D, Penman ID. Endoscopic ultrasound. Endoscopy. 2008;40:849–54. doi: 10.1055/s-2008-1077593. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–9. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 10.Tamerisa R, Irisawa A, Bhutani MS. Endoscopic ultrasound in the diagnosis, staging, and management of gastrointestinal and adjacent malignancies. Med Clin North Am. 2005;89:139–58. doi: 10.1016/j.mcna.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez-Sequeiros E, Levy MJ, Clain JE, et al. Routine vs. selective EUS-guided FNA approach for preoperative nodal staging of esophageal carcinoma. Gastrointest Endosc. 2006;63:204–11. doi: 10.1016/j.gie.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich CF, Ponnudurai R, Bachmann Nielsen M. Is there a need for new imaging methods for lymph node evaluation? Ultraschall Med. 2012;33:411–4. doi: 10.1055/s-0032-1325384. [DOI] [PubMed] [Google Scholar]
- 13.Dumonceau JM, Polkowski M, Larghi A, et al. European Society of Gastrointestinal Endoscopy. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 14.Polkowski M, Larghi A, Weynand B, et al. European Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 15.Araujo J, Bories E, Caillol F, et al. Distant lymph node metastases in gastroesophageal junction adenocarcinoma: Impact of endoscopic ultrasound-guided fine-needle aspiration. Endosc Ultrasound. 2013;2:148–52. doi: 10.7178/eus.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–92. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 17.Ardengh JC, Lopes CV, de Lima LF, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112–6. doi: 10.3748/wjg.v13.i22.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eloubeidi MA, Cerfolio RJ, Chen VK, et al. Endoscopic ultrasound-guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann Thorac Surg. 2005;79:263–8. doi: 10.1016/j.athoracsur.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, et al. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289–93. doi: 10.3748/wjg.v13.i2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: The M.D. Anderson Cancer Center experience. Cancer. 2002;96:174–80. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]
- 21.Giovannini M, Thomas B, Erwan B, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J Gastroenterol. 2009;15:1587–93. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hocke M, Ignee A, Topalidis T, et al. Back to the roots — Should gastroenterologists perform their own cytology? Z Gastroenterol. 2013;51:191–5. doi: 10.1055/s-0032-1313148. [DOI] [PubMed] [Google Scholar]
- 23.Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy. 2010;42:564–70. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich CF. Real-time tissue elastography. Multiple clinical applications. Multiple clinical solutions. Endo Heute. 2011;8:48–67. [Google Scholar]
- 25.Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European society of gastrointestinal endoscopy technical guidelines. Endosc Ultrasound. 2013;2:117–22. doi: 10.7178/eus.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich CF, Hocke M, Jenssen C. Ultrasound for abdominal lymphadenopathy. Dtsch Med Wochenschr. 2013;138:1001–18. doi: 10.1055/s-0032-1333027. [DOI] [PubMed] [Google Scholar]
- 27.Cui XW, Ignee A, Bachmann NM, et al. Contrast enhanced ultrasound of sentinel lymph nodes. J Ultrason. 2012;13:73–81. doi: 10.15557/JoU.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saftoiu A, Vilmann P, Gorunescu F, et al. European EUS Elastography Multicentric Study Group. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol. 2012;10:84–90. doi: 10.1016/j.cgh.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Shi J, Zeng X, et al. EUS elastography for the differentiation of benign and malignant lymph nodes: A meta-analysis. Gastrointest Endosc. 2011;74:1001–9. doi: 10.1016/j.gie.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Saftoiu A, Vilmann P, Ciurea T, et al. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291–300. doi: 10.1016/j.gie.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 31.Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol. 2015;41:1148–60. doi: 10.1016/j.ultrasmedbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Arcidiacono PG. Endoscopic ultrasound elastography. Gastroenterol Hepatol (N Y) 2012;8:48–67. [PMC free article] [PubMed] [Google Scholar]
- 33.Emerson K. Diseases of the breast. In: Thorn WW, editor. Principals of internal medicine. 7th ed. New York: McGraw-Hill; 1974. pp. 582–7. [Google Scholar]
- 34.Bhargava S, Bhargava SK, Sharma S, et al. Elastography: A new imaging technique and its application. JIMSA. 2013;26:25–30. [Google Scholar]
- 35.Dietrich CF, Saftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol. 2014;83:403–4. doi: 10.1016/j.ejrad.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich CF. Elastography, the new dimension in ultrasonography. Praxis (Bern 1994) 2011;100:1533–42. doi: 10.1024/1661-8157/a000735. [DOI] [PubMed] [Google Scholar]
- 38.Barr RG. U S Elastography: Applications in tumors. In: Luna A, editor. Functional Imaging in Oncology. Berlin Heidelberg: Springer-Verlag; 2014. pp. 459–88. [Google Scholar]
- 39.Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850–60. doi: 10.3748/wjg.v19.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietrich CF. Elastography Applications. Endo Heute. 2011;24:177–212. [Google Scholar]
- 41.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–47. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Rana SS, Vilmann P. Endoscopic ultrasound features of chronic pancreatitis: A pictorial review. Endosc Ultrasound. 2015;4:10–4. doi: 10.4103/2303-9027.151314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saftoiu A, Gheonea DI, Ciurea T. Hue histogram analysis of real-time elastography images for noninvasive assessment of liver fibrosis. AJR Am J Roentgenol. 2007;189:W232–3. doi: 10.2214/AJR.07.2571. [DOI] [PubMed] [Google Scholar]
- 44.Berg WA, Cosgrove DO, Dore CJ, et al. BE1 Investigators. Shear-wave elastography improves the specificity of breast US: The BE1 multinational study of 939 masses. Radiology. 2012;262:435–49. doi: 10.1148/radiol.11110640. [DOI] [PubMed] [Google Scholar]
- 45.Chiorean A, Duma M, Dudea S, et al. Short analysis on elastographic images of benign and malignant breast lesions based on color and hue parameters. Ultraschall Med. 2008;29:OP2_13. [Google Scholar]
- 46.Itoh A, Ueno E, Tohno E, et al. Breast disease: Clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 47.Krouskop TA, Wheeler TM, Kallel F, et al. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20:260–74. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 48.Thomas A, Fischer T, Frey H, et al. Real-time elastography — An advanced method of ultrasound: First results in 108 patients with breast lesions. Ultrasound Obstet Gynecol. 2006;28:335–40. doi: 10.1002/uog.2823. [DOI] [PubMed] [Google Scholar]
- 49.Thomas A, Kummel S, Fritzsche F, et al. Real-time sonoelastography performed in addition to B-mode ultrasound and mammography: Improved differentiation of breast lesions? Acad Radiol. 2006;13:1496–504. doi: 10.1016/j.acra.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–50. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Corpechot C, El Naggar A, Poujol-Robert A, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–24. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 52.Ferraioli G, Lissandrin R, Zicchetti M, et al. Diffuse liver diseases. In: Calliada F, et al., editors. Sonoelastography main clinical applications. Pavia: Edizioni Medico Scientifiche; 2012. [Google Scholar]
- 53.Gheonea DI, Saftoiu A, Ciurea T, et al. Real-time sono-elastography in the diagnosis of diffuse liver diseases. World J Gastroenterol. 2010;16:1720–6. doi: 10.3748/wjg.v16.i14.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lupsor M, Badea R, Stefanescu H, et al. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2010;19:53–60. [PubMed] [Google Scholar]
- 55.Lupsor Platon M, Stefanescu H, Feier D, et al. Performance of unidimensional transient elastography in staging chronic hepatitis C. Results from a cohort of 1,202 biopsied patients from one single center. J Gastrointestin Liver Dis. 2013;22:157–66. [PubMed] [Google Scholar]
- 56.Yu H, Wilson SR. New noninvasive ultrasound techniques: Can they predict liver cirrhosis? Ultrasound Q. 2012;28:5–11. doi: 10.1097/RUQ.0b013e31824a4fc9. [DOI] [PubMed] [Google Scholar]
- 57.Cui XW, Friedrich-Rust M, De MC, et al. Liver elastography, comments on EFSUMB elastography guidelines 2013. World J Gastroenterol. 2013;19:6329–47. doi: 10.3748/wjg.v19.i38.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui XW, Pirri C, Ignee A, et al. Measurement of shear wave velocity using acoustic radiation force impulse imaging is not hampered by previous use of ultrasound contrast agents. Z Gastroenterol. 2014;52:649–53. doi: 10.1055/s-0034-1366036. [DOI] [PubMed] [Google Scholar]
- 59.Dighe M, Bae U, Richardson ML, et al. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008;248:662–9. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 60.Lyshchik A, Higashi T, Asato R, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–11. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 61.Saftoiu A, Vilmann P, Gorunescu F, et al. European EUS Elastography Multicentric Study Group. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: A multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 62.Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–7. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 63.Iglesias-Garcia J, Dominguez-Munoz JE, Castineira-Alvarino M, et al. Quantitative elastography associated with endoscopic ultrasound for the diagnosis of chronic pancreatitis. Endoscopy. 2013;45:781–8. doi: 10.1055/s-0033-1344614. [DOI] [PubMed] [Google Scholar]
- 64.Fusaroli P, Eloubeidi MA. Endoscopic ultrasound elastography in diagnosing chronic pancreatitis: Has the strain ratio found its region of interest? Endoscopy. 2013;45:789–91. doi: 10.1055/s-0033-1344786. [DOI] [PubMed] [Google Scholar]
- 65.Dietrich CF, Hirche TO, Ott M, et al. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–20. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 66.Cochlin DL, Ganatra RH, Griffiths DF. Elastography in the detection of prostatic cancer. Clin Radiol. 2002;57:1014–20. doi: 10.1053/crad.2002.0989. [DOI] [PubMed] [Google Scholar]
- 67.Miyagawa T, Tsutsumi M, Matsumura T, et al. Real-time elastography for the diagnosis of prostate cancer: Evaluation of elastographic moving images. Jpn J Clin Oncol. 2009;39:394–8. doi: 10.1093/jjco/hyp026. [DOI] [PubMed] [Google Scholar]
- 68.Sommerfeld HJ, Garcia-Schurmann JM, Schewe J, et al. Prostate cancer diagnosis using ultrasound elastography. Introduction of a novel technique and first clinical results. Urologe A. 2003;42:941–5. doi: 10.1007/s00120-003-0297-4. [DOI] [PubMed] [Google Scholar]
- 69.Alam F, Naito K, Horiguchi J, et al. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. AJR Am J Roentgenol. 2008;191:604–10. doi: 10.2214/AJR.07.3401. [DOI] [PubMed] [Google Scholar]
- 70.Fritscher-Ravens A. Blue clouds and green clouds: Virtual biopsy via EUS elastography? Endoscopy. 2006;38:416–7. doi: 10.1055/s-2006-925277. [DOI] [PubMed] [Google Scholar]
- 71.Giovannini M, Hookey LC, Bories E, et al. Endoscopic ultrasound elastography: The first step towards virtual biopsy?. Preliminary results in 49 patients. Endoscopy. 2006;38:344–8. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 72.Janssen J, Schlorer E, Greiner L. EUS elastography of the pancreas: Feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–8. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 73.Saftoiu A, Vilman P. Endoscopic ultrasound elastography — A new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161–5. [PubMed] [Google Scholar]
- 74.Saftoiu A, Vilmann P, Hassan H, et al. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535–42. doi: 10.1055/s-2006-927117. [DOI] [PubMed] [Google Scholar]
- 75.Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol. 2014;44:956–62. doi: 10.1093/jjco/hyu105. [DOI] [PubMed] [Google Scholar]
- 76.Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 77.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 78.Lyshchik A, Higashi T, Asato R, et al. Cervical lymph node metastases: Diagnosis at sonoelastography – Initial experience. Radiology. 2007;243:258–67. doi: 10.1148/radiol.2431052032. [DOI] [PubMed] [Google Scholar]
- 79.Bhatia KS, Tong CS, Cho CC, et al. Shear wave elastography of thyroid nodules in routine clinical practice: Preliminary observations and utility for detecting malignancy. Eur Radiol. 2012;22:2397–406. doi: 10.1007/s00330-012-2495-1. [DOI] [PubMed] [Google Scholar]
- 80.Popescu A, Saftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound. 2014;3:109–17. doi: 10.4103/2303-9027.123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janssen J, Dietrich CF, Will U, et al. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952–7. doi: 10.1055/s-2007-966946. [DOI] [PubMed] [Google Scholar]
- 82.Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology — An overview. Best Pract Res Clin Gastroenterol. 2009;23:743–59. doi: 10.1016/j.bpg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Dietrich CF, Rudd L. The EFSUMB website, a guide for better understanding. Med Ultrason. 2013;15:215–23. doi: 10.11152/mu.2013.2066.153.cfd1lr2. [DOI] [PubMed] [Google Scholar]
- 84.Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J Nucl Med. 2004;45:1509–18. [PubMed] [Google Scholar]
- 85.Wallace MB, Ravenel J, Block MI, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg. 2004;77:1763–8. doi: 10.1016/j.athoracsur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 86.Schroder W, Baldus SE, Monig SP, et al. Lymph node staging of esophageal squamous cell carcinoma in patients with and without neoadjuvant radiochemotherapy: Histomorphologic analysis. World J Surg. 2002;26:584–7. doi: 10.1007/s00268-001-0271-5. [DOI] [PubMed] [Google Scholar]
- 87.Monig SP, Baldus SE, Zirbes TK, et al. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6:579–81. doi: 10.1007/s10434-999-0579-1. [DOI] [PubMed] [Google Scholar]
- 88.Monig SP, Zirbes TK, Schroder W, et al. Staging of gastric cancer: Correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol. 1999;173:365–7. doi: 10.2214/ajr.173.2.10430138. [DOI] [PubMed] [Google Scholar]
- 89.Jurgensen C, Dietrich CF. Role of endoscopic ultrasound (EUS) in the staging of rectal cancer. Z Gastroenterol. 2008;46:580–9. doi: 10.1055/s-2008-1027405. [DOI] [PubMed] [Google Scholar]
- 90.Prenzel KL, Holscher AH, Vallbohmer D, et al. Lymph node size and metastatic infiltration in adenocarcinoma of the pancreatic head. Eur J Surg Oncol. 2010;36:993–6. doi: 10.1016/j.ejso.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Prenzel KL, Holscher AH, Drebber U, Bollschweiler E, Gutschow CA, Stippel DL, et al. Extracapsular lymph node spread as a negative prognostic factor of adenocarcinoma of the pancreas and cancer of the papilla of vater. Pancreas. 2014;43:64–68. doi: 10.1097/MPA.0b013e3182a44a91. [DOI] [PubMed] [Google Scholar]
- 92.Prenzel KL, Monig SP, Sinning JM, et al. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463–7. doi: 10.1378/chest.123.2.463. [DOI] [PubMed] [Google Scholar]
- 93.Cui XW, Hocke M, Jenssen C, et al. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol. 2014;52:212–21. doi: 10.1055/s-0033-1356153. [DOI] [PubMed] [Google Scholar]
- 94.Talsma AK, Ong CA, Liu X, et al. Location of lymph node involvement in patients with esophageal adenocarcinoma predicts survival. World J Surg. 2014;38:106–13. doi: 10.1007/s00268-013-2236-x. [DOI] [PubMed] [Google Scholar]
- 95.Twine CP, Roberts SA, Rawlinson CE, et al. Prognostic significance of the endoscopic ultrasound defined lymph node metastasis count in esophageal cancer. Dis Esophagus. 2010;23:652–9. doi: 10.1111/j.1442-2050.2010.01072.x. [DOI] [PubMed] [Google Scholar]
- 96.Catalano MF, Sivak MV, Jr, Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–6. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 97.Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: A standard endobronchial ultrasound image classification system. Chest. 2010;138:641–7. doi: 10.1378/chest.09-2006. [DOI] [PubMed] [Google Scholar]
- 98.Memoli JS, El-Bayoumi E, Pastis NJ, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest. 2011;140:1550–6. doi: 10.1378/chest.11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts SA, Mahon BS, Evans R. Coagulation necrosis in malignant mediastinal nodes on endoscopic ultrasound: A new endosonographic sign. Clin Radiol. 2005;60:587–91. doi: 10.1016/j.crad.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 100.Bhutani MS, Saftoiu A, Chaya C, et al. Irregular echogenic foci representing coagulation necrosis: A useful but perhaps under-recognized EUS echo feature of malignant lymph node invasion. J Gastrointestin Liver Dis. 2009;18:181–4. [PubMed] [Google Scholar]
- 101.Sawhney MS, Debold SM, Kratzke RA, et al. Central intranodal blood vessel: A new EUS sign described in mediastinal lymph nodes. Gastrointest Endosc. 2007;65:602–8. doi: 10.1016/j.gie.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 102.Schmid-Bindert G, Jiang H, Kahler G, et al. Predicting malignancy in mediastinal lymph nodes by endobronchial ultrasound: A new ultrasound scoring system. Respirology. 2012;17:1190–8. doi: 10.1111/j.1440-1843.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- 103.Shafiek H, Fiorentino F, Peralta AD, et al. Real-time prediction of mediastinal lymph node malignancy by endobronchial ultrasound. Arch Bronconeumol. 2014;50:228–34. doi: 10.1016/j.arbres.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Gill KR, Ghabril MS, Jamil LH, et al. Endosonographic features predictive of malignancy in mediastinal lymph nodes in patients with lung cancer. Gastrointest Endosc. 2010;72:265–71. doi: 10.1016/j.gie.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schmulewitz N, Wildi SM, Varadarajulu S, et al. Accuracy of EUS criteria and primary tumor site for identification of mediastinal lymph node metastasis from non-small-cell lung cancer. Gastrointest Endosc. 2004;59:205–12. doi: 10.1016/s0016-5107(03)02692-0. [DOI] [PubMed] [Google Scholar]
- 106.Dhooria S, Agarwal R, Aggarwal AN, et al. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 107.Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593–8. doi: 10.1067/mge.2001.114060. [DOI] [PubMed] [Google Scholar]
- 108.Edey AJ, Pollentine A, Doody C, et al. Differentiating benign from malignant mediastinal lymph nodes visible at EBUS using grey-scale textural analysis. Respirology. 2015;20:453–8. doi: 10.1111/resp.12467. [DOI] [PubMed] [Google Scholar]
- 109.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: A prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628–33. doi: 10.1111/j.1572-0241.2004.04064.x. [DOI] [PubMed] [Google Scholar]
- 110.Jenssen C, Annema JT, Zels K, et al. Endosonographische evaluierung von lunge und mediastinum. Endoskopie Heute. 2014;27:37–44. [Google Scholar]
- 111.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: It's accuracy in evaluating mediastinal lymphadenopathy?. A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Filip M, Iordache S, Saftoiu A. Gastric Cancer Staging by Endoscopic Ultrasound — Contrast Enhancement and Real-Time Elastography. Video J Encyclopedia GI Endosc. 2014;1:164–6. [Google Scholar]
- 113.Okasha HH, Mansour M, Attia KA, et al. Role of high resolution ultrasound/endosonography and elastography in predicting lymph node malignancy. Endosc Ultrasound. 2014;3:58–62. doi: 10.4103/2303-9027.121252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol. 2012;18:889–95. doi: 10.3748/wjg.v18.i9.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jenssen C, Moller K, Wagner S, et al. Endoscopic ultrasound-guided biopsy: Diagnostic yield, pitfalls, quality management. Z Gastroenterol. 2008;46:897–908. doi: 10.1055/s-2008-1027419. [DOI] [PubMed] [Google Scholar]
- 116.Jenssen C, Moller M, Sarbia M, et al. Endosonographische Biopsie (EUS-FNA, EUS-TCB) — Fallstricke, Probleme und Problemlösungen. In: Dietrich CF, editor. Endosonographie. Lehrbuch und Atlas des endoskopischen Ultraschalls. Stuttgart: Thieme; 2007. pp. 87–140. [Google Scholar]
- 117.Knabe M, Gunter E, Ell C, et al. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc. 2013;27:1196–202. doi: 10.1007/s00464-012-2575-y. [DOI] [PubMed] [Google Scholar]
- 118.Ghajarzadeh M, Mohammadifar M, Azarkhish K, Emami-Razavi SH. Sono-elastography for Differentiating Benign and Malignant Cervical Lymph Nodes: A Systematic Review and Meta-Analysis. Int J Prev Med. 2014;5:1521–28. [PMC free article] [PubMed] [Google Scholar]
- 119.Edge S, Byrd DR, Compton CC, et al. 7th ed. New York: Springer; 2010. AJCC Cancer staging manual. [Google Scholar]
- 120.Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 121.Ganeshalingam S, Koh DM. Nodal staging. Cancer Imaging. 2009;9:104–11. doi: 10.1102/1470-7330.2009.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626–35. doi: 10.1053/j.gastro.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 123.Mortensen MB, Pless T, Durup J, et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy. 2001;33:478–83. doi: 10.1055/s-2001-14966. [DOI] [PubMed] [Google Scholar]
- 124.Giovannini M, Monges G, Seitz JF, et al. Distant lymph node metastases in esophageal cancer: Impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536–40. doi: 10.1055/s-1999-60. [DOI] [PubMed] [Google Scholar]
- 125.Marsman WA, Brink MA, Bergman JJ, et al. Potential impact of EUS-FNA staging of proximal lymph nodes in patients with distal esophageal carcinoma. Endoscopy. 2006;38:825–9. doi: 10.1055/s-2006-944611. [DOI] [PubMed] [Google Scholar]
- 126.Saltzman JR. Section III: Endoscopic and other staging techniques. Semin Thorac Cardiovasc Surg. 2003;15:180–6. [PubMed] [Google Scholar]
- 127.Gleeson FC, Kipp BR, Caudill JL, et al. False positive endoscopic ultrasound fine needle aspiration cytology: Incidence and risk factors. Gut. 2010;59:586–93. doi: 10.1136/gut.2009.187765. [DOI] [PubMed] [Google Scholar]
- 128.Levy MJ, Gleeson FC, Campion MB, et al. Prospective cytological assessment of gastrointestinal luminal fluid acquired during EUS: A potential source of false-positive FNA and needle tract seeding. Am J Gastroenterol. 2010;105:1311–8. doi: 10.1038/ajg.2010.80. [DOI] [PubMed] [Google Scholar]
- 129.Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2:CD009944. doi: 10.1002/14651858.CD009944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hassan H, Vilmann P, Sharma V. Impact of EUS-guided FNA on management of gastric carcinoma. Gastrointest Endosc. 2010;71:500–4. doi: 10.1016/j.gie.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 131.Larsen MH, Fristrup C, Hansen TP, et al. Endoscopic ultrasound, endoscopic sonoelastography, and strain ratio evaluation of lymph nodes with histology as gold standard. Endoscopy. 2012;44:759–66. doi: 10.1055/s-0032-1309817. [DOI] [PubMed] [Google Scholar]
- 132.Vilmann P, Annema J, Clementsen P. Endosonography in bronchopulmonary disease. Best Pract Res Clin Gastroenterol. 2009;23:711–28. doi: 10.1016/j.bpg.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 133.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 135.Singh P, Camazine B, Jadhav Y, et al. Endoscopic ultrasound as a first test for diagnosis and staging of lung cancer: A prospective study. Am J Respir Crit Care Med. 2007;175:345–54. doi: 10.1164/rccm.200606-851OC. [DOI] [PubMed] [Google Scholar]
- 136.Annema JT, Versteegh MI, Veselic M, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol. 2005;23:8357–61. doi: 10.1200/JCO.2005.01.1965. [DOI] [PubMed] [Google Scholar]
- 137.Larsen SS, Vilmann P, Krasnik M, et al. Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: Preliminary results from a randomised clinical trial. Lung Cancer. 2005;49:377–85. doi: 10.1016/j.lungcan.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: A randomized trial. Am J Respir Crit Care Med. 2008;177:531–5. doi: 10.1164/rccm.200708-1241OC. [DOI] [PubMed] [Google Scholar]
- 139.Trosini-Desert V, Jeny F, Taillade L, et al. Bronchial endoscopic ultrasound elastography: Preliminary feasibility data. Eur Respir J. 2013;41:477–9. doi: 10.1183/09031936.00124812. [DOI] [PubMed] [Google Scholar]
- 140.Andreo GF, Centeno Clemente CA, Sanz Santos J, et al. Initial experience with real-time elastography using an ultrasound bronchoscope for the evaluation of mediastinal lymph nodes. Arch Bronconeumol. 2015;51:e8–11. doi: 10.1016/j.arbres.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 141.Hirooka Y, Itoh A, Kawashima H, et al. Diagnosis of involvement of hepatoduodenal ligament and lymphnodes using EUS-elastography in the cases of biliary malignancies [Abstract] Gastrointest Endosc. 2009;69:AB240. [Google Scholar]
- 142.Gleeson FC, Rajan E, Levy MJ, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438–43. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 143.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–8. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tharian B, Tsiopoulos F, George N, et al. Endoscopic ultrasound fine needle aspiration: Technique and applications in clinical practice. World J Gastrointest Endosc. 2012;4:532–44. doi: 10.4253/wjge.v4.i12.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123:24–32. doi: 10.1053/gast.2002.34163. [DOI] [PubMed] [Google Scholar]
- 146.Shami VM, Parmar KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59–65. doi: 10.1007/s10350-003-0001-1. [DOI] [PubMed] [Google Scholar]
- 147.Gagliardi G, Bayar S, Smith R, et al. Preoperative staging of rectal cancer using magnetic resonance imaging with external phase-arrayed coils. Arch Surg. 2002;137:447–51. doi: 10.1001/archsurg.137.4.447. [DOI] [PubMed] [Google Scholar]
- 148.Cheng L, Zincke H, Blute ML, et al. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer. 2001;91:66–73. doi: 10.1002/1097-0142(20010101)91:1<66::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 149.Larsen MH, Fristrup CW, Mortensen MB. Intra- and interobserver agreement of endoscopic sonoelastography in the evaluation of lymph nodes. Ultraschall Med. 2011;32:E45–50. doi: 10.1055/s-0031-1273493. [DOI] [PubMed] [Google Scholar]
- 150.Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–50. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tschammler A, Heuser B, Ott G, et al. Pathological angioarchitecture in lymph nodes: Underlying histopathologic findings. Ultrasound Med Biol. 2000;26:1089–97. doi: 10.1016/s0301-5629(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 152.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) — update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 153.Goldberg BB, Merton DA, Liu JB, et al. Sentinel lymph nodes in a swine model with melanoma: Contrast-enhanced lymphatic US. Radiology. 2004;230:727–34. doi: 10.1148/radiol.2303021440. [DOI] [PubMed] [Google Scholar]
- 154.Stramare R, Scagliori E, Mannucci M, et al. The role of contrast-enhanced gray-scale ultrasonography in the differential diagnosis of superficial lymph nodes. Ultrasound Q. 2010;26:45–51. doi: 10.1097/RUQ.0b013e3181cf4469. [DOI] [PubMed] [Google Scholar]
- 155.Ishibashi N, Yamagata K, Sasaki H, et al. Real-time tissue elastography for the diagnosis of lymph node metastasis in oral squamous cell carcinoma. Ultrasound Med Biol. 2012;38:389–95. doi: 10.1016/j.ultrasmedbio.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 156.Fujiwara T, Tomokuni J, Iwanaga K, et al. Acoustic radiation force impulse imaging for reactive and malignant/metastatic cervical lymph nodes. Ultrasound Med Biol. 2013;39:1178–83. doi: 10.1016/j.ultrasmedbio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 157.Tamaki K, Tamaki N, Kamada Y, et al. Non-invasive evaluation of axillary lymph node status in breast cancer patients using shear wave elastography. Tohoku J Exp Med. 2013;231:211–6. doi: 10.1620/tjem.231.211. [DOI] [PubMed] [Google Scholar]
- 158.Jung WS, Kim JA, Son EJ, et al. Shear wave elastography in evaluation of cervical lymph node metastasis of papillary thyroid carcinoma: Elasticity index as a prognostic implication. Ann Surg Oncol. 2015;22:111–6. doi: 10.1245/s10434-014-3627-4. [DOI] [PubMed] [Google Scholar]
- 159.Götzberger M, Jenssen C. Lymph nodes, celiac ganglia, spleen: approach, normal findings, variants, characterization. In: Jenssen C, Gottschalk U, Schachschal G, Dietrich CF, editors. Endosonography Course Book. Stuttgart: Thieme; 2014. pp. S139–49. [Google Scholar]


