Abstract
Convex probe endobronchial ultrasound (CP-EBUS) was originally introduced as a diagnostic and staging tool for lung cancer and subsequently utilized for diagnosis of other malignant and benign mediastinal diseases such as melanoma, lymphoma, and sarcoidosis. More recently, CP-EBUS has been successfully used for the visualization and diagnosis of pulmonary emboli and other vascular lesions including primary and metastatic pulmonary artery (PA) tumors. In this review, we will underline the role of EBUS-guided transbronchial needle aspiration (EBUS-TBNA) for the diagnosis of pulmonary arterial tumors such as sarcomas and tumor emboli. We will concisely discuss the clinical applications of EBUS-TBNA and the types of pulmonary arterial tumors and their different diagnostic modalities. We searched the Cochrane Library and PubMed from 2004 to 2014 to provide the most comprehensive review. Only 10 cases of EBUS-TBNA for intravascular lesions were identified in the literature. Although many cases of EBUS and EUS-guided transvascular tumor biopsies were described in the literature, there were no reported cases of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for intravascular tumor biopsies. Except for one paper, all cases were published as case reports.
Keywords: Endobronchial ultrasound, pulmonary artery, sarcoma, transbronchial needle aspiration
INTRODUCTION
EBUS-guided transbronchial needle aspiration (EBUS-TBNA) is a relatively novel, rapidly growing diagnostic modality that allows precise sampling of mediastinal lymph nodes (LNs) and other peribronchial lesions. EBUS-TBNA is a minimally invasive, safe, and cost effective technique, with high diagnostic yield.[1] Although it is mainly used for diagnosis and LN staging of lung cancer, EBUS-TBNA is also useful for the diagnostic work up of unexplained mediastinal and hilar lymphadenopathy, as well as other mediastinal lesions.[2,3,4] Since its introduction in 2004,[3] the role of EBUS-TBNA has further expanded and now includes restaging lung cancer, diagnosing granulomatous lymphadenopathy including sarcoidosis, and evaluation of patients with extrathoracic malignancies. EBUS-TBNA has a high yield for diagnosing and staging lung cancer ranging from 89 to 98%,[2,3,4,5,6] and yield is higher when compared to computed tomography (CT) and positive emission tomography (PET).[5] It also reduces the need for surgical mediastinal staging and yield has been shown to be comparable to mediastinoscopy with the ability to sample more nodal stations than mediastinoscopy.[7] The ability to visualize the mediastinal vasculature including clots and masses within these vessels has raised questions about the efficacy and safety of sampling these lesions for accurate diagnosis, especially in cases where patients may not be good candidates for other procedure or interventions.
THE EBUS-TBNA PROCEDURE
Convex probe (CP)-EBUS was first introduced in 2004. The scope of EBUS is equipped with a conventional bronchoscopic fiberoptic component to identify airway anatomy, a linear US scanning probe for real-time imaging, and a biopsy channel for the aspiration needle. The integrated color Doppler allows easy identification of vascular structures. The scope consisted of a 7.5 MHz convex probe inside a saline-inflatable balloon at the tip of the bronchoscope. A newer generation EBUS scope permits multi-quad frequencies of 5, 7.5, 10, or 12 MHz; which allow changing the depth of penetration of the ultrasound image. The scope has an outer diameter insertion tube of 6.2 mm, with a tip end of 6.9 mm, and a working channel of 2.2 mm. The scope looks at a 35° forward oblique angle, with an angle of view of 80°. The scope angulation is 120° up and 90° down. EBUS-TBNA is usually performed using a dedicated disposable 22- or 21-gauge needle with an echogenic-dimpled tip. Both ultrasound and white-light bronchoscopy images are simultaneously visible on the monitor. CP-EBUS-TBNA is a real-time procedure that is usually performed under moderate or deep sedation or general anesthesia.
Technically, once the LN or mass has been clearly identified with EBUS, the needle is inserted through the working channel under real-time US guidance. The stylet of the needle is left in place on the first puncture; once the needle tip is inside the lesion, the stylet is used to clean the tip of the needle from any bronchial contamination. After removing the stylet, the standard of care is to apply suction using a negative pressure syringe and the needle is stabbed multiple times. The needle is removed and the specimen is expelled onto a glass slides using an air-filled syringe or by reinserting the stylet. The slides are then air-dried or alcohol-fixed or sent as cellblock specimen. In most cases, three to four passes per site are optimal to obtain enough tissue for accurate diagnosis.[8,9,10]
TYPES OF PULMONARY VASCULAR TUMORS
Pulmonary artery (PA) tumors consist of primary vascular sarcomas and metastatic tumors. There are several reported types of pulmonary artery sarcomas (PASs) including leiomyosarcoma, angiosarcoma, fibrosarcoma, rhabdomyosarcoma, myxosarcoma, and malignant fibrous histiocytoma.[11,12,13] Metastatic tumors to PA have been described; including metastatic leiomyosarcoma, lung cancer, hepatocellular carcinoma, and synovial sarcoma of the kidney among many others.[14,15]
Primary PAS is a rare tumor, typically grows insidiously, and patients remain asymptomatic until distant tumor embolism or severe PA occlusion ensues. The main PA is the most common location of PAS, followed by right PA, left PA, pulmonary valves, and right ventricular outflow. Although that most PAS tumors grow locoregionally, they have the propensity to metastasize predominantly to the lung and mediastinal LNs.[16]
DIAGNOSTIC MODALITIES FOR MEDIASTINAL VASCULAR TUMORS
Mediastinal vascular tumors are frequently misdiagnosed as thromboembolic disease and the diagnosis requires high level of suspicion. In many instances, vascular tumors of the mediastinum are suspected after failure of anticoagulation therapy with progression and extension of intravascular lesions [Figure 1].[17,18,19,20,21,22] In patients undergoing PET scan for cancer staging or surveillance, the finding of an intravascular fluorodeoxyglucose (FDG)-avid lesion should raise suspicion for intravascular metastasis over pulmonary embolic disease. PET/CT may be helpful to differentiate between PA tumor and PA thrombotic disease. Ito et al.,[23] studied 10 patients with either PAS or PA embolism. All patients had PET/CT done, and the standard uptake values (SUV) were measured. The mean SUV of 7.63 in patients with PASs was significantly higher than the mean SUV of 2.31 seen in cases of pulmonary embolism. Farsad et al.,[21] described a case of PA filling defect secondary to leiomyosarcoma with superimposed thromboembolism. In this case, the contrast-enhanced CT was able to identify the filling defect in the right main PA. The CT/PET showed an FDG-avid area in the lesion representing the sarcoma. The area with no FDG uptake represented the superimposed thromboembolism. CT and MRI characteristics may be helpful in differentiating PAS from thromboembolism.[24] PASs are heterogeneous lesions with high or low attenuation depending whether they have area of hemorrhage or necrosis. The PAS have soft tissue density with vascular distension and possible gadolinium enhancement. Thromboembolism tends to have homogenous soft tissue lesions, with abrupt cut-offs and vascular narrowing. Because PA tumors are very rare, there are no controlled studies or large data collection describing the sensitivity or specificity of the chest CT, PET, and MRI for detecting these tumors.
Figure 1.
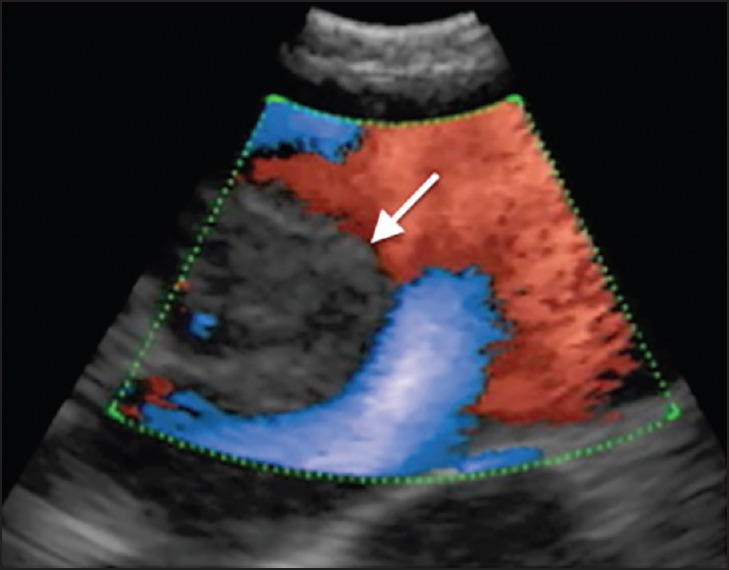
EBUS image of the right hilar area showing a vascular tumor of the right lower lobe pulmonary artery (arrow). Color Doppler showing blood flow around the intravascular tumor. EBUS: Endobronchial ultrasound
The confirmatory diagnosis of intravascular tumor is invasive and require vascular puncture to access the lesion or to obtain a diagnostic vascular sample from the targeted vessel (or surgical). Yamada et al.,[25] were the first to describe a case of catheter suction biopsy of a primary PA leiomyosarcoma. The patient was initially diagnosed with left pulmonary thromboembolism, which was treated with fibrinolysis and anticoagulation without any improvement. The diagnosis of primary pulmonary leiomyosarcoma was confirmed by transvenous suction biopsy that was performed using an embolectomy catheter. Prior to vascular catheterization era, the diagnosis of intravascular tumors was mainly postmortem or after surgery. More recently, pulmonary vascular tumors have been diagnosed using EBUS-guided transbronchial aspiration (EBUS-TBNA).[26]
EBUS-TBNA OF PA LESIONS
Intentional puncturing of the thoracic vasculature goes back to early 1900 with repetitive attempts to gain access to the heart through various approaches.[27,28] In 1959, Lemmon et al.,[29] published a series of patients who underwent coronary angiography using the suprasternal transaortic approach. In the mid-last century, multiple large series of transbronchial left heart catheter showed no fatalities and no significant complications.[30,31] Also, Scott et al., performed transbronchial left heart catheterization using the double needle technique in 20 patients without any complications.[32] Crymes et al., reported their experience with 76 cases of transbronchial left atrial catheterization.[33] Only three complications occurred with one fatality related to the procedure. They conducted a literature review and reported a mortality rate of 0.068% based on one death case out of 1,472 collected cases. Despite the very low morbidity and mortality rates related to the above-described procedures, transaortic and transpulmonary vascular procedures were generally abandoned and are now very rarely performed. In 1983, Wang and Terry[34] introduced the conventional TBNA procedure for the diagnosis and staging of lung cancer. It entails the insertion of the needle transbronchially into the targeted lesion. The targeted lesions are localized after reviewing the chest imaging and necessitate detailed anatomical understanding of the thoracic anatomy. The yield was reported to be high in some specialized centers, but was not reproducible in all centers. It was not a real time procedure and inadvertent puncture of thoracic vessels was probably not uncommon. In spite of well-documented safety of this procedure in several studies, it was performed only by a minor fraction of physicians likely due to issues related to training, yield, and concerns about unintended vascular puncture.
CP-EBUS continues to demonstrate a remarkable ability to outline thoracic anatomy. Its original role was to identify and sample mediastinal lymph for staging lung cancers and later extended for other malignant and benign mediastinal conditions such as sarcoidosis and lymphoma. Presently, there are increasing reports describing the role of CP-EBUS in the diagnosis of mediastinal vascular tumors. Some of these tumors were safely sampled with EBUS-TBNA. As the experience with EBUS combined with color Doppler continues to expand, bronchoscopists will strengthen their ability to characterize vascular anomalies and lesions, and perhaps uncover a CP-EBUS role in pulmonary vascular hemodynamics such as pulmonary hypertension. Future therapeutic role of CP-EBUS for treating pulmonary vascular emboli or tumors remains to be revealed.
The EBUS scope will not only be used as a diagnostic tool, but also acquire a therapeutic role.[35] In 2006, Seymour et al.,[36] published a review related to transbronchial needle injection (TBNI). In this review, the authors described the diagnostic and therapeutic role of conventional TBNI. The therapeutic role of TBNI consisted of injecting tumors with ethanol,[37] chemotherapeutic agents,[38] and modified genes therapy.[39] Moreover, TBNI has been used to guide surgical localization of peripheral lung nodule and sentinel LNs by injecting or placing markers. CP-EBUS has also been used to guide implantation of radiotherapy devices and for placing fiducial markers in lung cancers to guide stereotactic beam radiation therapy (SBRT).[40,41]
CP-EBUS has a unique ability of showing blood flow using the color Doppler ultrasound feature in addition to the ultrasound real-time images [Figure 2]. This helps the operators to distinguish LNs, necrotic areas, and mediastinal cysts from vascular structures. Evaluation of major thoracic vessels is an integral part of EBUS procedure as vascular landmarks are used to delineate several LNs stations in the current staging system. Additionally, this allows the identification of arterial and venous pulmonary arterial lesions such as primary or metastatic vascular tumors. In a report by Al-Ajam et al., EBUS was able to detect a left pulmonary venous tumor that was thought to be metastatic hepatocellular carcinoma.[14] Many other reports described the use of EBUS in the diagnosis of primary and metastatic pulmonary arterial tumors such as sarcomas, thyroid, and breast cancers.[22,42,43]
Figure 2.
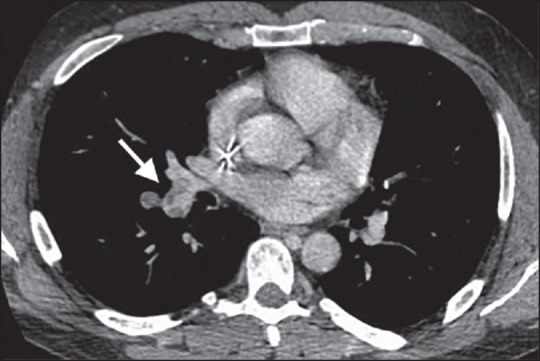
(a) Chest CT showing right pulmonary artery filling defect (arrow) extending to the right lower arterial branch consistent with vascular tumor that was initially diagnosed as thromboembolic disease. CT: Computed tomography
Detection of pulmonary emboli using CP-EBUS has been described and safely accomplished.[44,45,46,47,48,49,50,51,52] Additionally, CP-EBUS has been used for restaging lung cancer by detecting tumor vascular invasion.[53] With no reported complications, the aspiration of subaortic (aortopulmonary window) mediastinal LN has been successfully performed using EBUS-TBNA through a transarterial approach.[54] In this series, four patients with subaortic lymphadenopathy and contraindication for thoracoscopic biopsies were enrolled and EBUS-TBNA was performed using the 21-gauge EBUS needle. The malignant diagnosed was obtained in three patients and one patient required thoracoscopy procedure.
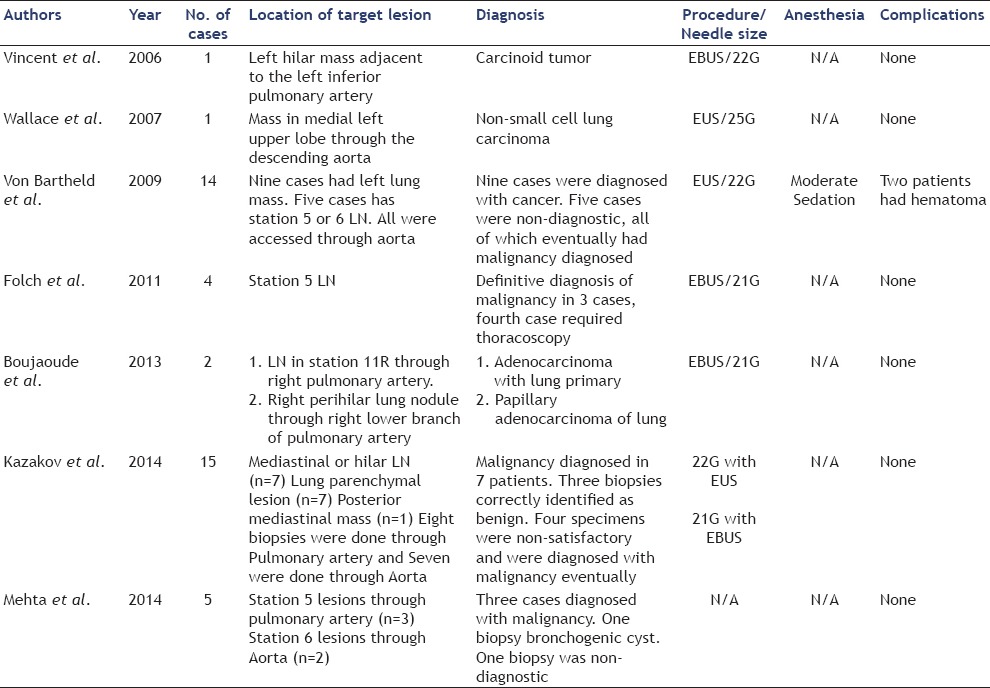
Dusemund et al.,[55] performed EBUS-TBNA of an occlusive mass of the left PA a year after treating a synovial sarcoma of the right kidney with nephrectomy and chemotherapy. CT showed occlusive solid mass of the left PA, which was FDG avid on PET scan. The patient was given a single dose of postoperative antibiotic to prevent infection and no complications were reported. Shingyoji et al.,[56] performed EBUS-TBNA of the left lower pulmonary tumor in a patient that presented with cough and CT showed mass lesions in bilateral pulmonary arteries. PET was positive with SUV of 12.4; EBUS-TBNA of the left PA mass was performed and the diagnosis of PAS was made without any procedure-related complication. Horowitz et al.,[57] performed EBUS-TBNA of the left PA mass lesion that was an extension of the left hilar mass. The diagnosis was consistent with lung adenocarcinoma and the patient was discharged postoperatively with no reported complications. A detailed review of the literature identified a few reported cases of EBUS-TBNA of intravascular tumors [Table 1 and Video 1].[15,22,26,42,43,55,56,57,58] To the best of our knowledge, there are no reported cases of fine needle aspiration (FNA) of intravascular thoracic tumors utilizing endoscopic ultrasound (EUS). Some authors have reported the use of EBUS-TBNA and EUS-FNA for transvascular tumor or LN sampling [Table 2].[54,59,60,61,62,63,64] von Bartheld et al.,[61] published the largest retrospective series that included 14 patients with para-aortic tumors (nine patients) or suspicious LNs (five patients). All the patients underwent EUS-FNA of which nine had lung cancer (64%), one reactive LN specimen, and four inconclusive materials. Of the five patients with no diagnosis, three were subsequently diagnosed with cancers after confirmatory surgical pathology (two lung cancers and one renal cancer). Two patients developed para-aortic hematoma immediately after the EUS-FNA. One patient remained asymptomatic and was discharged home. The other patient was observed overnight after developing postoperative chest discomfort. Thoracic CT showed evidence of para-aortic hematoma and excluded aortic dissection.
Table 1.
Reported cases of EBUS TBNA for intravascular lesions
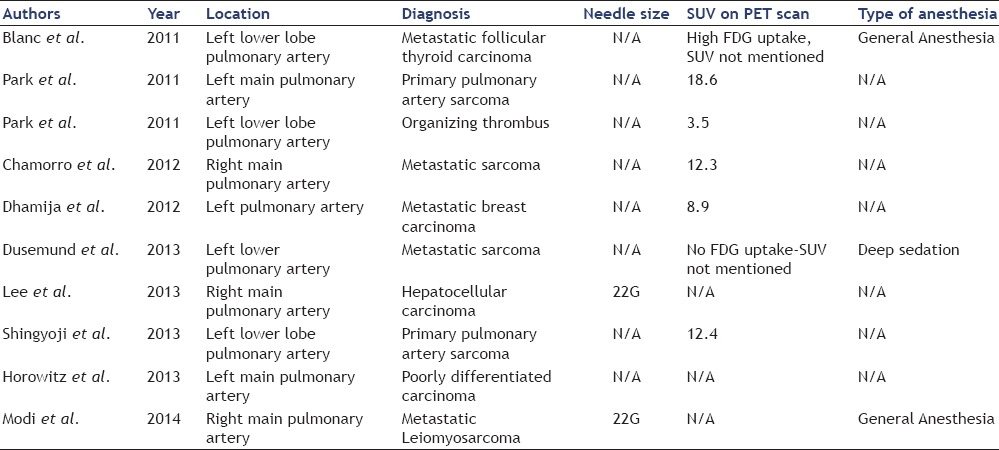
Table 2.
Reported cases of EBUS/EUS guided trans-vascular biopsies
Seven of the 10 patients who underwent EBUS-TBNA for intravascular lesions underwent PET [Table 1]. Six of these patients had PET-avid intravascular lesions of which one was diagnosed as organizing thrombus (SUV of 3.5). The other five patients (71.4%) were diagnosed with primary PAS (n = 2), metastatic sarcoma (n = 1), thyroid carcinoma (n = 1), and metastatic breast carcinoma (n = 1). One patient with metastatic PAS had no FDG uptake on PET. All 10 patients underwent chest contrast-enhanced CT scans that showed vascular lesions with differential diagnosis of thromboembolic disease versus tumors.
COMPLICATIONS
Although EBUS allows real-time sampling of lesions and avoidance of vascular puncture, accidental vascular punctures still occur. However, usually this does not result in any significant complication. Several case reports reported no major complications when hilar or mediastinal lesions located behind major thoracic vessels were successfully sampled by traversing the PA and aorta.[59,60,65]
Needle puncture of blood vessels are, however, not without potential complications. One report described bleeding and intramural hematoma and hemomediastinum after EBUS-guided PA puncture.[66] The artery was accidentally punctured during LN sampling, but the hematoma resolved spontaneously without any further intervention. After a detailed search of literature and review of several case reports of EBUS-TBNA for intravascular tumors, we did not find any description of any complications.[15,26,42,43,55,56,57,58] von Bartheld et al.,[61] published a series of 14 patients that underwent transaortic EUS-guided biopsies of para-aortic lesions. Two of these patients developed thoracic hematomas in the immediate postoperative period. One of the patients complained of chest pain, but both patients require no intervention. Although it is possible that the cases where complications occurred were not reported, based on the available literature, intentional or unintentional puncture of thoracic vessels can be safely accomplished in carefully selected cases.
CONCLUSION
EBUS-TBNA procedure for the diagnosis of intravascular mediastinal or hilar tumors appears to be feasible, safe, and minimally invasive. This procedure may be an acceptable alternative for more invasive procedure such as catheter suction biopsy and may avoid the potential delay in diagnosis, as reported in some cases due to the decision to observe. Similarly, based on few reports it appears that transvascular EBUS-TBNA is feasible and safe and in selected patients, may obviate invasive intervention. Nevertheless, until this question is studied in further detail in larger studies, the decision to puncture a major blood vessel to obtain diagnostic specimen should be made after a thorough consideration of the risks and benefits, and should be made on case-to-case basis.
Video Available on: www.eusjournal.com
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: A systematic review. Eur Respir J. 2009;33:1156–64. doi: 10.1183/09031936.00097908. [DOI] [PubMed] [Google Scholar]
- 2.Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50:347–54. doi: 10.1016/j.lungcan.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–8. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 4.Chalhoub M, Harris K. The use of endobronchial ultrasonography with transbronchial needle aspiration to sample a solitary substernal thyroid nodule. Chest. 2010;137:1435–6. doi: 10.1378/chest.09-2840. [DOI] [PubMed] [Google Scholar]
- 5.Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130:710–8. doi: 10.1378/chest.130.3.710. [DOI] [PubMed] [Google Scholar]
- 6.Herth FJ, Eberhardt R, Becker HD, et al. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: A prospective trial. Chest. 2006;129:147–50. doi: 10.1378/chest.129.1.147. [DOI] [PubMed] [Google Scholar]
- 7.Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3:577–82. doi: 10.1097/JTO.0b013e3181753b5e. [DOI] [PubMed] [Google Scholar]
- 8.Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: How many aspirations per target lymph node station? Chest. 2008;134:368–74. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 9.Diacon AH, Schuurmans MM, Theron J, et al. Transbronchial needle aspirates: How many passes per target site? Eur Respir J. 2007;29:112–6. doi: 10.1183/09031936.00055506. [DOI] [PubMed] [Google Scholar]
- 10.Chin R Jr, McCain TW, Lucia MA, et al. Transbronchial needle aspiration in diagnosing and staging lung cancer: How many aspirates are needed? Am J Respir Crit Care Med. 2002;166:377–81. doi: 10.1164/rccm.2106153. [DOI] [PubMed] [Google Scholar]
- 11.Huo L, Moran CA, Fuller GN, et al. Pulmonary artery sarcoma: A clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol. 2006;125:419–24. [PubMed] [Google Scholar]
- 12.Bleisch VR, Kraus FT. Polypoid sarcoma of the pulmonary trunk: Analysis of the literature and report of a case with leptomeric organelles and ultrastructural features of rhabdomyosarcoma. Cancer. 1980;46:314–24. doi: 10.1002/1097-0142(19800715)46:2<314::aid-cncr2820460217>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki M, Sumi Y, Sakakibara Y, et al. Pulmonary artery leiomyosarcoma diagnosed without delay. Case Rep Oncol. 2011;4:287–98. doi: 10.1159/000328994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Ajam MR, Kalanjeri S, Haas AR, Gillespie CT, Sterman DH. Endobronchial ultrasound. When to venture into the vasculature. Ann Am Thorac Soc. 2013;10:393–5. doi: 10.1513/AnnalsATS.201304-098OT. [DOI] [PubMed] [Google Scholar]
- 15.Chamorro N, Blanco I, Sanchez M, et al. The expanding horizons of endobronchial ultrasound: Diagnosis of a tumor embolism. Chest. 2012;142:1334–6. doi: 10.1378/chest.12-0181. [DOI] [PubMed] [Google Scholar]
- 16.Ramp U, Gerharz CD, Iversen S, et al. Sarcoma of the pulmonary artery: Report of two cases and a review of the literature. J Cancer Res Clin Oncol. 1992;118:551–6. doi: 10.1007/BF01225272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng J, Murday AJ. Leiomyosarcoma of the pulmonary artery. Ann Thorac Surg. 1992;53:905–6. doi: 10.1016/0003-4975(92)91468-o. [DOI] [PubMed] [Google Scholar]
- 18.Stella F, Davoli F, Brandolini J, et al. Pulmonary artery leiomyosarcoma successfully treated by right pneumonectomy. Asian Cardiovasc Thorac Ann. 2009;17:513–5. doi: 10.1177/0218492309348631. [DOI] [PubMed] [Google Scholar]
- 19.Akram K, Silverman ME, Voros S. A unique case of pulmonary artery leiomyosarcoma. J Natl Med Assoc. 2006;98:1995–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Croitoru AG, Klein MJ, Galla JD, et al. Primary pulmonary artery leiomyosarcoma. Cardiovasc Pathol. 2003;12:166–9. doi: 10.1016/s1054-8807(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 21.Farsad M, Pernter P, Triani A, et al. Thromboembolism in pulmonary artery sarcoma. Clin Nucl Med. 2009;34:239–40. doi: 10.1097/RLU.0b013e31819a1f7b. [DOI] [PubMed] [Google Scholar]
- 22.Modi K, Dhillon S, Kumar A, et al. Leiomyosarcoma of the pulmonary artery diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Endosc Ultrasound. 2014;3:249–51. doi: 10.4103/2303-9027.144547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K, Kubota K, Morooka M, et al. Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med. 2009;23:671–6. doi: 10.1007/s12149-009-0292-y. [DOI] [PubMed] [Google Scholar]
- 24.Kauczor HU, Schwickert HC, Mayer E, et al. Pulmonary artery sarcoma mimicking chronic thromboembolic disease: Computed tomography and magnetic resonance imaging findings. Cardiovasc Intervent Radiol. 1994;17:185–9. doi: 10.1007/BF00571532. [DOI] [PubMed] [Google Scholar]
- 25.Yamada N, Kamei S, Yasuda F, et al. Primary leiomyosarcoma of the pulmonary artery confirmed by catheter suction biopsy. Chest. 1998;113:555–6. doi: 10.1378/chest.113.2.555. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, Chung JH, Jheon S, et al. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J. 2011;38:1480–2. doi: 10.1183/09031936.00043211. [DOI] [PubMed] [Google Scholar]
- 27.Wickbom I. Thoracic aortography after direct puncture of the aorta from the jugulum. Acta Radiol. 1952;38:343–9. doi: 10.3109/00016925209139146. [DOI] [PubMed] [Google Scholar]
- 28.Radner S. Suprasternal puncture technique for left heart flow studies. Acta Chir Scandinav. 1954;108:54. [Google Scholar]
- 29.Lemmon WM, Lehman JS, Boyer RA. Suprasternal transaortic coronary arteriography. Circulation. 1959;19:47–54. doi: 10.1161/01.cir.19.1.47. [DOI] [PubMed] [Google Scholar]
- 30.Morrow AG, Braunwald E, Haller JA, Jr, et al. Left heart catheterization by the transbronchial route: Technic and applications in physiologic and diagnostic investigations. Circulation. 1957;16:1033–9. doi: 10.1161/01.cir.16.6.1033. [DOI] [PubMed] [Google Scholar]
- 31.Allison PR, Linden RJ. The bronchoscopic measurement of left auricular pressure. Circulation. 1953;7:669–73. doi: 10.1161/01.cir.7.5.669. [DOI] [PubMed] [Google Scholar]
- 32.Scott SM, Fish RG, Takaro T. A double-needle technic for transbronchial left heart catheterization. Circulation. 1960;22:976–8. doi: 10.1161/01.cir.22.5.976. [DOI] [PubMed] [Google Scholar]
- 33.Crymes TP, Fish RG, Smith DE, et al. Complications of transbronchial left atrial puncture. Am Heart J. 1959;58:46–52. doi: 10.1016/0002-8703(59)90272-8. [DOI] [PubMed] [Google Scholar]
- 34.Wang KP, Terry PB. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis. 1983;127:344–7. doi: 10.1164/arrd.1983.127.3.344. [DOI] [PubMed] [Google Scholar]
- 35.Schuhmann M, Bryant MG, Herth FJ, et al. The role of endobronchial ultrasound (EBUS) in radiographically occult mediastinal disease and the future of EBUS. Curr Respirat Care Rep. 2012;1:40–5. [Google Scholar]
- 36.Seymour CW, Krimsky WS, Sager J, et al. Transbronchial needle injection: A systematic review of a new diagnostic and therapeutic paradigm. Respiration. 2006;73:78–89. doi: 10.1159/000090994. [DOI] [PubMed] [Google Scholar]
- 37.Fujisawa T, Hongo H, Yamaguchi Y, et al. Intratumoral ethanol injection for malignant tracheobronchial lesions: A new bronchofiberscopic procedure. Endoscopy. 1986;18:188–91. doi: 10.1055/s-2007-1018369. [DOI] [PubMed] [Google Scholar]
- 38.Celikoglu F, Celikoglu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol. 2003;55:1441–8. doi: 10.1211/0022357021936. [DOI] [PubMed] [Google Scholar]
- 39.Swisher SG, Roth JA, Komaki R, et al. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res. 2003;9:93–101. [PubMed] [Google Scholar]
- 40.Tobias DR, Puchalski J. Convex probe endobronchial ultrasound for placement of intraparenchymal fiducial markers to guide stereotactic radiosurgery. Chest. 2011:164A. [Google Scholar]
- 41.McGuire R, Liming J, Ochran T, et al. Real-time endobronchial ultrasound-guided implantation of radiotherapy monitoring devices. J Bronchol. 2007;1:59–62. [Google Scholar]
- 42.Blanc AL, Jardin C, Faivre JB, et al. Pulmonary artery tumour-embolism diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Eur Respir J. 2011;38:477–9. doi: 10.1183/09031936.00182210. [DOI] [PubMed] [Google Scholar]
- 43.Dhamija A, Agarwal A, Basu A, et al. Hilar lymph node eroding into the pulmonary artery diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. BMJ Case Rep 2012. 2012 doi: 10.1136/bcr-2012-007438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy MP, Morice RC, Jimenez CA, et al. Endobronchial ultrasound appearance of pulmonary artery thrombus in a patient with thymic carcinoma. J Thorac Oncol. 2007;2:862. doi: 10.1097/JTO.0b013e31811f4764. [DOI] [PubMed] [Google Scholar]
- 45.Swartz MA, Gillespie CT. Pulmonary emboli detected by endobronchial ultrasound. Am J Respir Crit Care Med. 2011;183:1569. doi: 10.1164/rccm.201009-1477IM. [DOI] [PubMed] [Google Scholar]
- 46.Aumiller J, Herth FJ, Krasnik M, et al. Endobronchial ultrasound for detecting central pulmonary emboli: A pilot study. Respiration. 2009;77:298–302. doi: 10.1159/000183197. [DOI] [PubMed] [Google Scholar]
- 47.Harris K, Chalhoub M. Endobronchial ultrasound as a confirmatory tool for the diagnosis of pulmonary embolism. Ann Thorac Med. 2014;9:127–8. doi: 10.4103/1817-1737.128863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cetinkaya E, Yilmaz A, Ozgul A, et al. A case of pulmonary embolism confirmed by endobronchial ultrasound. Tuberk Toraks. 2011;59:318–20. doi: 10.5578/tt.2335. [DOI] [PubMed] [Google Scholar]
- 49.Casoni GL, Gurioli C, Romagnoli M, et al. Diagnosis of pulmonary thromboembolism with endobronchial ultrasound. Eur Respir J. 2008;32:1416–7. doi: 10.1183/09031936.00075208. [DOI] [PubMed] [Google Scholar]
- 50.Le Rouzic O, Terce G, Jardin C, et al. Pulmonary embolism diagnosed during an endobronchial ultrasound procedure. Rev Mal Respir. 2010;27:775–7. doi: 10.1016/j.rmr.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Sachdeva A, Lee HJ, Malhotra R, et al. Endobronchial ultrasound diagnosis of pulmonary embolism. J Bronchology Interv Pulmonol. 2013;20:33–4. doi: 10.1097/LBR.0b013e31827cc8e2. [DOI] [PubMed] [Google Scholar]
- 52.Senturk A, Arguder E, Babaoglu E, et al. Diagnostic imaging of pulmonary embolism using endobronchial ultrasound. Arch Bronconeumol. 2013;49:268–71. doi: 10.1016/j.arbres.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 53.MacEachern DB, Stather D, Tremblay A. Tumor invasion into pulmonary vessels viewed by endobronchial ultrasound. J Bronchol Interv Pulmonol. 2008;15:206–7. [Google Scholar]
- 54.Folch SJ, Machuzak M, Gildea T, et al. Safety and efficacy of ebus-guided tbna through the pulmonary artery: A preliminary report. Chest. 2011;140:600A. [Google Scholar]
- 55.Dusemund F, Schneider T, Zeisel C, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of an intravascular sarcoma metastasis. Respiration. 2013;86:430–2. doi: 10.1159/000354183. [DOI] [PubMed] [Google Scholar]
- 56.Shingyoji M, Ikebe D, Itakura M, et al. Pulmonary artery sarcoma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg. 2013;96:e33–5. doi: 10.1016/j.athoracsur.2013.01.080. [DOI] [PubMed] [Google Scholar]
- 57.Horowitz JC, Kleaveland K, Arenberg D. Endobronchial biopsy of an intrapulmonary arterial mass. J Bronchol Interv Pulmonol. 2013;20:93–5. doi: 10.1097/LBR.0b013e3182814b02. [DOI] [PubMed] [Google Scholar]
- 58.Lee SJ, Lee J, Yu SJ, Lee HJ, Kim KJ, Lee K-B, et al. Timely diagnosis of pulmonary artery tumor embolism by ultrasound-guided transbronchial needle aspiration. Thorac Cancer. 2014;5:184–7. doi: 10.1111/1759-7714.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent B, Huggins JT, Doelken P, et al. Successful real-time endobronchial ultrasound-guided transbronchial needle aspiration of a hilar lung mass obtained by traversing the pulmonary artery. J Thorac Oncol. 2006;1:362–4. [PubMed] [Google Scholar]
- 60.Wallace MB, Woodward TA, et al. Transaortic fine-needle aspiration of centrally located lung cancer under endoscopic ultrasound guidance: The final frontier. Ann Thorac Surg. 2007;84:1019–21. doi: 10.1016/j.athoracsur.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 61.von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest Endosc. 2009;69:345–9. doi: 10.1016/j.gie.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 62.Boujaoude Z, Pratter M, Abouzgheib W. Transpulmonary artery needle aspiration of hilar masses with endobronchial ultrasound: A necessary evil. J Bronchol Interv Pulmonol. 2013;20:349–51. doi: 10.1097/LBR.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 63.Jordan KV, Mohamed K, Pasquale F, et al. Endobronchial and endoscopic ultrasound guided trans-vascular biopsy of hilar, mediastinal, and lung lesions. B22. The golden gun? Advances in interventional bronchoscopy. Am Thorac Soc. 2014:A2501. [Google Scholar]
- 64.Ravindra AS, Rajani SB, Rohan A, et al. EBUS Guided Trans-Vascular Puncture (TV-EBUS) For Sampling Lesions Beyond The Great Vessels Pushing The Boundaries In The Mediastinum. B22. The golden gun? Advances in interventional bronchoscopy. 2014:A2519. [Google Scholar]
- 65.Meneses Hoyos J, Gomez Del Campo C. Angiography of the thoracic aorta and coronary vessels, with direct injection of an opaque solution into the aorta. Radiology. 1948;50:211–3. doi: 10.1148/50.2.211. [DOI] [PubMed] [Google Scholar]
- 66.Botana-Rial M, Nunez-Delgado M, Pallares-Sanmartin A, et al. Intramural hematoma of the pulmonary artery and hemopneumomediastinum after endobronchial ultrasound-guided transbronchial needle aspiration. Respiration. 2012;83:353–6. doi: 10.1159/000332925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


