Significance
Exopolysaccharides and extracellular DNA are important structural components that contribute to the self-assembly of large aggregates or microcolonies that are characteristic of biofilms. Pseudomonas aeruginosa is capable of producing multiple exopolysaccharides, including alginate, Psl, and Pel. At present, little is known about Pel’s chemical structure and its role in microcolony formation. Our results demonstrate that Pel is composed of cationic amino sugars. Using this knowledge, we have developed a Pel-specific lectin stain to directly visualize Pel in biofilms. We show that the positive charge on Pel facilitates its binding to extracellular DNA in the biofilm stalk, and that Pel can compensate for lack of Psl in the biofilm periphery.
Keywords: biofilms, exopolysaccharide, extracellular DNA, Pel, Psl
Abstract
Biofilm formation is a complex, ordered process. In the opportunistic pathogen Pseudomonas aeruginosa, Psl and Pel exopolysaccharides and extracellular DNA (eDNA) serve as structural components of the biofilm matrix. Despite intensive study, Pel’s chemical structure and spatial localization within mature biofilms remain unknown. Using specialized carbohydrate chemical analyses, we unexpectedly found that Pel is a positively charged exopolysaccharide composed of partially acetylated 1→4 glycosidic linkages of N-acetylgalactosamine and N-acetylglucosamine. Guided by the knowledge of Pel’s sugar composition, we developed a tool for the direct visualization of Pel in biofilms by combining Pel-specific Wisteria floribunda lectin staining with confocal microscopy. The results indicate that Pel cross-links eDNA in the biofilm stalk via ionic interactions. Our data demonstrate that the cationic charge of Pel is distinct from that of other known P. aeruginosa exopolysaccharides and is instrumental in its ability to interact with other key biofilm matrix components.
Biofilm infections are inherently difficult to eradicate, owing to increased resistance to antimicrobials and host defenses (1–3). Biofilms are microbial communities embedded in an extracellular matrix (4), composed of exopolysaccharides, extracellular DNA (eDNA), and proteins (5–7). Pseudomonas aeruginosa is an opportunistic pathogen that causes chronic biofilm infections (1) and has the capacity to synthesize three exopolysaccharides implicated in biofilm formation: alginate, Psl, and Pel (8, 9). Psl is a neutral polysaccharide consisting of a pentasaccharide repeat containing glucose, mannose, and rhamnose (10), and alginate is a negatively charged polymer of guluronic and mannuronic acid (8). Although previous reports suggest that glucose may be the primary component of Pel, its structure is unknown.
The role of Pel in biofilm formation was first identified in a screen for mutants deficient in pellicle formation (i.e., biofilms forming at the air–liquid interface of standing cultures) (11). Pel was later shown to be important for initiating and maintaining cell–cell interactions in biofilms (12, 13). In some circumstances, Pel also can play a role in adherence of cells to a surface (14). Finally, Pel affords biofilms protection against certain aminoglycoside antibiotics (12).
The importance of Pel in biofilm formation is strain-dependent. Nonmucoid strains (i.e., strains that make little alginate), use Pel and/or Psl as the primary structural scaffold. In the common laboratory strain PAO1, Psl is the primary exopolysaccharide produced in the biofilm matrix, although some Pel is produced as well (13, 15, 16). Deletion of pel genes in PAO1 does not have a significant impact on biofilm development (16). In contrast, Pel is the primary biofilm matrix exopolysaccharide in another commonly used laboratory strain, PA14, which is incapable of Psl production (16). Some strains appear to rely on both exopolysaccharides. Strains characterized by hyperadherence and hyperaggregation, called rugose small colony variants (RSCVs), are frequently isolated from in vivo and in vitro biofilms (17, 18). RSCVs harbor mutations (such as in wspF) that result in constitutive overexpression of both Pel and Psl (19, 20).
Biofilm formation is an ordered and sequential process that often begins with adherence of cells to a surface, followed by formation of large aggregates or microcolonies (21, 22). Polymers within the biofilm matrix exhibit spatial localization that is coordinated with the stages of biofilm development. In mature biofilms, controlled cell death and lysis occur in the interior of the microcolony, releasing eDNA that contributes to the stability of the biofilm structure (23–27). eDNA and Psl are spatially separated in mature microcolonies, with eDNA localized primarily to the base of the microcolony (27) and Psl located at the periphery of the microcolony (25).
Current data relating to the structure of Pel are limited and conflicting. It has been reported that PA14 pellicles were glucose-rich compared with a pel deletion mutant (11); however, the predominance of glucose was later attributed to cyclic glucans and not to the pel locus (28). In another study, analysis of extracellular material from PA14 pellicles identified lipopolysaccharide (LPS)-like material, leading to speculation that Pel is a modified form of LPS (29). We previously reported that the periplasmic PelA protein has de-N-acetylase activity in vitro, suggesting that some of the Pel sugars are deacetylated before being exported from the cell (30).
In the present study, we used glycosyl sugar and linkage analyses with optimized glycosidic cleavage conditions to investigate the composition of Pel. We combined lectin staining with confocal microscopy to confirm structural analyses and to directly visualize Pel in biofilms produced by distinct P. aeruginosa strains. These surprising results indicate that Pel is positively charged, and composed of 1→4 linked partially acetylated galactosamine and glucosamine sugars. We provide evidence that Pel cross-links eDNA in the biofilm stalk and can structurally compensate for the absence of Psl in the biofilm periphery. Thus, P. aeruginosa has a chemically diverse and flexible suite of matrix exopolysaccharides. Knowledge of the composition and localization of Pel is essential to delineate its functional role in biofilms, and ultimately to facilitate the development of therapeutic strategies aimed at eradicating the biofilm matrix.
Results
LPS Biosynthesis and Uridine 5′-Diphosphate–Glucose Are Not Required for Pel Production.
Polysaccharide biosynthesis requires nucleotide-sugar precursors that are incorporated into the elongating polysaccharide chain (8, 31). There is precedence for a functional overlap of LPS and exopolysaccharide biosynthesis enzymes (10), and previous work suggests that Pel and LPS biosynthesis may be linked (29). Therefore, we mutated pathways involved in LPS biosynthesis and evaluated whether this impacted Pel production. These mutations impaired A band and B band O-antigen production and the LPS core. Pel immunoblots indicated that mutant strains deficient in LPS precursors reacted with the Pel antisera, whereas the corresponding mutants deficient in Pel did not (Fig. S1). Our data show that Pel production occurs even in the absence of different steps of LPS biosynthesis, demonstrating that Pel is not a modified form of LPS.
Fig. S1.

α-Pel immunoblot indicates that LPS biosynthesis enzymes and UDP-Glc are not required for Pel production. Cell-associated and secreted (supernatant) Pel extracts from PBADpel (+) and Δpel (−) in a PAO1 ΔwspF Δpsl ΔR2 background were blotted. LPS is a major constituent of the outer membrane in Gram-negative bacteria and is composed of three parts: lipid A, a core containing KDO, and O-antigen polysaccharides (A and B bands). Mutant phenotypes were as follows: ΔwbpX, deficient in A band LPS; ΔwbpJ and ΔwbpM, deficient in B band LPS; ΔwbpL, deficient in A and B bands; ΔalgC, deficient in A band with a truncated LPS core; and ΔgalU, unable to synthesize UDP-Glc, deficient in A and B bands with a truncated LPS core.
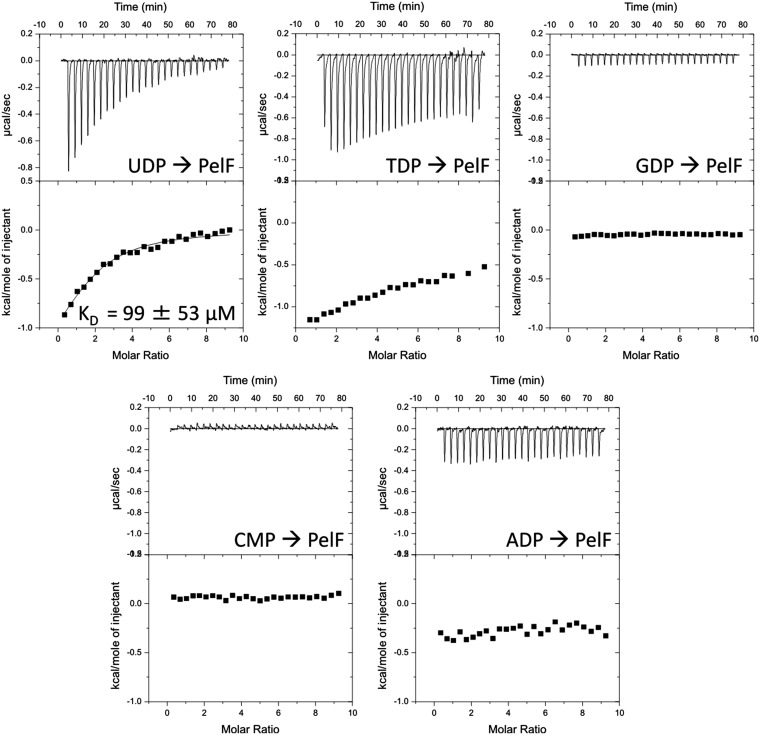
Glycosyltransferases catalyze the formation of glycosidic bonds by transferring the sugar moiety from a sugar nucleotide to a specific acceptor molecule (32). To gain insight into the activated sugar moiety that is used to generate Pel, we purified PelF, the sole predicted glycosyltransferase encoded by the pel operon. We used isothermal titration calorimetry (ITC) to show that PelF specifically binds uridine 5′-diphosphate (UDP) with micromolar affinity (Fig. S2), suggesting that a UDP-sugar nucleotide is required for Pel production.
Fig. S2.
PelF, the predicted glycosyltransferase, binds UDP with micromolar affinity. Isothermal titration calorimetry of PelF with the indicated nucleosides. For each nucleoside, the heats of injection (Upper) and normalized integration as a function of the molar syringe and cell concentrations (Lower) are shown. The calculated dissociation constant (KD) is indicated where binding was observed.
Pel was previously reported as a glucose-rich polysaccharide (11). GalU is critical for the production of the nucleotide sugar UDP-glucose (Glc) (33). If Pel were a glucose-rich polysaccharide, then disrupting UDP-Glc generation should prevent Pel biosynthesis (10). Immunoblots of extracts from a galU mutant revealed robust Pel production relative to a Δpel ΔgalU double mutant (Fig. S1). The results demonstrate that Pel production occurs even in the absence of UDP-Glc, thus suggesting that glucose is not a primary sugar component of Pel.
Pel Is Cationic and Has a Cell-Associated and Secreted Form.
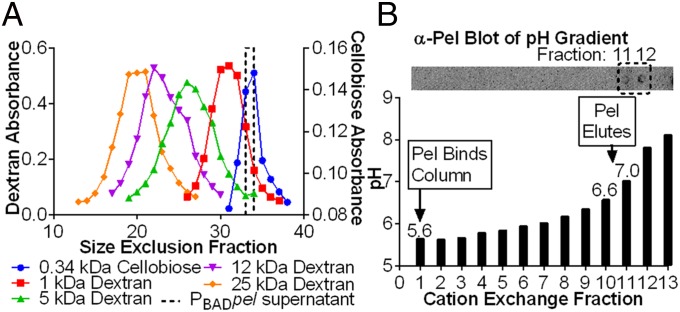
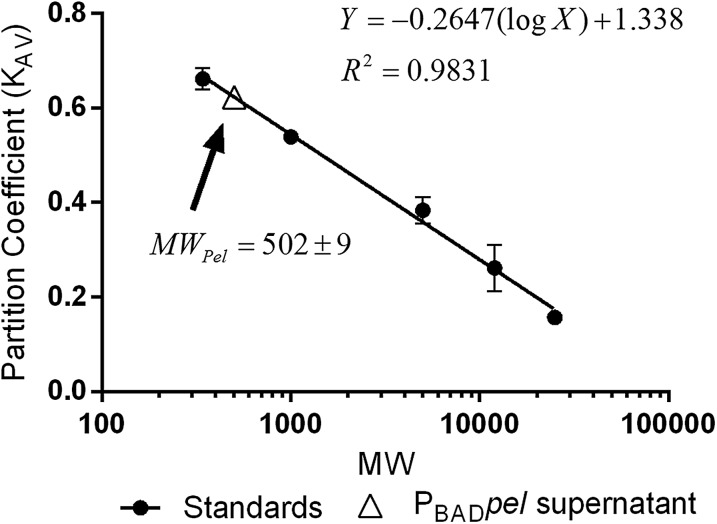
We began the characterization of Pel by investigating the molecular weight and potential charge of the polysaccharide using a strain with the pel operon under control of the arabinose-inducible PBAD promoter (PAO1 ΔwspF Δpsl PBADpel, herein designated PBADpel). This strain maximizes Pel production. Size-exclusion chromatography indicated that Pel has two forms: cell-associated and secreted. Cell-associated Pel, which was prepared by EDTA extraction of PBADpel cell pellets, eluted in the void volume, indicating a size >80 kDa. Secreted Pel, collected from PBADpel culture supernatants, eluted near the total column volume. We calculated the size of secreted Pel as 0.5 kDa by comparison with dextran standards (Fig. 1A and Fig. S3). In this study, we characterized the chemical composition of secreted Pel, because this form is more stable than cell-associated Pel.
Fig. 1.
Secreted Pel is a low molecular weight cation. (A) The average molecular weight of secreted Pel is 0.5 kDa and was determined by comparison of Pel, detected with α-Pel immunoblot in size-exclusion fractions, to dextran and cellobiose standards, detected with a colorimetric assay for neutral sugars. A single replicate is shown for clarity, but a duplicate replicate from an independent experiment behaved similarly. (B) A pH gradient applied to a cation column with secreted Pel bound indicated that Pel, detected with α-Pel immunoblot, had an isoelectric point of 6.7–6.9.
Fig. S3.
Size-exclusion calibration curve for molecular weight determination of secreted Pel. The partition coefficient (KAV) versus molecular weight (log scale) of standards and PBAD pel supernatant is shown. KAV is defined as (Ve− Vo)/(Vt − Vo), where Ve is the elution volume, Vo is the column void volume, and Vt is the total bed volume. Error bars represent SD (n = 2).
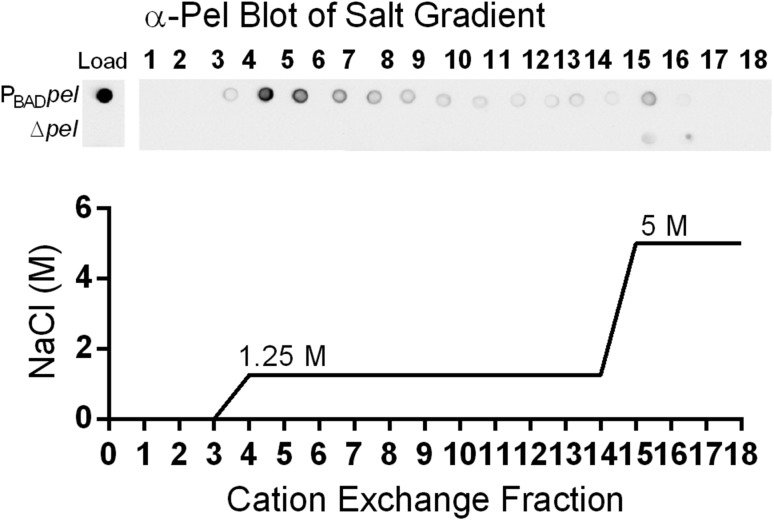
To determine whether Pel carries a net charge, we performed ion-exchange chromatography on filtered supernatant of PBADpel and PAO1 ΔwspF Δpsl Δpel (herein designated Δpel) cultures. Culture supernatant was applied to a cation-exchange column preequilibrated with a pH 5.5 buffer. Examination of the cation-exchange fractions using Pel antiserum indicated that Pel bound the column and eluted at 1.25 M NaCl (Fig. S4). No binding of Pel was observed to an anion-exchange column equilibrated with a pH 7.7 buffer. These results suggest that Pel is positively charged at pH 5.5.
Fig. S4.
Secreted Pel has an overall positive charge. Supernatant from PBADpel, but not from Δpel, bound a strong-cation exchange column at pH 5.5 and eluted at 1.25 M NaCl. Fractions from the cation column were probed with Pel antiserum (Top). Load indicates immunoblot of samples loaded to the column. Results shown are from a step gradient (Bottom), but a continuous gradient of salt concentration was initially used to determine the minimum salt concentration needed to elute Pel.
The isoelectric point of Pel was determined by applying supernatant from a PBADpel culture to a cation-exchange column under acidic conditions that facilitated binding (pH 5.5), and then gradually increasing the pH until Pel eluted (Fig. 1B). Pel was first detected in the effluent at pH 7.0. This suggests that the isoelectric point of Pel is between 6.7 and 6.9, and thus that Pel carries a net positive charge at pH values below this range. The isoelectric point for Pel is consistent with that of a polysaccharide containing amino sugars; for example, the amino group of chitosan (poly-β-1,4-N-acetylglucosamine <50% acetylated) has a pKa of 6.5.
Pel Is Rich in 1→4 Glycosidic Linkages of N-Acetylgalactosamine and N-Acetylglucosamine.
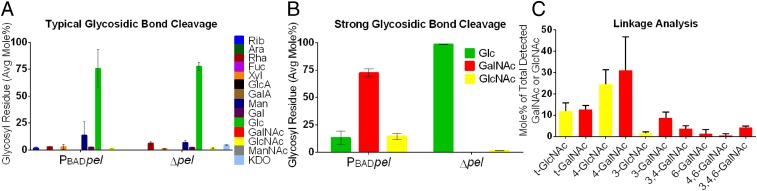
We hypothesized that if Pel contains amino sugars, that detection in previous work might have been hampered by inadequate glycosidic bond cleavage (11), because the hydrolysis of glycosidic bonds connecting amino sugars requires stronger acidic conditions than needed for neutral sugars (34). To test this hypothesis, we performed glycosyl composition analysis on ethanol precipitations of PBADpel and Δpel culture supernatants using two different methods of glycosidic bond cleavage. The first condition, which is typically used for glycosyl composition analysis, was the weaker of the two and involved no acid hydrolysis before methanolysis (Fig. 2A). The second and stronger cleavage condition included acid hydrolysis before methanolysis (Fig. 2B).
Fig. 2.
Glycosyl composition and linkage analyses of PBADpel and Δpel culture supernatant indicates that Pel is composed of 1→4 glycosidic linkages of GalNAc and GlcNAc. (A) Typical glycosidic cleavage conditions (no acid hydrolysis before methanolysis) were not sufficient to cleave Pel into monosaccharide components and resulted in detection of an abundance of glucose (Glc). (B) Strong glycosidic cleavage conditions (acid hydrolysis followed by methanolysis) enabled detection of amino sugars GalNAc and GlcNAc. (C) Glycosyl linkage analysis indicated that 4-linked GalNAc and GlcNAc were the most abundant residues detected of the total GalNAc and GlcNAc linkages. t, terminal. Error bars represent SD (n = 2).
The use of strong glycosidic bond cleavage conditions revealed that PBADpel is rich in N-acetylgalactosamine (GalNAc; 72.5 ± 3.6 mol %) and N-acetylglucosamine (GlcNAc; 14.5 ± 2.6 mol %). In Δpel, GalNAc was below detection, and GlcNAc was 1.4 ± 0.3 mol %. The ratio of GalNAc to GlcNAc was 5:1 (± 0.7) and could indicate either a minimum repeating unit or a random incorporation of GlcNAc into the polysaccharide. The sugar composition analysis involved the chemical re-N-acetylation of amino sugars and thus does not indicate the degree of acetylation of such sugars; however, in secreted Pel, the amino sugars cannot be 100% acetylated, because otherwise the polysaccharide would not carry a charge. The presence of partially acetylated amino sugars is consistent with the observed de-N-acetylase in vitro enzyme activity of PelA (30).
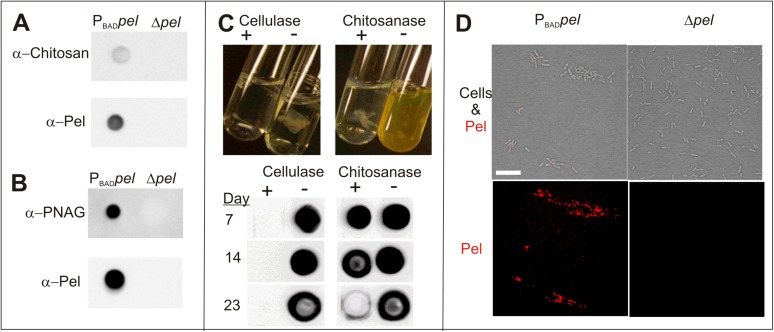
Glycosyl linkage analysis of ethanol precipitations of PBADpel supernatant revealed that among the residues detected from GalNAc and GlcNAc linkages, 4-linked GalNAc and GlcNAc were the most abundant (Fig. 2C). These results suggest that Pel is a linear exopolysaccharide containing 1→4 glycosidic linkages of partially acetylated GalNAc and GlcNAc. Consistent with glucosamine as a secondary component of Pel, monoclonal antibodies raised against poly-β-1,6-N-acetylglucosamine (PNAG) and chitosan (poly-β-1,4-N-acetylglucosamine) bound samples from a PBADpel strain (Fig. S5 A and B). Chitosanase, which catalyzes the specific cleavage of β-1,4 acetylated and nonacetylated glucosamine linkages, showed some activity on pellicle biofilms (Fig. S5C).
Fig. S5.
Antibody binding, enzyme digestion, and lectin staining provide supportive evidence that Pel contains partially acetylated GalNAc and GlcNAc. (A) Monoclonal antibodies for chitosan bind secreted Pel from a cation-exchange purification of PBADpel, but not Δpel, culture supernatant. (B) Monoclonal antibodies for PNAG bind cell-associated Pel from EDTA extraction of cell pellets in PBADpel, but not in Δpel. (C) Chitosanase has activity on pellicles. PBADpel pellicles were grown statically before liquid was removed and replaced with buffer (−) or buffer plus enzyme (+). (Upper) Images of enzyme digest taken after 2 d. (Lower) α-Pel immunoblot of enzyme digests show chitosanase results in slow degradation of Pel-antisera signal compared with more rapid degradation by cellulase. (D) WFL, which recognizes GalNAc sugars, specifically binds PBADpel cell clusters (red), but not Δpel cells. A merge of bright field and red channel (Upper) and red channel only (Lower) are shown. (Scale bar: 10 µm.)
Given that GalNAc was the most abundant sugar detected, we screened lectins that recognized GalNAc sugars to identify a Pel-specific lectin. Fluorescein-labeled Wisteria floribunda lectin (WFL), which has specificity to GalNAc moieties, bound clusters of planktonic PBADpel cells, but not from Δpel cells (Fig. S5D). This finding provides evidence that in addition to secreted Pel, GalNAc is also a component of cell-associated Pel.
Pel Localization in Biofilms Is Strain-Dependent.
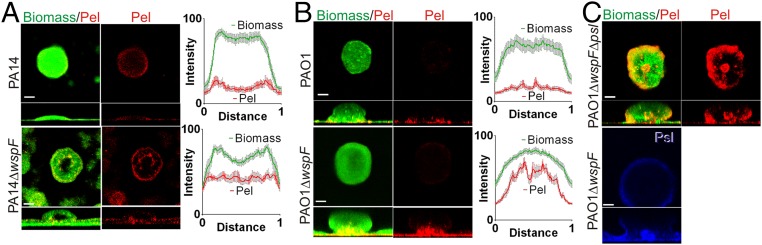
To directly visualize Pel in developing biofilms, we coupled Pel-specific lectin staining with confocal microscopy. Biofilms were grown in continuous-flow chambers (flow cells) inoculated with strains that differ in their exopolysaccharide use/dependence. Pel localization in PA14 is similar to that previously reported for Psl localization in PAO1 (25), in that Pel was found at the periphery of cell aggregates (Fig. 3A). The peripheral staining pattern was enhanced in the Pel-overproducing strain PA14 ΔwspF; however, PAO1 and PAO1 ΔwspF, strains that produce significant amounts of peripherally localized Psl, exhibited minimal Pel staining at the periphery of cell aggregates (Fig. 3B). We hypothesized that Psl might prevent or supersede Pel production in the periphery. To test this, we generated a psl-deficient mutation in PAO1 ΔwspF (PAO1 ΔwspF Δpsl), and found a strong increase in Pel staining at the periphery of the biofilm (Fig. 3C).
Fig. 3.
Pel localization in biofilms (A–C). Representative confocal images are from biofilms cultivated for 3–4 d in flow cells and imaged with the Pel-specific lectin (WFL, red), Psl-specific lectin (HHA, blue), and/or biomass stain (Syto62, green). Horizontal optical cross-sections (large square) captured in the middle of the microcolony and side view (rectangle) are shown. (Scale bars: 30 µm.) Line profiles were quantified from a horizontal line drawn in the stalk (bottom quarter of the microcolony). The average normalized fluorescence signal intensity (100 × [intensity/maximum intensity]) from 12 to 16 microcolonies from at least two independent experiments is plotted versus normalized distance (distance/total length of the microcolony).
Localization of Pel to the center of the microcolony “stalk” near the attachment surface was observed in all strains tested (Fig. 3). This staining pattern was most prominent in PAO1 ΔwspF, which overproduces Pel. A similar but less-intense staining pattern was observed in PAO1. Although this staining pattern was seen in most cell aggregates, it was occasionally absent in PA14 or PA14 ΔwspF. Minimal to no background staining was detected in biofilms produced by pel mutants cultured on glucose minimal media, verifying that the WFL lectin is specific to Pel (Fig. S6A). A PAO1 ΔwspF and PA14 biofilm development time course resulted in consistent localization patterns regardless of the relative age of the biofilm (Fig. S6 B and C).
Fig. S6.
Localization patterns of Pel are consistent in biofilm time course. (A) Pel lectin was specific for Pel and showed minimal staining in PAO1 ΔwspF Δpel cultivated for 4 d on glucose minimal media. (B) Pel was localized to the stalk with minimal periphery staining in mature PAO1 ΔwspF microcolonies. (C) In PA14, Pel was localized to the stalk and periphery of the mushroom cap. For both strains, Pel staining was uniform in younger microcolonies (days 1–2). Shown are representative confocal images of flow cell biofilms cultivated for 1–4 d before staining with Pel-specific lectin (WFL, red) and biomass stain (Syto62, green). (D) Pel was localized in induced strain to the periphery and stalk, but Psl was uniformly distributed throughout the entire biofilm population. Shown are representative confocal images of flow cell biofilms from PBADpel and PBADpsl cultivated for 2 d in the presence of arabinose before staining with biomass stain (Syto62, green) and Pel-specific lectin (WFL, red) or Psl-specific lectin (HHA, blue). (Scale bars: 30 µm.)
Transcriptional Control Is Not Sufficient to Account for Pel Localization.
One potential explanation of the observed localization of exopolysaccharides in biofilms is that only cells in the stalk and periphery of these aggregates synthesize Pel. To test this possibility, we used two strains, PAO1 ΔwspF Δpel PBADpsl (herein referred to as PBADpsl) and PAO1 ΔwspF Δpsl PBADpel, in which either Psl or Pel expression is under the control of an arabinose-inducible promoter. The strategy was to grow biofilms in the presence of arabinose and then assess which regions were capable of exopolysaccharide production using lectin staining.
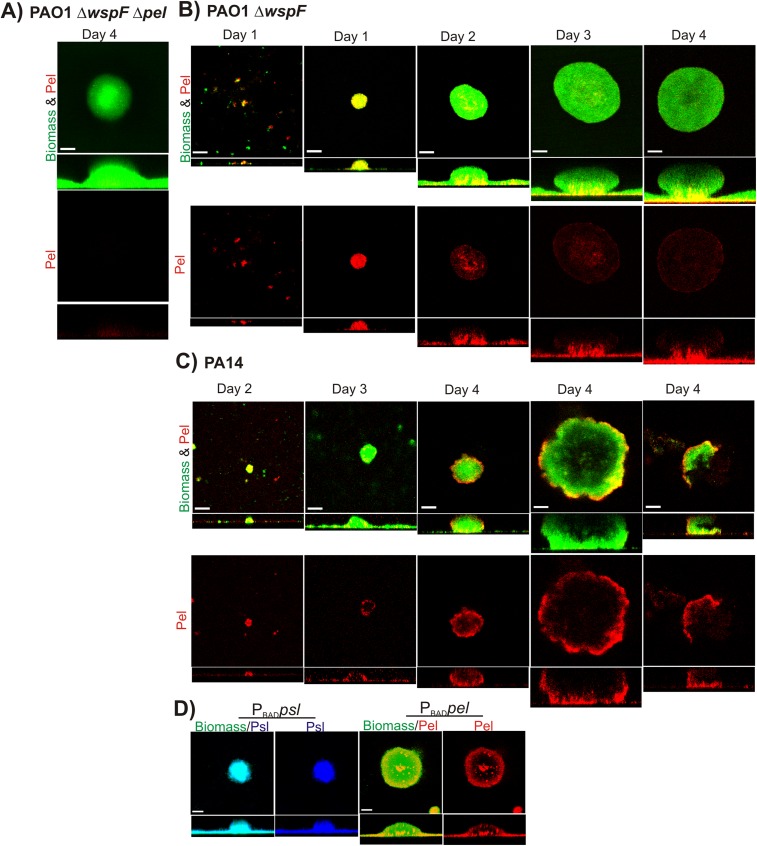
In a PBADpsl biofilm stained with Hippeastrum hybrid (HHA; Psl-specific lectin) (25), Psl staining was uniform throughout the biofilm (Fig. S6D). This is in contrast to uninduced strains, such as PAO1 (25) and PAO1 ΔwspF biofilms (Fig. 3C), in which Psl staining occurs primarily at the surface and periphery of large aggregates. This suggests that although cells in the interior of the aggregates of PAO1 biofilms have the capacity to produce Psl, some sort of regulatory control prevents this.
Surprisingly, in a PBADpel biofilm stained with Pel-specific lectin, Pel was present only in the stalk and periphery of the large aggregates, leaving large unstained regions in the interior of the microcolony (Fig. S6D). This pattern of staining is similar to that observed in PA14 and PA14 ΔwspF biofilms. Staining was uniform in younger PBADpel microcolonies, indicating that the localization in mature microcolonies was not likely an artifact of a staining idiosyncrasy. Assuming that the pel transcript is expressed uniformly throughout the biofilm (which certainly appears to be the case for psl, as described above), these data suggest that some form of posttranscriptional control is preventing Pel production in the unstained regions of the biofilm community. We also cannot rule out the possibility that the Pel lectin is sensitive to the presence of other matrix components in the biofilm (although this appears not to be the case for the Psl lectin).
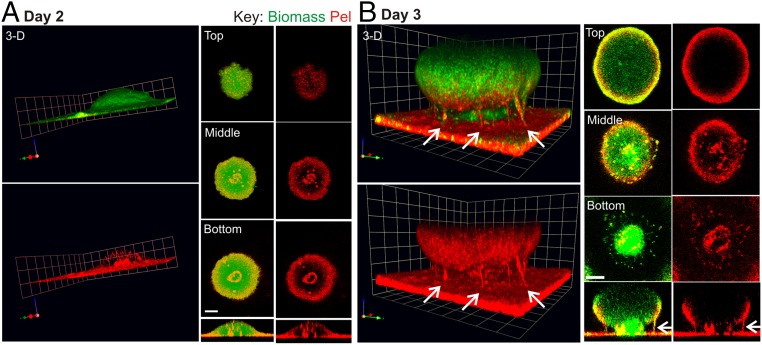
The Pel localization pattern in a 2-d PBADpel biofilm was such that staining of the periphery and stalk were clearly evident (Fig. 4A). At day 2, Pel staining in the stalk was visible as a small concentric circle in the interior of the microcolony near the substratum. By day 3, a large microcolony developed, and Pel staining material in the stalk had expanded until it was visible at the exterior of the biofilm (Fig. 4B). The images (Fig. 4B and Movie S1) illustrate that the stalk consists of intertwined fibers or columns of Pel extending from the substratum to the mushroom-shaped cap, tethering the latter in place. From these images, it is suggestive that Pel can play a major structural role in holding different regions of the biofilm together.
Fig. 4.
Pel is localized to the periphery and stalk in a PBADpel biofilm and is a major structural component of the stalk. Shown are representative confocal images of flow cell biofilms cultivated for 2 d (A) and 3 d (B) before staining with biomass stain (Syto62, green) and Pel-specific lectin (WFL, red). Arrows indicate columns of Pel in the biofilm stalk. (Scale bars: 30 µm.) For 3D images, 1 U = 22.6 µm for 2 d biofilms and 1 U = 14.15 µm for 3 d biofilms. The gamma was adjusted on the red channel of the 3D images to reduce periphery staining and enhance visualization of Pel in the microcolony interior (day 2, γ = 3; day 3, γ = 2).
Pel Colocalizes with eDNA in the Biofilm Stalk.
We hypothesized that localization of Pel in biofilms could be influenced by its positive charge. Previous reports indicate that negatively charged eDNA is present in the stalk of developing biofilm aggregates (27); thus, we predict that Pel would interact with eDNA. To investigate whether Pel and eDNA staining patterns colocalized, we treated biofilms with the Pel-specific lectin and the eDNA stain propidium iodide (PI). PAO1 ΔwspF and PBADpel biofilms stained separately with PI or WFL indicate that eDNA and Pel colocalize to the stalk of the microcolony (Fig. 5A). When we attempted to stain Pel and eDNA in the same biofilm, PI and WFL interfered with the staining of each other (Fig. S7A).
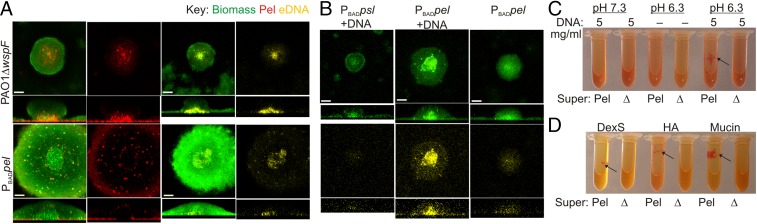
Fig. 5.
Pel cross-links eDNA in the biofilm stalk via an ionic binding mechanism. (A and B) Representative confocal images of flow cell biofilms before staining with Pel-specific lectin (WFL, red), biomass stain (Syto62, green), and/or eDNA stain (PI, yellow). Horizontal cross-sectional views were captured in the biofilm stalk (lower quarter of the microcolony). (Scale bars: 30 µm.) (A) Pel and eDNA colocalized in PAO1 ΔwspF and PBADpel biofilms. (B) Salmon sperm DNA incubated for 15 min with mature PBADpel biofilms concentrated in the stalk compared with PBADpel without added DNA. Exogenous DNA was not localized in PBADpsl biofilms. (C) Cross-linking of secreted Pel from PBADpel supernatant (Pel) to salmon sperm DNA resulted in visible aggregates that bind Congo red (arrow). (D) Aggregation of the anionic polymers dextran sulfate (DexS), hyaluronan (HA), and mucin with positively charged Pel from PBADpel supernatant (Pel) at pH 6.3 (arrows). Aggregation was not observed in Δpel supernatant (Δ).
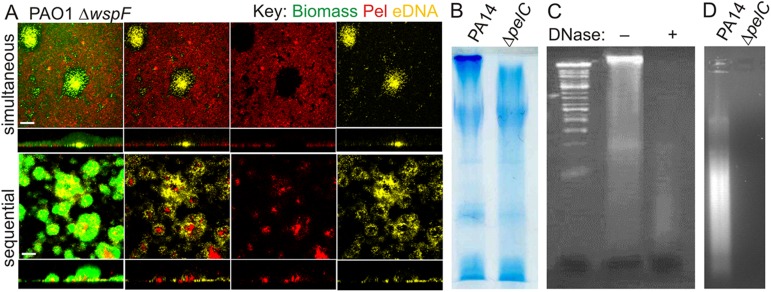
Fig. S7.
(A) Pel and eDNA interfere with staining of each other when stained with WFL and PI simultaneously in the same microcolony (top row) or sequentially with WFL followed by PI (bottom row). Shown are representative confocal images of flow cell biofilms from PAO1 ΔwspF cultivated for 4 d before staining. (Scale bars: 30 µm.) (B) An HMW band is visible in DOC-PAGE gel profiles of the phenol extracts from PA14 cells, but not from ΔpelC cells. The gel was stained with alcian blue. (C) An HMW band in DOC-PAGE gel profile of the phenol extracts from PA14 before (−) and after (+) DNase treatment. The gel was stained with ethidium bromide. A DNA ladder is shown in leftmost lane. (D) Abundant DNA is present in 2% agarose gel profiles of the phenol extracts from PA14 cells, but not from ΔpelC cells.
We hypothesized that exogenously added DNA to the bulk liquid might directly bind to Pel in biofilms. To test this, we introduced salmon sperm DNA to mature PBADpel biofilms for 15 min before staining with PI. We found greater PI staining concentrated in the stalk of PBADpel biofilms when exogenous DNA was added, relative to the control lacking exogenously added DNA (Fig. 5B). PI staining in the PBADpsl biofilm was minimal, suggesting that DNA binds something in the biofilm stalk in a Pel-dependent manner. Why exogenous DNA does not bind peripherally localized Pel is unclear, although it is possible that the pH at the exterior of the biofilm is too high to produce positively charged Pel.
Pel Cross-Links DNA via an Ionic-Binding Mechanism.
Gel electrophoresis of phenol extracts of PA14 standing cultures indicated the presence of a high molecular weight band that was absent from the corresponding profile of PA14Δpel (Fig. S7B). This band stained with ethidium bromide and was DNase-sensitive (Fig. S7 C and D). These results indicate that a phenol extraction of a PA14 pellicle is enriched in DNA, possibly due to interactions with Pel.
Cross-linking can occur by ionic or covalent bonding of two polymers to each other. To investigate the mechanism of Pel/DNA cross-linking, we added DNA to supernatant from PBADpel 24-h planktonic cultures (Fig. 5C). The cross-linking of secreted Pel to exogenous DNA resulted in visible aggregates that stained with Congo red, a dye known to bind Pel (11). No aggregation was observed with Δpel supernatant. A small amount of aggregation was observed in samples with PBADpel supernatant and no added DNA, presumably owing to endogenous eDNA.
Cross-linking of Pel to DNA was found to be pH-dependent, supporting the role of the positively charged amino groups of Pel in this process. In vitro aggregation (cross-linking) occurred at pH 6.3, where Pel is positively charged, but not above the isoelectric point of Pel at pH 7.3, where the polysaccharide carries no charge. The cross-linking of Pel to DNA was reversible. Pel /DNA aggregates could be resolubilized by increasing the pH to ∼9 or increasing the salt concentration of the solution to ∼1.4 M NaCl. Aggregates would spontaneously reform once the pH was dropped below the isoelectric point.
We reasoned that Pel could participate in the cross-linking of other anionic polymers, such as dextran sulfate, hyaluronan (an important component of the host extracellular matrix), or mucin (the primary component of mucous). Supporting this notion, positively charged Pel from PBADpel supernatant cross-linked and formed visible aggregates with dextran sulfate, hyaluronan, and mucin (Fig. 5D). Collectively, these results suggest that Pel interacts with DNA and other anionic polymers via an ionic-binding mechanism. The cross-linking of Pel to host relevant polymers, such as hyaluronan and mucin, suggests that this mechanism of adhesion could have implications in disease pathogenesis.
Discussion
Herein we provide multiple complementary lines of data indicating that Pel is composed of positively charged amino sugars. More specifically, our data suggest that Pel is a cationic exopolysaccharide consisting of partially acetylated GalNAc and GlcNAc. The positive charge imparts important functional characteristics on Pel, as it is capable of cross-linking eDNA in the biofilm matrix. Finally, our findings allowed us to devise an approach to directly visualize Pel in biofilms with the WFL lectin. Our data illustrate that (i) Pel binds eDNA in the biofilm stalk region and (ii) Pel can compensate for the lack of Psl in the biofilm periphery.
It is known that P. aeruginosa can produce the neutral and anionic exopolysaccharides Psl and alginate, respectively. In this study, we have shown that Pel is positively charged under neutral to mildly acidic conditions. The ability to produce three exopolysaccharides, each with a different charge at physiological pH, may afford P. aeruginosa biofilms increased flexibility to maintain biofilm structure and/or protect cells from antimicrobials under different conditions. We imagine gaining further insight into this question as we start to unravel how environmental conditions regulate the production of these polymers. We predict that the positive charge comes from the partial de-N-acetylation of GalNAc and GlcNAc sugars. In Staphylococcus species, partial deacetylation (10–20%) of the amino groups from the exopolysaccharide PNAG introduces a net positive charge (35). Nondeacetylated PNAG does not attach to the cell surface, presumably due to loss of cationic charge, and thus strains in which the PNAG deacetylation machinery is impaired are deficient for biofilm formation (36).
There is precedence for exopolysaccharides (e.g., Psl) to have a secreted and cell-associated form that can differ slightly in structure and modifications (10). NMR structural analysis of Pel likely would indicate whether cell-associated Pel is chemically distinct from secreted Pel, but attempts to obtain an NMR spectrum of Pel have thus far been unsuccessful. NMR also would indicate whether there is a small glucose component to Pel, or whether the cellulase sensitivity of pellicles (11) and use of UDP-Glc as a substrate by PelF (37) is related to nonspecific enzyme activity.
Cell death and the corresponding eDNA release is a coordinated process in the biofilm lifecycle (24). Cell lysis occurs in the central region of microcolonies, releasing abundant amounts of eDNA that concentrate primarily in the stalk (25, 27). Although eDNA can act as a structural component of biofilms (23, 26, 27), the idea that it might directly bind to other matrix polysaccharides was never clearly appreciated. In the present study, we have demonstrated that secreted Pel cross-links DNA via an ionic-binding mechanism. pH gradients in P. aeruginosa biofilms, reported to range from pH 5.5 at the center of the microcolony to pH 7 near the bulk fluid (38), indicate that Pel likely is positively charged in the biofilm stalk, facilitating ionic interactions. We propose that Pel localization to the stalk is partially driven by the presence of eDNA, and that Pel/eDNA interactions form the structural core of the biofilm stalk. Research investigating the correlation between Pel-dependent strains and the importance of eDNA is currently underway.
It is possible that the cross-linking of eDNA to other cationic exopolysaccharides (e.g., PNAG) may be a general mechanism underlying the structural integrity of biofilms. Furthermore, the cross-linking of Pel to host polymers (i.e., hyaluronan and mucin) may have implications in disease pathogenesis. Hyaluronan and mucin are abundant at sites of chronic infection, such as the lungs of patients with the genetic disorder cystic fibrosis. P. aeruginosa’s capacity to produce a polysaccharide that binds diverse host polymers may be a key mechanism by which the bacterial pathogen initiates and sustains cystic fibrosis lung infections.
Both Psl and Pel can serve as key structural components of the biofilm matrix (16). In PAO1, Psl is localized to the periphery of the microcolonies (25). In this study, we have shown that either Pel or Psl can localize to the periphery of microcolonies. In the absence of Psl, Pel production increases in the periphery, suggesting that Pel can compensate for the lack of Psl. We propose that in general, the structural integrity of a biofilm microcolony depends on the presence of an exopolysaccharide at the aggregate’s periphery. Psl appears to be the dominant matrix component suited to fill this role, because in strains that have the genetic capability to produce both exopolysaccharides, only Psl is observed at the periphery. The ability of Pel to compensate for Psl is consistent with previous findings demonstrating increased Pel production in pellicles in the absence of Psl (15). The regulatory mechanism that ensures that Pel compensates for the absence of Psl in the periphery is unknown, but it is tempting to speculate that the Psl polysaccharide is the environmental signal that down-regulates Pel production in the biofilm periphery.
In conclusion, our study demonstrates that the repertoire of exopolysaccharide types that can be used by P. aeruginosa is surprisingly diverse in its chemistries. We hypothesize that a primary role of Pel is to provide structural stability to the interior/core of biofilm microcolonies. Our data also suggest that peripherally localized exopolysaccharide may be an important general feature of biofilm microcolonies. Knowledge of the spatial localization of conserved biofilm matrix components may impact treatment regimens for biofilm infections.
Materials and Methods
More detailed information is provided in SI Materials and Methods.
Culturing and Purification of Pel Polysaccharide.
Bacterial strains are listed in Table S1. Planktonic cultures were maintained on Jensen’s defined medium (pH 7.3). Biofilms were grown in continuous-flow cell chambers on glucose minimal media (pH 7). Cell-associated Pel was extracted from PBADpel and Δpel cell pellets with EDTA. Secreted Pel was collected from culture supernatants and in some cases purified further using cation-exchange or ethanol precipitation.
Table S1.
Bacterial strains used in this study
| Strains | Description | Source |
| PAO1 | Wild type | (30) |
| PAO1 ΔwspF | wspF, nonpolar mutation | (30) |
| PAO1 ΔwspF Δpsl | wspF, nonpolar mutation; pslBCD, polar mutant of the psl operon | (30) |
| PAO1 ΔwspF Δpel PBADpsl | arabinose-inducible psl operon | This study |
| PAO1 ΔwspF Δpsl PBADpel | wspF, nonpolar mutation; pslBCD, polar mutant of the psl operon; arabinose-inducible pel operon | (30) |
| PAO1 ΔwspF Δpsl Δpel | wspF, nonpolar mutation; pelA, polar mutant of the pel operon; pslBCD, polar mutant of the psl operon | (30) |
| PA14 ΔwspF | This study | |
| PA14 PBADpel | Chromosomal replacement of the native promoter with araC-PBAD promoter | (12) |
| PA14 | Wild type | (30) |
| PA14 ΔpelF | This study | |
| PAO1 ΔwspF Δpsl ΔwbpJ ΔR2 PBADpel | Deficient in B band LPS and R2 pyocin production (40) | This study |
| PAO1 ΔwspF Δpsl ΔwbpX ΔR2 PBADpel | Deficient in A band LPS (32, 40) | This study |
| PAO1 ΔwspF Δpsl ΔwbpL ΔR2 PBADpel | Deficient in A and B band LPS (32, 40) | This study |
| PAO1 ΔwspF Δpsl ΔwbpM ΔR2 PBADpel | Deficient in B band LPS (32, 40) | This study |
| PAO1 ΔwspF Δpsl ΔalgC ΔR2 PBADpel | Deficient in A band LPS and with a truncated LPS core (31, 40) | This study |
| PAO1 ΔwspF Δpsl ΔgalU ΔR2 PBADpel | Deficient in A and B band and UDP-Glc, and with a truncated LPS core (33, 40) | This study |
| PAO1 ΔwspF Δpsl ΔwbpJ ΔR2 Δpel | This study | |
| PAO1 ΔwspF Δpsl ΔwbpX ΔR2 Δpel | This study | |
| PAO1 ΔwspF Δpsl ΔwbpL ΔR2 Δpel | This study | |
| PAO1 ΔwspF Δpsl ΔwbpM ΔR2 Δpel | This study | |
| PAO1 ΔwspF Δpsl ΔalgC ΔR2 Δpel | This study | |
| PAO1 ΔwspF Δpsl ΔgalU ΔR2 Δpel | This study |
Glycosyl Composition and Linkage Analyses.
Carbohydrate analyses were conducted at the University of Georgia’s Complex Carbohydrate Research Center (CCRC) on ethanol precipitated supernatant from PBADpel and Δpel. Samples for composition analysis were subjected to (i) typical glycosidic cleavage (no acid hydrolysis before methanolysis) or (ii) strong glycosidic cleavage (hydrolysis with TFA and hydrochloric acid, followed by methanolysis). GC/MS was used to analyze per-O-trimethylsilyl methyl glycosides for composition analysis and partially methylated alditol acetates for linkage analysis.
Lectin Staining and Confocal Microscopy.
Flow cell biofilms were stained and then imaged on a confocal microscope. To determine whether exogenous DNA bound Pel in biofilms, salmon sperm DNA was added to mature biofilms and incubated statically for 15 min, and then rinsed, stained with Syto62 and PI, rinsed again, and finally imaged on the confocal microscope.
In Vitro Pel Cross-Linking Experiments.
PBADpel and Δpel supernatant were mixed with anionic polymers including DNA, dextran sulfate, hyaluronan, and mucin until dissolved. The pH of the polymer solution was adjusted to 7.3 or 6.3. Congo red was added, and aggregates resulting from the cross-linking of the Pel and anionic polymers were visualized.
SI Materials and Methods
Bacterial Strains and Culturing.
The bacterial strains used in this study are listed in Table S1. LPS sugar nucleotide allelic replacement strains were constructed using an unmarked, nonpolar deletion strategy described previously (39). Planktonic cultures were routinely grown on Jensen’s chemically defined medium at 37 °C with constant shaking (225 rpm) unless indicated otherwise. Jensen’s medium contained NaCl (85.6 mM), K2HPO4 (14.4 mM), sodium glutamate (92 mM), valine (24 mM), phenylalanine (8 mM), glucose (70 mM), MgSO4 (1.33 mM), CaCl2 (0.14 mM), FeSO4 (0.0039 mM), ZnSO4 (0.0085 mM). The first five ingredients were mixed, the pH was adjusted to 7.3, and resulting solution was subsequently autoclaved. Jensen’s medium was supplemented with glucose and metals after autoclaving. Glucose minimal media (pH 7) was made by modifying the Jensen’s glucose recipe as follows: the glucose concentration was reduced to 0.3 mM, ammonium sulfate was added (15.1 mM final concentration), and the amino acids valine, phenylalanine, and glutamic acid were removed. Media for induced strains was supplemented with arabinose at 0.5% (vol/vol).
Pel Polysaccharide Purification.
Secreted Pel was prepared for glycosyl composition and linkage analysis by inoculating 50 mL of Jensen’s medium with 0.5 mL of overnight culture of PAO1 ΔwspF Δpsl PBADpel (herein designated PBADpel) and PAO1 ΔwspF Δpsl Δpel (herein designated Δpel). Cultures were grown for 20 h at 37 °C with constant shaking. The supernatant was harvested by centrifugation (8,300 × g for 15 min at 22 °C). Polysaccharides were precipitated for 1 h at 4 °C with ethanol (final concentration 75% vol/vol). The precipitate was washed three times with 95–100% (vol/vol) ethanol, resuspended in 2 mL of buffer (1 mM CaCl2 and 2 mM MgCl2 in 50 mM Tris, pH 7.5), and treated with 5 mg DNase I and 5 mg RNase A for 2 h at 37 °C, followed by 5 mg of proteinase K overnight at 37 °C. Lyophilized samples were submitted to the CCRC at the University of Georgia.
Crude secreted Pel in culture supernatant was collected from the centrifugation of overnight cultures. Purified secreted Pel was prepared by cation exchange of culture supernatant. Filtered culture supernatant from PBADpel and Δpel was applied to a 1-mL HiTrap SP-FF strong cation exchange column (GE Healthcare) preequilibrated with 50 mM acetic acid buffer at pH 5.5. The column was washed with equilibration buffer, and Pel was eluted with buffer containing 50 mM acetic acid and 1.25 M NaCl at pH 5.5.
Cell-associated Pel was prepared by extraction with EDTA. PBADpel and Δpel cells were harvested by centrifugation from overnight cultures grown at 37 °C with constant shaking. Cell pellets were resuspended in EDTA (0.5 M, pH 8) and heated to 95 °C for 20 min to remove the polysaccharide from the cell surface. Extracts were centrifuged, and the resulting supernatant was retained and thereafter referred to as cell-associated Pel.
Immunoblot Analysis.
Cell lysates for adsorption of Pel antiserum were generated from strains PA14 ΔpelF and PAO1 ΔwspF Δpsl ΔpelF grown to late log phase in 100 mL of LB medium. Cells were harvested by centrifugation (5,000 × g for 15 min at 4 °C). Pelleted cells were resuspended in 3 mL of buffer (50 mM Tris, 10 mM EDTA, pH 8). Cells were lysed by three freeze/thaw cycles, sonicated at full power for four periods of 20 s on ice, and finally centrifuged (12,000 × g for 15 min at 4 °C). In addition, a third lysate was produced by an ethanol precipitation of the PA14 ΔpelF supernatant, followed by DNase I, RNase A, and proteinase K treatment to simulate conditions under which the Pel antiserum was created. The Pel antiserum was adsorbed for 3–4 h at room temperature with constant rotation using the following reaction mixture: 25 µL of α-Pel antiserum, 33 μL of PA14 ΔpelF lysate, 33 μL of PAO1 ΔwspF Δpsl ΔpelF lysate, and 33 μL of ethanol- precipitated PA14 ΔpelF lysate in 300 µL of 5% (wt/vol) nonfat milk in TBST buffer (50 mM Tris, 150 mM NaCl, and 0.05% Tween 20).
Pel immunoblots were conducted essentially as described by Colvin et al. (30). In brief, samples were treated with proteinase K (60 °C for 60 min, followed by 80 °C for 30 min) before blotting 5 μL on a nitrocellulose membrane. Membranes were blocked with 5% (wt/vol) dry skim milk in TBST for 1 h at room temperature. The membrane was probed with α-Pel at a 1:1,000 dilution for 1 h at room temperature. Blots were washed twice for 5 min and once for 10 min with TBST, probed with goat α-rabbit HRP-conjugated secondary antibody (Thermo Scientific) for 1 h at room temperature, and then washed again. The signal for all immunoblots was developed using a SuperSignal West Pico chemiluminescent detection kit (Thermo Scientific) following the manufacturer’s recommendations.
The α-PNAG immunoblotting was conducted as follows. Cell-associated Pel (5 μL) from EDTA-treated cell pellets of PBADpel and Δpel cultures was blotted on nitrocellulose membranes and dried for 5 min. Membranes were blocked for 1 h with 5% (wt/vol) skim milk in PBST (PBS containing 0.05% Tween 20). The blots were probed for 1 h with murine IgM MAb 2F3.1D4 (α-PNAG, diluted 1:10,000 in PBST plus 1% BSA) raised against Escherichia coli PNAG. Membranes were washed twice for 5 min and once for 10 min with PBST and treated with the secondary antibody (HRP-conjugated anti-murine Ig) at a 1:20,000 dilution for 1 h and then washed again before developing.
The α-chitosan immunoblots were created by blotting a cation-exchange purification of culture supernatant (5 μL) from PBADpel and Δpel cultures onto a nitrocellulose membrane and then blocking with 3% (wt/vol) skim milk in PBST buffer for 1 h. Blocked membranes were probed with IgM, mAbG7 (α-chitosan, 1:1,000 dilution of 1 μg/mL stock) in PBST containing 1% BSA. After washing, the membrane was treated for 1 h with the secondary antibody used for α-PNAG at a 1:10,000 dilution in PBST.
Size Exclusion Chromatography.
An overnight culture of PBADpel and Δpel was grown on Jensen’s media at 37 °C with constant shaking. The supernatant was separated from the cell pellet by centrifugation. Cell-associated Pel was purified from the cell pellet by EDTA extraction. Filtered EDTA extract and filtered supernatant were applied to a HiPrep 16/60 Sephacryl S-200 HR size-exclusion column (GE Healthcare) preequilibrated with water to obtain a rough estimate of size. The average molecular weight of secreted Pel was determined by separation of PBADpel supernatant and dextran (Sigma-Aldrich) and cellobiose (Fluka) standards on a Superdex 75 10/300 GL size-exclusion column (GE Healthcare) preequilibrated with water and 0.02% sodium azide at room temperature. The void volume of the column was estimated using filtered blue dextran (2,000 kDa). Fractions (2 mL) were assayed using (i) Pel antiserum to detect Pel and (ii) a phenol-sulfuric acid assay to detect total carbohydrate in the standards. The size of secreted Pel was interpolated from a plot of the partition coefficient (KAV) versus the molecular weight (log scale) of standards. KAV is defined as (Ve − Vo)/(Vt − Vo), where Ve is the elution volume, Vo is the column void volume, and Vt is the total bed volume.
Phenol-Sulfuric Acid Assay for Total Carbohydrate.
Neutral sugars were quantified using a phenol-sulfuric acid colorimetric assay adapted for a microtiter plate. For this, 25 μL of sample or standard was added to a clear, untreated, flat-bottomed 96-well plate (Nunc) on ice. In the fume hood, 25 µL of 5% (wt/vol) phenol was added to each well and mixed at slow speed on a vortex mixer for 30 s. Then the plate was returned to the bed of ice, and 125 µL of concentrated sulfuric acid was added, followed by mixing at slow speed for 30 s. The reaction was incubated at 80 °C for 30 min and at room temperature for 5 min. Hexoses were detected by measuring absorbance at 490 nm.
Ion-Exchange Chromatography.
For a preliminary assessment of the charge of Pel, filtered culture supernatant from PBADpel and Δpel was applied to strong anion- and cation-exchange spin columns (Pierce) equilibrated with 25 mM Tris⋅HCl pH 7.7 and 25 mM acetic acid pH 5.5, respectively, at room temperature. Pel was eluted with buffer containing 5 M NaCl. Spin fractions were evaluated on α-Pel immunoblots. The minimum salt concentration required to elute Pel (1.25 M NaCl) was determined using a Atka FPLC system (GE Healthcare) to apply a salt gradient (0–5 M NaCl) to a 1-mL HiTrap SP FF strong cation-exchange column (GE Healthcare) equilibrated with 50 mM acetic acid buffer at pH 5.5 at room temperature and loaded with filtered supernatant. To estimate the isoelectric point of Pel, a pH gradient was applied from 5.5 to 8.1 until Pel eluted. Fractions were evaluated with Pel antiserum.
Glycosyl Sugar Composition and Linkage Analyses.
Glycosyl composition analysis was performed at the CCRC by combining GC/MS of the per-O-trimethyl (TMS) derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis. More specifically, samples (300–500 µg) were dialyzed and subjected to two different glycosidic cleavage conditions: (i) typical glycosidic cleavage, which did not include any acid hydrolysis before methanolysis, and (ii) strong glycosidic cleavage, which involved hydrolysis with 2 M TFA for 6 h and 6 M HCl for 4 h. Methyl glycosides were prepared from the dry samples by methanolysis in 1 M HCl in methanol at 80 °C for 17 h, followed by re–N-acetylation with pyridine and acetic anhydride in methanol (for detection of amino sugars). The samples were per-O-trimethylsilylated by treatment with Tri-Sil (Pierce) at 80 °C for 0.5 h. GC/MS analysis of TMS methyl glycosides was performed on an Agilent 6890N GC interfaced to a 5975B MSD, using an Agilent DB-1 fused silica capillary column (30 m × 0.25 mm i.d.) and inositol as an internal standard.
Glycosyl linkage analysis was conducted at the CCRC. Samples (1 mg) were acetylated with pyridine/acetic anhydride, dried, suspended in DMSO (200 µL), and stirred for 2 d. Samples were permethylated by treatment with sodium hydroxide for 15 min, followed by methyl iodide in dry DMSO for 45 min. The hydroxide and methyl iodide treatment was repeated to ensure complete methylation of the polymer. The permethylated material was hydrolyzed using 2 M TFA (2 h in a sealed tube at 121 °C), reduced with NaBD4, and acetylated using acetic anhydride/pyridine. The resulting partially methylated alditol acetates were analyzed by the same GC/MS used for composition analysis. Separation of neutral sugars was performed on a 30-m Supelco 2331 bonded-phase fused silica capillary column. Amino sugars were separated on a Zebron ZB1-MS column.
Purification of PelF.
The nucleotide sequence of pelF from P. aeruginosa PA01 was used to design primers specific for the gene that encodes for the full-length protein. The forward primer contains an NdeI restriction site, whereas the reverse primer contains an XhoI restriction site. The amplified PCR products were digested with NdeI and XhoI restriction endonucleases and then cloned into a pET28a vector (Novagen). The fidelity of the nucleotide sequence was verified using DNA sequencing (ACGT DNA Technologies, Toronto, Canada). The resulting expression vector encodes residues 1–507 of PelF fused to a cleavable N-terminal His6 tag for purification purposes.
Expression of PelF was achieved through the transformation of the vector into E. coli BL21 (DE3)-competent cells, which were grown in 4 L of LB medium containing 50 μg/mL kanamycin at 37 °C. The cells were grown to an OD600 of 0.6, after which protein expression was induced by the addition of isopropyl-β-d-1-thiogalacto pyranoside to a final concentration of 1.0 mM. The induced cells were incubated for 20 h at 25 °C before being harvested via centrifugation (6,260 × g for 20 min at 4 °C). The resulting cell pellet was stored at −20 °C until needed.
To purify PelF, the cell pellet from 4 L of bacterial culture was thawed and resuspended in 160 mL of buffer A [50 mM Tris⋅HCl pH 7.5, 300 mM NaCl, 10% (vol/vol) glycerol, 1 mM DTT] containing one SigmaFAST protease inhibitor EDTA-free mixture tablet (Sigma-Aldrich). Because of the presence of six cysteines in PelF, DTT was included to prevent intermolecular cross-linking of the protein. These cysteines are not expected to be involved in disulfide bond formation, owing to the cytoplasmic localization of the native protein. The resuspension was then lysed by homogenization using an EmulsiFlex-C3 homogenizer (Avestin) at a pressure of 10,000–15,000 psi, until the resuspension appeared translucent. Insoluble cell lysate was removed by centrifugation (25,000 × g for 45 min at 4 °C). The supernatant was loaded onto a 5-mL Ni2+-NTA column preequilibrated with buffer A containing 5 mM imidazole to reduce background binding. To remove any contaminants, the column was washed with 10 column volumes of buffer A containing 20 mM imidazole. Bound protein was eluted from the column with five column volumes of buffer A containing 250 mM imidazole. Fractions containing PelF were pooled and concentrated to a volume of 2 mL by centrifugation (2,200 × g at 4 °C) using an Amicon Ultra centrifugal filter device (EMD Millipore) with a 30-kDa molecular weight cutoff. PelF was further purified and buffer- exchanged into buffer B [20 mM Hepes pH 7.5, 150 mM NaCl, 10% (vol/vol) glycerol] with 1 mM DTT by size-exclusion chromatography using a HiLoad 16/60 Superdex 200 gel-filtration column (GE Healthcare). PelF eluted as a single Gaussian peak; all PelF-containing fractions were pooled, and the protein was concentrated by centrifugation (2,200 × g at 4 °C) using an Amicon Ultra centrifugal filter device (EMD Millipore) with a 30-kDa molecular weight cutoff and stored at 4 °C. SDS/PAGE analysis revealed that the resulting PelF was ∼95% pure and appeared at its expected molecular weight of 56 kDa.
Isothermal Titration Calorimetry.
PelF protein samples and nucleosides or nucleotide sugars were prepared in buffer B with 10 mM DTT, and each solution was degassed before experimentation. Isothermal titration calorimetry (ITC) measurements were performed with a microcalorimeter (VP-ITC; MicroCal). Titrations were carried out with 3 mM ligand in the syringe and 60 μM PelF in the cuvette. Each titration experiment consisted of 25 10-μL injections with an 180-s interval between each injection at 10 °C. The ITC data were analyzed using Origin version 5.0 (MicroCal) and fitted using a single-site binding model.
Enzyme Digestion of Pellicles.
PBADpel pellicles were generated for enzyme digestion by inoculation of 3 mL of Jensen’s medium with 3 μL of overnight culture, followed by static incubation at 37 °C for 4 d. Pellicles were washed to remove spent media and resuspended in buffer with and without enzyme. Enzyme digestion buffer contained 50 mM sodium acetate at pH 5 for cellulose and pH 5.5 for chitosanase. Sodium azide (0.02%) was included in the buffer to reduce bacterial growth. Enzyme concentrations used were 2 mg/3 mL for cellulase (Sigma-Aldrich; C1184, from Aspergillus niger) and 1 μL/3 mL for chitosanase (EMD Millipore; 220477, from Streptomyces sp. N174). Pellicle integrity was evaluated by visual inspection and α-Pel immunoblot of the enzyme/buffer mixture over time.
Flow Cell Biofilms and Confocal Microscopy.
Biofilms were cultivated in flow cell chambers essentially as described by Colvin et al. (16) with some modifications. Flow cells were inoculated from a mid-log LB culture that was diluted with glucose minimal media to an OD600 of 0.05 for PA14 and 0.01 for all other strains. Strains that aggregated in mid-log phase were homogenized before dilution for inoculation. Cells were allowed to attach under static conditions to an inverted flow cell for 1 h before induction of flow. Biofilms were grown on glucose minimal media for 1–7 d at room temperature under a constant flow rate (10 mL/h). Biofilms were stained for 15 min with fluorescein-labeled WFL lectin (100 μg/mL; Vector Laboratories) for Pel, TRITC-labeled HHA (100 μg/mL; EY Laboratories) for Psl (25), Syto62 red fluorescent nucleic-acid stain (5 μM; Life Technologies) for biomass, and PI (30 μM; Life Technologies) for eDNA. After staining, flow cells were washed with media at 10 mL/h for 5 min and then visualized on a Zeiss LSM 510 scanning confocal laser microscope. Image analysis was conducted using Velocity software (Improvision). Microscopy images were artificially colored as follows: Pel WFL lectin, red; Psl HHA lectin, blue; Syto62 biomass, green; and PI eDNA, yellow.
To determine whether exogenous DNA bound Pel in biofilms, salmon sperm DNA (5 mg/mL; USB) was added to 2-d PBADpel and PBADpsl biofilms. The mature biofilms were incubated statically with the DNA for 15 min, washed with 1.5 mL of glucose minimal media at pH 6.3, stained with PI and Syto62, washed again, and then imaged on the confocal microscope.
To ensure that the microscopy images shown in Fig. 3 were representative, line profiles were generated as follows. A horizontal cross-sectional image was captured in the biofilm stalk (bottom quarter of the microcolony), and a 5-µm-wide line was drawn through the microcolony in the cross-sectional image. The normalized fluorescence signal intensity (100 × [intensity/maximum intensity]) was averaged from 12–16 microcolonies from at least two independent experiments and then plotted against the normalized distance (distance/total length of the microcolony).
Gel Electrophoresis.
PA14 and PA14 ΔpelC were grown statically in 75 mL of T medium (10 g/L bacto peptone, 5 g/L NaCl) at 30 °C for 6 d. Standing cultures were inoculated with plate-grown bacteria to an OD600 of 0.0025, as recommended by Friedman and Kolter (11). PA14, but not the PA14 ΔpelC, formed pellicle biofilms. Cells were collected by centrifugation, gently washed with water, and extracted with 50% (vol/vol) aqueous phenol at 70–75 °C for 30 min with intensive stirring. The mixture was cooled on ice, and the phases separated by centrifugation (9,000 × g for 15 min). The water phase was further deproteinated by an additional extraction with 90% (vol/vol) phenol, followed by two extractions with chloroform. The phenol extracts were analyzed by sodium deoxycholate (DOC)-PAGE, as described previously (29), and by electrophoresis on 2% (wt/vol) agarose gels (75 mA constant voltage). DOC-PAGE gels were stained with alcian blue (0.005% in fixing solution of acetic acid-ethanol-water 5:40:55) overnight, and with ethidium bromide.
In Vitro Pel/Anionic Polymer Cross-Linking Experiments.
To determine whether Pel bound DNA or the anionic polymers dextran sulfate, hyaluronan, and mucin in vitro, 1.5 mL of supernatant from 24-h PBADpel and Δpel planktonic cultures at a pH of 8.2 was added to anionic polymer (5 mg/mL) and shaken until completely dissolved. Subsequently, the pH of the solution was adjusted to 7.3 or 6.3 with HCl. Congo red was added at a final concentration of 40 µg/mL. Aggregates resulting from the cross-linking of the Pel to anionic polymers were detected by visual inspection.
Supplementary Material
Acknowledgments
We thank T. Romeo and S. Schillberg for antibodies, and M. Vedadi and G. Senisterra (Structural Genomics Consortium, Toronto) for ITC assistance. This work was supported by National Institutes of Health Grants 2R01AI077628 (to M.R.P.) and R01AI097511 (to D.J.W.), and Canadian Institutes of Health Research Operating Grant 13337 (to P.L.H.). L.K.J. is the recipient of an American Heart Association Postdoctoral Fellowship (14POST20130017). P.R.S. and B.S.T. are recipients of Cystic Fibrosis Foundation Postdoctoral Fellowships. L.S.M. is supported by graduate scholarships from the Natural Sciences and Engineering Research Council of Canada, the Ontario Graduate Scholarship Program, and the Hospital for Sick Children Foundation Student Scholarship Program. P.L.H is the recipient of a Canada Research Chair.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503058112/-/DCSupplemental.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 3.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49(1):711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 5.Flemming H-C, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland IW. The biofilm matrix: An immobilized but dynamic microbial environment. Trends Microbiol. 2001;9(5):222–227. doi: 10.1016/s0966-842x(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 7.Pamp SJ, Gjermansen M, Tolker-Nielsen T. 2007. The biofilm matrix: A sticky framework. The Biofilm Mode of Life: Mechanisms and Adaptations, eds Kjelleberg S, Givskov M (Horizon Bioscience, Norfolk, UK), pp 37–69.
- 8.Franklin MJ, Nivens DE, Weadge JT, Howell PL. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol. 2011;2(167):167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann EE, Wozniak DJ. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev. 2012;36(4):893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd MS, et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol. 2009;73(4):622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman L, Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol. 2004;51(3):675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- 12.Colvin KM, et al. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7(1):e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, et al. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ Microbiol. 2011;13(7):1705–1717. doi: 10.1111/j.1462-2920.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- 14.Vasseur P, Vallet-Gely I, Soscia C, Genin S, Filloux A. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology. 2005;151(Pt 3):985–997. doi: 10.1099/mic.0.27410-0. [DOI] [PubMed] [Google Scholar]
- 15.Ghafoor A, Hay ID, Rehm BHA. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl Environ Microbiol. 2011;77(15):5238–5246. doi: 10.1128/AEM.00637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colvin KM, et al. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol. 2012;14(8):1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Häussler S, et al. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J Med Microbiol. 2003;52(Pt 4):295–301. doi: 10.1099/jmm.0.05069-0. [DOI] [PubMed] [Google Scholar]
- 18.Kirisits MJ, Prost L, Starkey M, Parsek MR. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2005;71(8):4809–4821. doi: 10.1128/AEM.71.8.4809-4821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci USA. 2005;102(40):14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starkey M, et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol. 2009;191(11):3492–3503. doi: 10.1128/JB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56(1):187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 22.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54(1):49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Rice KC, et al. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci USA. 2007;104(19):8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb JS, et al. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol. 2003;185(15):4585–4592. doi: 10.1128/JB.185.15.4585-4592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, et al. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5(3):e1000354. doi: 10.1371/journal.ppat.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 27.Allesen-Holm M, et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol. 2006;59(4):1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 28.Sadovskaya I, et al. High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: the ndvB gene is involved in the production of highly glycerol-phosphorylated β-(1→3)-glucans, which bind aminoglycosides. Glycobiology. 2010;20(7):895–904. doi: 10.1093/glycob/cwq047. [DOI] [PubMed] [Google Scholar]
- 29.Coulon C, Vinogradov E, Filloux A, Sadovskaya I. Chemical analysis of cellular and extracellular carbohydrates of a biofilm-forming strain Pseudomonas aeruginosa PA14. PLoS One. 2010;5(12):e14220. doi: 10.1371/journal.pone.0014220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colvin KM, et al. PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa. J Bacteriol. 2013;195(10):2329–2339. doi: 10.1128/JB.02150-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocchetta HL, Burrows LL, Lam JS. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 1999;63(3):523–553. doi: 10.1128/mmbr.63.3.523-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocchetta HL, Burrows LL, Pacan JC, Lam JS. Three rhamnosyltransferases responsible for assembly of the A-band D-rhamnan polysaccharide in Pseudomonas aeruginosa: A fourth transferase, WbpL, is required for the initiation of both A-band and B-band lipopolysaccharide synthesis. Mol Microbiol. 1998;28(6):1103–1119. doi: 10.1046/j.1365-2958.1998.00871.x. [DOI] [PubMed] [Google Scholar]
- 33.Dean CR, Goldberg JB. Pseudomonas aeruginosa galU is required for a complete lipopolysaccharide core and repairs a secondary mutation in a PA103 (serogroup O11) wbpM mutant. FEMS Microbiol Lett. 2002;210(2):277–283. doi: 10.1111/j.1574-6968.2002.tb11193.x. [DOI] [PubMed] [Google Scholar]
- 34.Merkle RK, Poppe I. Carbohydrate composition analysis of glycoconjugates by gas-liquid chromatography/mass spectrometry. Methods Enzymol. 1994;230:1–15. doi: 10.1016/0076-6879(94)30003-8. [DOI] [PubMed] [Google Scholar]
- 35.Cerca N, et al. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect Immun. 2007;75(7):3406–3413. doi: 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuong C, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279(52):54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- 37.Ghafoor A, Jordens Z, Rehm BHA. Role of PelF in Pel polysaccharide biosynthesis in Pseudomonas aeruginosa. Appl Environ Microbiol. 2013;79(9):2968–2978. doi: 10.1128/AEM.03666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter RC, Beveridge TJ. Application of a pH-sensitive fluoroprobe (C-SNARF-4) for pH microenvironment analysis in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2005;71(5):2501–2510. doi: 10.1128/AEM.71.5.2501-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212(1):77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 40.Penterman J, Singh PK, Walker GC. Biological cost of pyocin production during the SOS response in Pseudomonas aeruginosa. J Bacteriol. 2014;196(18):3351–3359. doi: 10.1128/JB.01889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.