Significance
Gram-negative bacteria use contact-dependent growth inhibition (CDI) systems to bind neighboring bacteria and deliver diverse nuclease toxins that inhibit target-cell growth. This process requires toxin transport across the outer and inner membranes of target bacteria to reach DNA and RNA substrates in the cytoplasm. Our data indicate that CDI toxins contain a variable domain that specifies the entry pathway into target bacteria. These “translocation domains” exploit specific integral membrane proteins to deliver linked nuclease domains into the cytoplasm. We also find that CDI translocation domains can be exchanged between CdiA C-terminal toxin domains to deliver nucleases via different routes. These findings reveal a versatile protein-transport mechanism that could potentially be harnessed to deliver other antimicrobial cargoes into Gram-negative bacteria.
Keywords: bacterial cell envelope, DNase activity, genetic selection, toxin/immunity genes, tRNase activity
Abstract
Contact-dependent growth inhibition (CDI) systems function to deliver toxins into neighboring bacterial cells. CDI+ bacteria export filamentous CdiA effector proteins, which extend from the inhibitor-cell surface to interact with receptors on neighboring target bacteria. Upon binding its receptor, CdiA delivers a toxin derived from its C-terminal region. CdiA C-terminal (CdiA-CT) sequences are highly variable between bacteria, reflecting the multitude of CDI toxin activities. Here, we show that several CdiA-CT regions are composed of two domains, each with a distinct function during CDI. The C-terminal domain typically possesses toxic nuclease activity, whereas the N-terminal domain appears to control toxin transport into target bacteria. Using genetic approaches, we identified ptsG, metI, rbsC, gltK/gltJ, yciB, and ftsH mutations that confer resistance to specific CdiA-CTs. The resistance mutations all disrupt expression of inner-membrane proteins, suggesting that these proteins are exploited for toxin entry into target cells. Moreover, each mutation only protects against inhibition by a subset of CdiA-CTs that share similar N-terminal domains. We propose that, following delivery of CdiA-CTs into the periplasm, the N-terminal domains bind specific inner-membrane receptors for subsequent translocation into the cytoplasm. In accord with this model, we find that CDI nuclease domains are modular payloads that can be redirected through different import pathways when fused to heterologous N-terminal “translocation domains.” These results highlight the plasticity of CDI toxin delivery and suggest that the underlying translocation mechanisms could be harnessed to deliver other antimicrobial agents into Gram-negative bacteria.
Bacteria are constantly in competition for environmental resources and have evolved a number of systems to suppress the growth of competing cells. Research during the past decade has revealed that Gram-negative bacteria commonly use type V and type VI secretion systems to deliver protein toxins into neighboring cells (1, 2). The type V mechanism was the first to be identified and has been termed contact-dependent growth inhibition (CDI) because inhibitor cells must make direct contact with target bacteria to transfer toxins (3, 4). CDI+ bacteria express CdiB/CdiA two-partner secretion (TPS) systems, which assemble as a complex on the cell surface. CdiB is an outer-membrane β-barrel protein required for the export and presentation of toxic CdiA effectors. CdiA proteins are very large (180–630 kDa depending on bacterial species) and are presented as individual β-helical filaments that emanate several hundred angstroms from the inhibitor-cell surface (5). CdiA binds to specific outer-membrane receptors on susceptible bacteria and transfers its C-terminal toxin domain (CdiA-CT) into the target cell (6, 7). CDI+ bacteria also produce CdiI immunity proteins to protect themselves from toxin delivered by neighboring sibling cells. The immunity protein binds to the CdiA-CT and neutralizes its toxin activity (6, 8). Notably, CdiA-CT/CdiI sequences are highly variable between bacteria and even between different strains of the same species (6, 8). For example, isolates of Escherichia coli contain at least 20 CDI toxin/immunity sequence types. These toxin/immunity protein families are distinct from one another and form specific CdiA-CT/CdiI cognate pairs. Because CdiI immunity proteins do not protect against noncognate toxins, CDI provides a mechanism for self/nonself recognition between bacteria.
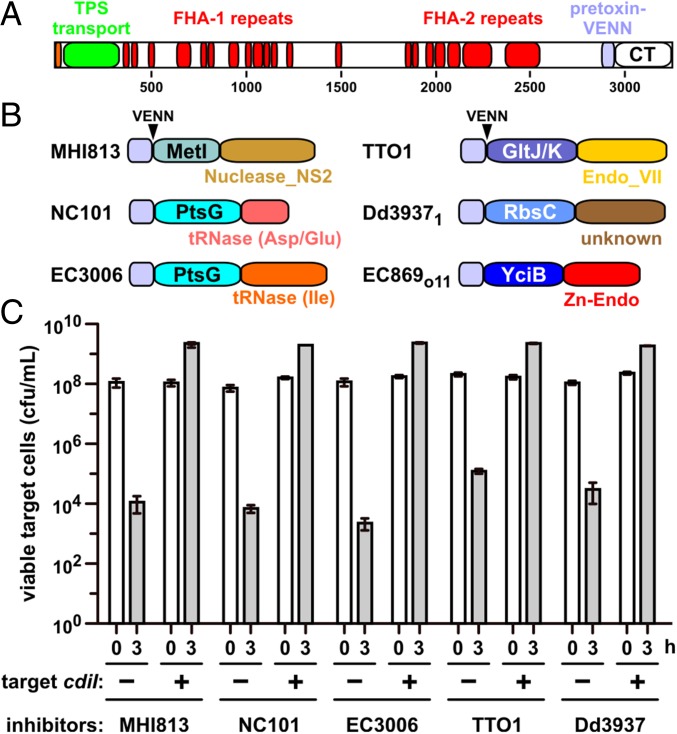
A remarkable feature of CDI is the modularity of CdiA-CT toxins, which can be exchanged between different CdiA proteins to generate functional chimeras. All CdiA proteins have a similar architecture consisting of an N-terminal TPS transport domain, an extended central region of filamentous hemagglutinin peptide repeats, and the CdiA-CT toxin region (Fig. 1A). In many bacteria, the variable CdiA-CT region is demarcated by the VENN peptide motif, which forms the C-terminal boundary of the pretoxin-VENN domain (Fig. 1A) (6, 9). Heterologous CdiA-CTs can be delivered into E. coli target cells when fused to the VENN sequence of CdiAEC93 from E. coli EC93 (6, 10–13). Closer examination of the CdiA-CT region reveals that it is often composed of two variable domains that assort independently to form CdiA-CT composites (Fig. 1B). For example, the CdiA-CTEC536 from uropathogenic E. coli 536 and CdiA-CTECL from Enterobacter cloacae American Type Culture Collection 13047 (ECL) share nearly identical N-terminal domains but carry different C-terminal nucleases (10, 14). The function of the CdiA-CT N-terminal domain has not been examined, but biochemical studies show this region is not required for nuclease activities in vitro (8, 11, 14). Here, we provide evidence that the N-terminal domain of the CdiA-CT region plays a critical role in toxin translocation during CDI. Using a genetic approach, we identified a collection of CDI-resistance (CDIR) mutations that protect E. coli target cells from specific CDI toxins. Each CDIR mutation disrupts expression of an inner-membrane protein (IMP) and confers resistance to CdiA-CTs that share homologous N-terminal domains. We also demonstrate that the N- and C-terminal domains of CdiA-CT regions can be recombined to produce novel hybrids that are functional in cell-mediated CDI. We propose that the N-terminal domain of the CdiA-CT region binds to specific IMP receptors and mediates toxin transport across the inner membrane.
Fig. 1.
Activity of CdiA chimeras. (A) CdiA proteins contain an N-terminal TPS transport domain and two filamentous hemagglutinin (FHA)-peptide repeat regions. The pretoxin-VENN domain is adjacent to and demarcates the variable CdiA-CT region. (B) Predicted CdiA-CT domain structures. Toxins from E. coli MHI813 and Photorhabdus luminescens TTO1 carry predicted C-terminal Nuclease_NS2 (Pfam database ID: PF13930) and Endonuclease_VII (PF14411) domains, respectively. The C-terminal nuclease domains from E. coli NC101 and 3006 cleave tRNAAsp/tRNAGlu and tRNAIle, respectively. The nuclease domain from E. coli EC869 is a Zn2+-dependent DNase, and the activity of the Dickeya dadantii 3937 toxin is unknown. N-terminal domains are labeled according to their putative membrane receptors. The pretoxin-VENN domain and the conserved VENN motif are also depicted. (C) CDI competitions. E. coli target cells were cocultured with the indicated CDI inhibitors. Average target-cell counts (±SEM) are presented for three independent experiments. Where indicated, target cells were provided with the cognate cdiI immunity gene.
Results
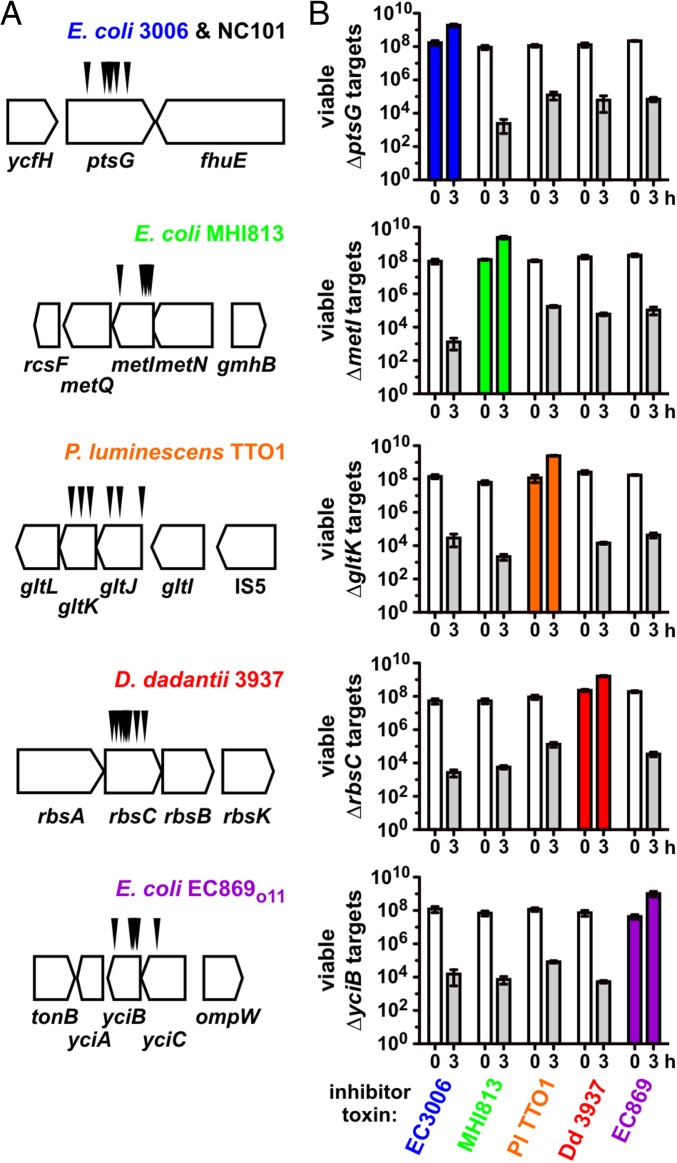
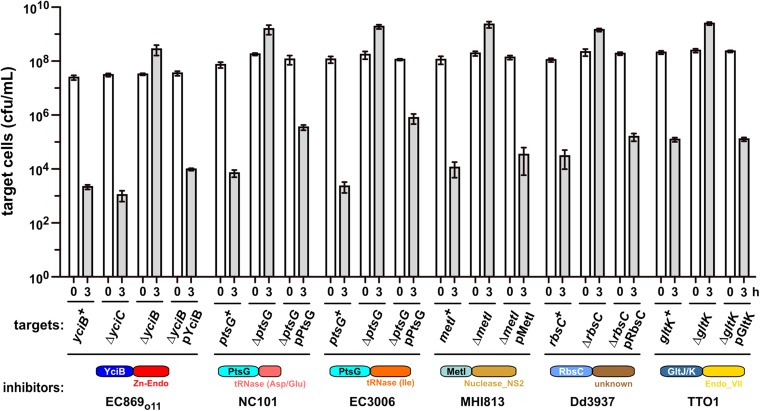
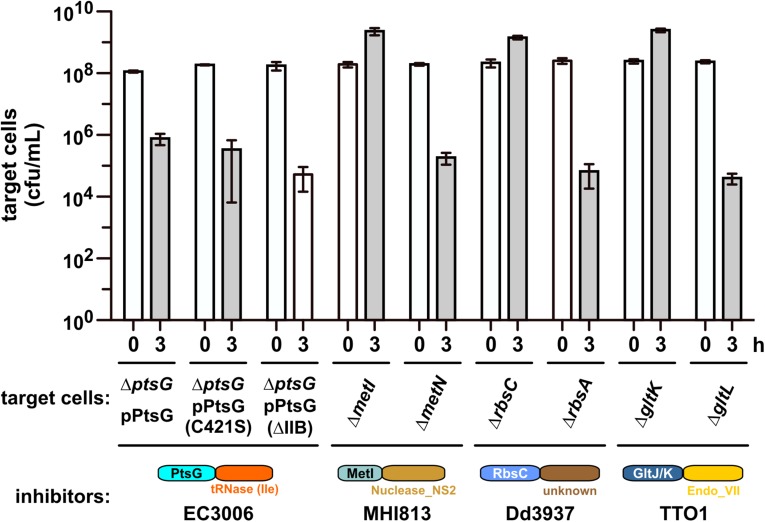
We performed a series of selections for CDI-resistant (CDIR) E. coli mutants, reasoning that protective mutations would disrupt genes required for toxin import and/or activation. Plasmid-borne chimeric CDI systems were constructed in which heterologous cdiA-CT/cdiI coding sequences were fused at the VENN encoding region of cdiAEC93 (Fig. 1A). Each chimeric fusion was functional in CDI, reducing target-cell viability between 103- and 106-fold during coculture (Fig. 1C). Moreover, target bacteria were protected when provided with the appropriate cognate cdiI immunity gene (Fig. 1C), indicating that the grafted CdiA-CTs are responsible for growth inhibition. Inhibitor strains were then used to enrich CDIR target cells from a pool of mariner transposon-insertion mutants. CDIR mutants were selected with iterative cycles of competition coculture until the target-cell population was fully resistant. We isolated individual target-cell clones from independent experiments and tested CDIR phenotypes in competitions. Linkage of CDIR to each transposon insertion was confirmed by transduction. Identification of the transposon-insertion sites revealed that resistance to a given CdiA-CT toxin was due to disruption of one or two genes. For example, CDIMHI813-resistant mutants contained independent insertions in metI, whereas the nine CDI1Dd3937-resistant mutants had multiple insertions within rbsC (Fig. 2A). CDITTO1-resistant mutants were disrupted in gltK or gltJ, and CDIo11EC869-resistant mutants carried insertions in yciC or yciB (Fig. 2A). Interestingly, ptsG mutations were isolated from selections for resistance to CDINC101 and CDIEC3006 (Fig. 2A). Notably, each disrupted gene encodes an integral membrane protein. MetI, RbsC, and GltJ/GltK are ABC transporter membrane permeases for d/l-methionine, d-ribose, and l-glutamate/l-aspartate, respectively (15–17). PtsG is the main phosphotransferase system permease for d-glucose (18). The functions of YciC and YciB are unknown, but both are predicted integral IMPs. In-frame deletions were constructed for each gene to confirm its role in CDIR (Fig. 2B). This analysis showed that ∆yciC mutants are not resistant to CDIo11EC869 (Fig. S1), indicating that the original yciC insertion exerts a polar effect on yciB (Fig. 2A). Complementation analysis confirmed the role of yciB in the CDIo11EC869 pathway, and showed the metI, rbsC, gltK, and ptsG are required for their respective CDI pathways (Fig. S1). We also tested each in-frame deletion strain in competitions against other inhibitor strains and found that resistance was specific, such that ∆metI cells were resistant to CDIMHI813 but susceptible to other CDI systems (Fig. 2B). Thus, each CDI system requires a specific IMP to inhibit target cells.
Fig. 2.
Specificity of CDIR mutations. (A) Transposon-insertion sites are shown for each selection toxin. No other verified CDIR mutations were identified during the selections. (B) CDIR mutations are toxin-specific. The indicated target cell strains were cocultured with inhibitors that deploy CdiA-CTEC3006, CdiA-CTMHI813, CdiA-CTTTO1, CdiA-CT1Dd3937, or CdiA-CTo11EC869 toxins. Average target-cell counts (±SEM) per mL are presented for three independent experiments.
Fig. S1.
Complementation of CDIR mutations. E. coli target cells of the indicated genotypes were cocultured for 3 h at a 1:1 ratio with inhibitor strains that deploy the indicated CdiA-CT toxins. For each CDIR mutation, expression of the WT gene from a plasmid (e.g., pYciB) restores sensitivity to growth inhibition. Average target-cell counts (±SEM) are presented for three independent experiments.
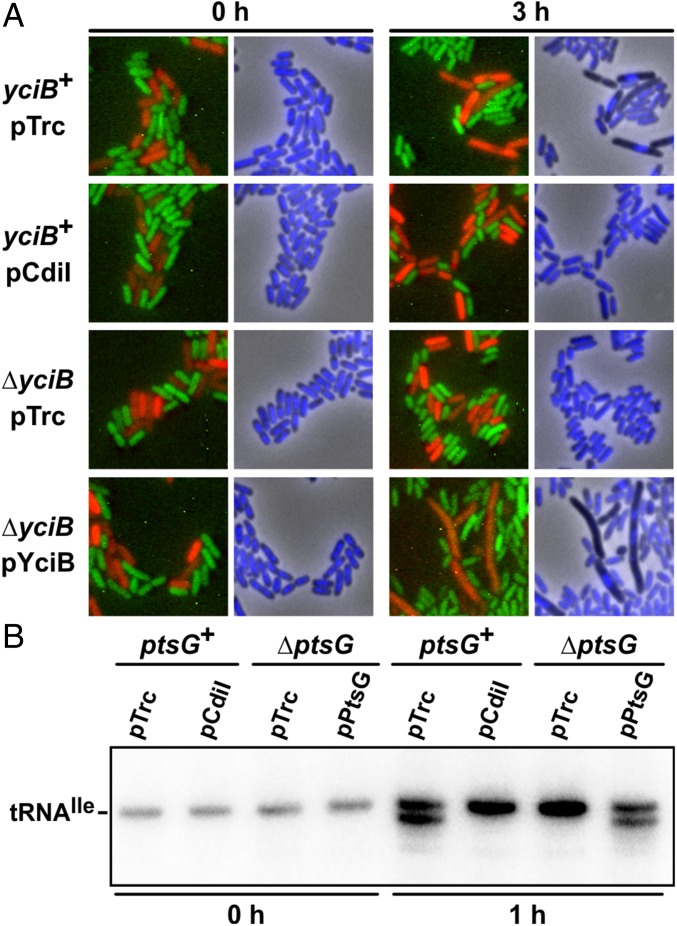
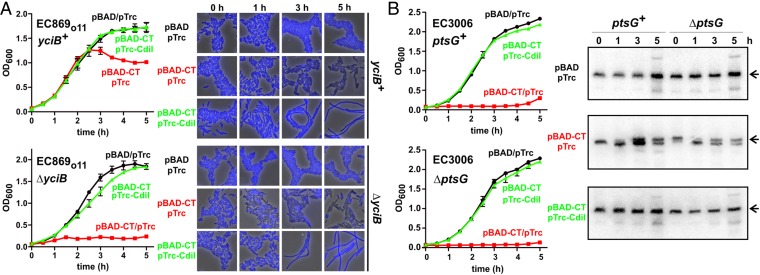
Given that CDIR was invariably associated with disruption of IMPs, we hypothesized that CDI toxins exploit these proteins to enter target bacteria. We tested whether CDI toxins are delivered into CDIR target cells by monitoring nuclease activities in competition cocultures. CdiA-CTo11EC869 toxin has a potent DNase activity that produces anucleate target cells (Fig. 3A) (11). However, ∆yciB mutants retained normal nucleoid morphology during coculture with CDIo11EC869 inhibitors and appeared similar to immune target cells that express the CdiIo11EC869 immunity protein (Fig. 3A). We also examined the tRNase activity of CdiA-CTEC3006, which specifically cleaves tRNA1Ile. Cleaved tRNA was detected in ptsG+ cells after 1 h incubation with CDIEC3006 inhibitors, but no tRNase activity was observed in cocultures with ∆ptsG targets (Fig. 3B). These results suggest that toxin is excluded from the cytoplasm of CDIR target cells. Alternatively, the IMPs could function as so-called permissive factors, which activate CDI toxins after entry into target bacteria (14). This latter model predicts that CDIR mutants should also be resistant to toxin produced internally. To test this model, we used controllable proteolysis to degrade ssrA(DAS)-tagged immunity proteins and thereby activate toxins inside the cell (8, 19, 20). CdiA-CTo11EC869 activation was slow in yciB+ cells, with growth inhibition and in vivo DNase activity observed after 3 h (Fig. 4A). In contrast, ∆yciB cell growth was inhibited immediately upon toxin activation, and DNase activity was apparent within 1 h (Fig. 4A). Similar results were obtained when CdiA-CTEC3006 was activated in ptsG+ and ∆ptsG cells, in which growth inhibition was immediate and tRNase activity was identical in both backgrounds (Fig. 4B). These results show that CdiA-CTo11EC869 and CdiA-CTEC3006 retain full toxicity when expressed inside CDIR mutant strains, excluding the toxin-activation model. Together, these data support a role for IMPs in the delivery of CDI toxins into the target-cell cytoplasm.
Fig. 3.
Toxin nuclease activities inside target bacteria. (A) Fluorescence microscopy of CDIo11EC869 competition cocultures. Inhibitor cells (YFP-labeled) were incubated with the indicated yciB+ or ∆yciB target cells (mRFP-labeled), and nucleoids were visualized with DAPI staining. (B) Northern blot analysis of CDIEC3006 competition cocultures. Target cells (ptsG+ or ∆ptsG) were incubated with CDIEC3006 inhibitor cells and RNA isolated for Northern blot analysis of tRNA1Ile. The migration position of uncleaved tRNA1Ile is indicated.
Fig. 4.
Toxin expression inside CDIR mutants. (A) CdiA-CTo11EC869 was induced at 0 h from a pBAD vector in yciB+ and ∆yciB cells as described previously (20). Cell growth was monitored by measuring the OD at 600 nm of the culture. CdiIo11EC869 immunity protein was coexpressed from a pTrc vector where indicated. (Right) DAPI-stained cells sampled at 0, 1, 3, and 5 h of culture. (B) CdiA-CTEC3006 was induced at 0 h from a pBAD vector in ptsG+ and ∆ptsG cells, and growth was monitored by measuring the OD600 of the culture. CdiIEC3006 immunity protein was coexpressed from a pTrc vector where indicated. (Right) Northern blot analysis of RNA isolated at 0, 1, 3, and 5 h. The arrows indicate the migration position of full-length tRNA1Ile.
Most of the CDIR mutations disrupt metabolite permeases, raising the possibility that transport activity could play a general role in CDI toxin import. We tested mutants lacking the cytoplasmic ATP-binding components of the Met (∆metN), Rbs (∆rbsA), and Glt (∆gltL) ABC transporters and found that each strain was still sensitive to CDI (Fig. S2). We also tested PtsG proteins that carry the Cys421Ser mutation and lack the entire cytoplasmic IIB domain, both of which are unable to transport d-glucose (18, 21). Each transport-defective PtsG protein rendered ∆ptsG cells sensitive to CDIEC3006 (Fig. S2). Therefore, the membrane permeases are required for CDI-mediated growth inhibition, but their metabolite transport activities are not.
Fig. S2.
Metabolite transport activity is not required for CDI. E. coli target cells of the indicated genotypes were cocultured for 3 h at a 1:1 ratio with inhibitor strains that deploy the indicated CdiA-CT toxins. Average target-cell counts (±SEM) are presented for three independent experiments.
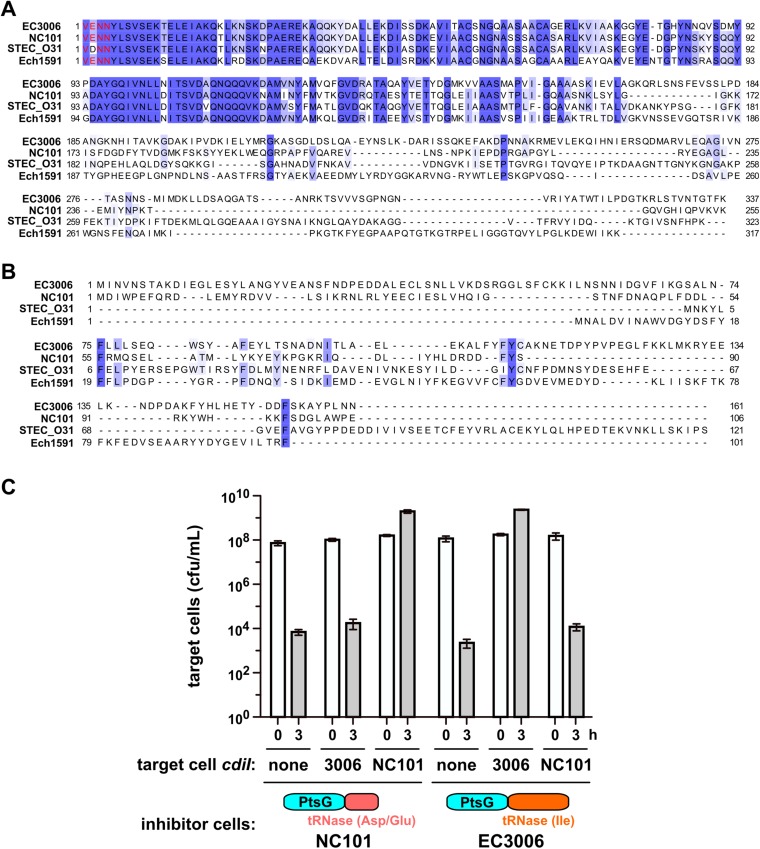
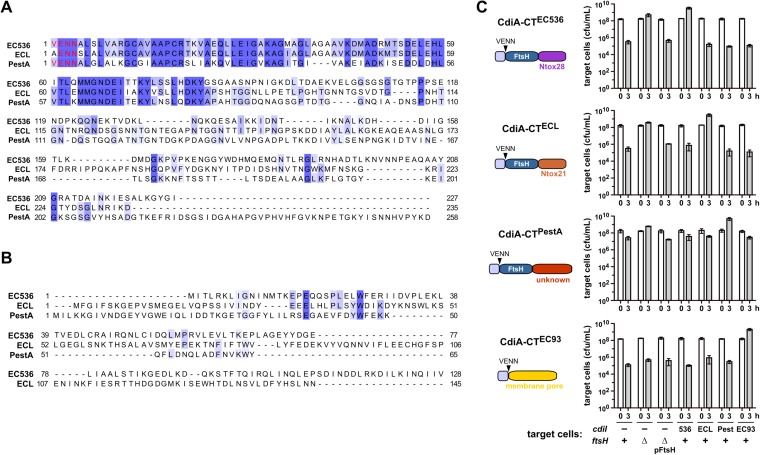
ptsG mutants were isolated in selections for resistance to CDINC101 and CDIEC3006 (Fig. 2A). These CdiA-CT sequences are 73.7% identical over the first 167 residues, but the C-terminal nuclease domains are unrelated (Fig. S3A). The CdiI immunity proteins also share no significant homology (Fig. S3B). In accord with this divergence, neither immunity protein protects against inhibition by the heterologous system (Fig. S3C). Together with previous analyses of CDI toxins (8, 11, 14), these observations indicate that CdiA-CT regions are often composed of two domains, with the extreme C-terminal domain containing the actual growth inhibition activity. Moreover, the genetic interaction between PtsG and the N-terminal sequences of CdiA-CTNC101 and CdiA-CTEC3006 suggests that the shared domain specifies the cell-entry pathway. The CdiA-CTs from uropathogenic E. coli 536 (EC536) and ECL also share N-terminal domains, but carry different C-terminal RNase domains and have distinct immunity proteins (Fig. S4 A and B) (10, 14). Based on reports that E. coli ∆ftsH mutants are resistant to multiple colicin nucleases (22–24), we screened ∆ftsH cells in CDI competitions and discovered that they are resistant to CDIEC536 and CDIECL, but sensitive to inhibition by CDIEC93 (Fig. S4C). FtsH is a hexameric AAA+ unfoldase/protease that is tethered to the inner membrane through two transmembrane helices, again suggesting that each CDI system exploits a specific IMP. ∆ftsH mutants are also resistant to the CdiA-CTPestA toxin from Yersinia pestis Pestoides A, which shares the N-terminal domain with CdiA-CTEC536 and CdiA-CTECL (Figs. S4 A and B). Together, these data show that CdiA-CT regions are commonly composed of two variable domains and suggest that the N-terminal domain may dictate the cell-entry pathway.
Fig. S3.
The N-terminal domain of the CdiA-CT region dictates the specificity of resistance. (A) Alignment of CdiA-CT sequences from E. coli strains 3006 (EC3006_4140), NC101 (ECNC101_09164), STEC_O31 (ECSTECO31_4009), and Dickeya zeae Ech1591 (Dd1591_2008). The conserved VENN peptide motif is presented in red type. Sequences were aligned by using Clustal Omega on the Uniprot server (www.uniprot.org/align/), and the results rendered at 30% sequence identity by using Jalview (www.jalview.org). (B) Alignment of CdiI immunity protein sequences from E. coli strains 3006 (EC3006_4139), NC101 (ECNC101_09169), and STEC_O31 (ECSTECO31_4008), and D. zeae Ech1591 (Dd1591_2009). (C) E. coli target cells expressing the indicated cdiI immunity genes were cocultured for 3 h at a 1:1 ratio with inhibitor strains that deploy CdiA-CTNC101 or CdiA-CTEC3006. Average target-cell counts (±SEM) are presented for three independent experiments.
Fig. S4.
Analyses of FtsH-dependent CDI toxins. (A) Alignment of CdiA-CT sequences from E. coli EC536 (ECP_4580), ECL (ECL_04451), and Y. pestis Pestoides A (YPS_3004). The conserved VENN peptide motif is presented in red type. Sequences were aligned by using Clustal Omega on the Uniprot server (www.uniprot.org/align/), and the results rendered at 30% sequence identity by using Jalview (www.jalview.org). (B) Alignment of CdiI immunity protein sequences from E. coli EC536 (ECP_4579), ECL (unannotated), and Y. pestis Pestoides A (YPS_3003). (C) Inhibitor strains that deploy indicated CdiA-CT toxins were cocultured for 3 h at a 1:1 ratio with target cells of the indicated genotypes. Plasmid-borne ftsH (pFtsH) complements the ∆ftsH mutation and restores sensitivity to FtsH-dependent CDI toxins. Average target-cell counts (±SEM) are presented for three independent experiments.
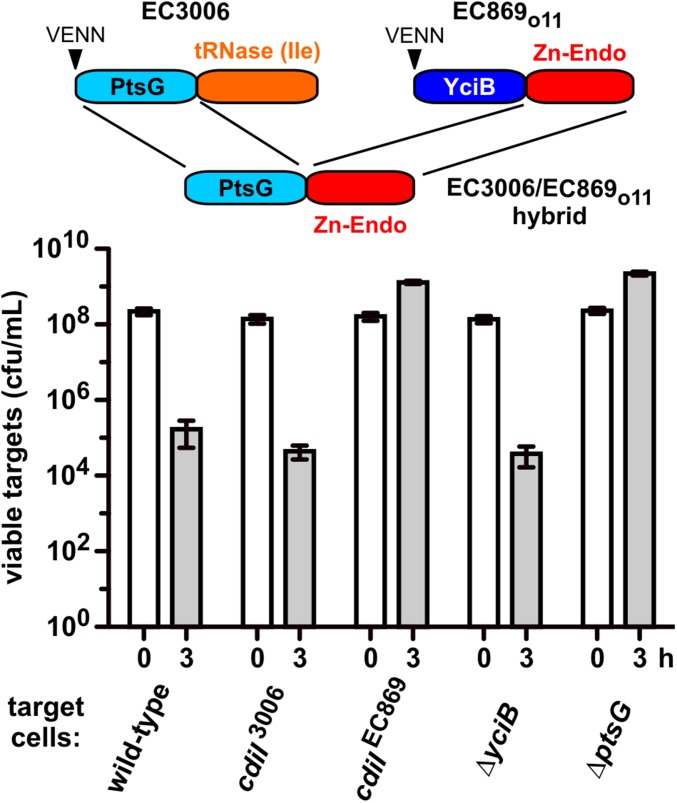
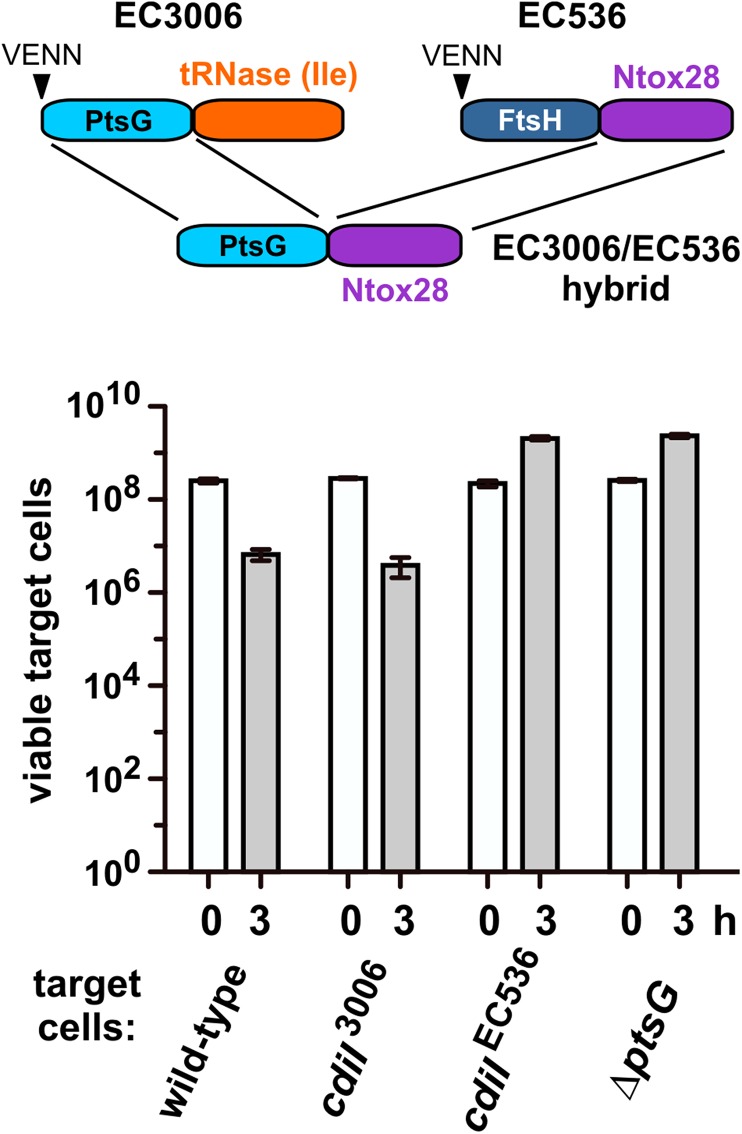
Analyses of naturally occurring CdiA-CTs suggest that the N- and C-terminal domains can be rearranged to deliver nucleases through different pathways. We tested this prediction with novel CdiA-CT hybrid constructs. We fused the N-terminal domain of CdiA-CTEC3006 (Val1–Leu167, numbered from Val1 of the VENN motif; Fig. S3A) to the DNase domain of CdiA-CTo11EC869 (Ala154–Lys297) (11) (Fig. 5), and then grafted the hybrid onto CdiAEC93 to generate a chimeric CDI system. The resulting triple chimera reduced target-cell viability ∼100-fold in coculture, and target cells were protected when they expressed cdiIo11EC869 but not the cdiIEC3006 immunity gene (Fig. 5). We then tested the EC3006/EC869o11 hybrid against ∆yciB and ∆ptsG target cells and found that only ∆ptsG mutants were resistant (Fig. 5). We used the same approach to deliver the CdiA-CTEC536 tRNase domain (Lys127–Ile227) into target cells with the CdiA-CTEC3006 N-terminal domain (Fig. S5). As expected, CdiIEC536 protein protected target cells from the EC3006/EC536 hybrid, and growth inhibition required PtsG (Fig. S5). These results show that the two CdiA-CT domains are modular, and nucleases can be delivered through different pathways specified by the N-terminal domain.
Fig. 5.
CdiA-CT constituent domains are modular. The N-terminal domain of CdiA-CTEC3006 was fused to the C-terminal DNase domain of CdiA-CTo11EC869. EC3006-EC869o11 hybrid inhibitors were cocultured with the indicated target strains. Average target-cell counts (±SEM) are presented for three independent experiments.
Fig. S5.
CdiA-CT constituent domains are modular. The N-terminal domain of CdiA-CTEC3006 was fused to the C-terminal tRNase domain of CdiA-CTEC536. EC3006-EC536 hybrid inhibitors were cocultured with the indicated target strains. Average target-cell counts (±SEM) are presented for three independent experiments.
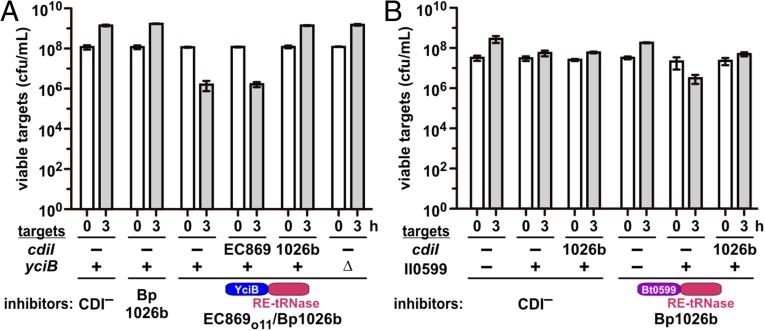
CDI systems are generally conserved between bacteria, but Burkholderia systems have an alternative gene order, and the CdiA-CT region is demarcated by a distinct ELYN peptide motif (8, 25). We found that fusion of CdiA-CTIIBp1026b (Glu1–Asn297, numbered from Glu1 of ELYN) from Burkholderia pseudomallei 1026b to CdiAEC93 produces a nonfunctional chimera (Fig. 6A). However, the C-terminal tRNase domain from CdiA-CTIIBp1026b (Thr162–Asn297) can be delivered efficiently when fused to the N-terminal domain of CdiA-CTo11EC869 (Fig. 6A). Moreover, as predicted from the delivery domain model, E. coli ∆yciB mutants are resistant to the EC869o11/Bp1026b hybrid CdiA-CT (Fig. 6A). This latter result shows that the Burkholderia tRNase domain can be delivered into E. coli target cells, raising the possibility that E. coli lacks the pathway required for native CdiA-CTIIBp1026b import. We recently discovered that Burkholderia thailandensis ∆BTH_II0599 mutants are resistant to the CDIIIBp1026b system (26). BTH_II0599 encodes a member of the major facilitator superfamily (MFS), which are integral membrane transporters of small metabolites and antibiotics (27). BTH_II0599 is highly conserved among Burkholderia species, but homologs are absent from enterobacteria. Therefore, we provided E. coli cells with plasmid-borne BTH_II0599 and tested them as targets in competitions against inhibitors that deploy the native CdiA-CTIIBp1026b. Remarkably, cells that express BTH_II0599 became sensitized to growth inhibition, and showed a ∼15-fold decrease in viable cell counts after 3 h (Fig. 6B). Moreover, sensitized target cells were protected when they expressed the cognate cdiIIIBp1026b immunity gene (Fig. 6B), indicating that the CdiA-CTIIBp1026b tRNase domain mediated growth inhibition. Collectively, these data reveal a genetic interaction between the N-terminal domain of CdiA-CTIIBp1026b and BTH_II0599 and suggest that this MFS protein is required for toxin translocation into target bacteria.
Fig. 6.
BTH_II0599 allows CdiA-CTIIBp1026b delivery into E. coli cells. (A) Inhibitor strains (mock CDI−, Bp1026b, and hybrid EC869o11-Bp1026b) were cocultured with target bacteria of the indicated cdiI and yciB genotypes. Viable target bacteria were quantified at 0 and 3 h. (B) Inhibitor strains (mock CDI− and Bp1026b) were cocultured with target cells that express BTH_II0599 and cdiIIIBp1026b where indicated. Average target-cell counts (±SEM) are presented for three independent experiments.
Discussion
We previously reported that variable CdiA-CT regions are often composed of two domains (8, 10, 11, 14). The extreme C-terminal domain typically has nuclease activity and is sufficient to inhibit growth when expressed inside E. coli cells (10, 11, 14). In contrast, the N-terminal domain of the CdiA-CT has no inhibition activity, and its function has not been explored. The findings presented here suggest that the N-terminal domain is critical for nuclease toxin translocation during CDI. This model is based on the identification of multiple CDIR mutations that disrupt integral membrane proteins and concomitantly protect target bacteria from specific CdiA-CT toxins. In principle, these membrane proteins could function as permissive factors that bind and activate CdiA-CT toxins after delivery (14). However, CdiA-CTo11EC869 and CdiA-CTEC3006 nuclease domains have full activity when expressed inside CDIR mutants, excluding permissive factor function. Moreover, ptsG, yciB, and ftsH mutants are resistant to CdiA-CTs based on the identity of the nontoxic N-terminal domains. These genetic interactions suggest that N-terminal domains use specific IMPs as receptors during CDI. Although our data do not demonstrate direct toxin–IMP interactions, this model is supported by experiments showing that BTH_II0599 expression sensitizes E. coli to the native CdiA-CTIIBp1026b toxin. Because BTH_II0599 is completely heterologous, with no homologs in γ-proteobacteria, the simplest explanation is that CdiA-CTIIBp1026b binds directly to this IMP to translocate into the cytoplasm. Moreover, the N-terminal domain of CdiA-CTIIBp1026b is limited to B. pseudomallei systems, arguing that these effectors target only other Burkholderia that contain BTH_II0599 homologs.
Crystal structures are available for three CDI toxin/immunity protein complexes, but the N-terminal domain is resolved in only one model (10, 11). Residues Met86–Thr153 of CdiA-CTo11EC869 form a small helical bundle that packs against the C-terminal DNase domain (11). The tertiary contacts with the nuclease domain probably facilitated the resolution of this domain. For many other CdiA-CT regions, the N- and C-terminal domains are connected by flexible peptide linkers, suggesting the domains have few or no tertiary contacts and move independently of one another. Additionally, the N-terminal domains do not make direct contacts with CdiI immunity proteins (10, 11, 14). Together, these observations suggest that the N- and C-terminal domains are autonomous units that can be recombined in virtually any combination. Although this hypothesis is supported by the functional hybrid CdiA-CTs constructed in this work, we note that the reengineered toxins are less effective than their naturally occurring counterparts. Thus, a given nuclease domain may have a preferred translocation pathway that is more efficient than others.
CdiA-CT nuclease domains are delivered into the target-cell cytoplasm (10–13), but the molecular details of CDI toxin translocation remain obscure. Most of the IMPs identified here are metabolite transporters, but our data indicate that transporter activity is not required for toxin import. Moreover, it seems unlikely that protein toxin domains could be transported in the same manner as small molecules. Instead, we hypothesize that CDI toxins exploit IMPs as receptors to bring nuclease domains into close proximity with the membrane, thereby allowing the toxin to enter and penetrate the lipid bilayer. Further, because the target-cell proton motive force is required for CDI (12), we postulate that this electrochemical gradient provides the driving force to transport toxins into the cytosol. This mechanism is similar to that proposed for colicin E3 and E9 nuclease toxins, which spontaneously enter lipid micelles and mediate their own transport across membranes (28, 29). However, colicins do not appear to require IMP receptors, and nearly all CDIR mutations provide no protection against colicins (30). The one exception is ftsH, which was originally identified as the tolZ mutation and confers resistance to nuclease toxins of group A and B colicins (22, 31). FtsH is a membrane-associated AAA+ superfamily member with ATP-dependent metalloprotease activity. Two models have been proposed for the role of FtsH in colicin import. De Zamaroczy and coworkers have shown that FtsH is required for the release of colicin nucleases into the cell, and they hypothesize that the protease directly cleaves the domain (23, 32). Kleanthous and coworkers have proposed that the ATP-dependent unfoldase activity of FtsH is used to pull the nuclease domain into the cell (24). AAA+ proteases are processive enzymes that actively unfold and cleave proteins into small peptides, so, in these models, the colicin nuclease domain must resist complete degradation during transport. Intriguingly, bacteriophages are also known to exploit IMPs to transfer their genomes into host cells. Phage λ requires the ManY component of the mannose phosphotransferase system to infect E. coli cells (33), and it was recently reported that PtsG is required for infection by E. coli phage HK97 (34). Thus, CDI and phages may use similar strategies to transport macromolecules into bacterial targets.
Other toxin-delivery systems, including Neisseria MafB proteins (35), type VI secretion-associated Rhs proteins (9, 20, 36), and predicted type VII secretion toxins from Bacillus and Mycobacteria (37), carry C-terminal nuclease domains that are related to those in CdiA proteins. Like CDI, the genetic organization of these other toxin-delivery systems is modular, allowing toxin interchange at the C terminus of conserved delivery proteins. These observations imply that toxin/immunity coding sequences are subject to frequent horizontal gene transfer between systems. Therefore, widely distributed toxin domains must be active against multiple clades of bacteria. Perhaps this explains why so many of these toxins are nucleases, which should be effective against any bacterium provided the domain can be delivered into the cytoplasm. It seems likely that each competition system uses a different mechanism to deliver toxins into target bacteria. For example, type VI secretion is thought to mechanically penetrate the target cell envelope, which could explain why analogous translocation domains are not found adjacent to the C-terminal toxin domains of Rhs effectors. The physical basis for CDI toxin translocation is unknown, but the mechanism appears to be quite versatile, allowing a nuclease domain to be transported through multiple independent pathways. Further, CDI exploits several membrane protein families, suggesting that, in principle, any IMP could be hijacked as a translocation receptor. Given this plasticity, we speculate that the mechanism could be harnessed to transport other cargos into Gram-negative bacteria and perhaps form the basis of novel antibacterial therapies.
Materials and Methods
Bacterial strains are listed in Table S1. E. coli EPI100 cells carrying plasmid-borne cdi gene clusters were used as inhibitors, and E. coli MC4100 and MG1655 derivatives were used as target cells. E. coli MC4100 was subjected to mariner-mediated mutagenesis by using plasmid pSC189 (38). Gene disruptions were from the Keio collection (39) and were transferred into E. coli MC4100 by using phage P1-mediated general transduction. Plasmids and oligonucleotides are listed in Tables S2 and S3, respectively. The details of all plasmid constructions are provided in SI Materials and Methods. Competition cocultures were performed at a 1:1 inhibitor to target cell ratio in shaking lysogeny broth medium at 37 °C as described in SI Materials and Methods. Competitions with ∆ftsH target cells were performed at 30 °C, and chimeric EC93-Bp1026b inhibitors were used in 10-fold excess over target bacteria. Viable target cells were enumerated as cfu counts per milliliter and expressed as the average ± SEM for three independent experiments. RNA was isolated by guanidinium isothiocyanate-phenol extraction (40). Northern blots were performed with 10 µg of total RNA using a probe for E. coli tRNA1Ile. In vivo DNase activity was assessed by fluorescence microscopy of DAPI-stained bacteria as described in SI Materials and Methods.
Table S1.
Bacterial strains
| Strain | Description | Source |
| X90 | F´ lacIq lac´ pro´/ara ∆(lac-pro) nal1 argE(amb) rifr thi-1, RifR | — |
| MG1655 | WT strain | — |
| DA28100 | galK::sYFP2opt-cat, CmR | Sanna Koskiniemi |
| EPI100 | F–mcrA ∆(mrr-hsdRMS-mcrBC) φ80dlacZ∆M15 ∆lacXcZ∆M15 ∆lacX recA1 endA1 araD139 ∆(ara, leu)7697 galU galK λ– rpsL nupG, StrR | Epicentre |
| DY378 | W3110 λcI857 ∆(cro-bioA) | Ref. 41 |
| MFDpir | MG1655 RP4-2-Tc::[∆Mu1::aac(3)IV-∆aphA-∆nic35-∆Mu2::zeo] dapA::(erm-pir) ∆recA, AprR ZeoR ErmR | Ref. 43 |
| CH43 | MG1655 ∆ftsH3::kan lpxC | Ref. 44 |
| CH2505 | X90 galK::sYFP2opt-kan, RifR KanR | This study |
| CH2550 | EPI100 galK::sYFP2opt-kan, KanR | This study |
| CH7157 | X90 ∆clpX ∆clpA::kan, RifR KanR | Ref. 8 |
| CH8119 | DH5αpir+ | Biomedal |
| CH8251 | MC4100 rifr, RifR | This study |
| CH9401 | CH8251 ∆yciC::kan, RifR KanR | This study |
| CH9404 | CH8251 ∆yciB::kan, RifR KanR | This study |
| CH9405 | X90 ∆yciB::kan, RifR KanR | This study |
| CH10013 | JCM158 rifr, RifR | Ref. 6 |
| CH10229 | JCM158 rifr ∆wzb::cat, RifR CmR | Ref. 6 |
| CH11843 | CH8251 ∆metI::kan, RifR KanR | This study |
| CH11844 | CH8251 ∆metN::kan, RifR KanR | This study |
| CH11845 | CH8251 ∆ptsG::kan, RifR KanR | This study |
| CH12000 | CH8251 ∆rbsA::kan, RifR KanR | This study |
| CH12002 | CH8251 ∆rbsC::kan, RifR KanR | This study |
| CH12008 | CH8251 ∆gltJ::kan, RifR KanR | This study |
| CH12009 | CH8251 ∆gltK::kan, RifR KanR | This study |
| CH12010 | CH8251 ∆gltL::kan, RifR KanR | This study |
| CH12741 | X90 ∆ptsG::kan, RifR KanR | This study |
AmpR, ampicillin-resistant; AprR, aprimycin-resistant; CmR, chloramphenicol-resistant; ErmR, erythromycin-resistant; KanR, kanamycin-resistance; RifR, rifampicin-resistant; TetR, tetracycline-resistant; ZeoR, zeocin-resistant.
Table S2.
Plasmids
| Plasmid | Description | Source |
| pTrc99a | IPTG-inducible expression plasmid, AmpR | GE Healthcare |
| pTrc99KX | Derivative of pTrc99a that contains a 5′-KpnI restriction site and 3′-SpeI and XhoI sites, AmpR | Ref. 10 |
| pCH450 | pACYC184 derivative with E. coli araBAD promoter for arabinose-inducible expression, TetR | Ref. 12 |
| pNAK | pBluescript derivative with FRT-flanked kanamycin-resistance cassette, AmpR KanR | This study |
| pSC189 | Mobilizable plasmid with R6Kγ replication origin. Carries the mariner transposon containing kanamycin-resistance cassette, AmpR KanR | Ref. 38 |
| pSIM6 | Heat-inducible expression of the phage λ Red recombinase proteins, AmpR | Ref. 41 |
| pCH172 | pEL3C17-pJ23110-mRFP-cat, CmR | Sanna Koskiniemi |
| pCH360 | pTrc99a::ftsH, AmpR | This study |
| pCH361 | pTrc(CM)::BTH_II0599, CmR | This study |
| pCH1417 | Constitutive expression of chimeric cdiAEC93-CTPestA and cdiIPestA genes, CmR | This study |
| pCH2156 | pTrc99KX::cdiIPestA, IPTG-inducible expression of cdiIPestA immunity gene, AmpR | This study |
| pCH2500 | pNAK::galM´, AmpR KanR | This study |
| pCH2503 | pNAK::galT´-yfp-galM´, AmpR KanR | This study |
| pCH9305 | Constitutive expression of chimeric cdiAEC93-CTo11EC869 and cdiIo11EC869 genes, CmR | Ref. 11 |
| pCH9433 | Constitutive expression of chimeric cdiAEC93-CTIIBp1026b and cdiIIIBp1026b genes, CmR | This study |
| pCH7959 | pCH450::cdiA-CT/cdiIo11EC869-DAS, produces CdiIo11EC869 with C-terminal ssrA(DAS) epitope for controllable proteolysis, TetR | Ref. 20 |
| pCH9315 | pTrc99a::cdiIo11EC869, IPTG-inducible expression of cdiIo11EC869 immunity gene, AmpR | Ref. 11 |
| pCH9577 | pTrc99a::cdiIIIBp1026b, IPTG-inducible expression of cdiIIIBp1026b immunity gene, AmpR | Ref. 8 |
| pCH9922 | pTrc99KX::yciB, IPTG-inducible expression of yciB, AmpR | This study |
| pCH10163 | CosmidpCdiA-CT/pheS* that carries a kan-pheS* cassette in place of the E. coli EC93 cdiA-CT/cdiI coding sequence. Used for allelic exchange and counter selection. CmR KanR | Ref. 11 |
| pCH10415 | Constitutive expression of chimeric cdiAEC93-NTo11EC869-CTIIBp1026b and cdiIIIBp1026b genes, CmR | Ref. 12 |
| pCH10445 | Constitutive expression of chimeric cdiAEC93-CTECL and cdiIECL genes, CmR | Ref. 10 |
| pCH10673 | Constitutive expression of chimeric cdiAEC93-CTEC536 and cdiIEC536 genes, CmR | Ref. 12 |
| pCH11434 | Constitutive expression of chimeric cdiAEC93-CTNC101 and cdiINC101 genes, CmR | This study |
| pCH11446 | Constitutive expression of chimeric cdiAEC93-CTMHI813 and cdiIMHI813 genes, CmR | This study |
| pCH11483 | Constitutive expression of chimeric cdiAEC93-CTEC3006 and cdiIEC3006 genes, CmR | This study |
| pCH11840 | pTrc99a::ptsG, IPTG-inducible expression of ptsG, AmpR | This study |
| pCH11949 | Constitutive expression of chimeric cdiAEC93-CTTTO1 and cdiITTO1 genes, CmR | This study |
| pCH11950 | Constitutive expression of chimeric cdiAEC93-CT1Dd3937 and cdiI1Dd3937 genes, CmR | This study |
| pCH12021 | pTrc99KX::ptsG(C421S), IPTG-inducible expression of ptsG(C421S), AmpR | This study |
| pCH12022 | pTrc99KX::metI, IPTG-inducible expression of metI, AmpR | This study |
| pCH12024 | pTrc99KX::rbsC, IPTG-inducible expression of rbsC, AmpR | This study |
| pCH12025 | pTrc99KX::gltK, IPTG-inducible expression of gltK, AmpR | This study |
| pCH12042 | pTrc99KX::cdiINC101, IPTG-inducible expression of cdiINC101 immunity gene, AmpR | This study |
| pCH12043 | pTrc99KX::cdiIMHI813, IPTG-inducible expression of cdiIMHI813 immunity gene, AmpR | This study |
| pCH12045 | pTrc99KX::cdiIEC3006, IPTG-inducible expression of cdiIEC3006 immunity gene, AmpR | This study |
| pCH12077 | Constitutive expression of chimeric cdiAEC93-NTEC3006-CTEC536 and cdiIEC536 genes, CmR | This study |
| pCH12082 | pTrc99KX::cdiI13937, IPTG-inducible expression of cdiI13937 immunity gene, AmpR | This study |
| pCH12202 | pTrc99a::ptsG(E387Oc), IPTG-inducible expression of PtsG lacking the C-terminal IIB domain, AmpR | This study |
| pCH12205 | pTrc99KX::cdiITTO1, IPTG-inducible expression of cdiITTO1 immunity gene, AmpR | This study |
| pCH12237 | Constitutive expression of chimeric cdiAEC93-NTEC3006-CTo11EC869 and cdiIo11EC869 genes, CmR | This study |
| pCH12599 | pCH450::cdiA-CT/cdiIEC3006-DAS, produces CdiIEC3006 with C-terminal ssrA(DAS) epitope for controllable proteolysis, TetR | This study |
AmpR, ampicillin-resistant; CmR, chloramphenicol-resistant; KanR, kanamycin-resistance; RifR, rifampicin-resistant; TetR, tetracycline-resistant.
Table S3.
Oligonucleotides
| Oligonucleotide | Sequence* | Source |
| DL1527 | 5′-GAA CAT CCT GGC ATG AGC G | Ref. 11 |
| DL1663 | 5′-CCC AAA GGT TAG ACA CCA GAC C | Ref. 11 |
| DL2368 | 5′-GTT GGT AGT GGT GGT GCT G | Ref. 11 |
| DL2470 | 5′-ATT ATT CTC AAC CGA GTT CCT ACC TG | Ref. 11 |
| CH106 (Kan-1) | 5′-TGT GTA GGC TGG AGC TGC TTC | This study |
| CH107 (Kan-2) | 5′-CAT ATG AAT ATC CTC CTT AGT TCC | This study |
| CH577 (Ile-1 probe) | 5′-ACC GAC CTC ACC CTT ATC AG | This study |
| CH2139 (yciB-Kpn-for) | 5′-AAT GGT ACC ATG AAG CAG TTT CTT GAT TTT TTA C | This study |
| CH2140 (yciB-Xho-rev) | 5′-GCA CTC GAG TTA GGA TTT ATC TTC CTG CGG | This study |
| CH2260 (mariner rev seq) | 5′-CAA GCT TGT CAT CGT CAT CC | This study |
| CH2501 (EC93-1026b) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATG CAC TGG GCA ACG ACC CCC AAA AAA CG | This study |
| CH2504 (EC93-1026b) | 5′-GGT CTG GTG TCT AAC CTT TGG GTT ACC TCC GGT ATT CGT TAT CTT GC | This study |
| CH2525 (BTH0599-Kpn-for) | 5′-AAT GGT ACC ATG CAA CTG ATC GAA GTC TCC | This study |
| CH2526 (BTH0599-Xho-rev) | 5′-ATA CTC GAG TCA TCG ATC GGA GGT GTT CGG | This study |
| CH2636 (ftsH-Eco-for) | 5′-GTT TTG AAT TCA GTT GTA ATA AGA GG | This study |
| CH2637 (ftsH-Hind-rev) | 5′-AAA AAG CTT CAT GAT GTT ATC CCT GG | This study |
| CH3172 (3006) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATT ATC TTA GCG TGT CTG AAA AGA CAG AGC | This study |
| CH3173 (3006) | 5′-GGT CTG GTG TCT AAC CTT TGG GTT AAT TAT TCA GAG GAT AAG CTT TTG AAA AAT CAT CG | This study |
| CH3174 (MHI813) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATT TTT TGA CCG CAG ATC AGA TCG ATA GC | This study |
| CH3175 (MHI813) | 5′-GGT CTG GTG TCT AAC CTT TGG GTT ATA GTT CAT CATCAT ATT GAA AGT TTA TGC TAA | This study |
| CH3176 (NC101) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATT ACC TGA GCG TGT CTG AAA AGA CAG | This study |
| CH3177 (NC101) | 5′-GGT CTG GTG TCT AAC CTT TGG GTT ATT CAG GCC ATG CCA ATC CAT C | This study |
| CH3238 (NC101-cdiI-for) | 5′-CTG GTA CCA TGG ATA TTT GGC CTG | This study |
| CH3239 (NC101-cdiI-rev) | 5′-GAC TCG AGT TAT TCA GGC CAT GCC AAT C | This study |
| CH3240 (MHI-cdiI-for) | 5′-CTG GTA CCA TGA ACG AAT TAG ATG | This study |
| CH3241 (MHI-cdiI-rev) | 5′-GAC TCG AGT TAT AGT TCA TCATCA TAT TG | This study |
| CH3244 (3006-cdiI-for) | 5′-CTG GTA CCA TGA TAA ATG TGA ATA G | This study |
| CH3245 (3006-cdiI-rev) | 5′-GAC TCG AGT TAA TTA TTC AGA GGA TAA GC | This study |
| CH3269 (PSI-univ-Nco-for) | 5′-TTT AAG AAG GAG TCT CTC CCA TGG-3′ | This study |
| CH3422 (NC101-cdiI-Spe-rev) | 5′-GAA CTA GTT TCA GGC CAT GCC AAT C | This study |
| CH3425 (3006-cdiI-Spe-rev) | 5′-GAA CTA GTA TTA TTC AGA GGA TAA GCT TTT G | This study |
| CH3477 (metI-for) | 5′-CTG GTA CCA TGT CTG AGC CGA TGA TGT G | This study |
| CH3478 (metI-rev) | 5′-GAC TCG AGT TAC TTG CGA GTG ACA GCC | This study |
| CH3481 (ptsG-C421S-for) | 5′-CAT TAC TAA CCT CGA CGC AAG TAT TAC CCG TCT GC | This study |
| CH3513 (Dd3937-1) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATT TCC TGA ACA AAG GAA GAC CG | This study |
| CH3514 (Dd3937-1) | 5′-GGT CTG GTG TCT AAC CTT TGG GTT AAC TCC ACT TCC ATT TTA TGA TCA AAT | This study |
| CH3519 (PlumTTO1) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATG CGC TGG CCT CGC GAA ATC | This study |
| CH3520 (PlumTTO1) | 5′-GGT CTG GTG TCT AAC CTT TGG GTT AAT TAC CTT CTA TCC ATA CTT GC | This study |
| CH3530 (Pestoides A) | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATG CGC TGG GTC TGG CTC TGA AG | This study |
| CH3531 (Pestoides A) | 5′-GGT CTG GTG TCT AAC CTT TGG TTA ATA CCA TTT TAC ATT AAA ATC AGC | This study |
| CH3570 (pUC rev Xho) | 5′-AAA CTC GAG GCC TCT GCA GTC G | This study |
| CH3571 (pUC for Xho) | 5′-AAA CTC GAG TCG CGA ATG CAT C | This study |
| CH3572 (Pest cdiI for) | 5′-GGA GGT ACC ATG ATC TTG AAA AAA G | This study |
| CH3612 (ptsG-Eco-for) | 5′-GTT CCG AAT TCA AGA ATG CAT TTG CTA ACC TG | This study |
| CH3613 (ptsG-Pst-rev) | 5′-GAC TGC AGT TAG TGG TTA CGG ATG TAC TC | This study |
| CH3629 (gltK-for) | 5′-CTG GTA CCA TGT ACG AGT TTG ACT GGA G | This study |
| CH3630 (gltK-rev) | 5′-GAC TCG AGT TAT GCT GTC CTT CTT TTC AAG | This study |
| CH3631 (rbsC-for) | 5′-CTG GTA CCA TGA CAA CCC AGA CTG TCT C | This study |
| CH3632 (rbsC-rev) | 5′-GAC TCG AGT TAC TGC TTT TTG TTG TCT ACC | This study |
| CH3633 (gltJ-for) | 5′-CTG GTA CCA TGT CTA TAG ACT GGA ACT GG | This study |
| CH3634 (gltJ-rev) | 5′-GAC TCG AGT TAT TTG CCC CCC ATG TTG | This study |
| CH3674 (3937-cdiI2-Kpn-for) | 5′-GAA GGT ACC ATG AAA TGT AAT GAT TTT | This study |
| CH3675 (3937-cdiI2-Xho-rev) | 5′-TTT CTC GAG CTA ACT CCA CTT CCA TT | This study |
| CH3676 (TTO1-cdiI-Kpn-for) | 5′-TTT GGT ACC ATG AAT ACT AAA CTT AAT G | This study |
| CH3677 (TTO1-cdiI-Xho-rev) | 5′-TTT CTC GAG CTA ATT ACC TTC TAT CC | This study |
| CH3682 (536-K127-for) | 5′-AAA ACT GTA GAT AAG CTT AAT CAG AAG | This study |
| CH3683 (3006-L167-OE-rev) | 5′-CTT CTG ATT AAG CTT ATC TAC AGT TTT CAG AAC TTC TAT CTT ACT GGC C | This study |
| CH3722 (ptsG-R386-Xho-rev) | 5′-GAC TCG AGT TAA CGA CCC GGC GTT TTC | This study |
| CH3723 (o11CT-T151-for) | 5′-ACA GCG ACA GCG ACG | This study |
| CH3724 (3006NT-L167-o11CT-T151-rev) | 5′-CGT CGC TGT CGC TGT CAG AAC TTC TAT CTT ACT GGC C | This study |
| CH3787 (galT-Kpn-for) | 5′-CAC GGT ACC ATT TGG GCA AAT AGC TTC C | This study |
| CH3788 (yfp-Eco-rev) | 5′-CT GAA TTC GCG GCC GCT TCT AGA | This study |
| CH3789 (galM-Bam-for) | 5′-CGC GGA TCC CGG AAG AGC TGG | This study |
| CH3790 (galM-Sac-rev) | 5′-TCT GAG CTC AGG GCA AAC AGC ACC | This study |
Restriction endonuclease sites are underlined.
SI Materials and Methods
Plasmid Constructions.
Plasmid-borne chimeric CDI systems were constructed by allelic exchange of the counter selectable pheS* marker from plasmid pCH10163 as described previously (11). The various cdiA-CT/cdiI sequences were amplified by PCR by using the following primer pairs: Escherichia coli NC101 (ECNC101_09164/09169), CH3176/CH3177; E. coli 3006 (EC3006_4140/4139), CH3172/CH3173; E. coli MHI813 (ECSTECMHI813_1064/1065), CH3174/CH175; P. luminescens TTO1 (plu0548/plu0547), CH3519/CH3520; D. dadantii 3937 (Dda3937_04704/02929), CH3513/CH3514; Yersinia pestis Pestoides A (YPS_3004/YPS_3003), CH3530/CH3531; and Burkholderia pseudomallei 1026b (BP1026B_II2207/cdiI is unannotated), CH2501/CH2504. Each cdiA-CT/cdiI fragment was fused to upstream and downstream homology regions amplified from the cdiAEC93 gene. The cdiAEC93 upstream homology fragment was amplified by using primers DL1527/DL2470, and the downstream fragment was amplified with primers DL1663/DL2368. The three products (cdiA-CT/cdiI, upstream cdiAEC93, and downstream cdiAEC93) were then fused to each other through overlapping-end PCR (OE-PCR) by using primers DL1527/DL2368. The final DNA product (100 ng) was electroporated together with plasmid pCH10163 (300 ng) into E. coli strain DY378 cells as described previously (11). Clones with recombinant plasmids were selected on yeast extract glucose-agar supplemented with 33 µg/mL chloramphenicol and 10 mM d/l-p-chlorophenylalanine.
The EC3006/EC536 hybrid cdiA-CT/cdiI sequence was generated by OE-PCR. A fragment encoding the N-terminal domain of CdiA-CTEC3006 was amplified from plasmid pCH11483 by using primers DL1527/CH3683, and a fragment encoding the C-terminal tRNase domain of CdiA-CTEC536 and its immunity protein was amplified from plasmid pCH10673 by using primers CH3682/DL2368. The two PCR products were combined into one fragment through OE-PCR by using primers DL1527/DL2368, and the resulting product was recombined into plasmid pCH10163 as described earlier. The EC3006/EC869o11 hybrid construct was generated in the same manner. A fragment encoding the N-terminal domain of CdiA-CTEC3006 was amplified from plasmid pCH11483 by using primers DL1527/CH3724, and a fragment encoding the C-terminal DNase domain of CdiA-CTo11EC869 and its immunity protein was amplified from plasmid pCH9305 by using primers CH3723/DL2368.
The cdiI immunity genes were amplified by PCR by using the following primer pairs: E. coli NC101, CH3238/CH3239; E. coli 3006, CH3244/CH3245; E. coli MHI813, CH3240/CH3241; P. luminescens TTO1, CH3676/CH3677; D. dadantii 3937, CH3674/CH3675; and Y. pestis Pestoides A, CH3572/CH3571. All PCR products were digested with KpnI/XhoI and ligated to plasmid pTrc99KX (10). Genes encoding CDIR membrane proteins were amplified with the following primer pairs: yciB, CH2139/CH2140; BTH_II0599, CH2525/CH2526; ftsH, CH2636/CH2637; metI, CH3477/CH3478; ptsG, CH3612/CH3613; gltK, CH3629/3630; rbsC, CH3631/CH3632; and gltJ, CH3633/CH3634. The yciB, BTH_II0599, metI, gltK, gltJ, and rbsC PCR products were digested with KpnI/XhoI and ligated to plasmid pTrc99KX. The ftsH and ptsG products were digested with EcoRI/HindIII and EcoRI/PstI (respectively) and ligated to plasmid pTrc99A digested with the appropriate restriction endonucleases. The Cys421Ser mutation was introduced into ptsG by using megaprimer PCR. The 3′ region of ptsG was amplified with primers CH3481/CH3613, and the resulting product was used as a megaprimer in a second reaction with primer CH3612. The ptsG(E387Oc) ochre mutation that deletes the C-terminal IIB domain was made by PCR by using primers CH3612/CH3722. The CdiA-CT/CdiIEC3006-DAS controllable proteolysis construct was made by amplifying the cdiA-CT/cdiIEC3006 gene pair with primers CH3269/CH3425 and ligating the product into NcoI/SpeI-digested plasmid pCH9460.
YFP-labeled E. coli cells were generated by integrating the yfp coding sequence at the gal locus. First, a genomic integration construct was made by amplifying the kanamycin-resistance cassette from plasmid pKAN (10) with primers CH106/CH107, followed by blunt-end ligation to SmaI-digested plasmid pBluescript. A clone was identified that had the kanamycin-resistance cassette in the opposite orientation as pKAN, and this plasmid was termed pNAK. A fragment of galM was then amplified by using primers CH3789/CH3790, and the product was ligated to SacI/BamHI-digested plasmid pNAK to produce plasmid pCH2500. A yfp-galT fragment was amplified from E. coli DA28100 (gift from Sanna Koskiniemi, Uppsala University, Uppsala, Sweden) by using primers CH3787/CH3788, and the product was digested with KpnI/EcoRI and then ligated into pCH2500 to yield plasmid pCH2503. The large KpnI/SacI fragment from pCH2503 was recombined into E. coli EPI100 and X90 cells that harbor plasmid pSIM6 as described previously (41). Target bacteria were labeled with mRaspberry by using plasmid pEL3C17 (pJ23110-mRFP-cat).
Transposon Library Construction and Selection for CDIR Mutants.
The mariner transposon was introduced into E. coli CH10229 cells through conjugation with E. coli MFDpir donor cells carrying plasmid pSC189. Donor and recipient cells were grown to midlog phase in lysogeny broth (LB) medium supplemented with 150 µg/mL ampicillin and 30 µM diaminopimelic acid (donors) or 33 µg/mL chloramphenicol (recipients). Donors (∼6.0 × 108 cfu) and recipients (∼3 × 108 cfu) were mixed and collected by centrifugation for 2 min at 6,000 × g in a microcentrifuge. The supernatant was removed by aspiration and the cell pellet resuspended in 100 µL of 1× M9 salts. Cell mixtures were spotted onto 0.45-µm nitrocellulose membranes, and the filters were then incubated on LB agar (without inversion) for 4 h at 37 °C. The cells were then harvested from the filters by using 2 mL of 1× M9 salts. Transposon insertion mutants were selected by plating 10-fold serial dilution on LB-agar supplemented with 50 µg/mL of kanamycin.
More than 20,000 colonies from each transposon library were collected from the agar plates in 1× M9 salts, then inoculated into 50 mL of LB medium in a 250-mL baffled flask. CDI+ inhibitor strains were grown in a parallel 50 mL LB medium culture. Both cultures were grown at 37 °C until midlog phase, then mixed at approximately a 1:1 ratio and cultured for 3 h with shaking at 37 °C. Viable target cells from the transposon mutant library were enumerated as cfu counts per milliliter on LB agar supplemented with 50 µg/mL of kanamycin. The survivors of the first round of CDI competition were harvested from the plates with 1× M9 salts and used to inoculate 50 mL LB medium culture for a second round of CDI selection. After the third round of selection, the target cell population was usually completely resistant to the CDI inhibitor cells. Individual clones were then isolated and the CDIR phenotype confirmed in competition cocultures. The transposon mutations were then transferred into CDI-sensitive cells by bacteriophage P1-mediated transduction, and the resulting transductants were tested for the CDIR phenotype.
Transposon insertions that were linked to CDIR were identified by rescue cloning. Chromosomal DNA was prepared from each CDIR mutant by using phenol/chloroform extraction and ethanol precipitation. Genomic DNA (1 µg) was digested with AgeI and XmaI restriction endonucleases for 2 h at 37 °C and then the enzymes were inactivated at 65 °C for 10 min. ATP and T4 DNA ligase were added and the reaction was incubated for 2 h at room temperature. The ligated DNA was precipitated with 95% (vol/vol) ethanol, washed once with 75% (vol/vol) ethanol, and dissolved in 50 µL water. The ligated DNA was electroporated into E. coli DH5α pir+ cells, and transformants were selected on LB agar supplemented with 50 µg/mL kanamycin. Plasmid DNA was isolated from selected transformants, and the transposon insertion junctions were identified by DNA sequencing by using primer CH2260.
Competition Cocultures.
Competition coculture assays were carried out as previously described (10, 11). Briefly, inhibitor cells (E. coli EPI100 carrying CDI expression constructs) and target cells (E. coli MC4100 or MG1655 carrying pTrc99a derivatives) were grown in LB medium supplemented with appropriate antibiotics overnight at 37 °C. The next day, cells were inoculated into fresh LB medium without antibiotics in baffled flasks. Individual cultures were grown with shaking until early log phase, and then the populations were mixed at a 1:1 ratio in fresh prewarmed LB in baffled flasks. The competitions between EC93-Bp1026b inhibitors and targets that express BTH_II0599 were conducted at a 10:1 inhibitor-to-target cell ratio. A sample of each coculture was taken at initial mixing to enumerate viable target cells as cfu counts per milliliter. The cocultures were incubated with shaking for 3 h at 37 °C. Viable target cell counts are presented as the average ± SEM of three independent competition experiments.
Activation of CdiA-CT Toxins Inside E. coli Cells.
E. coli X90 cells (WT and ∆yciB and ∆ptsG mutants) were cotransformed with pTrc99a constructs that express CdiIo11EC869 or CdiIEC3006 and pCH450 derivatives that express CdiA-CT/CdiIo11EC869-DAS or CdiA-CT/CdiIEC3006-DAS protein pairs. Transformants were selected overnight on LB agar supplemented with 150 µg/mL Amp, 15 µg/mL Tet, and 0.4% d-glucose. The following day, transformants were inoculated into 25 mL of LB medium supplemented with Amp, Tet, and glucose. Cells were grown to midlog phase, then diluted to an OD600 of 0.05 in fresh LB supplemented with Amp, Tet, 0.2% arabinose, and 0.15 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) to induce expression from both plasmids. Cell growth was monitored by measuring the OD600 of the culture every 30 min for 5 h. The presented growth curves show the average ± SEM for three independently performed experiments. Culture samples were removed at 0, 1, 3, and 5 h for microscopy or RNA isolation.
RNA Isolation and Analysis.
Cells from internal expression experiments and CDI competition cocultures were poured into an equal volume of ice-cold methanol, and cells were collected by centrifugation in a Sorvall RC 5B centrifuge at 15,000 × g for 15 min at 4 °C. Cell pellets were frozen at −80 °C and RNA was isolated by using guanidine isothiocyanate-phenol as described previously (40). RNA (10 µg) was resolved on denaturing 8 M urea/10% (wt/vol) polyacrylamide gels and then electrotransferred onto nylon membrane (Nytran Supercharge). tRNA1Ile was detected by Northern blot hybridization by using radiolabeled oligonucleotide CH577 as a probe. Blots were visualized on a Bio-Rad PhosphorImager by using Quantity One software.
Microscopy.
Cells (equivalent to an OD600 of 0.2) were collected by centrifugation in a microcentrifuge for 2 min at 6,000 × g. Cells were fixed in freshly prepared 4% (vol/vol) formaldehyde in 1× PBS solution for 15 min, and the reaction was quenched with 125 mM glycine (pH 7.5). Cells were washed three times with 1× PBS solution, resuspended in 100 μL 1× PBS solution, and spotted onto poly-d-lysine–treated slides. Excess liquid was removed with a Kimwipe, and slides were dried and gently rinsed with Nanopure water to remove nonadherent bacteria. Slides were sealed with Fluorogel II with DAPI (Fisher Scientific/EMS) and a glass coverslip. Images were acquired on an Olympus fluorescent microscope with a 100× oil objective by using an Optronics MacroFire digital microscope camera. Light-field images were taken with a 12-ms exposure (gain 2). DAPI-stained images were acquired with a 48-ms exposure (gain 2). Fluorescent images were recorded in grayscale by using a 502-ms exposure (gain 5). Images were false-colored, overlaid by using FIJI (42), and cropped to 200 × 200 pixels using GIMP.
Acknowledgments
We thank Mary Raven for microscopy assistance and Sanna Koskiniemi and Dan Andersson for bacterial strains. This work was supported by National Institutes of Health Grant U01 GM102318 (to D.A.L. and C.S.H.) and National Science Foundation Graduate Research Fellowship DGE-1144085 (to J.L.E.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512124112/-/DCSupplemental.
References
- 1.Ruhe ZC, Low DA, Hayes CS. Bacterial contact-dependent growth inhibition. Trends Microbiol. 2013;21(5):230–237. doi: 10.1016/j.tim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman JM, Brunet YR, Cascales E, Mougous JD. Structure and regulation of the type VI secretion system. Annu Rev Microbiol. 2012;66:453–472. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki SK, et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol. 2008;70(2):323–340. doi: 10.1111/j.1365-2958.2008.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki SK, et al. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309(5738):1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 5.Kajava AV, et al. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol Microbiol. 2001;42(2):279–292. doi: 10.1046/j.1365-2958.2001.02598.x. [DOI] [PubMed] [Google Scholar]
- 6.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468(7322):439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. MBio. 2013;4(4):e00480–e00413. doi: 10.1128/mBio.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolakakis K, et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol Microbiol. 2012;84(3):516–529. doi: 10.1111/j.1365-2958.2012.08039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck CM, et al. CdiA from Enterobacter cloacae delivers a toxic ribosomal RNase into target bacteria. Structure. 2014;22(5):707–718. doi: 10.1016/j.str.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse RP, et al. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc Natl Acad Sci USA. 2012;109(52):21480–21485. doi: 10.1073/pnas.1216238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhe ZC, Nguyen JY, Beck CM, Low DA, Hayes CS. The proton-motive force is required for translocation of CDI toxins across the inner membrane of target bacteria. Mol Microbiol. 2014;94(2):466–481. doi: 10.1111/mmi.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb JS, et al. Delivery of CdiA nuclease toxins into target cells during contact-dependent growth inhibition. PLoS One. 2013;8(2):e57609. doi: 10.1371/journal.pone.0057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diner EJ, Beck CM, Webb JS, Low DA, Hayes CS. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI) Genes Dev. 2012;26(5):515–525. doi: 10.1101/gad.182345.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart JB, Hermodson MA. Topology of RbsC, the membrane component of the Escherichia coli ribose transporter. J Bacteriol. 2003;185(17):5234–5239. doi: 10.1128/JB.185.17.5234-5239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gál J, Szvetnik A, Schnell R, Kálmán M. The metD D-methionine transporter locus of Escherichia coli is an ABC transporter gene cluster. J Bacteriol. 2002;184(17):4930–4932. doi: 10.1128/JB.184.17.4930-4932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlin C, Gardiner G, Durand S, Masters M. The Escherichia coli metD locus encodes an ABC transporter which includes Abc (MetN), YaeE (MetI), and YaeC (MetQ) J Bacteriol. 2002;184(19):5513–5517. doi: 10.1128/JB.184.19.5513-5517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buhr A, Flükiger K, Erni B. The glucose transporter of Escherichia coli. Overexpression, purification, and characterization of functional domains. J Biol Chem. 1994;269(38):23437–23443. [PubMed] [Google Scholar]
- 19.McGinness KE, Baker TA, Sauer RT. Engineering controllable protein degradation. Mol Cell. 2006;22(5):701–707. doi: 10.1016/j.molcel.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Poole SJ, et al. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 2011;7(8):e1002217. doi: 10.1371/journal.pgen.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meins M, et al. Cysteine phosphorylation of the glucose transporter of Escherichia coli. J Biol Chem. 1993;268(16):11604–11609. [PubMed] [Google Scholar]
- 22.Qu JN, et al. The tolZ gene of Escherichia coli is identified as the ftsH gene. J Bacteriol. 1996;178(12):3457–3461. doi: 10.1128/jb.178.12.3457-3461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauleau M, Mora L, Serba J, de Zamaroczy M. FtsH-dependent processing of RNasecolicins D and E3 means that only the cytotoxic domains are imported into the cytoplasm. J Biol Chem. 2011;286(33):29397–29407. doi: 10.1074/jbc.M111.242354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker D, Mosbahi K, Vankemmelbeke M, James R, Kleanthous C. The role of electrostatics in colicin nuclease domain translocation into bacterial cells. J Biol Chem. 2007;282(43):31389–31397. doi: 10.1074/jbc.M705883200. [DOI] [PubMed] [Google Scholar]
- 25.Anderson MS, Garcia EC, Cotter PA. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLoS Genet. 2012;8(8):e1002877. doi: 10.1371/journal.pgen.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koskiniemi S, et al. Genetic analysis of the CDI pathway from Burkholderia pseudomallei 1026b. PLoS One. 2015;10(3):e0120265. doi: 10.1371/journal.pone.0120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law CJ, Maloney PC, Wang DN. Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol. 2008;62:289–305. doi: 10.1146/annurev.micro.61.080706.093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosbahi K, et al. The cytotoxic domain of colicin E9 is a channel-forming endonuclease. Nat Struct Biol. 2002;9(6):476–484. doi: 10.1038/nsb797. [DOI] [PubMed] [Google Scholar]
- 29.Mosbahi K, Walker D, James R, Moore GR, Kleanthous C. Global structural rearrangement of the cell penetrating ribonuclease colicin E3 on interaction with phospholipid membranes. Protein Sci. 2006;15(3):620–627. doi: 10.1110/ps.051890306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma O, et al. Genome-wide screens: Novel mechanisms in colicin import and cytotoxicity. Mol Microbiol. 2009;73(4):571–585. doi: 10.1111/j.1365-2958.2009.06788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teff D, Koby S, Shotland Y, Ogura T, Oppenheim AB. A colicin-tolerant Escherichia coli mutant that confers hfl phenotype carries two mutations in the region coding for the C-terminal domain of FtsH (HflB) FEMS Microbiol Lett. 2000;183(1):115–117. doi: 10.1111/j.1574-6968.2000.tb08943.x. [DOI] [PubMed] [Google Scholar]
- 32.Mora L, de Zamaroczy M. In vivo processing of DNase colicins E2 and E7 is required for their import into the cytoplasm of target cells. PLoS One. 2014;9(5):e96549. doi: 10.1371/journal.pone.0096549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams N, Fox DK, Shea C, Roseman S. Pel, the protein that permits lambda DNA penetration of Escherichia coli, is encoded by a gene in ptsM and is required for mannose utilization by the phosphotransferase system. Proc Natl Acad Sci USA. 1986;83(23):8934–8938. doi: 10.1073/pnas.83.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cumby N, Reimer K, Mengin-Lecreulx D, Davidson AR, Maxwell KL. The phage tail tape measure protein, an inner membrane protein and a periplasmic chaperone play connected roles in the genome injection process of E. coli phage HK97. Mol Microbiol. 2015;96(3):437–447. doi: 10.1111/mmi.12918. [DOI] [PubMed] [Google Scholar]
- 35.Jamet A, et al. A new family of secreted toxins in pathogenic Neisseria species. PLoS Pathog. 2015;11(1):e1004592. doi: 10.1371/journal.ppat.1004592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koskiniemi S, et al. Rhs proteins from diverse bacteria mediate intercellular competition. Proc Natl Acad Sci USA. 2013;110(17):7032–7037. doi: 10.1073/pnas.1300627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holberger LE, Garza-Sánchez F, Lamoureux J, Low DA, Hayes CS. A novel family of toxin/antitoxin proteins in Bacillus species. FEBS Lett. 2012;586(2):132–136. doi: 10.1016/j.febslet.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang SL, Rubin EJ. Construction of a mariner-based transposon for epitope-tagging and genomic targeting. Gene. 2002;296(1-2):179–185. doi: 10.1016/s0378-1119(02)00856-9. [DOI] [PubMed] [Google Scholar]
- 39.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garza-Sánchez F, Janssen BD, Hayes CS. Prolyl-tRNA(Pro) in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. J Biol Chem. 2006;281(45):34258–34268. doi: 10.1074/jbc.M608052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrières L, et al. Silent mischief: Bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J Bacteriol. 2010;192(24):6418–6427. doi: 10.1128/JB.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akiyama Y, Shirai Y, Ito K. Involvement of FtsH in protein assembly into and through the membrane. II. Dominant mutations affecting FtsH functions. J Biol Chem. 1994;269(7):5225–5229. [PubMed] [Google Scholar]