Significance
The nucleus accumbens (NAc) is a key neural substrate that controls aversive learning through D1 receptor-expressing direct pathway neurons and D2 receptor-expressing indirect pathway neurons. We explored how aversive learning is controlled by intracellular PKA signaling in these two types of neurons in the NAc. We approached this issue not only by pathway-specific inhibition of PKA in either direct or indirect pathway neurons, but also by microendoscopic analysis of FRET responses of the PKA biosensor distinctly expressed in the two types of neurons. We obtained consistent findings from these two different approaches, and demonstrate that activation of PKA in the indirect pathway neurons plays a pivotal role in both the formation and the retention of aversive memory.
Keywords: basal ganglia, in vivo FRET imaging, transmission blockade, cAMP-PKA signal, aversive behavior
Abstract
The nucleus accumbens (NAc) serves as a key neural substrate for aversive learning and consists of two distinct subpopulations of medium-sized spiny neurons (MSNs). The MSNs of the direct pathway (dMSNs) and the indirect pathway (iMSNs) predominantly express dopamine (DA) D1 and D2 receptors, respectively, and are positively and negatively modulated by DA transmitters via Gs- and Gi-coupled cAMP-dependent protein kinase A (PKA) signaling cascades, respectively. In this investigation, we addressed how intracellular PKA signaling is involved in aversive learning in a cell type-specific manner. When the transmission of either dMSNs or iMSNs was unilaterally blocked by pathway-specific expression of transmission-blocking tetanus toxin, infusion of PKA inhibitors into the intact side of the NAc core abolished passive avoidance learning toward an electric shock in the indirect pathway-blocked mice, but not in the direct pathway-blocked mice. We then examined temporal changes in PKA activity in dMSNs and iMSNs in behaving mice by monitoring Förster resonance energy transfer responses of the PKA biosensor with the aid of microendoscopy. PKA activity was increased in iMSNs and decreased in dMSNs in both aversive memory formation and retrieval. Importantly, the increased PKA activity in iMSNs disappeared when aversive memory was prevented by keeping mice in the conditioning apparatus. Furthermore, the increase in PKA activity in iMSNs by aversive stimuli reflected facilitation of aversive memory retention. These results indicate that PKA signaling in iMSNs plays a critical role in both aversive memory formation and retention.
Aversive stimuli induce not only rapid avoidance behavior, but also memory formation to escape from uncomfortable environments, and thus strongly influence animal behavior (1–3). The mesolimbic dopaminergic (DA) system plays a critical role in both rapid aversive reaction and memory formation (3–5). The nucleus accumbens (NAc) receives DA inputs from the ventral tegmental area (VTA) and serves as a key neural substrate for the control of aversive learning (6–8). The NAc consists of two subpopulations of medium-sized spiny neurons (MSNs) (9–11). The MSNs of the direct pathway (dMSNs) send their axons to the substantia nigra pars reticulata (SNr) and VTA, and selectively express dopamine D1 receptors, whereas the MSNs of the indirect pathway (iMSNs) indirectly project to the SNr and VTA via the ventral pallidum (VP) and predominantly express D2 receptors (12, 13). D1 receptors stimulate the cAMP-dependent protein kinase A (PKA) signaling cascade via Gs and exhibit a low affinity for DA (14–16). Conversely, D2 receptors inhibit the cAMP-PKA cascade via Gi and show a high affinity for DA (14–16). Thus, these two distinct types of MSNs, constituting two parallel pathways, contribute to the dynamic modulation of neuronal cell excitability and synaptic plasticity in the NAc circuitry (14–16).
Although accumulated evidence indicates that DA modulation of the NAc is critical for both reward-based and aversive reactions (3, 5, 6, 17), the response of DA neurons in the VTA to aversive stimuli is not uniform; that is, some DA neurons are stimulated in response to aversive stimuli, whereas most others react by transiently suppressing their firing (18–22). Recent optogenetic studies have revealed that not only activation of iMSNs, but also inactivation of the VTA neurons, which down-regulates DA levels in the NAc, evoke an aversive reaction and learning (23–26); however, how intracellular cAMP-PKA signaling is involved in the induction and retention of aversive memory in a cell type-dependent manner in the NAc circuit remains largely elusive.
In the present investigation, we addressed this issue using two approaches. We first used asymmetric reversible neurotransmission blocking (aRNB) techniques (27, 28), in which either the direct or indirect pathway at one side of the NAc was selectively blocked by the pathway-specific expression of transmission-blocking tetanus toxin and the other intact side was manipulated by injection of PKA inhibitors. In the second approach, we examined temporal changes in PKA activities of these two pathways in the formation of aversive memory by monitoring Förster resonance energy transfer (FRET) responses of PKA selective for either dMSNs or iMSNs with the aid of in vivo microendoscopic analysis (29, 30). These two different approaches explicitly demonstrated that the activation of PKA in iMSNs plays a key role in both the formation and the retention of aversive memory.
Results
Aversive Memory Formation by PKA Activation in the Indirect Pathway, but Not in the Direct Pathway.
It has been reported that aversive learning not only is induced by aversive stimuli that decrease DA release in the NAc core, but also is impaired by selective lesioning of this nucleus (7, 31). We thus focused on the PKA signaling cascade in the NAc core in both aRNB experiments and the subsequent microendoscopic analysis. In the NAc circuitry, its function becomes defective only when both sides of the NAc are simultaneously impared in the brain hemispheres (32, 33). We applied the aRNB technique, in which the transmission of either the direct pathway (D-aRNB) or the indirect pathway (I-aRNB) was unilaterally blocked by the pathway-specific expression of transmission-blocking tetanus toxin, which was driven by the interaction of the tetracycline-repressive transcription factor (tTA) with the tetracycline-responsive element (27, 28). The specific expression of tTA in either the direct pathway or the indirect pathway was driven by the substance P or the enkephalin promoter, respectively, with use of the adeno-associated virus (AAV)-mediated expression system (27, 28).
At 2–3 wk after viral injection, the intact side of the NAc core was infused with one of two different PKA inhibitors [PKI 14–22 amide, myristoylated (PKI) or adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-cAMPS)] or an inactive cAMP analog [2′-deoxyadenosine-3′,5′-cyclic monophosphate (2′-dcAMP)] through an implanted cannula (Fig. 1A). This aRNB technique allowed us to address whether the PKA signaling cascade is involved in controlling aversive learning in a pathway-specific manner.
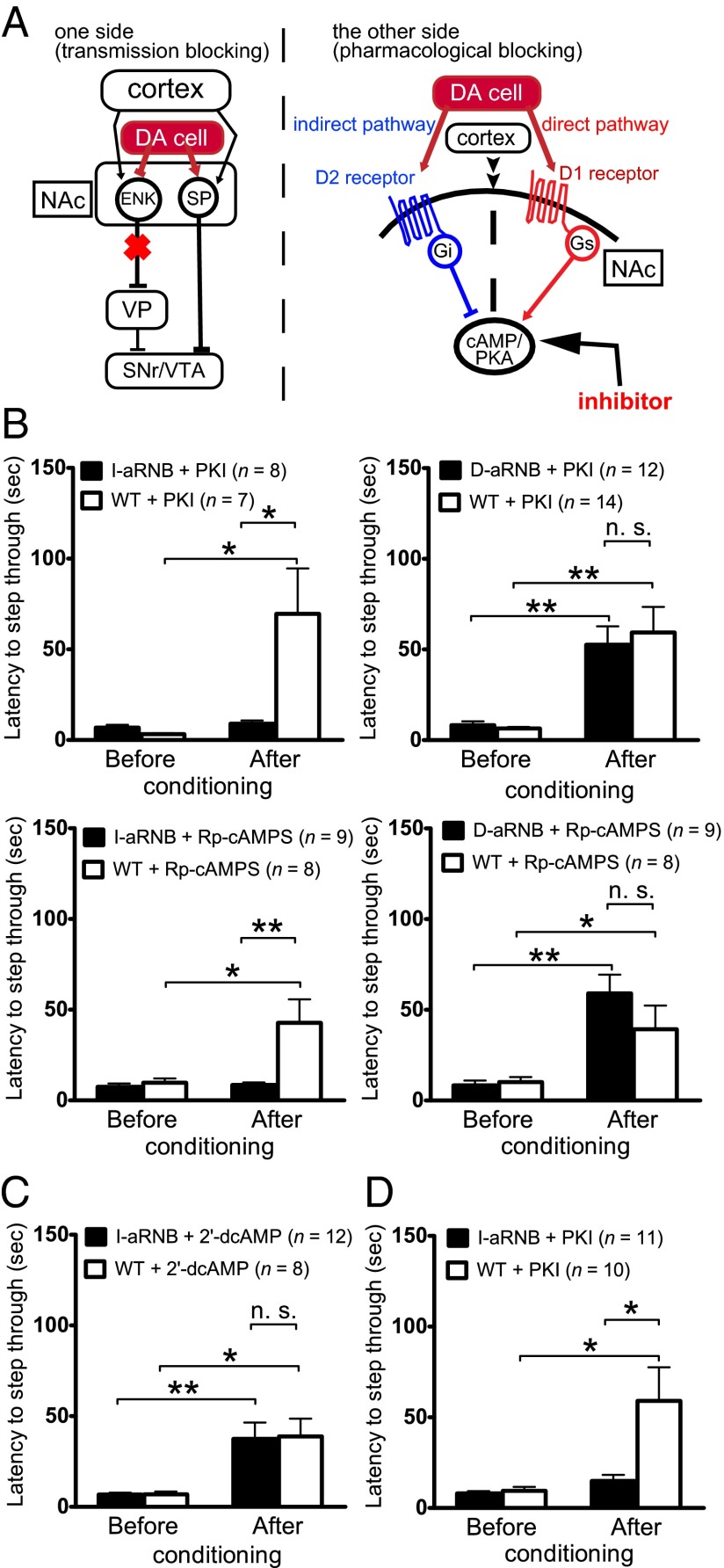
Fig. 1.
Formation of aversive learning by PKA activation in the indirect pathway. (A) Schema of the aRNB technique combined with application of PKA inhibitors. Transmission blockade is indicated by a red cross. The line with the arrowhead and the line with a block denote activation and inhibition of neuronal cells or signaling molecules, respectively. ENK, enkephalin; SP, substance P. (B and C) Either a PKA inhibitor (PKI or Rp-cAMPS) or an inactive analog (2′-dcAMP) was injected into the NAc core. At 20 min later, the mice were subjected to a single electric footshock (0.6 mA, 60 Hz, 2 s) once they had entered the dark chamber. They were then transferred to their home cages within 1 min. Passive avoidance learning was tested 24 h later by measuring time latencies of the mice to enter the dark chamber. (D) Animals were treated as described in B, except that PKI was injected into the NAc core at 1 min after the mice had entered the dark chamber. Columns and bars indicate the mean and SEM, respectively. The numbers of mice analyzed in this and subsequent experiments are indicated in parentheses. Time latencies to enter the dark chamber were compared before and after aversive conditioning (paired t test) and between WT and D-aRNB or I-aRNB mice (two-way ANOVA). *P < 0.05; **P < 0.01; n.s., not significant.
Aversive learning was tested by performing a one-trial inhibitory avoidance task (27, 28). In this test, mice received a single electric footshock (0.6 mA, 60 Hz, 2 s) after entry into the preferred dark chamber from a lighted chamber and were subsequently kept in the home cage for 24 h. Aversive memory formation was evaluated by measuring the time latency to entry of the dark chamber at 24 h after aversive conditioning. In the absence of aversive conditioning, all three groups of animals [wild type (WT), D-aRNB, and I-aRNB] quickly entered the preferred dark chamber, with no statistical difference regardless of the infusion of PKA inhibitors (Fig. 1B). Infusion of PKI or Rp-cAMPS into the NAc core at 20 min before aversive conditioning significantly impaired avoidance learning of the I-aRNB mice, but not of the D-aRNB and WT mice (Fig. 1B). No such impairment was elicited by the infusion of 2′-dcAMP into the WT and I-aRNB mice (Fig. 1C). These results indicate that activation of PKA in the indirect pathway, but not in the direct pathway, is indispensable for the formation of aversive memory.
We also examined whether the PKA inhibition after exposure to aversive stimuli would affect the formation of aversive memory by shifting the time point of PKA inhibitor application from the conditioning period to the transition period of memory formation. Animals were exposed to an electric footshock, then subjected to PKI infusion and kept in their home cages for 24 h. The I-aRNB mice, but not the WT mice, showed significant impairment in aversive learning the next day (Fig. 1D), suggesting that the activation of D2-PKA that occurred after aversive stimuli is necessary for the formation of aversive memory.
In our previous studies (27, 28), we showed that aversive learning in the avoidance test was significantly impaired in bilaterally blocked I-RNB mice and in I-aRNB mice pretreated with D2 receptor agonists that counteracted the D2 receptor inactivation necessary for aversive learning. The injection of the PKA inhibitors into the I-aRNB mice prevented passive avoidance learning, comparable to the learning deficit seen in the I-RNB mice and the D2 receptor agonist-treated I-aRNB mice. These findings led to a consistent concept to explain the DA-PKA signaling mechanism underlying passive avoidance learning. Aversive stimuli reduce DA levels in the NAc and inactivate postsynaptic D2 receptors in iMSNs (14, 17). This inactivation of D2 receptors in turn activates the cAMP-PKA signaling cascade in iMSNs by relieving the inhibitory action of Gi (14, 17). Previous electrophysiological studies found that when the D2 receptors in iMSNs were inhibited, elaborate synaptic modulation via NMDA receptors, A2a adenosine receptors, and endocannabinoid CB1 receptors was critically involved in inducing long-term potentiation in glutamatergic transmission of iMSNs (34, 35). Furthermore, our behavioral studies using aRNB techniques revealed that all of these LTP-inducing key receptors, when pharmacologically manipulated, prevented passive avoidance learning specific for the I-aRNB mice (17, 28). Thus, the PKA signaling cascade via D2 receptors and the sequential mechanistic events mediated by the key receptors play a pivotal role in the induction of aversive learning in an indirect pathway-dependent manner of the NAc circuit.
Temporal Dynamics of PKA Activity in dMSNs and iMSNs in Aversive Learning.
To explore how the cell type-specific PKA signaling cascade is involved in the process of aversive learning, we applied in vivo microendoscopic analysis, in which time-lapse changes in PKA activities were pursued by measuring FRET responses of the PKA biosensor in behaving animals (30). In this methodology, the genetically encoded FRET biosensor of PKA was specifically expressed in either dMSNs or iMSNs by crossing floxed PKA biosensor-expressing transgenic mice with D1-Cre or D2-Cre bacterial artificial chromosome (BAC) transgenic mice, respectively (30, 36). Cell-specific fluorescence changes between the active and inactive forms of PKA in dMSNs (D1-PKA) and iMSNs (D2-PKA) were monitored by microendoscopic analysis of freely moving mice.
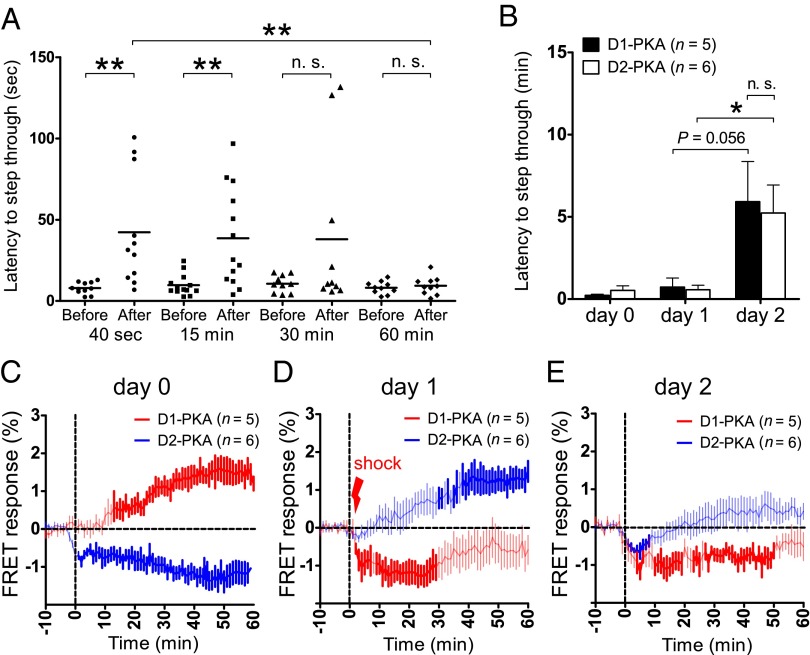
Aversive learning was examined by performing a one-trial inhibitory avoidance test (27). In this test, FRET responses were monitored for at least 60 min after delivery of an electric shock, but we found that aversive memory was significantly reduced when mice were kept in the conditioning dark chamber for longer periods. Thus, we addressed the time dependency of memory retention by keeping the animals in the electrical conditioning dark chamber for different durations and then transferring them into their home cages (Fig. 2A). This analysis showed that aversive memory was retained when the animals were transferred to their home cages immediately after the electric shock, but was significantly reduced or lost when they were kept in the dark chamber for 30–60 min after the shock (Fig. 2A).
Fig. 2.
Temporal changes in PKA activity in dMSNs and iMSNs after aversive learning. (A) Mice were conditioned with a single electric shock (0.6 mA, 60 Hz, 2 s) once they had entered the dark chamber. They were kept in the dark chamber for 40 s, 15 min, 30 min, or 60 min and then transferred to their home cages. Before and at 24 h after conditioning, the time latency of each mouse to enter the dark chamber was measured. Bars indicate the mean of time latencies to step through before and after conditioning. **P < 0.01; n.s., not significant, paired t test (before vs. after) or unpaired t test (40 s after vs. 60 min after). (B) On day 0, no electric shock was applied when the mice moved to the dark chamber in the test apparatus. On day 1, mice received an electric shock (1.6 mA, 50 Hz, 3 s) immediately after their entry into the dark chamber. On day 2, the electric shock was omitted when they entered the dark chamber. At every session, the mice were transferred to their home cages within 1 min after their entry into the dark chamber. Time latencies of unconditioned mice and those of mice before and at 24 h after conditioning with an electric shock on day 1 were measured on day 0 and on days 1 and 2, respectively. Columns and bars indicate the mean and SEM, respectively. *P < 0.05; n.s., not significant, paired t test (day 1 vs. day 2) or unpaired t test (D1-PKA vs. D2-PKA on day 2). (C–E) Temporal changes in FRET responses of D1-PKA and D2-PKA were monitored at each session of the aversive learning test. The vertical dotted line indicates the time of door opening. The vertical lines in traces show SEM, and darker colors denote statistically significant changes in PKA activity from the basal activity (P < 0.05, paired t test).
Consequently, we imposed the following protocol to examine time-lapse changes in PKA activity during the induction of aversive memory. On day 0, the animals did not receive any electric shock in the test apparatus as the control condition. On day 1, when the mice had entered the dark chamber from a lighted chamber, they immediately received an electric footshock and were then returned to their home cages within 1 min after the shock. The electric stimulus was strengthened (1.6 mA, 50 Hz, 3 s) to enhance aversive memory formation. On day 2, avoidance learning was tested without the electric shock. Simultaneously, temporal changes in FRET responses of D1-PKA and D2-PKA were continuously monitored in animals that stayed in the test apparatus and subsequently in their home cages.
We found no differences in the ability of the D1-PKA and D2-PKA mice to prefer the dark chamber and to avoid the electrical conditioning chamber in the avoidance test (Fig. 2B); however, D1-PKA and D2-PKA showed opposite changes in activity when the mice were handled and placed in the test apparatus on day 0 (Fig. 2C). D1-PKA and D2-PKA slowly and continuously increased and decreased, respectively, during the stay of these animals in their home cages. Because the similar change in activity of D2-PKA was observed in animals that remained in the test apparatus (see Fig. 4B), the changes in D1-PKA and D2-PKA on day 0 might have resulted from handling of the mice to initiate the avoidance test; this finding was not further explored in this investigation, however. Importantly, changes in the activity of D1-PKA and D2-PKA after the electric shock on day 1 were in marked contrast to those observed without the electric shock on day 0 (Fig. 2D). The electric shock caused a progressive increase in D2-PKA activity but resulted in a rapid decrease in D1-PKA activity, which remained low thereafter (Fig. 2D). A similar temporal change in the activity of both D1-PKA and D2-PKA was observed on day 2, when the conditioned mice encountered the aversive context of the test apparatus (Fig. 2E). Thus, the synergistic and reciprocal changes in PKA activity in dMSNs and iMSNs were evoked not only in the induction, but also in the retrieval, of aversive memory.
Fig. 4.
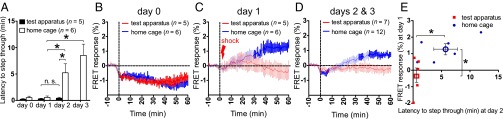
Activation of D2-PKA by an electrical stimulus and its relationship with memory retention. (A) Animals were conditioned with an electric shock by two different procedures. In the test apparatus group, each mouse was kept in the conditioning dark chamber for 60 min after an electric shock and then transferred to its home cage. In the home cage group, a mouse was transferred to its home cage within 1 min after the electric conditioning. Memory retention of each group was examined by measuring the time latencies to step through into the dark chamber on days 2 and 3. Columns and bars indicate the mean and SEM, respectively. *P < 0.05; n.s., not significant, paired t test (comparison between days) or unpaired t test (test apparatus vs. home cage). (B–D) Temporal changes in FRET responses of D2-PKA were monitored for two groups of animals. Data for day 0, day 1, and combined days 2 and 3 are presented in B, C, and D, respectively. The vertical lines in traces indicate SEM, and darker colors indicate statistically significant changes (P < 0.05, paired t test). (E) FRET responses of D2-PKA at the late stage (40–60 min) of day 1 were plotted against latencies to enter the dark chamber on day 2. Filled circles and rectangles represent the mean of FRET responses of D2-PKA at the late stage (40–60 min) of day 1 in the animals in the home cage and test apparatus, respectively. The open circle and open rectangle represent the mean of the two groups of animals, respectively. The bars indicate ± SEM. *P < 0.05, unpaired t test.
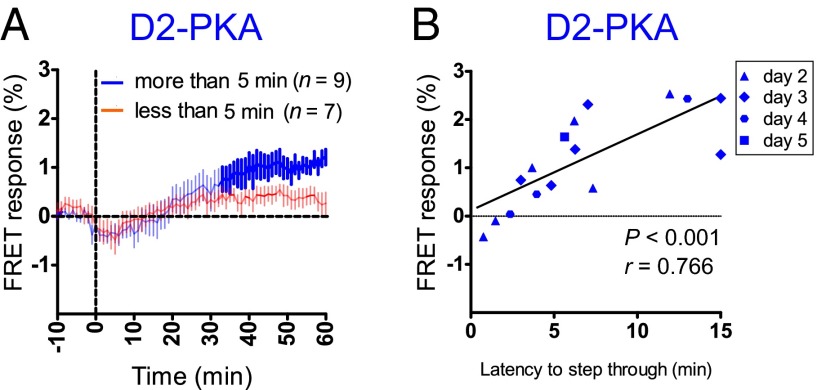
Mice retained the ability to avoid the conditioned chamber for at least 5 d once they had experienced a single electric shock on day 1. Thus, we extended our analysis of time-lapse changes in the D2-PKA activity of the conditioned mice to days 2–5. We divided the tested mice into two groups, those entering the conditioning chamber in less than 5 min and those entering it more than 5 min after initiation of the avoidance test, and assessed time-lapse changes in the D2-PKA activity of each group of these conditioned animals (Fig. 3A). This analysis revealed significantly higher D2-PKA activity in the highly responding mice than in the poorly responding mice at the late stage (40–60 min) of the avoidance test.
Fig. 3.
Positive correlation between D2-PKA activation and avoidance learning. (A) Conditioned D2-PKA mice were divided into two groups, one entering the dark chamber within 5 min and the other entering after a lapse of 5 min on days 2–5 after conditioning with an electric shock on day 1. FRET responses of D2-PKA were monitored and averaged for each group of conditioned mice. (B) FRET responses of D2-PKA were plotted against latencies of the corresponding animals to enter the dark chamber on days 2–5 after conditioning with an electric shock on day 1. A significant positive correlation was noted between memory retention and peak amplitudes of FRET responses of conditioned D2-PKA mice on each day. r values were calculated by Pearson correlation analysis.
To further assess the relationship between the D2-PKA activity and the avoidance learning ability of the D2-PKA mice, we plotted the D2-PKA activity on days 2–5 against time latencies to avoid the conditioning chamber at the corresponding days (Fig. 3B). There was a significant positive correlation between the activation of D2-PKA and the strength of memory-based avoidance ability (Fig. 3B). These results indicate that elevated PKA activity in iMSNs is critical to the capability of the conditioned mice to avoid an uncomfortable place.
Role of Stimulus-Induced D2-PKA Activation in Retention of Aversive Memory.
Because the ability of conditioned mice to form aversive memory depended on whether or not they were forced to stay in the contextual environment without further conditioning (test apparatus or home cage), we addressed whether the activity changes in D2-PKA also may have been influenced by these different environmental conditions after aversive stimuli. When the D2-PKA mice did not receive an electric shock in the test apparatus on day 0, the D2-PKA activity decreased comparably under the two environmental conditions (Fig. 4B). When the D2-PKA mice received an electric shock, the D2-PKA activity tended to increase initially in both groups of mice (Fig. 4C). Interestingly, D2-PKA activity increased continuously under the condition of the home cage, but gradually decreased to basal levels under the condition of the test apparatus. On days 2 and 3, when the electric shock was omitted, significant avoidance memory was retained when the conditioned mice were returned to their home cages after the avoidance test, but was lost when they were kept in the test apparatus for 60 min (Fig. 4A). Notably, D2-PKA activity gradually increased under the home cage condition, but demonstrated no obvious change under the condition of the test apparatus (Fig. 4D).
Importantly, plotting the elevated levels of D2-PKA activity after the electric shock on day 1 against time latencies to avoid the conditioned chamber on day 2 revealed a significant relationship between the activation of D2-PKA on day 1 and the aversion-avoiding ability on day 2 (Fig. 4E). This indicates that the activation of D2-PKA is also involved in the retention of acquired aversive memory.
Discussion
In this study, we explored the DA-mediated intracellular signaling mechanism underlying aversive learning by combining the pathway-specific blockade of NAc transmission and pharmacologic analysis. The inhibition of PKA in the indirect pathway, but not in the direct pathway, abolished passive avoidance memory, demonstrating that activation of PKA in the indirect pathway is indispensable for the formation of aversive memory. This conclusion is strengthened by our microendoscopic analysis, in which the pathway-specific changes in PKA activity were examined by monitoring FRET responses of the PKA biosensor in the process of aversive memory formation and retention in behaving mice. The D2-PKA activity gradually increased in response to an aversive electric shock, and this increase was reflected in long-lasting memory retention. Furthermore, the activated D2-PKA returned to basal levels of activity, in accordance with the loss of aversive memory, when the conditioned mice were kept stimulus-free in the stimulus-preexposed environment. These results demonstrate that the PKA signaling cascade in iMSNs plays a critical role in aversive memory formation and learning.
In the NAc circuit, the high-affinity D2 receptors are expressed predominantly in iMSNs and negatively control cAMP-PKA signaling through coupling to inhibitory Gi (10, 14, 15). Recently reported evidence indicates that the inactivation of D2 receptors in iMSNs is necessary for the induction of aversive memory (23, 25, 28). In previous studies using the aRNB technique, D2 agonists impaired passive avoidance learning against electric shock, and this impairment was caused selectively not only by administration of D2 agonists among the DA receptor agonists and antagonists analyzed, but also specifically by transmission blockade of the indirect pathway in the NAc circuitry (27, 28). In optogenetic studies, the inactivation of DA neurons in the VTA promptly lowered DA levels in the NAc and immediately evoked an avoidance response to entering the preferred dark room (25). Furthermore, the suppression of DA neurons in the VTA promoted aversive learning toward the optogenetically conditioned place in a D2 receptor-dependent manner (24, 25). The foregoing findings support the conclusion that the inactivation of D2 receptors in iMSNs stimulate the cAMP-PKA signaling that is critical for memory formation and retention.
Administration of PKA inhibitors to the D-aRNB mice had no appreciable impairing effects on aversive learning. Interestingly, however, our microendoscopic analysis revealed that the PKA activity of dMSNs was not only suppressed, but also lowered, by aversive stimuli when the D1-PKA mice encountered the electrically conditioned environment. Although, unlike in the case of D2-PKA, no significant correlation was noted between reduced D1-PKA activity and the level of aversive memory retention, the suppression of D1-PKA activity also might have contributed to stimulus-dependent and effective induction of aversive learning. DA neurons in the VTA exhibit phasic firings and stimulate D1 receptors in dMSNs during positively acting behaviors, such as motivational and reward-seeking behaviors (3, 10, 17). This direction of activation of D1-PKA may frequently occur during animal behaviors and could disturb the suppressive state required for the aversive reaction and memory formation. Thus, although the PKA signaling cascade in the direct pathway is not involved in the triggering of aversive memory formation, the rapid and continuous suppression of this pathway could be important in stabilizing suppressive DA modulation in the NAc for animals to accurately and effectively respond to aversive environments. Also noteworthy is the fact that the passive avoidance learning tested in this investigation represents the innate animal behavior to quickly avoid uncomfortable environments. In aversive behavior, active aversive learning is also induced by repeated exposure to aversive stimuli, and this learning is more complicated by the involvement of other mental activities, such as motivational and intentional activities, for animals to more positively avoid uncomfortable environments (8, 37). Thus, it would be interesting to explore how active aversive learning is controlled in a cell type-specific and DA-dependent manner. Also of interest is the fact that D2 receptor antagonists are widely used as effective therapeutic drugs for certain psychiatric disorders, such as schizophrenia (38). Thus, further investigation of PKA and its downstream signaling in D2 receptor-expressing iMSNs would be useful in better understanding the mechanisms underlying psychiatric disorders.
Materials and Methods
Animals.
Male mice aged ∼2–3 mo were used in all experiments. The D-aRNB and I-aRNB mice were generated by unilateral injection of the recombinant D- and I-AAV viruses, respectively, into four sites at one side of the NAc core by a stereotaxic technique as described previously (27, 28). Virus-injected WT littermates served as controls. Two lines of D1-Cre BAC transgenic mice (EY262 and FK150) and 1 line of D2-Cre BAC mice (ER44) were obtained from Mutant Mouse Regional Resource Centers. The FRET biosensor of PKA, AKAR3EV, was generated (29), and the Cre-dependent expression of the FRET biosensor of PKA in dMSNs and iMSNs was achieved by crossing the floxed-stop AKAR3EV reporter mice with the D1-Cre or D2-Cre transgenic mice (30). The pathway-specific expression of the FRET biosensor of PKA was verified by visualizing FRET fluorescence in the corresponding pathway (30). All mice were maintained by breeding with C57BL/6J founder mice. All animal handling procedures were approved by the Animal Research Committee of Osaka Bioscience Institute.
Drug Infusion in aRNB Mice.
For drug infusion, the intact side of the NAc core of D-aRNB and I-aRNB mice was implanted with a cannula aimed toward the NAc, as described previously (28). Then 100 nM Rp-cAMPS, 100 nM 2′-dcAMP (both from Biolog), and 0.82 μM PKI (Tocris) were delivered in a volume of 1 μL into the intact side of the NAc core. After behavioral analysis, the drug injection sites were confirmed by direct visualization of a series of slice sections of the NAc region. When conditioned WT and aRNB mice entered the dark chamber more than 200 s after the door had been opened or when the injection site of PKA inhibitor was outside of the NAc core, the data were discarded (∼10% of the animals analyzed).
Microendoscopic Analysis.
A microendoscope was fabricated, and microendoscopic analysis to monitor FRET responses of PKA in dMSNs and iMSNs was performed as described previously (30). For in vivo imaging, the scalp was opened, and a hole was drilled in the skull (1.2 mm anterioposterior and 1.1 mm mediolateral from bregma). Two skull screws (M1.0; 4 mm long) were first implanted as anchors, after which the endoscope with its μ-drive (30) was implanted into the NAc core (3.5 mm below the surface of the brain) and cemented in place with dental acrylic. Animals were allowed to recover for at least 4 d before optical recordings were made.
The time-lapse FRET recordings were made by monitoring 20–25 frames of raw endoscope images at 15 frames/s. Data were stored as a noncompressed AVI file every 1 min, offline-averaged, smoothed, and FRET-calculated using ImageJ. The mice connected to the microendoscope were allowed to move around the entire test apparatus and home cage. Once the endoscope placement was fixed at the session on day 0, the endoscope was not moved from its fixed position.
In the inhibitory avoidance test, care was taken to ensure that the mouse had calmed down sufficiently in the home cage and that the trace of FRET responses had become stable. For quantitative analysis of FRET responses, the fluorescence intensities of CFP and FRET were measured in the entire field of a view that corresponded to the diameter of a microendoscopic fiber bundle (300 μm), and averaged values of both CFP and FRET were calculated without subtraction of their background fluorescence (30). The effect of bleaching of fluorophores was evaluated as negligible by time-lapse imaging without stimulation (30). The cell viability and the location of a microendoscopic tip in the NAc core was confirmed by DAPI staining and GFP immunostaining of serial sections of NAc after microendoscopic analysis (30) (Fig. S1).
Fig. S1.
Histological verification of fiber placement. The location of the microendoscopic tip in the NAc core (enclosed by a dashed line) was confirmed by DAPI and indicated by blue and red dots. Numbers on the right denote anteroposterior coordinates rostral to bregma.
Avoidance Test of aRNB Mice with Application of PKA Inhibitors.
The behavioral apparatus used in the experiments with the aRNB mice was composed of a lighted chamber (10 × 13 × 13 cm) and a dark chamber (18 × 13 × 13 cm). The lighted chamber, which was illuminated by a lamp, had a plastic floor, clear walls, and an entrance (5 cm wide) to the dark chamber. The dark chamber had a metal grid floor for electric shock, walls, and a roof, which were all black in color.
At 20 min after drug infusion, the animals were subjected to an electric shock (0.6 mA, 60 Hz, 2 s), when they had stepped with all four paws into the dark chamber. At 1 min after the shock, the animals were returned to their home cages, except when subjected to experiments to determine the time dependency of memory formation, in which case they were kept in the conditioning dark chamber for 40 s, 15 min, 30 min, or 60 min and then returned to their home cages. In some experiments, drug infusion was done within 1 min after delivery of the electric shock. Memory retention was tested 24 h later by measuring the latency to enter the dark chamber.
Avoidance Test with Microendoscopic Recording.
The test apparatus used in this experiment was composed of a dark chamber (20 × 17 × 21 cm) and a lighted chamber (10 × 17 × 21 cm). The lighted chamber had a plastic floor and white walls without a roof and was illuminated by a lamp. The dark chamber had a metal grid floor, a roof, and walls, all of which were colored black. The lighted chamber was linked to the dark chamber through a sliding door.
The avoidance test consisted of three sessions. On day 0, the mouse was placed in a lighted chamber, and the door leading to the dark chamber was then opened. Once the mouse had stepped with all four paws into the dark chamber, the door was closed. The mouse was then returned to its home cage within 1 min after it had entered the dark chamber (home cage condition) or kept in the dark chamber for 60 min (test apparatus condition). On day 1, the mouse was placed in the lighted chamber of the test apparatus. Once the mouse had entered the dark chamber, the door was closed, and an electric footshock was delivered (1.6 mA, 50 Hz, 3 s). The mouse was returned to its home cage 1 min after receiving the footshock in the dark chamber (home cage condition) or kept in the dark chamber for 60 min (test apparatus condition). On days 2–5, memory retention was tested every 24 h by repeating the foregoing procedures without electric shock and measuring the time latencies for the mice to step into the dark chamber in which they had received an electric shock on day 1. When a mouse did not enter the dark chamber within 15 min, it was returned to its home cage.
Statistical Analysis.
Statistical analyses, including paired and unpaired t tests, two-way ANOVA, and Pearson correlation analysis, were conducted using GraphPad Prism 5.0 and are described in the figure legends.
Acknowledgments
This work was supported by Research Grants-in-Aid 2222005 (to S.N.), 24111552 (to K.F.), 22300136 (to K.F.), 26560470 (to K.F.), 23120011 (to T.H., S.Y., and S.N.) and 26830022 (to T.Y.) from the Ministry of Education Culture, Sports, Science and Technology of Japan; by the Takeda Science Foundation (S.N.); and by the Uehara Memorial Foundation (K.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514731112/-/DCSupplemental.
References
- 1.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 3.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11(12):1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badrinarayan A, et al. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32(45):15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci. 2012;32(42):14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 10.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 12.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90(4):385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23(4):546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95(1):1–13. doi: 10.1016/j.pneurobio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi S, Hikida T, Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience. 2014;282C:49–59. doi: 10.1016/j.neuroscience.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 19.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 20.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482(7383):85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan KR, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73(6):1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2014;111(17):6455–6460. doi: 10.1073/pnas.1404323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilango A, et al. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34(3):817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Hikida T, et al. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci USA. 2013;110(1):342–347. doi: 10.1073/pnas.1220358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komatsu N, et al. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22(23):4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto A, et al. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc Natl Acad Sci USA. 2015;112(21):6718–6723. doi: 10.1073/pnas.1507121112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 33.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17(7):1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 34.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13(8):958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27(37):9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilango A, Shumake J, Wetzel W, Scheich H, Ohl FW. The role of dopamine in the context of aversive stimuli with particular reference to acoustically signaled avoidance learning. Front Neurosci. 2012;6:132. doi: 10.3389/fnins.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses. 2010;4(1):56–73. [PubMed] [Google Scholar]