Significance
There is a growing understanding that inflammation impairs synaptic plasticity and cognition and that the aged brain has an elevated sensitivity to cognitive impairment by the proinflammatory cytokine interleukin 1β (IL-1β). IL-1β activates different pathways via AcP (proinflammatory) or AcPb (prosurvival) IL-1 receptor subunits. This study demonstrates that the IL-1 receptor subunit system undergoes an age-dependent reconfiguration in hippocampal synapses. This previously undescribed reconfiguration, characterized by an increase in the AcP/AcPb ratio, is responsible for potentiating impairments of synaptic plasticity and memory by IL-1β. Our data reveal a previously unidentified mechanism that explains the age-related vulnerability of hippocampal function to impairment by inflammation and adds another dimension beyond glia to understanding how inflammation causes cognitive decline in aging.
Keywords: AcP, AcPb, neuroinflammation, receptor sensitivity, LTP
Abstract
In the aged brain, synaptic plasticity and memory show increased vulnerability to impairment by the inflammatory cytokine interleukin 1β (IL-1β). In this study, we evaluated the possibility that synapses may directly undergo maladaptive changes with age that augment sensitivity to IL-1β impairment. In hippocampal neuronal cultures, IL-1β increased the expression of the IL-1 receptor type 1 and the accessory coreceptor AcP (proinflammatory), but not of the AcPb (prosurvival) subunit, a reconfiguration that potentiates the responsiveness of neurons to IL-1β. To evaluate whether synapses develop a similar heightened sensitivity to IL-1β with age, we used an assay to track long-term potentiation (LTP) in synaptosomes. We found that IL-1β impairs LTP directly at the synapse and that sensitivity to IL-1β is augmented in aged hippocampal synapses. The increased synaptic sensitivity to IL-1β was due to IL-1 receptor subunit reconfiguration, characterized by a shift in the AcP/AcPb ratio, paralleling our culture data. We suggest that the age-related increase in brain IL-1β levels drives a shift in IL-1 receptor configuration, thus heightening the sensitivity to IL-1β. Accordingly, selective blocking of AcP-dependent signaling with Toll–IL-1 receptor domain peptidomimetics prevented IL-1β–mediated LTP suppression and blocked the memory impairment induced in aged mice by peripheral immune challenge (bacterial lipopolysaccharide). Overall, this study demonstrates that increased AcP signaling, specifically at the synapse, underlies the augmented vulnerability to cognitive impairment by IL-1β that occurs with age.
Interleukin 1β (IL-1β) is a key proinflammatory cytokine associated with age-related cognitive decline (1–3). A growing body of evidence indicates that synaptic plasticity (4), learning, and memory (5) are more vulnerable to impairment by IL-1β with age. After systemic immune activation (e.g., Escherichia coli or trauma), aged, but not young, rodents show deficits selective for hippocampal-dependent memory (6–11), long-term potentiation (LTP) (12, 13), and brain-derived neurotrophic factor (BDNF) signaling (14), all of which are blocked by brain infusion of the IL-1 receptor antagonist (IL-1ra) (6, 7, 12, 14). Although investigation of underlying mechanisms has largely focused on inflammatory responses of glia (15), it is possible that synapses themselves may also undergo maladaptive changes with age that augment vulnerability to inflammation. We explored the hypothesis that the suppression of BDNF signaling, LTP, and memory may be driven by an increased sensitivity to IL-1β that occurs directly at synapses.
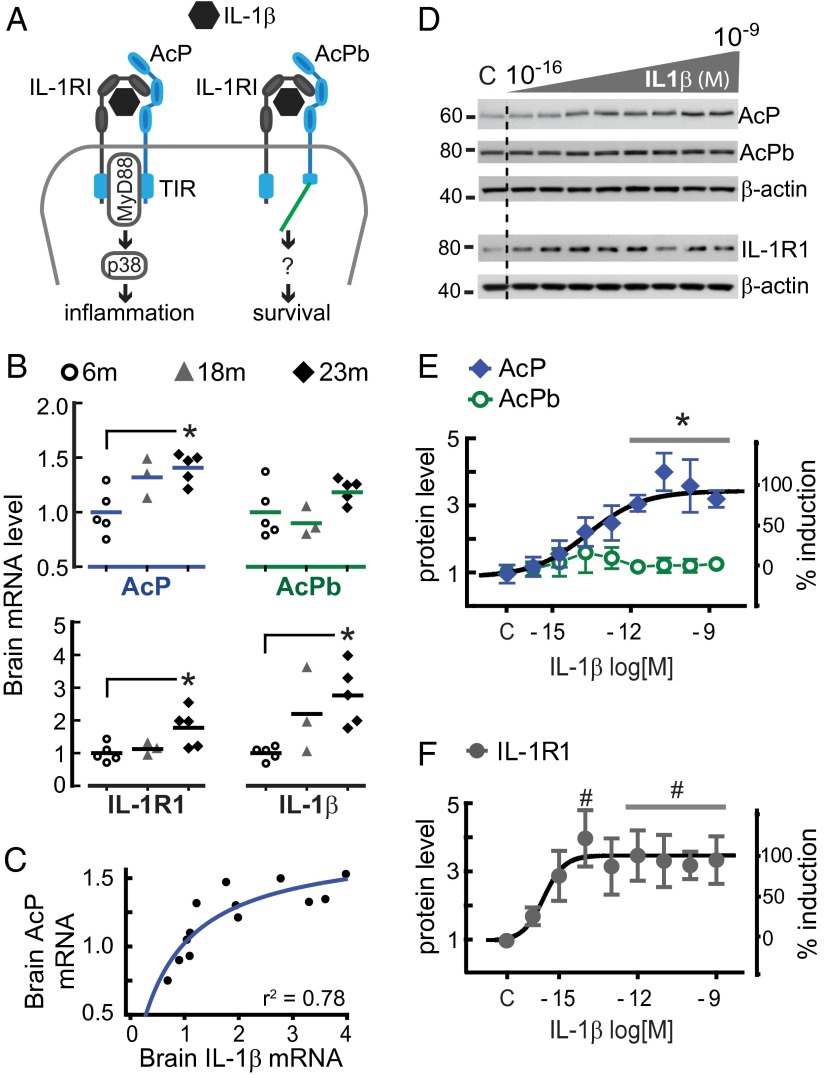
Canonical IL-1β signaling promotes inflammation through a heterodimeric receptor comprising the ligand-binding subunit, IL-1 receptor type 1 (IL-1R1), and the accessory protein subunit (AcP), which functions as an essential coreceptor (16). IL-1β binding to IL-1R1 is followed by AcP recruitment (17), and the ensuing juxtaposition of the Toll/IL-1 receptor (TIR) domains of IL-1R1 and AcP engages the adapter protein MyD88 (18), a fundamental step for activation of downstream effectors of inflammation (e.g., stress kinase p38) (19). In the CNS, however, IL-1β signaling can be additionally mediated by a second accessory protein, AcPb—an alternative splice variant of AcP (20) that is expressed only in neurons (21, 22). Although AcP and AcPb have identical extracellular segments, the intracellular C-terminal tail is extended in AcPb, potentially affecting the TIR domain structure (21). Indeed, although both AcP and AcPb physically interact with IL-1R1 in response to IL-1β, only AcP recruits MyD88, such that AcPb is unable to activate canonical downstream effectors of IL-1β proinflammatory signaling (20, 21). As would be predicted, CNS induction of inflammatory cytokines in response to bacterial lipopolysaccharide (LPS) is absent in AcP-deficient mice, but intact in AcPb-deficient mice (21), demonstrating that only AcP activates the proinflammatory response. Consistent with the emerging data that AcP and AcPb serve different functions, studies using AcP- and AcPb-knockout mice demonstrate that AcP mediates inflammation and neuronal damage in a model of multiple sclerosis, whereas AcPb serves a protective role, promoting neuronal survival after the induction of acute inflammation (21) and excitotoxicity (23) (Fig. 1A).
Fig. 1.
Aging and IL-1β reconfigure the IL-1 receptor system. (A) Neuronal IL-1 receptor system. Note that AcPb has a long C-terminal region potentially blocking MyD88 recruitment (see text). (B) Whole-brain mRNA expression of AcP, AcPb, IL-1R1, and IL-1β in mice at 6 (n = 5), 18 (n = 3), and 23 (n = 5) mo old, determined by qPCR. Levels were normalized with values from 6-mo-old mice and are presented in dot plots (mean indicated by a bar). GAPDH level was used as internal control. *P < 0.05 (Kruskal–Wallis, Dunn’s post hoc test). (C) Correlation between AcP and IL-1β relative expression in the whole set of brain samples (r2 = 0.78; n = 13). (D) Western blot analysis of AcP, AcPb, and IL-1R1 in primary rat hippocampal neurons (5–7 DIV) treated with IL-1β (3 h, 0.3 fM to 3 nM, in 10-fold increments). β-actin was used as loading control. (E and F) AcP, AcPb (E; n = 5), and IL-1R1 (F; n = 4) densitometry values were normalized to vehicle-treated cells (c, control); results are from five independent experiments (neurons from different embryo litters). AcP vs. AcPb, P < 0.0001 (two-way ANOVA main effect of receptor, F1,54 = 46.1); *P < 0.01 (Bonferroni post hoc test). IL-1R1 vs. AcPb, P < 0.0001 (two-way ANOVA main effect of receptor, F1,51 = 46.8); #P < 0.05 (Bonferroni post hoc test). Concentration–response relationships were fitted to the Hill equation (black traces), and the highest induction of AcP (E) and IL-1R1 (F) was set as the Emax (maximal effect, 100%). Data are presented as mean ± SEM.
Here, we demonstrate that the balance between AcP and AcPb levels in hippocampal neurons is a fundamental determinant of the downstream consequences of IL-1β exposure. Using an assay that we developed to track LTP directly in synaptosomes, we demonstrate that IL-1β impairs LTP directly at the synapse. Synaptic sensitivity to IL-1β is augmented in the aged hippocampus and is due to an increased AcP relative to AcPb expression, which biases signaling toward AcP-dependent (proinflammatory) signaling. We extend our findings in vivo to specifically investigate the role of AcP-dependent signaling in the memory impairments induced by peripheral immune challenge in aged mice.
Results
IL-1β Induces AcP, but Not AcPb, Expression in Hippocampal Neurons.
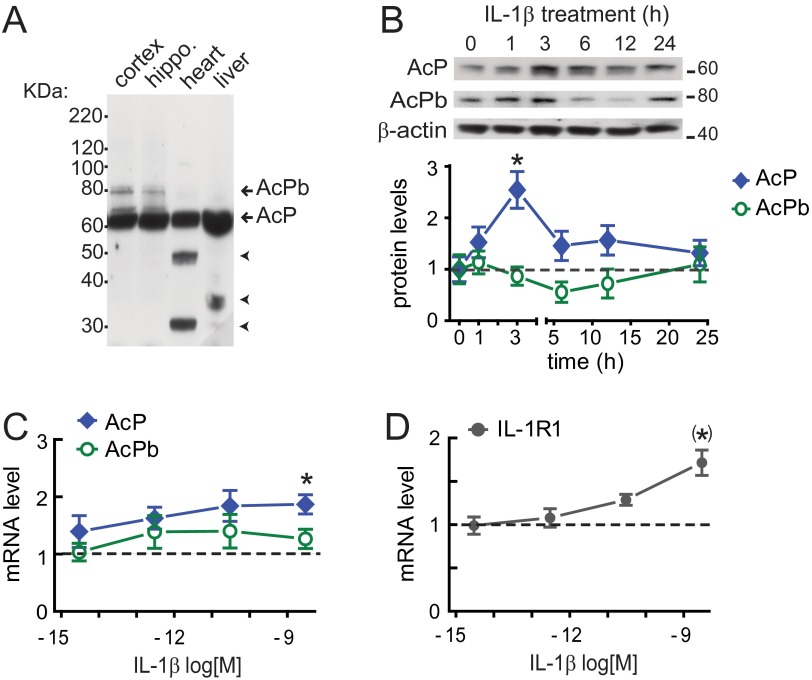
We first explored whether chronic IL-1β signaling in the aged brain was associated with altered expression of IL-1 receptor subunits, because it is known that IL-1β amplifies its own inflammatory responses by inducing gene expression of IL-1 system components (19), including IL-1β (24) and IL-1R1 (25, 26). Analysis of whole brain homogenates from 6-, 18-, and 23-mo-old mice revealed an age-dependent up-regulation of AcP, IL-1R1, and IL-1β mRNA, but not AcPb mRNA (Fig. 1B). The rise in IL-1β gene expression across age positively correlated with AcP (r2 = 0.78; n = 13; Fig. 1C), but not with AcPb mRNA levels (r2 = 0.29), suggesting that age-related elevations of IL-1β selectively increase AcP, but not AcPb. To directly test whether IL-1β modulates neuronal expression of IL-1 receptor components, we evaluated AcP, AcPb, and IL-1R1 protein levels after IL-1β treatment in cultured rat hippocampal neurons, an experimental system devoid of nonneuronal brain cells, notably microglia and astrocytes, which are responsive to IL-1β. Cultured hippocampal neurons (5–7 d in vitro; DIV) were treated with 3 nM (50 ng/mL) IL-1β and harvested at different time points (0, 1, 3, 6, 12, and 24 h). AcP (∼62 kDa) and AcPb (∼80 kDa) were identified by Western blot by their molecular masses (Fig. S1). No significant change in AcPb protein expression was found at any time point after IL-1β treatment. However, after 3 h of IL-1β treatment, there was a significant increase in AcP (P < 0.05; Fig. S1). Additional experiments demonstrated that IL-1β treatment (3 h) across a range of concentrations (0.3 fM to 3 nM) increased AcP protein expression in a concentration-dependent manner (IC50: 17 fM; r2 = 0.97), whereas AcPb levels were not significantly changed at any concentration tested (Fig. 1 D and E). In parallel with the effect on AcP levels, IL-1β treatment (3 h) increased IL-1R1 protein levels in a dose-dependent manner (IC50: 0.26 fM; r2 = 0.94; Fig. 1 D and F). Overall, these data indicate that IL-1β reconfigures the IL-1 receptor system by increasing IL-1R1 and AcP (but not AcPb) protein levels in hippocampal neurons, a series of findings confirmed by mRNA quantification using quantitative RT-PCR (RT-qPCR) (Fig. S1). These results suggest that IL-1β exposure may bias IL-1β signaling toward IL-1R1–AcP proinflammatory responses.
Fig. S1.
AcP induction by IL-1β is time- and concentration-dependent. (A) CNS-restricted expression of AcPb. AcP (∼62 KDa) and AcPb (∼80 KDa) detection by Western blot (full-length blot) is shown. Unidentified proteins (arrowheads) were detected in heart and liver. (B) AcP and AcPb by Western blotting in cultured hippocampal neurons (5–7 DIV) treated with 3 nM IL-1β for the indicated times; β-actin served as loading control. AcP and AcPb densitometry values were quantified at each time point and normalized to vehicle-treated cells (time = 0). *P < 0.05 [AcP vs. control; ANOVA, Tukey’s post hoc test; n = 6 per group; six independent experiments (neurons from different embryo litters)]. (C and D) mRNA levels in neurons treated with IL-1β (3 fM to 3 nM; 100-fold increase) for 3 h; shown are AcP (C; n = 7), AcPb (C; n = 5), and IL-1R1 (D; n = 7), with results from seven independent experiments. Values were normalized to vehicle-treated cells (dotted line), and GAPDH was used as internal control. *P < 0.05 (AcP vs. control); (*)P < 0.01 (IL-1R1 vs. control) (Kruskal–Wallis, Dunn post hoc test). Data are presented as mean ± SEM.
Increased AcP/AcPb Ratio Sensitizes the IL-1β Response in Hippocampal Neurons.
We next hypothesized that IL-1R1 and AcP up-regulation by IL-1β may sensitize hippocampal neuronal responses to subsequent IL-1β challenge. We tested this idea using BDNF signaling as an endpoint, based on our previous work demonstrating that IL-1β suppresses BDNF signaling in hippocampal neuronal cultures (27, 28).
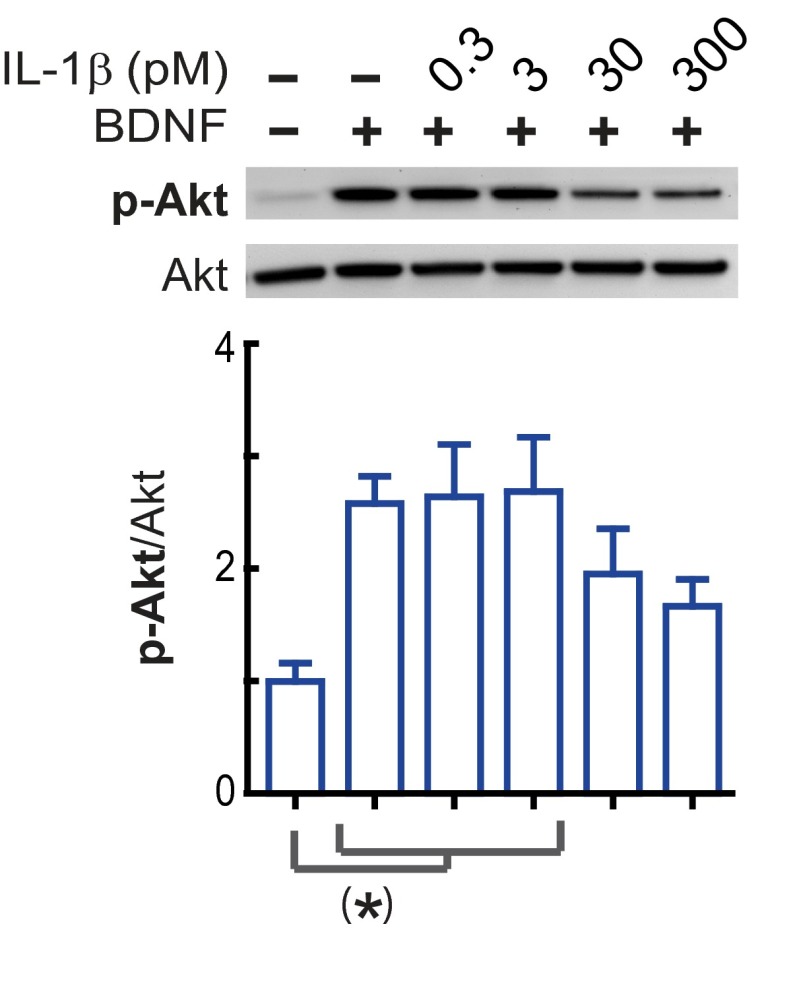
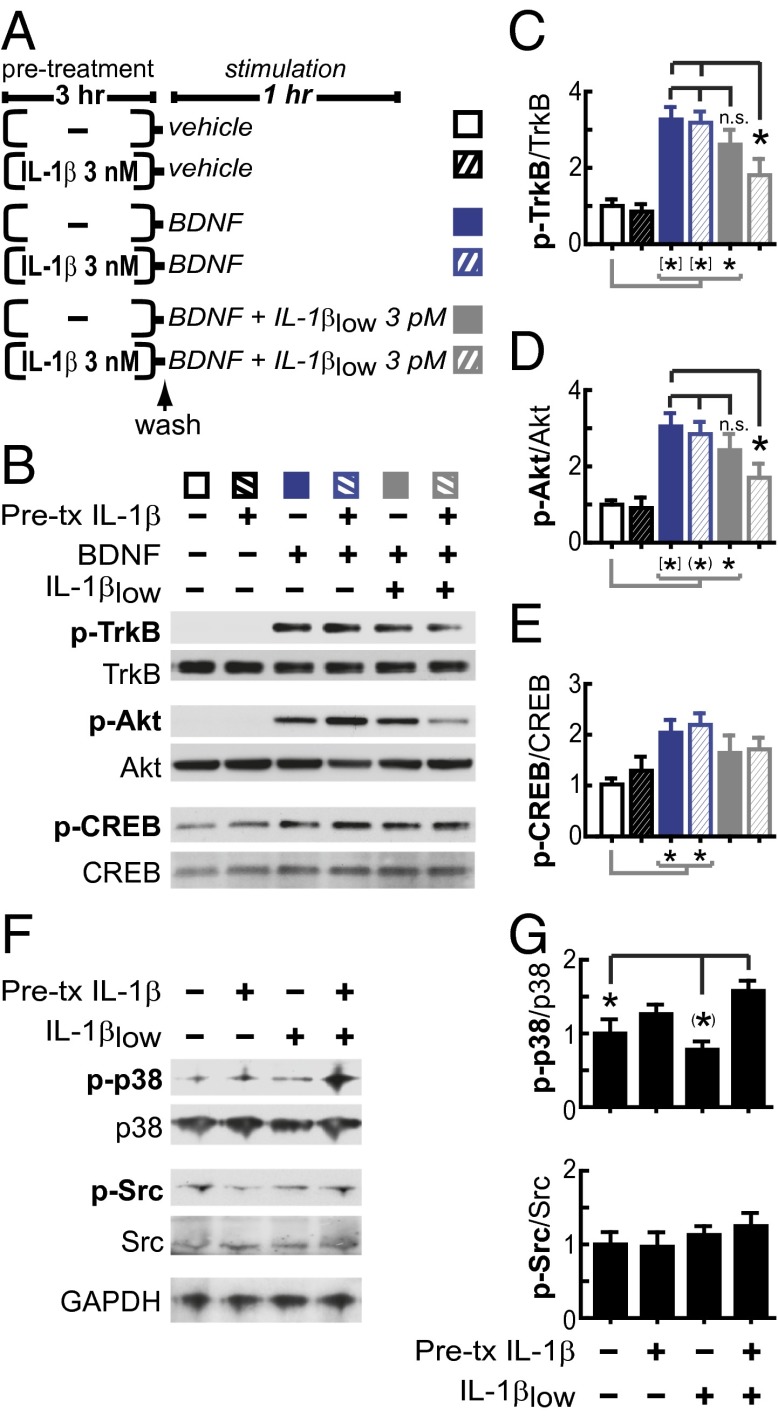
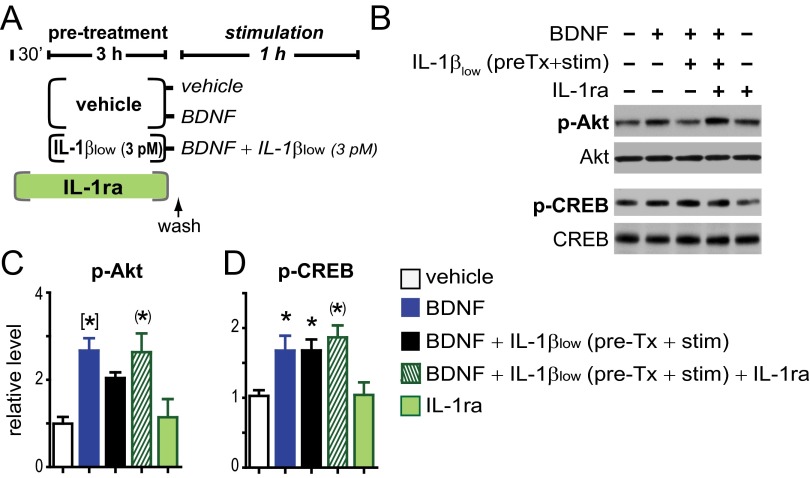
We have consistently found that 3 nM IL-1β significantly impairs neuronal BDNF signaling (27–29). Fig. S2 shows that BDNF signaling in primary rat hippocampal neurons (5–7 DIV) is also reduced by 30 and 300 pM IL-1β, but not by 0.3 or 3 pM IL-1β. Thus, to test whether treatment with IL-1β sensitizes neuronal responses to subsequent IL-1β exposure, cultured rat hippocampal neurons were preincubated with IL-1β (3 nM, 3 h), washed, and immediately treated with BDNF (50 ng/mL) in the presence of 3 pM IL-1β (hereafter referred to as IL-1βlow) (Fig. 2A). In control neurons not exposed to IL-1β (neither pretreatment nor cotreatment), BDNF induced the phosphorylation of its receptor, TrkB, as well as phosphorylation of the downstream targets Akt and CREB (Fig. 2 B–E). Consistent with our previous findings that BDNF signaling is only suppressed in the presence of IL-1β (27–29), BDNF signaling was not affected by IL-1β pretreatment alone. Similarly, BDNF signaling was not affected by IL-1βlow exposure in the absence of IL-1β pretreatment. In contrast, after IL-1β pretreatment, BDNF signaling was significantly impaired by IL-1βlow (Fig. 2 B–E), supporting the idea that IL-1β exposure sensitizes neurons to subsequent IL-1β challenge, evoking responses at levels of IL-1β that are normally ineffective for impairing BDNF signaling. To directly test the possibility that IL-1β can potentiate its own signaling, we examined the stress kinase p38, a downstream effector of the AcP-dependent IL-1β signaling (21). We found that IL-1βlow activates p38 in neurons pretreated with 3nM IL-1β (3 h), whereas no changes were detected in the activation levels of Src, a downstream kinase recently associated with AcPb-dependent IL-1β signaling (22) (Fig. 2 F and G). These findings further support the idea that IL-1β exposure biases IL-1β signaling toward AcP proinflammatory responses.
Fig. S2.
Inhibition of BDNF signaling by IL-1β is dose-dependent. Primary rat hippocampal neurons (5–7 DIV) were incubated with IL-1β (0.3, 3, 30, or 300 pM) or vehicle for 1 h and stimulated with BDNF (50 ng/mL) for an additional 1 h. Phosphorylated and total levels of Akt were assessed. Relative levels of p-Akt(Ser-473)/Akt were calculated, normalizing to p-Akt/Akt levels from vehicle-treated neurons (control) [n = 5 per group; five independent experiments (neurons from different embryo litters)]. (*)P < 0.01 (ANOVA, Tukey’s post hoc test). Data are presented as mean ± SEM.
Fig. 2.
IL-1β pretreatment sensitizes the inflammatory branch of IL-1β signaling in rat hippocampal neurons. (A) Experimental design: Primary rat hippocampal neurons (5–7 DIV) were preincubated with 3 nM IL-1β or vehicle for 3 h, washed, and stimulated with BDNF with or without IL-1βlow for 1 h, as indicated. (B) Western blot analysis of phosphorylated and total levels of TrkB, Akt, and CREB after the above treatments. (C–E) Relative levels of p-TrkB (Tyr-490)/TrkB (C), p-Akt (Ser-473)/Akt (D), and p-CREB (Ser-133)/CREB (E). Data were normalized with vehicle-treated neurons (control) (n = 9; nine independent experiments). *P < 0.05; (*)P < 0.01; [*]P < 0.001; n.s., not significant (ANOVA, Tukey’s post hoc test). (F and G) Primary rat hippocampal neurons (5–7 DIV) were preincubated with 3 nM IL-1β or vehicle for 3 h, washed, and stimulated with IL-1βlow for 20 min, as indicated. Western blot analysis of phosphorylated and total levels of p38 and Src followed the above treatments. (G) Relative levels of p-p38 (Thr-180/Tyr-182)/p38 and p-Src (Tyr-416)/Src. Data were normalized with vehicle-treated neurons (control) (n = 8; eight independent experiments). *P < 0.05; (*)P < 0.01 (ANOVA, Tukey’s post hoc test). Data are presented as mean ± SEM.
Because 3 h treatment with IL-1βlow (3 pM) enhanced IL-1R1 and AcP expression (Fig. 1 D–F), we hypothesized that even IL-1βlow may increase sensitivity to IL-1β. As predicted, we found that pretreatment with IL-1βlow (3 h) is sufficient to sensitize the IL-1β response to subsequent IL-1βlow challenge (Fig. S3), thus supporting a causal link between the IL-1R–AcP up-regulation and the enhanced inflammatory potency of IL-1β to suppress BDNF signaling in hippocampal neurons. Further, to confirm a role of IL-1R1 in the sensitization of the IL-1β response, the impairment of BDNF signaling by IL-1βlow was blocked by IL-1ra, a cytokine that blocks the binding site for IL-1β on IL-R1 (16) (Fig. S3). Although BDNF activates multiple signaling pathways (PI3K/Akt, MAPK/ERK, and PLC/CaMK) (30), we found that the sensitized IL-1β response suppressed BDNF-induced activation of Akt, but not phosphorylation of CREB (Fig. S3 and Fig. 2 B, D, and E).
Fig. S3.
IL-1βlow sensitizes its own response via IL-1R1. (A) Experimental design: Primary hippocampal neurons at 5–7 DIV were preincubated with IL-1βlow (3 pM) or vehicle for 3 h. After washing, vehicle-treated neurons were stimulated with vehicle or BDNF only (50 ng/mL, 1 h), whereas neurons pretreated (pre-Tx) with IL-1βlow were stimulated with BDNF in the presence of IL-1βlow for 1 h. To specifically test the role of IL-1R1 on the induction of sensitivity, IL-1ra (300 nM) was added 30 min before IL-1βlow pretreatment, but was absent during stimulation. Control groups consisted of a vehicle-only group (vehicle) and an IL-1ra–vehicle group (IL-1ra). (B) Phosphorylated and total levels of Akt and CREB were evaluated by using Western blots. (C and D) Relative levels of p-Akt(Ser-473)/Akt (C) and p-CREB(Ser-33)/CREB (D) were normalized with vehicle-only treated controls [n = 5 per group; five independent experiments (neurons from different embryo litters)]. *P < 0.05; (*)P < 0.01; [*]P < 0.001 vs. vehicle control (ANOVA, Tukey’s post hoc test). Data are presented as mean ± SEM.
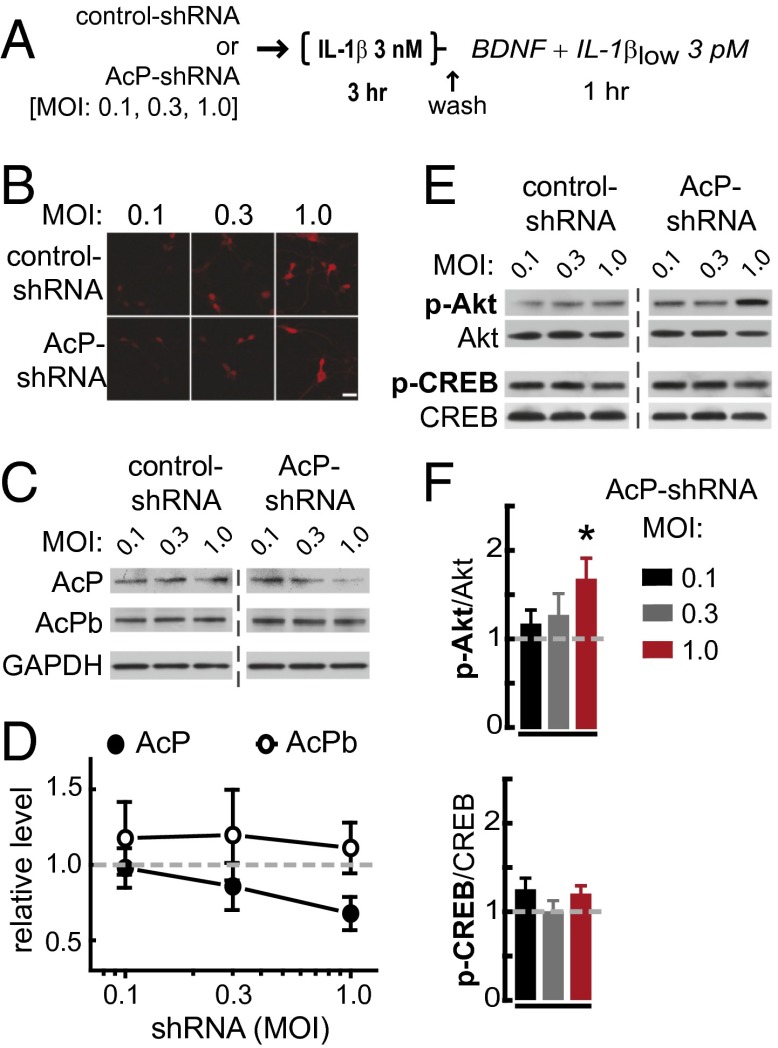
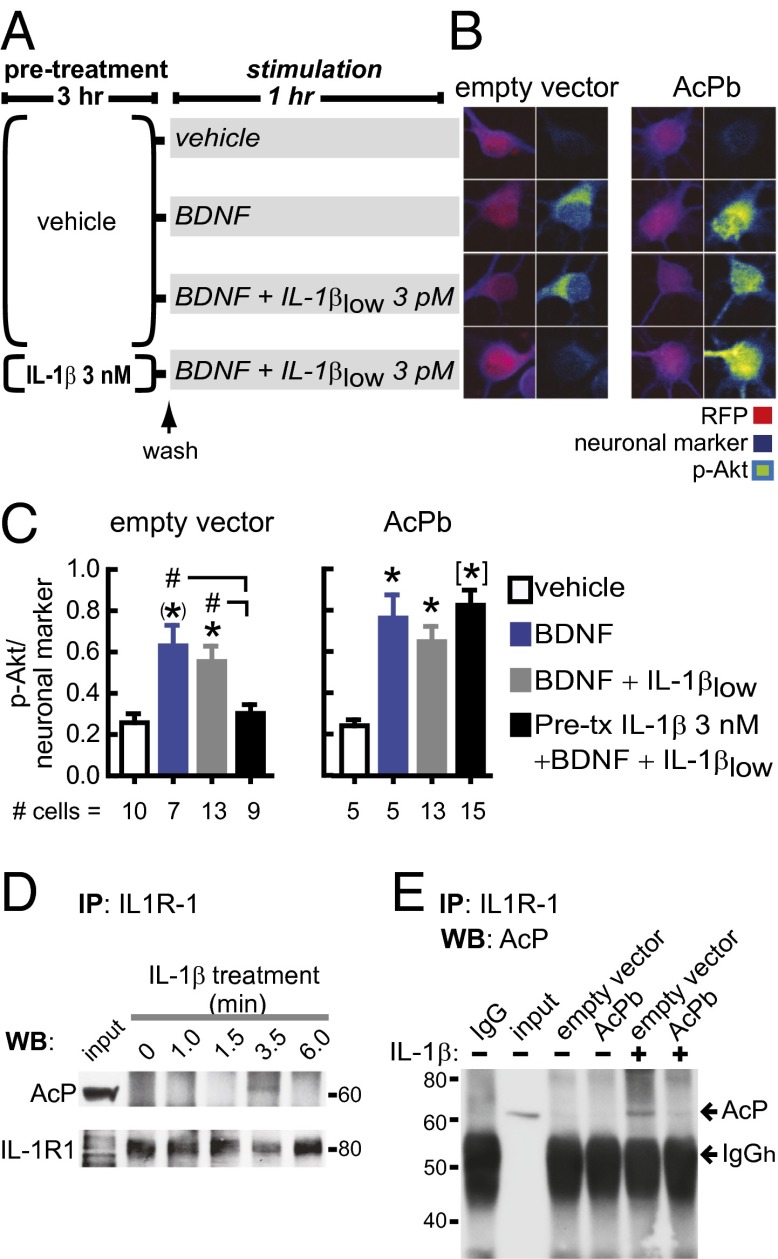
To determine whether the increased AcP relative to AcPb expression is responsible for the potentiated inflammatory response to IL-1β in neurons that had been preincubated in IL-1β, we investigated whether the impairment of BDNF signaling by IL-1β can be blocked by reducing the AcP/AcPb ratio, either by inhibiting AcP expression or by increasing AcPb levels. First, we reduced the AcP/AcPb ratio by using short-hairpin RNA (shRNA) to specifically knock down AcP expression, without affecting AcPb levels (P = 0.04; Fig. 3 A–D). As predicted, compared with control-shRNA transfection, AcP knockdown reduced the IL-1β impairment of BDNF-dependent Akt activation in primary rat hippocampal neurons preincubated with 3 nM IL-1β (3 h) and challenged with IL-1βlow (Fig. 3 E and F). Similarly, in AcPb-overexpressing neurons, BDNF induced a significant increase in p-Akt levels, despite IL-1β pretreatment and subsequent IL-1βlow challenge (Fig. 4 A–C). Overall, these results indicate that the IL-1β inflammatory response in hippocampal neurons depends on AcP and can be attenuated by AcPb, thus supporting the idea that the AcP/AcPb ratio modulates downstream effects of IL-1β signaling.
Fig. 3.
AcP knockdown attenuates the neuronal IL-1β inflammatory response. (A) Experimental design. Both control– and AcP–shRNA-transfected neurons (at 3 DIV) were treated identically (at 6–7 DIV): All cultures were preincubated with 3 nM IL-1β for 3 h, washed, and stimulated with BDNF (50 ng/mL) with IL-1βlow (3 pM) for 1 h, as indicated. AcP–shRNA targets a sequence in the unique 3′ terminal segment of the rat AcP mRNA, a sequence absent in rat AcPb mRNA (SI Methods). (B) RFP detection in primary rat hippocampal neurons transfected with increasing MOI of lentivirus. (Scale bar: 50 μm.) (C) Western blot analysis of AcP and AcPb levels; GAPDH was used as loading control. (D) Quantification: AcP (n = 8) and AcPb (n = 8) levels in AcP–shRNA-transfected neurons were normalized with corresponding levels from neurons transfected with equivalent MOI of control shRNA (dashed line). Results are from five independent experiments (neurons from different embryo litters). AcP vs. AcPb levels, P = 0.04 (two-way ANOVA main effect of shRNA; F3,44 = 2.99). (E) Western blot analysis of p-Akt (Ser-473) and p-CREB (Ser-133) levels (Akt and CREB levels as loading controls). (F) Quantification: After densitometry analysis, p-Akt (n = 8) and p-CREB (n = 7) levels in AcP–shRNA-transfected neurons were normalized with corresponding levels from neurons transfected with equivalent MOI of control shRNA (dashed line); results are from five independent experiments. AcP–shRNA vs. control-shRNA (two way ANOVA): p-Akt, P = 0.01, effect of shRNA, F1,41 = 7.07; p-CREB, P = 0.17, effect of shRNA concentration, F1,36 = 1.96. *P < 0.05 (Bonferroni post hoc test for p-Akt). Data are presented as mean ± SEM.
Fig. 4.
AcPb attenuates the neuronal IL-1β inflammatory response. (A) Primary rat hippocampal neurons were transfected with empty or AcPb-containing vectors at 3 DIV (AcPb gene sequence under the CMV promoter). After 3–4 d, neurons were preincubated with 3 nM IL-1β or vehicle for 3 h, washed, and stimulated with BDNF with or without IL-1βlow for 1 h, as indicated. (B) Phosphorylated levels of Akt (p-Akt; Ser-473) were assessed by immunofluorescence in RFP (reporter gene)-positive cells treated as indicated in A. Pan neuronal marker staining was used for cell-volume normalization. Representative images are shown. (Scale bar: 10 μm.) (C) p-Akt/pan-neuronal marker levels following the above treatments. The number of analyzed neurons is shown at the bottom of each treatment. Transfection itself did not interfere with BDNF signaling. Akt activation by BDNF was not impaired by IL-1βlow treatment alone (i.e., in the absence of IL-1β pretreatment) in either control- or AcPb-transfected neurons. Consistent with experiments in nontransfected cells (Fig. 2D), BDNF induction of p-Akt was prevented by IL-1βlow challenge after IL-1β pretreatment in control-transfected neurons. Bar graphs show data from a representative experiment (one out of four independent experiments). *P < 0.05; (*)P < 0.01; [*]P < 0.001 vs. control; #P < 0.05 (ANOVA, Tukey’s post hoc test). (D) Neurons were treated with 3 nM IL-1β for the indicated times. IL-1R1 immunoprecipitation (IP) was followed by Western blot (WB) for AcP and IL-1R1 (n = 3; three independent experiments). IL-1R1–AcP interaction was detected after 3.5 min, but not after 6 min, of IL-1β treatment, possibly due to MyD88 binding to the C-terminal domain of IL-1R1 (18), the same region recognized by the antibody used for IL-1R1 IP. (E) IL-1R1 IP was performed in neurons transfected with empty or AcPb-containing vectors. Neurons were treated with vehicle or IL-1β (3 nM, 3.5 min), as indicated. Membrane was probed for AcP; shown is a representative experiment (n = 3; three independent experiments). Data are presented as mean ± SEM.
Because both AcP and AcPb are recruited to IL-1R1 in response to IL-1β (20, 21), AcPb may attenuate AcP-dependent responses by competing for IL-1R1 binding, thus attenuating IL-1β–induced suppression of BDNF signaling (Fig. 2). Confirming that AcP is recruited to the IL-1R1–IL-1β complex (17), AcP coimmunoprecipitated with IL-1R1 after IL-1β treatment of cultured rat hippocampal neurons (Fig. 4D). To test whether AcPb competes with AcP for IL-1R1 binding, IL-1R1 was immunoprecipitated after IL-1β treatment of AcPb- and control-transfected neurons. Compared with controls, IL-1R1–AcP coimmunoprecipitation was reduced in neurons transfected with AcPb (Fig. 4E). This result strongly suggests that AcP and AcPb compete for the ligand-binding chain of the IL-1 receptor, consistent with our hypothesis that the relative abundance of AcP/AcPb modulates the extent to which IL-1β triggers proinflammatory cascades.
Our in vitro data indicate that up-regulation of AcP and IL-1R1, which occurs in the hippocampus during the course of aging (Fig. 1B), potentiates the IL-1β inflammatory response in rat hippocampal neurons. Because AcP and IL-1R1 have been detected in postsynaptic density (PSD) fractions from adult rat hippocampus (31), we hypothesized that both the reconfiguration and sensitization of the IL-1 receptor may occur directly at synapses in the aged hippocampus.
Age-Dependent Increase in the Level of AcP Relative to AcPb in Hippocampal Synapses.
To investigate whether age-related changes in IL-1 receptor components occur specifically at the synapse, we quantified AcP, AcPb, and IL-1R1 protein levels in hippocampal synaptosomes [presynaptic terminals attached to postsynaptic dendritic spines (32)] from young (6–7 mo) and middle-aged (13–15 mo) mice. We verified the enrichment of synaptic proteins in our synaptosomal preparation (Fig. 5A). Data analysis revealed a striking age-dependent change in the expression pattern of IL-1 receptor subunits. Notably, in synaptosomes from young mice, AcPb was the most abundant subunit and was present at significantly higher levels than either AcP (P < 0.001) or IL-1R1 (P < 0.01) (Fig. 5 B and C), whereas in synaptosomes from middle-aged mice, the three subunits were expressed at similar levels (Fig. 5 B and C). Thus, although both AcP and AcPb levels in hippocampal synaptosomes changed with aging, the change was in opposite directions. As a result, the AcP/AcPb ratio shifted with age, with aging associated with a nearly threefold increased AcP/AcPb ratio in hippocampal synapses (P = 0.033; Fig. 5D).
Fig. 5.
Aging reconfigures the IL-1 receptor in mouse hippocampal synaptosomes. (A) Hippocampal synaptosomes from young and middle-aged mice were purified by density gradient. Purity was evaluated by Western blot analysis of the synaptic markers synaptophysin (Syp) and PSD95 in nuclear (N) and synaptosome (S) fractions. Expression of astrocyte (glial fibrillary acidic protein; GFAP) and oligodendrocyte (2′,3′-cyclic nucleotide 3′-phosphodiesterase; CNPase) markers was not detected in synaptosome fractions. (B) AcP, AcPb, and IL-1R1 proteins detected by Western blot in hippocampal synaptosome samples. (C) Quantification of AcP, AcPb (n = 11 samples/group), and IL-1R1 (n = 7 samples per group) protein levels in synaptosomes from young (7–8 mo) and middle-aged (13–15 mo) mice. Fresh hippocampi from two mice were pooled for each sample. Values were normalized with corresponding GAPDH levels. AcP and AcPb detection for each sample was performed in duplicate, and values were averaged before statistical analysis. *P < 0.01; [*]P < 0.001 vs. AcPb in young mice (ANOVA, Tukey’s post hoc test). (D) AcP/AcPb protein ratio. *P < 0.05 (Mann–Whitney test). Data are presented as mean ± SEM.
Building on our synaptosomal data demonstrating that the AcP/AcPb ratio increases specifically in synapses with age, along with our in vitro data that AcP and IL-1R1 up-regulation by IL-1β sensitizes hippocampal neurons to subsequent low-level IL-1β challenge and impairs BDNF signaling (Figs. 1 and 2), we next investigated whether aging is accompanied by increased IL-1β sensitivity directly at synapses and whether the increased IL-1β sensitivity compromises synaptic plasticity.
Aging and IL-1β Interact to Suppress LTP in Synapses via IL-1R–AcP.
We assessed the effect of IL-1β on synaptic plasticity in hippocampal synaptosomes from young (6–7 mo) and middle-aged (13–15 mo) mice using a novel approach that we have developed to track LTP in freshly isolated synaptosomes. Our method induces LTP in synaptosomes by chemical stimulation (chemical LTP; cLTP) (33–39) and tracks the insertion of glutamate AMPA receptors (GluR1) into the postsynaptic surface, the first critical step for potentiation of synaptic transmission (40). Using flow cytometry, we quantify GluR1 surface expression in synaptosomal preparations at the single-synaptosome level. We refer to this approach as “Fluorescence Analysis of Single-Synapse Long-Term Potentiation” (FASS-LTP). Our method first identifies synaptosomes by size using calibrated beads (Fig. 6 A and B), as described (41). Consistent with the average size of synaptosomes (32), the subset of particles between 0.5 and 3.0 μm in P2 synaptosomal fractions (42) is highly enriched in synaptosomes, as demonstrated in our preparation by the high proportion of size-gated particles that coexpress synaptophysin and PSD95 (> 60%) and synapsin-I and PSD95 (> 70%) (Fig. 6C). Next, to identify potentiated synapses, our method uses antibodies specific for extracellular epitopes on GluR1 and neurexin-1β (Nrx1β) (Fig. 6D), a presynaptic adhesion molecule stabilized at the membrane surface by synaptic activity (43). GluR1+Nrx1β+ double-labeling ensures that we are analyzing intact synaptosomes that contain both presynaptic and postsynaptic elements. Fluorescence analysis of the size-gated population after cLTP induction (45 min, glycine–KCl stimulation; Methods) identifies an increased proportion of GluR1+Nrx1β+ double-positive events over basal levels (Fig. 6D), demonstrating that FASS-LTP detects activity-dependent plasticity in synaptosomes. Importantly, the cLTP response in isolated synaptosomes is sustained (159.8 ± 23.0% GluR1+Nrx1β+ events over basal after 75 min; P = 0.04; n = 6; Mann–Whitney test) and depends on NMDA receptor activation, because it is blocked by the NMDA receptor antagonist AP5 [50 µM] (P = 0.02; n = 5; Mann–Whitney test).
Fig. 6.
Sensitized IL-1β response in aged synapses suppresses cLTP via IL-1R1–AcP signaling. (A) Forward scatter (FSC) vs. side scatter (SSC) dot plot showing the size–complexity profile of gated particles (inside rectangle, size-gated synaptosomes). (B) FSC–SSC dot plots using 0.5-, 0.75-, 1.0-, and 3.0-μm calibrated beads. Gray area represents the size range used to select putative synaptosomes particles: 0.5 μm < gated particles ≤ 3.0 μm. (C) Two-color parameter density plots showing synaptophysin–PSD95 (Left) and synapsin–I-PSD95 (Right) double-labeling in size-gated synaptosomes. Thresholds for endogenous/nonspecific fluorescence for each marker are set by using secondary antibody staining only (lower left quadrant). Note that a high proportion of particles coexpress presynaptic and postsynaptic markers (>60%). Representative plots of one experiment out of five are shown. (D, Left and Center) Two-color parameter density plots showing Nrx1β and GluR1 surface detection in size-gated synaptosomes before and after cLTP. GluR1–Nrx1β double-positive events (upper right quadrant) increase after cLTP. (D, Right) The model illustrates the insertion of Nrx1β- and GluR1-containing endosomes after cLTP detected in single events by FASS-LTP. (E) FASS-LTP was performed in fresh synaptosome fractions obtained from young (6–7 mo; n = 7) and middle-aged (13–15 mo; n = 6) mice. IL-1βlow or external solution was added 5 min before cLTP induction. EM-163 (20 µM), IL-1ra (3 µM), or equivalent volumes of external solution were added 10 min before IL-1βlow (3 pM) treatment. The upper right quadrant shows the proportion of GluR1–Nrx1β double-positive events detected after 45 min of cLTP in each experimental condition. (F) Overall data presented as mean ± SEM. *P < 0.05 (ANOVA, Tukey’s post hoc test).
Using FASS-LTP, we tested whether chronic IL-1β signaling in aging (Fig. 1B and ref. 3) sensitizes the IL-1β response in hippocampal synapses. Synaptosomal fractions isolated from hippocampi of young (6–7 mo) and middle-aged (13–15 mo) mice were treated with or without IL-1βlow before inducing cLTP. FASS-LTP analysis revealed that, in the absence of IL-1βlow treatment, the proportion of GluR1+Nrx1β+ events in cLTP-stimulated samples was significantly increased over basal levels in synaptosomes from both young (185 ± 22%; P < 0.05) and middle-aged (156 ± 11%; P < 0.05) mice (Fig. 6 E and F). A FASS-LTP time course (0, 15, 25, and 45 min) showed that cLTP responses in synaptosomes from middle-aged mice are significantly reduced relative to young animals (P = 0.01; two-way ANOVA effect of age; F1,45 = 6.4), a finding consistent with the age-dependent reduction of “conventional” electrically induced LTP in middle-aged mice (44, 45) and rats (46). Interestingly, treatment with IL-1βlow did not significantly affect cLTP-induced changes in synaptosomes isolated from young mice, but totally suppressed the cLTP response in synaptosomes from middle-aged mice (P < 0.05; Fig. 6 E and F). These data, to our knowledge, demonstrate for the first time that the IL-1β response is sensitized in synapses from the aged hippocampus. Importantly, because glial cells are absent in the synaptosomal preparation, our finding strongly suggests that IL-1β impairs LTP by direct actions on neuronal synapses and does not require an intermediary response from IL-1β–activated glia.
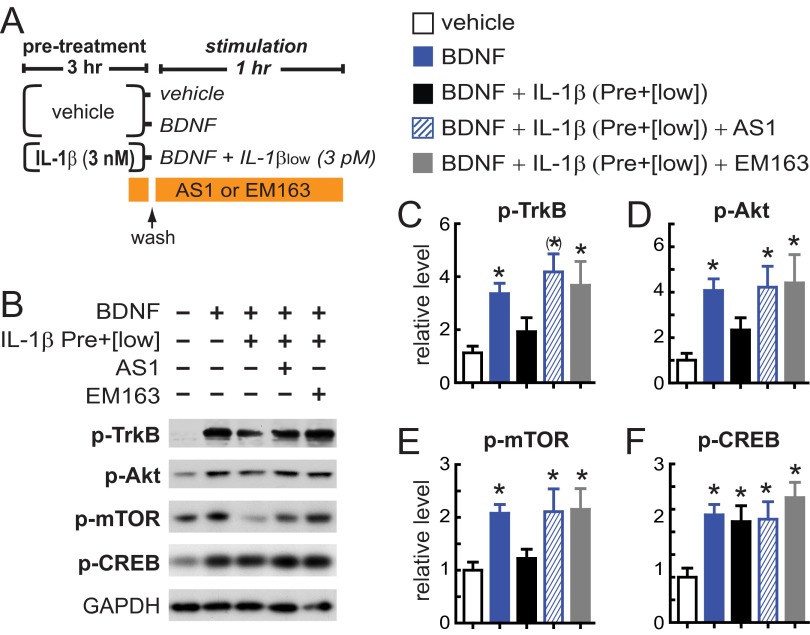
Based on our data that the IL-1β inflammatory response in hippocampal neurons depends on AcP (Fig. 3), and because the IL-1R1–AcP (but not IL-1R1–AcPb) receptor recruits MyD88 via TIR domains (18, 20, 21) [canonical IL-1β signaling (19)], we hypothesized that the potentiated IL-1β suppression of cLTP in aged synaptosomes is TIR-domain–dependent. To block TIR domain signaling, we used TIR mimetics, synthetic compounds that mimic the BB-loop of the TIR domain and specifically block TIR-domain–dependent IL-1β signaling in multiple cell types (47, 48) including neurons (49). We found that the TIR mimetics AS1 (47) and EM163 (48) specifically block the sensitized signaling through the IL-1R1–AcP complex and rescued BDNF signaling in hippocampal cultures exposed to our experimental paradigm of IL-1β sensitization (Fig. S4), thus demonstrating main roles of IL-1R1 and AcP in the sensitization of the IL-1β response. Using FASS-LTP, we then tested whether the TIR mimetic EM163 or IL-1ra can block the suppression of cLTP induced by IL-1βlow in aged synaptosomes. Consistent with the rescue of BDNF signaling by TIR mimetics and IL-1ra in hippocampal cultures (Figs. S3 and S4), the cLTP response of aged synaptosomes was not impaired by IL-1βlow when synaptosomes were preincubated with EM163 or IL-1ra (P < 0.05 vs. basal; Fig. 6 E and F).
Fig. S4.
The potentiated IL-1β inflammatory response is blocked by TIR mimetics. (A) Experimental design: Primary rat hippocampal neurons (5–7 DIV) were preincubated with vehicle or IL-1β (3 nM, 3 h). After washing, vehicle-treated neurons were exposed to BDNF (50 ng/mL, 1 h) or vehicle, and neurons pretreated with IL-1β were stimulated with BDNF in the presence of IL-1βlow (1 h). The effect of TIR mimetics was tested in neurons pretreated with IL-1β and stimulated with BDNF/IL-1βlow, with TIR mimetics AS1 or EM163 (20 μM) added during the last 15 min of IL-1β pretreatment and during the BDNF/IL-1βlow cotreatment. (B) Western blot analysis of phosphorylated levels of TrkB, Akt, mTOR and CREB (n = 8 per group) with GAPDH used as loading control. (C–F) Western blot quantification: p-TrkB (C), p-Akt (D), p-mTOR (E), and p-CREB (F) levels were normalized with vehicle-treated neurons (vehicle). *P < 0.05; (*)P < 0.01 vs. control (one-way ANOVA, Tukey’s post hoc test). Data are presented as mean ± SEM.
Together, our data from synaptosomes show that an age-dependent increase in the AcP/AcPb ratio potentiates the IL-1β–induced suppression of cLTP directly at synapses. Consistent with our hypothesis that increased activation of the AcP-dependent branch of IL-1β signaling drives the age-related heightened sensitivity to impairment by IL-1β, the cLTP deficit in middle-aged animals was rescued by a TIR-domain blocker, which specifically inhibits AcP-dependent signaling. Based on our data and the demonstration that the TIR mimetic AS1 blocks brain IL-1β signaling in vivo (47), we reasoned that AS1 may protect memory function in the aged animal from impairment after acute inflammation.
Acute Inflammation Impairs Hippocampal-Dependent Memory in Aged Mice via TIR Domain Signaling.
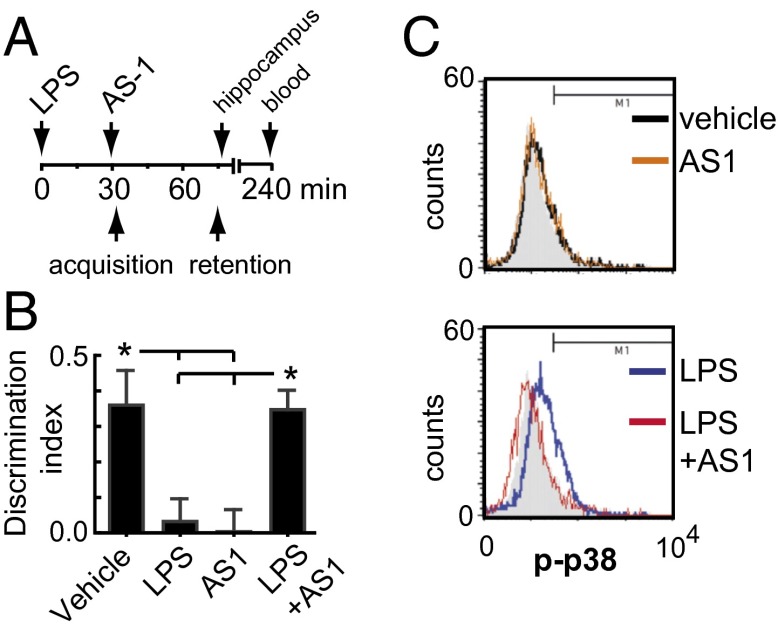
To determine whether the AcP-dependent branch of IL-1β signaling is responsible for the cognitive impairing effects of acute inflammation in aged animals (6, 7, 9, 11), mice were treated with the TIR mimetic AS1 after an acute immune challenge. We challenged 20- to 22-mo-old mice with bacterial LPS (0.3 mg/kg, i.p.) based on previous reports that have consistently shown spatial memory impairments and increased central IL-1β induction by LPS in 18- to 24-mo-old mice (9, 11). Thus, aged mice were injected with LPS or saline, followed 30 min later by administration of the TIR mimetic AS1 immediately before the acquisition trial of the object location memory (OLM) task (50) (Fig. 7A). In the retention trial (45 min after acquisition), saline-treated animals exhibited a preference for the novel location over the familiar location (calculated as the discrimination index; P < 0.05; Fig. 7B), demonstrating intact spatial memory. In contrast, animals challenged with LPS exhibited significantly reduced discrimination indices compared with saline-treated controls (P < 0.05; Fig. 7B). Consistent with our prediction, administration of the TIR mimetic AS1 prevented the OLM impairment, demonstrating that blocking the AcP-dependent branch of IL-1β signaling prevents LPS-induced spatial memory deficits in aged mice (P < 0.05 vs. LPS; Fig. 7B). Interestingly, administration of AS1 in the absence of LPS treatment also impaired OLM (P < 0.05 vs. vehicle; Fig. 7B). This finding is consistent with the notion that endogenous IL-1β at physiologically low levels may be essential for hippocampal memory function (51–53).
Fig. 7.
TIR (IL-1R1–AcP)-dependent IL-1β signaling mediates the memory impairment induced by LPS in old mice. (A) Experimental design: 20- to 22-mo-old mice were injected with saline or LPS (0.3 mg/kg, i.p.) and 30 min later with AS1 (200 mg/kg, i.p.) or an equivalent volume of DMSO. OLM task was undertaken immediately after AS1 injection. The acquisition trial (5 min) was followed 45 min later by the retention test trial (5 min). All mice spent equal time exploring both objects in the acquisition trial, indicating no preference for either location (P = 0.40; ANOVA). Mice were euthanized immediately after test trial to obtain the hippocampus. (B) Discrimination index (n = 7 per group). *P < 0.05 (ANOVA, Tukey’s post hoc test). Data are presented as mean ± SEM. (C) Levels of p-p38 (Thr-180/Tyr-182) in size-gated hippocampal synaptosomes from mice used for OLM task. Background fluorescence was determined by using a PE-conjugated control isotype antibody (gray filled histogram) (n = 4 per group). P < 0.01, LPS vs. LPS+AS1 (Kolmogorov–Smirnov test).
Because p38 is a pivotal stress kinase downstream of the AcP-dependent pathway (19) by which IL-1β inhibits hippocampal-dependent memory (54), LTP (29, 55), and BDNF signaling (29), we assessed p38 activation in size-gated synaptosomes from aged mice treated in accordance with the design used to evaluate OLM. Flow cytometry analysis showed that LPS significantly increased p-p38 levels in hippocampal synaptosomes. Importantly, synaptosomes from mice treated with AS1 after LPS challenge showed p-p38 levels comparable to saline-treated mice (Fig. 7C), a finding consistent with the rescue of hippocampal-dependent memory by AS1.
Together, our data provide a mechanism for the impairment of hippocampal-dependent memory after immune activation in aged animals, with a key role of AcP-dependent IL-1β signaling and its downstream effector p38 acting directly at synapses.
Discussion
In the hippocampus, aging and inflammation interact to induce memory deficits via IL-1β (6, 7). We demonstrate a previously unidentified mechanism to explain the increased hippocampal sensitivity to IL-1β with age. Our analysis at the synapse level together with in vivo behavior indicate that AcP-dependent signaling is a key pathway mediating the age-related impairment of synaptic plasticity and memory by IL-1β and that synapses themselves are the site of the potentiated IL-1R1–AcP response. We further identify TIR domains as a drug target to selectively block the sensitized IL-1R1–AcP signaling and demonstrate that administration of TIR mimetics can prevent IL-1β–driven impairment of synaptic function and hippocampal-dependent memory in aged animals.
We had hypothesized that chronically elevated IL-1β signaling, such as occurs with age and neurodegenerative disease, can affect the IL-1 receptor system directly in neurons. Here we demonstrate that, along with up-regulating IL-1R1, IL-1β also increases expression of AcP (but not the splice variant AcPb) in hippocampal cultured neurons, thus modulating the relative expression of AcP and AcPb, which are gatekeepers driving different functional outcomes of IL-1β signaling (AcP, proinflammatory, vs. AcPb, neuroprotective). We show that the IL-1β–induced up-regulation of IL-1R1–AcP potentiates the suppression by IL-1β of BDNF signaling in hippocampal neurons, such that BDNF signaling can subsequently be suppressed by exposure to low-level, normally subthreshold IL-1β challenge. The key role of AcP in the potentiated IL-1β signaling was established directly by shRNA knock down of AcP and was supported by AcPb overexpression to reduce AcP interaction with IL-1R1, both of which reduced the IL-1β impairment of BDNF-dependent signaling.
In addition to suppressing BDNF signaling, IL-1β influences a variety of neuronal properties and functions (e.g., excitability, transmitter release) via multiple biochemical pathways (16, 19, 56, 57), which might also be modulated by the relative expression of AcP and AcPb. For instance, it has been shown that high IL-1β concentrations (1–10 nM) activate p38 and require AcP, whereas low IL-1β concentrations (≤0.6 pM) may improve neuronal activity via AcPb-dependent activation of Src kinase (22). Our finding that AcP and AcPb compete for IL-1R1 binding suggests that the relative expression of these IL-1 coreceptors may change the effective concentration at which IL-1β switches from Src to p38 activation. Indeed, we found that a low IL-1β concentration (3 pM) induced the activation of p38 in neurons with an elevated AcP/AcPb ratio. Thus, our data suggest that chronically elevated IL-1β signaling can shift the effects of low IL-1β concentrations from facilitating neuronal activity via Src (22) to suppression of BDNF signaling (29) and synaptic plasticity (29, 55) via p38.
Previous reports have shown that hippocampal-dependent memory is vulnerable to IL-1 after infection-like immune challenges in aged animals (6, 7), and it has recently been found that IL-1 signaling also mediates the age-related cognitive decline associated with low-grade sterile inflammation, an innate immune response induced by the accumulation of endogenous “danger signals” in the absence of overt infection (3). Because intact synaptic activity is essential for learning and memory, we explored the hypothesis that IL-1β directly impacts synapse functionality. Using synaptosomes isolated from the hippocampus of young and middle-aged animals, we demonstrated that the AcP/AcPb ratio in synapses shifts with age, with a nearly threefold increase in the ratio in middle-aged synapses. Paralleling the neuronal culture data, we found that an increased AcP/AcPb ratio is associated with a sensitized IL-1β response with increasing age. FASS-LTP revealed that low levels of IL-1β (3 pM) did not impair cLTP-induced changes in synaptosomes isolated from young mice, but totally suppressed the cLTP response in synaptosomes from middle-aged mice. Thus, aging biased IL-1β signaling toward AcP-dependent (proinflammatory) responses directly at hippocampal synapses and thereby impaired LTP. Our data are consistent with the early impairments of hippocampal-dependent memory associated with deficits in CA1 late LTP (45) and with increased hippocampal expression of inflammatory genes (58) in middle-aged mice. Moreover, middle-aged mice also exhibit an increased Nlrp3 inflammasome-dependent activation of caspase-1 (IL-1–converting enzyme), a significant finding because Nlrp3 inflammasome is a major immune sensor, causing age-related sterile inflammation via caspase-1 and IL-1β in both periphery and CNS (3). We suggest that chronically elevated brain IL-1β levels generate increased sensitivity to IL-1β at the synapse, which may be an early factor driving hippocampal dysfunction.
Our data, together with the literature, indicate that inflammation wields a double-edged sword in the aged brain, not only exaggerating microglia responses (15) to immune challenge, but also heightening IL-1β sensitivity at the synapse. Consistent with the in vitro finding that microglia-derived IL-1β can reduce spine density (59), our data demonstrate that hippocampal synapses contain the biochemical IL-1β–signaling pathway(s) impairing plasticity (e.g., p38) (29, 54). To our knowledge, we demonstrate for the first time that AcP-dependent IL-1β signaling at the synapse can itself underlie the suppression of plasticity by IL-1β. The synaptic response to IL-1β is amplified with age, and IL-1β can suppress LTP in the aged brain by acting directly on sensitized synapses, an effect that is not dependent on an intermediary response from IL-1β–activated glia. Our results are consistent with the interaction of IL-1β and aging on LTP previously identified in more complex systems (e.g., hippocampal slices and in vivo) (4, 12) and extend the growing understanding that synaptic dysfunction is a major cause of cognitive decline in aging (60) and many brain diseases (61), including Alzheimer’s disease (AD) (62).
One of our goals was to identify therapeutic interventions that can counteract the cognitive impairments caused by IL-1β in the aged animal. Our data identify AS1, a TIR mimetic that blocks MyD88 recruitment to TIR domains on the IL-1R1–AcP receptor and prevents IL-1R1–AcP signaling (47), as such a therapeutic agent. AS1 inhibited the following: (i) the potentiated IL-1β inflammatory response that suppresses BDNF signaling in cultured hippocampal neurons; (ii) the age-related impairment of cLTP by IL-1β in synaptosomes; and (iii) the impairment of hippocampal-dependent memory and p38 activation that occur in aged animals after peripheral immune challenge with LPS. In vitro experiments demonstrate a direct action of AS1 on neurons and synapses; however, the beneficial effects of AS1 on memory and p38 activation in animals challenged with LPS might be reflecting the inhibition of IL-1β signaling in many cell types in the periphery and in the brain, including neurons and glia.
Overall, we provide, to our knowledge, the first evidence that the IL-1–receptor system undergoes age-related reconfiguration in neurons. We demonstrate that the IL-1–receptor subunit reconfiguration sensitizes the IL-1R1–AcP-dependent response, occurs directly at synapses, and renders synaptic plasticity and memory vulnerable to impairment by IL-1β challenge. It stands to reason that the increased IL-1R1–AcP receptor sensitivity at the synapse is likely a common mechanism increasing neuronal vulnerability in brain diseases associated with IL-1β–driven neuroinflammation, such as depression (63), chronic stress (64), and AD (65). Finally, supporting the notion that the relative abundance of AcP and its splice variant AcPb modulates the strength and direction of neuronal IL-1β signaling, our work opens a road toward the search for selective therapeutic strategies to alleviate cognitive decline in the elderly population.
Methods
A complete description of experimental procedures is available in SI Methods.
Animals.
Both mice and rats were housed with food and water ad libitum. Lights were maintained on a 12:12 light/dark cycle, and behavior testing was carried out during the light phase of the cycle. All procedures used in the present study followed the Principles of Laboratory Animal Care from the NIH and were approved by the University of California, Irvine, Institutional Animal Care and Use Committee.
Hippocampal Cell Cultures.
Primary cultures were prepared from embryonic day 18 (E18) Sprague–Dawley rats as described (27). After 5–7 DIV, neurons were treated at 37 °C, O2/CO2 (95%/5%). Immunostaining in cell cultures was performed as described (27). The fluorescence analysis was performed in RFP-positive cells. It should be noted that we used E18 rats for all culture studies and mice for all other experiments to open the possibility of follow-up studies in transgenic models. Rat cultures provided great quantities of tissue for concentration–response, transgene-expression, and signaling-pathway studies and other large pilot culture experiments. We, however, did confirm IL-1β sensitivity after IL-1β pretreatment in cultured neurons from E16 mice. It is also noteworthy that the localization of IL-1R1 and AcP in the mouse brain is similar to that observed in the rat brain (66) and that IL-1β similarly activates p38 in primary hippocampal neurons from rat (67, 68) and mouse (22).
Biochemistry.
Homogenates (from neuronal cultures, tissue, and hippocampal synaptosomes) and immunoblotting were prepared as described (27). Immunoprecipitation was performed with a commercially available kit (Active Motif). Antibodies are listed in Table S1. Quantitative densitometric analyses were performed on images of immunoblots with ImageJ (National Institutes of Health). Total RNA extraction, cDNA synthesis, and qPCR were performed with commercially available kits (Qiagen).
Table S1.
Sources and dilutions of primary antibodies
| Target | Supplier, catalog no. | Method, dilution, [conc.] |
| AcP | Abcam, ab8110 | WB, 1:1,000 |
| AcP | R&D, BAF676 | WB, 1:250 |
| Akt (rabbit) | Cell Signaling, 9272 | WB, 1:1,000 |
| Akt (mouse) | Cell Signaling, 2920 | WB, 1:1,000 |
| CNPase | Covance, SMI-91R | WB, 1:200 |
| CREB | Cell Signaling, 9104 | WB, 1:1,000 |
| GAPDH | GeneTex, GTX100118 | WB, 1:5,000 |
| GFAP | Millipore, MAB360 | WB, 1:1,000 |
| GluR1 (n-Term) | Millipore, ABN241 | FC, 1:400 |
| IL-1R1 (n-Term) | Epitomics, 1695-1 | WB, 1:1,000 |
| IL-1R1 (c-Term) | Santa Cruz, sc-689 | IP, 5 μg/mL |
| Isotype control IgG-PE | Cell Signaling, 2975 | FC, [0.0625 μg/mL] |
| mTOR | Cell Signaling, 2983 | WB, 1:1,000 |
| p38 (biotinylated) | Cell Signaling, 2387 | WB, 1:1,000 |
| Pan Neuronal Marker (MilliMark) | Millipore, MAB2300 | IFC, 1:200 |
| Nrx1β | Neuromab, 75-216 | FC, 1:400 |
| Src | Cell Signaling, 2110 | WB, 1:1,000 |
| TrkB | Millipore, 07-225 | WB, 1:1,000 |
| p-p38 (Thr-180/Tyr-182) | Cell Signaling, 9215 | WB, 1:1,000 |
| p-p38-PE | Cell Signaling, 6908 | FC, [0.0625 μg/mL] |
| p-Akt (Ser-473) | Cell Signaling, 4060 | WB, 1:1,000; IF, 1:200 |
| p-CREB (Ser-133) | Cell Signaling, 9198 | WB, 1:1,000 |
| p-mTOR (Ser-2448) | Cell Signaling, 5536 | WB, 1:1,000 |
| p-Src (Tyr-416) | Cell Signaling, 2101 | WB, 1:1,000 |
| p-TrkB (Tyr-490) | Cell Signaling, 9141 | WB, 1:1,000 |
| PSD-95 (mouse) | Millipore, MAB1598 | WB, 1:1,000; FC, 1:400 |
| PSD-95 (rabbit) | Abcam, ab18258 | FC, 1:400 |
| Rabbit IgG (IP control) | Santa Cruz | IP, 5 μg/mL |
| Secondary Alexa Fluor 488 (anti-rabbit) | Life Technologies, A-11034 | 1:400 |
| Secondary Alexa Fluor 488 (anti-mouse) | Life Technologies, A-11029 | 1:400 |
| Secondary Alexa Fluor 647 (anti-rabbit) | Life Technologies, A-21245 | 1:400 |
| Secondary Alexa Fluor 647 (anti-mouse) | Life Technologies, A-21236 | 1:400 |
| Secondary HRP (anti-mouse) | Pierce, 31438 | 1:5,000 |
| Secondary HRP (anti-rabbit) | Pierce, 31460 | 1:10000 |
| Streptavidin HRP-conjugated | Millipore, SA202 | 1:500 |
| Synapsin-I | Millipore, AB1543 | FC, 1:160 |
| Synaptophysin | Millipore, MAB5258 | FC, 1:400 |
| β-actin | Sigma, A2066 | WB, 1:3,000 |
Transfections.
AcP knockdown and AcPb overexpression were performed by using Cellecta lentiviral particles encoding RFP as reporter. Neurons were infected at 3 DIV according to the manufacturer’s protocol. For AcP knockdown, we used vectors containing either a negative control shRNA sequence or a shRNA to target rat AcP mRNA. Control–shRNA (targeting luciferase, shLuc): target sequence, 5′-CGCTGAGTACTTCGAAATGTC-3′; insert (hairpin) sequence, 5′- ACCGGCGCTGAGTACTTTGAAATGTTGTTAATATTCATAGCGACATTTCGAAGTACTCAGCGTTTT -3′; construct, pRSI16-U6-shLuc-UbiC-TagRFP-2A-Puro. AcP-shRNA: target sequence, 5′-GAGACCCTGAGCTTCATTCAG-3′; insert sequence 5′-ACCGGGAGACCCTGAGTTTCATTTAGGTTAATATTCATAGCCTGAATGAAGCTCAGGGTCTCTTTT-3′; construct, pRSI16-U6-shAcP-UbiC-TagRFP-2A-Puro (SI Text). For AcPb overexpression experiments, vectors contained either the rat AcPb sequence (RefSeq: GU123169.1; vector: pR-CMV-AcPb-EF1-TagRFP) or an empty vector as control. Sequencing quality-control data verified the identity of AcPb rat gene.
Synaptosome Preparation by Ficoll/Sucrose Gradient.
Synaptosomes from B6129SF2/J mice were prepared by using a discontinuous Ficoll gradient as described (69).
RNA Extraction and qPCR.
Total RNA extraction from rat neuronal cultures, mouse (B6129SF2/J) whole brain (no cerebellum), and mouse (B6129SF2/J) hippocampal synaptosomes was performed by using TRIzol (Molecular Research Center) and the Qiagen RNeasy Mini Kit. The RNA Integrity Number of RNA samples was ≥7, as evaluated by the Genomics High-Throughput Facility at the University of California, Irvine. In neuronal cultures, gene expression was quantified by using TaqMan assays (Applied Biosystems) using appropriate primer sets and probes, as listed in Table S2 [AcP (21), AcPb (21), IL-1R1 (70), and GAPDH (70)]. Data were analyzed by the 2−ΔΔCt method and expressed as fold change over control after normalizing with control samples. In mouse brain, qPCR was performed by using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies). Primer sets (71) are described in Table S3. Data were analyzed by the 2−ΔΔCt method and expressed as fold change over young mice (6 mo) after normalizing with input samples.
Table S2.
Primer and probes for qPCR TaqMan assays
| Gene | Forward 5′–3′ [nM] | Reverse 5′–3′ [nM] | Probe (FAM) [nM] |
| AcP | GGGCAGGTTCTGGAAGCA [900] | GCTAGACCACCTGGGACTCT [900] | TCACTGGCATGGCCACCTGCAG [200] |
| AcPb | GGAGTTTAAGCTGGGTGTCATGT [50] | TGCTCAAGCGGACGGTACT [50] | CCATTGCCACTAAGC [200] |
| IL-1R1 | GCCCACGGAATGAGACGAT [100] | GCCCGTGACGTTGCAGA [100] | AGCTGACCCAGGATCCACGATACAACTG [50] |
| GAPDH | GAACATCATCCCTGCATCCA [100] | CCAGTGAGCTTCCCGTTCA [100] | CTTGCCCACAGCCTTGGCAGC [50] |
Table S3.
Primers for SYBR Green assays
| Gene | Forward 5′–3′ [ 200 nM] | Reverse 5′–3′ [200 nM] |
| AcP | GGGCAACATCAACGTCATTTTAG | CAGCTCTTTCACCTTCATGTCCTT |
| AcPb | GGAGTTTAAGCTGGGTGTCATGT | TGCTCAAGCGGACGGTACT |
| IL-1R1 | AACGTGAGCTTCTTCGGAGT | CCGGAACGTATAGGACATAC |
| IL-1β | CAACCAACACGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
GAPDH: RT2 qPCR Primer Assay for Mouse GAPDH: PPM02946E [500nM] (Qiagen).
FASS-LTP.
Fresh hippocampal synaptosome P2 fractions were obtained from B6129SF2/J mice (42) and stimulated (cLTP) as described (72–74). Briefly, hippocampi were rapidly dissected from a single mouse and homogenized in 320 mM sucrose containing Hepes (10 mM) and protease/phosphatase inhibitors mixture (Pierce) at pH 7.4. The homogenate was centrifuged at 1,200 × g (10 min). Supernatant was transferred into two microfuge tubes and centrifuged at 13,000 × g (20 min). Supernatants (S2) were removed, and pellets (P2 crude synaptosome fraction) were resuspended by gently pipetting up and down in 1.5 mL of extracellular (tube 1) or cLTP (tube 2) solutions. Extracellular solution contains (in mM): 120 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 15 glucose, and 15 Hepes, pH 7.4; whereas cLTP solution is Mg2+-free and contains (in mM): 150 NaCl, 2 CaCl2, 5 KCl, 10 Hepes, and 30 glucose, pH 7.4 (73). Synaptosome P2 fractions were incubated in a cell culture dish (30 mm) with agitation at room temperature for 15–30 min for recovery. After recovery, synaptosomes maintained in cLTP solution were supplemented with 0.001 mM strychnine and 0.02 mM bicuculline methiodide, and 180 µL (50–100 µg of protein determined by BCA assay) of this suspension was transferred to cytometry tubes. As control, an equal volume (180 µL) of synaptosome maintained in external solution was also transferred to a cytometry tube. Synaptosomes in external solution were used to determine basal levels of potentiated synaptosomes (see below). For stimulation, 20 µL of external solution was added to control synaptosomes (in external solution), whereas 20 µL of glycine (5 mM in cLTP solution) was added to synaptosomes in cLTP solution (final [glycine] = 500 µM; ref. 74). After 15 min of glycine treatment, synaptosomes were depolarized with 200 µL of a [high] KCl solution consisting of (in mM): 50 NaCl, 2 CaCl2, 100 KCl, 10 Hepes, 30 glucose, 0.5 glycine, 0.001 strychnine, and 0.02 bicuculline methiodide, pH 7.4 (final [KCl] = 50 mM) and incubated for 30 min; 200 µL of external solution were added to control synaptosomes. For cytokine treatment, 3 pM IL-1β was added 5 min before glycine, whereas EM-163 or IL-1ra was added 10 min before IL-1β in the corresponding tubes. After KCl incubation, stimulation was stopped by sequential addition of 0.5 mL of ice-cold 0.1 mM EGTA–PBS (pH 7.4) and 4 mL of ice-cold blocking buffer [5% (wt/vol) FBS in PBS]. Tubes were chilled on ice and centrifuged at 2,500 × g for 5 min at 4 °C (Sorvall RT6000B). After centrifugation, the pellet was resuspended and incubated with primary-antibody solution contained rabbit anti-GluR1 and mouse anti-Nrx1β antibodies (listed in Table S1), both at a concentration of 2.5 µg/mL in blocking buffer. After incubation, synaptosomes were washed (×2) with 4 mL of ice-cold blocking buffer, centrifuged (2,500 × g for 5 min at 4 °C), and pellet-resuspended in secondary-antibody solution [400 µL; anti-rabbit–Alexa 488 and anti-mouse–Alexa 647 antibodies (Life Sciences), both at 2.5 µL/mL]. After 30 min on ice with agitation and protected from light, synaptosomes were washed as described and resuspended in 400 µL of 2% paraformaldehyde in PBS. Samples were acquired by using a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences). Relative size and granularity was determined by forward scatter (FSC) and side scatter (SSC) properties. FSC, SSC, and fluorescence [FL1 (530 ± 15 nm) and FL4 (650 ± 25 nm)] signals were collected by using log amplification. Identical FSC settings were used for acquiring data on bead standards and samples. Small fragments and debris were excluded by establishing a FSC-H threshold (325). Synaptosome integrity was assessed with Calcein AM by using a FL1 detector. A total of 10,000 size-gated particles were collected and analyzed for each sample by using CellQuest Pro software (BD Biosciences).
OLM Test, p-p38 Detection in Synaptosomes, and Cytokine Quantification.
Mouse (C57BL/6J) cognition was evaluated by using the OLM test, adapted from described tasks (50). The relative exploration time was recorded and expressed as a discrimination index [DI = (tnovel − tfamiliar)/(tnovel + tfamiliar)]. All animals were euthanized immediately after OLM testing, and hippocampi were removed and immediately processed for Western blot or flow cytometry assays. Immunolabeling for p-p38 detection by flow cytometry in synaptosomes was performed according to a method for staining of intracellular antigens. Cytokines in plasma were quantified by Multiplexing LASER Bead Technology (Eve Technologies).
Statistical Analysis.
Sample sizes were chosen on the basis of pilot experiments (FASS-LTP) and previous experience with similar types of in vitro (27, 28) and in vivo (75) experiments. Mann–Whitney test (two-tailed) was used as nonparametric t test for unpaired data. ANOVA (parametric test) was used where assumptions of normality (Kolmogorov–Smirnov) and equal variance (Bartlett's test) were met and was replaced by Kruskal–Wallis (nonparametric test) where appropriate. One-way ANOVA was followed by post hoc Tukey’s test for mean comparisons of three or more groups, whereas Kruskal–Wallis was followed by Dunn’s post hoc test. Two-way ANOVAs were followed by Bonferroni’s post hoc test. Statistical tests and the nonlinear fit shown in Fig. 1C were performed by using GraphPad Prism (Version 5.0). In Fig. 7D, sample distribution comparison was performed by the Kolmogorov–Smirnov test using CellQuest Pro software. Data are presented as mean ± SEM. P < 0.05 was considered significant.
SI Text
SI Experimental strategy to knock down AcP expression
To knock down AcP expression in rat-cultured neurons, the AcP–shRNA was designed to target a sequence in the unique 3′-terminal segment of the rat AcP mRNA (see AcP sequence below). By alternative splicing, the prototypical AcP C-terminal exon 12 is skipped, and a novel exon 12b is used for AcPb (21). Thus, AcP mRNA contains a unique 3′ terminal segment (415 nt). This AcP sequence is not present in AcPb mRNA, which contains a novel and longer 3′ terminal sequence (see AcPb sequence below).
SI Methods
Hippocampal Cell Cultures.
Cells were maintained in Neurobasal medium supplemented with B27, GlutaMAX, and penicillin/streptomycin (all culture reagents from Life Sciences). After 5–7 DIV, neurons were treated at 37 °C with 50 ng/mL BDNF, IL-1β (different concentrations) (PeproTech), 20 µM AS1 (Millipore), 20 µM EM163 (provided by J.R.), and 300 nM IL-1ra (PeproTech), with control neurons receiving equal volumes of vehicle.
Biochemistry.
After treatments, cultured neurons were washed in ice-cold PBS, lysed in radioimmunoprecipitation assay (RIPA) buffer [RIPA/Nonidet P-40 buffer containing protease and phosphatase inhibitor mixtures (Pierce, Thermo Fisher Scientific)], and immediately frozen. Neurons were harvested, and protein concentration was determined by BCA assay (Pierce; 23227). Membrane fraction homogenates from rat cortex, hippocampus, heart, and liver (Fig. S1) were obtained by homogenization of fresh tissue in ice-cold buffer containing 4 mM Hepes, 320 mM sucrose, 5 mM EDTA, and and protease and phosphatase inhibitor mixtures (pH 7) by using a glass–Teflon tissue grinder. Homogenates were centrifuged for 10 min at 2,000 × g at 4 °C; supernatants were centrifuged for 1 h at 100,000 × g at 4 °C. The pellet (which contains tissue membranes) was resuspended in 2 vol of lysis buffer and briefly homogenized by gentle vortex. Protein concentration was determined by BCA assay. Ficoll-gradient purified synaptosomes were sonicated in RIPA buffer, and protein concentration was determined by the Lowry method. All samples were supplemented with Laemmli buffer, boiled (7 min), run on Criterion XT gels, and then transferred to PVDF membranes according to the manufacturer's instructions (Bio-Rad). Membranes were blocked in 5% BSA or 5% milk (AcP antibody) for 1 h and then probed with primary antibody (4 °C, overnight). The membranes were washed (4 × 10 min in TBS with 0.1% Tween-20, vol/vol) and probed with HRP-conjugated secondary antibody for 1 h. The membranes were washed and developed by using Pierce Chemiluminescent Substrate (Pierce; 32106) or Luminata Crescendo Western HRP substrate (Millipore). Multiple film exposures were used to verify linearity. Blots were washed, stripped, and reprobed with antibodies to GAPDH, β-actin, or total levels of specific proteins. Membranes were incubated in stripping buffer according to the manufacturer's instructions. Stripping buffers were from Pierce (46430), Bioland Scientific (SB01-01), and Millipore (WB59). Stripping buffer was selected based on the effectiveness to remove the original signal, tested in pilot experiments. Antibodies used are listed in Table S1. AcP and AcPb were detected by using an antibody (Abcam) raised against a peptide corresponding to amino acids 525–540 of rat AcP, highly conserved in AcPb (20, 21), or with an AcP antibody against the N-terminal domain of human AcP (R&D). Quantitative densitometric analyses were performed on images of immunoblots with ImageJ (National Institutes of Health).
Immunoprecipitation was performed with a commercially available kit (Active Motif). Briefly, cultured neurons were washed in ice-cold PBS containing phosphatase inhibitors and lysed in IP-Buffer containing protease and phosphatase inhibitors, and protein concentration was determined by BCA assay. Protein concentration was adjusted with IP-Buffer to 0.1 µg/µL, and 500 µL was incubated overnight with rotation at 4 °C, with 2.5 µg of the anti–IL-1R1 antibody (C-terminal; Santa Cruz) or with a control rabbit IgG (2.5 µg). After incubation, protein-G magnetic beads were added and incubated for 1 h with rotation at 4 °C. Protein complexes were magnetically precipitated according to the manufacturer's instructions, lysed with Laemmli buffer (2×), boiled (7 min), run on Criterion XT gels, and transferred to PVDF membranes. Antibodies used for immunoprecipitation and blotting are listed in Table S1.
Immunocytochemistry.
After treatment, neurons were washed in cold PBS, fixed with paraformaldehyde (4%) for 20 min at room temperature (RT), permeabilized for 15 min in 100% methanol at −20 °C, and incubated for 30 min at RT in blocking buffer (5% goat serum and 0.1% Triton X-100 in PBS). Cells were incubated with primary antibodies [p-Akt and neuronal marker (Millimark; Millipore)] in blocking buffer overnight. Primary antibodies are listed in Table S1. After washes (×3, blocking buffer), secondary antibodies conjugated to Alexa Fluor 488 and 647 (Life Sciences) were incubated 1 h at RT. After washes (×3, PBS), coverslips were mounted by using Fluoromount (Vector Laboratories) followed by microscopic observations on an Olympus Fluoview FV1000 motorized inverted laser-scanning confocal microscope with Plan-Apo ×40/1.3-NA, in which the laser power and confocal settings were kept constant within experiments. ImageJ software was used for analysis. First, transfected cells were identified in the red channel (594 nm) as those with cell body RFP intensity at least 2 SDs greater than the mean. Next, the p-Akt/neuronal marker (green channel/blue channel) ratio was quantified in RFP-positive neurons from five to six fields per condition, acquired from a consistent starting point within each well.
Synaptosome Preparation by Ficoll/Sucrose Gradient.
Synaptosomes were prepared by using a discontinuous Ficoll gradient (69). In brief, dissected hippocampus from B6129SF2/J mice (7–8 and 13–15 mo) was homogenized (using a Dounce homogenizer) in 320 mM sucrose, 10 mM Tris⋅HCl (buffer A; pH 7.4) containing complete protease and phosphatase inhibitor mixtures. After homogenization, the crude synaptosomal fraction (synaptosomes plus mitochondria) was isolated by two sequential centrifugations (1,500 × g for 10 min, followed by 12,500 × g for 20 min at 4 °C). The pellet after the first centrifugation (P1) was discarded, and the supernatant (S1) was centrifuged at 12,500 × g. The P2 pellet, corresponding to the synaptosomal P2 fraction, was resuspended in 13% (final concentration) Ficoll 400 (in buffer A) and layered on the bottom of a discontinuous gradient, composed of buffer A and 7% Ficoll (in buffer A). The gradients were centrifuged at 100,000 × g for 45 min at 4 °C, and the synaptosomes were isolated at the 7.5–13% interface. After washing (×2, buffer A), synaptosome protein content was quantified.
RNA Extraction and qPCR.
Total RNA extraction from rat neuronal cultures, mouse whole brain (no cerebellum), and mouse hippocampal synaptosomes was performed by using TRIzol (Molecular Research Center) and the Qiagen RNeasy Mini Kit. The RNA Integrity Number of RNA samples was ≥7, as evaluated by using the Agilent Bioanalyzer at the Genomics High-Throughput Facility at the University of California, Irvine. In rat neuronal cultures, gene expression was quantified using TaqMan assays (Applied Biosystems). Briefly, 2–4 µg of total RNA was used for one-cycle reverse-transcriptase reaction to make cDNA according to the manufacturer’s protocol (QuantiTect Reverse Transcription Kit; Qiagen). For qPCR TaqMan assays, 25–100 ng of cDNA was mixed with appropriate primer sets and probes listed in Table S2 [AcP (21), AcPb (21), IL-1R1 (70), and GAPDH (70)]. GAPDH expression levels were used to normalize gene expression in each treatment. Reactions were run in a Stratagene MX3005P thermocycler (50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles: 95 °C for 15 s, 60 °C for 1 min) followed by a dissociation curve in the final step to verify the amplification of a single product. Each qPCR run included all samples run in triplicate. Data were analyzed by using the 2−ΔΔCt method and expressed as fold change over control after normalizing with control samples.
In total mouse brain (no cerebellum), 4 µg of total RNA was used for one-cycle reverse transcriptase reaction to make cDNA in accordance with the manufacturer’s protocol (QuantiTect Reverse Transcription Kit; Qiagen). qPCR was performed with 100 ng of cDNA using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies). Primer sets (71) are described in Table S3. GAPDH (primers at 500 nM; Qiagen; PPM02946E) expression levels were used for normalizing gene expression in each sample. Reactions were run in a Stratagene MX3005P thermocycler (95 °C for 3 min, 40 cycles; 95 °C for 20 s, and 60 °C for 20 s), and a dissociation curve in the final step to verify the amplification of a single product. Data were analyzed by the 2−ΔΔCt method and expressed as fold change over young mice (6 mo) after normalizing with input samples.
FASS-LTP.
Synaptosomes isolation.
Fresh crude synaptosome P2 fractions were obtained from whole mouse hippocampus (B6129SF2/J mice) by using our long-standing protocol (42). All steps for the synaptosome P2 fraction isolation were carried out at 4 °C, with the sucrose buffer, grinder, pestle, and microfuge tubes all precooled on ice. Hippocampi were rapidly dissected from a single mouse and homogenized in 320 mM sucrose (1.5 mL) containing Hepes (10 mM) and protease/phosphatase inhibitors mixture (Pierce), pH 7.4. Homogenization consisted of six to eight manual strokes in a glass–Teflon grinder; clearance between plunger and glass was 0.15–0.25 mm. Plunger was gently rotated during strokes, while the grinder was kept on ice. The homogenate was centrifuged at 1,200 × g for 10 min. Supernatant (S1, containing mitochondria and synaptosomes) was transferred into two clean microfuge tubes and centrifuged at 13,000 × g for 20 min. Supernatants (S2) were carefully removed by using a plastic tip and vacuum. Pellets (P2; corresponding to the crude synaptosome fraction) were resuspended by gently pipetting up and down in 1.5 mL of extracellular (tube 1) or cLTP (tube 2) solutions. Extracellular solution contained (in mM): 120 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 15 glucose, and 15 Hepes, pH 7.4; whereas cLTP solution was Mg2+-free and contained (in mM): 150 NaCl, 2 CaCl2, 5 KCl, 10 Hepes, and 30 glucose, pH 7.4 (73). Synaptosome P2 fractions were filtered with a 40-µm pore cell strainer (BD Biosciences) and incubated in a cell culture dish (30 mm) with agitation at RT for 15–30 min for recovery.
Stimulation.
After recovery, synaptosomes maintained in cLTP solution were supplemented with 0.001 mM strychnine and 0.02 mM bicuculline methiodide, and 180 µL of this suspension (containing 50–100 µg of protein, determined by BCA assay using BSA as a standard) were transferred to cytometry tubes. As control, an equal volume (180 µL) of synaptosome maintained in external solution was transferred to a cytometry tube. Synaptosomes in external solution were used to determine basal levels of potentiated synaptosomes (see below). All cytometry tubes were prewarmed in a 37 °C bath (5 min) before stimulation. External, glycine, and KCl solutions were also prewarmed at 37 °C. Next, 20 µL of external solution was added to control synaptosomes (in external solution), whereas 20 µL of glycine (5 mM in cLTP solution) was added to synaptosomes in cLTP solution (final [glycine] = 500 µM; ref. 74). Glycine was incubated for 15 min to prime synaptic NMDAR. For cytokine treatment, 3 pM IL-1β was added 5 min before glycine, whereas EM-163 or IL-1ra were added 10 min before IL-1β in the corresponding tubes. After glycine treatment, synaptosomes were depolarized with 200 µL of a [high] KCl solution consisting of (in mM): 50 NaCl, 2 CaCl2, 100 KCl, 10 Hepes, 30 glucose, 0.5 glycine, 0.001 strychnine, and 0.02 bicuculline methiodide, pH 7.4 (final [KCl] = 50 mM) and were incubated for 30 min. A total of 200 µL of external solution was added to control synaptosomes. KCl is used to depolarize and induce the release of endogenous glutamate from presynaptic terminals. Because the presynaptic active zone is adjacent to PSD, this protocol preferentially activates synaptic NMDARs. After KCl incubation, stimulation was stopped by sequential addition of 0.5 mL of ice-cold 0.1 mM EGTA–PBS (pH 7.4) and 4 mL of ice-cold blocking buffer (5% FBS in PBS). Tubes were chilled on ice and immediately centrifuged at 2,500 × g for 5 min at 4 °C (Sorvall RT6000B). After centrifugation, the pellet was resuspended by gentle finger agitation (no vortex) and kept on ice.
Immunolabeling.
Primary antibody solution (400 µL) was added to the resuspended pellet and incubated for 30 min on ice with agitation. Primary antibody solution contained rabbit anti-GluR1 and mouse anti-Nrx1β antibodies (listed in Table S1), each at a concentration of 2.5 µg/mL in blocking buffer (5% FBS in PBS). After incubation, synaptosomes were washed (×2) with 4 mL of ice-cold blocking buffer and centrifuged (2,500 × g for 5 min at 4 °C). Supernatant was discarded, and the pellet was gently resuspended. Secondary antibody solution (400 µL) was added to each tube and incubated for 30 min on ice with agitation and protected from light. After incubation, synaptosomes were washed as described. Secondary antibody solution contained anti-rabbit–Alexa 488 and anti-mouse–Alexa 647 antibodies (Life Sciences), each at a concentration of 2.5 µL/mL Endogenous/nonspecific background fluorescence for each marker was determined by using secondary antibody staining only in a tube containing synaptosomes in external solution. No differences in background fluorescence were found when comparing synaptosomes in external solution (basal state) or after cLTP stimulation. After the last wash, the pellet was resuspended as described, and 400 µL of 2% paraformaldehyde in PBS was added to each tube. Samples were protected from light, maintained at 4 °C, and run on a flow cytometer within 48 h.
Flow synaptometry.
Samples were acquired by using a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences) equipped with argon 488-nm and helium–neon 635-nm lasers. Relative size and granularity was determined by FSC and SSC properties. FSC, SSC, and fluorescence [FL1 (530 ± 15 nm) and FL4 (650 ± 25 nm)] signals were collected by using log amplification. FSC–SSC plots were used to select particles matching the size of synaptosomes (0.5–3.0 μm) by using calibrated beads (Fig. 6 A and B), as described (41). Identical FSC settings were used for acquiring data on bead standards and samples. Small fragments and debris were excluded by establishing a FSC-H threshold (325). Settings for fluorescence amplification on FL1 and FL4 photomultiplier tube detectors were based on the emission detected on size-based gated particles. Alexa 488 and 647 fluorochromes were detected by the FL1 and FL4 detectors, respectively. Synaptosome integrity was assessed with Calcein AM by using a FL1 detector. A total of 10,000 size-gated particles were collected and analyzed for each sample (event rate: ∼300 per s). Analysis was performed by using CellQuest Pro software (BD Biosciences).
OLM Test and p-p38 Detection in Synaptosomes.
Aged (22 mo old; n = 28) C57BL/6J (National Institute on Aging, Bethesda) mice were used for this study. All animals were handled for 2 min/d for 5 d, followed by habituation to the experimental apparatus (white rectangular open field measuring 30 × 23 × 21.5 cm) for 5 min/d for 4 consecutive days before training. On the test day, mice were injected with saline or LPS (0.3mg/kg; i.p.) and 30 min later with 20 μM AS1 or an equivalent volume of DMSO. Immediately after AS1 injection, mice were given a 5-min acquisition trial by exposing them to two objects placed in the test arena. At 45 min after the acquisition, animals were given a test trial (retention, 5 min) where one object was moved to a novel location, whereas the location of the other object remained constant. Animals were allowed to explore the objects freely, and the time spent exploring each object was recorded. Exploration was scored when a mouse's head was oriented toward the object within a distance of 1 cm or when the nose was touching the object. The relative exploration time was recorded and expressed as a discrimination index [DI = (tnovel − tfamiliar)/(tnovel + tfamiliar)]. All animals were euthanized immediately after OLM testing, and hippocampi were removed and immediately processed for Western blot or flow cytometry assays.
Immunolabeling of synaptosomes for p-p38 after OLM test was performed by using a method to specifically stain intracellular antigens. Immediately after OLM, the synaptosome P2 fraction was obtained, and 200 µL (50–100 µg of protein determined by BCA assay using BSA as a standard) of this suspension was transferred to prechilled cytometry tubes and washed with 4 mL of ice-cold PBS as described above (FASS-LTP). After centrifugation, the pellet was resuspended and fixed with PFA ([final] = 2%) for 20 min at 4 °C. Permeabilization was performed by adding ice-cold 100% methanol slowly (while gently vortexing) to a final concentration of 90% methanol and incubating 15 min on ice. Samples were washed with ice-cold PBS, blocked with 200 µL of Blocking/Perm buffer (5% FBS, 0.1% Triton-X in PBS) for 10 min, and stained by adding 200 µL of rabbit anti-p-p38–Phycoerythrin (PE) antibody (Table S1) solution (5 µg/mL; 4 °C, 30 min on agitation and protected from light). Samples were washed (×2) with ice-cold Blocking/Perm buffer, and pellets were resuspended in 400 µL of 2% PFA. Endogenous/nonspecific background fluorescence was determined by using a rabbit IgG antibody (isotype control) PE-conjugated (Table S1).
Acknowledgments
We thank Michelle Nguyen for assistance in performing imaging experiments; and Dr. Andrea Tenner and Michael Hernandez for use of the flow cytometer and CellQuest Pro software. This work was supported by National Institutes of Health Grants P01-AG000538, R01-AG34667 (to C.W.C.), and AG027544-06 (to F.M.L.); and Larry Hillblom Foundation Grant 2013-A-016-FEL (to D.B.-V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514486112/-/DCSupplemental.
References
- 1.Lynch MA. Age-related impairment in long-term potentiation in hippocampus: A role for the cytokine, interleukin-1 beta? Prog Neurobiol. 1998;56(5):571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 2.Trompet S, et al. PROSPER Group Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain. 2008;131(Pt 4):1069–1077. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- 3.Youm YH, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18(4):519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18(8):2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology. 2015;96(Pt A):11–18. doi: 10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32(42):14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank MG, et al. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24(2):254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrientos RM, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, et al. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Y, et al. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS One. 2014;9(9):e106837. doi: 10.1371/journal.pone.0106837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarr AJ, et al. The effects of age on lipopolysaccharide-induced cognitive deficits and interleukin-1β expression. Behav Brain Res. 2011;217(2):481–485. doi: 10.1016/j.bbr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: Peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30(22):7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31(11):4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas C, Bazan JF, Garcia KC. Structure of the activating IL-1 receptor signaling complex. Nat Struct Mol Biol. 2012;19(4):455–457. doi: 10.1038/nsmb.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: An adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7(6):837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 19.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3(105):cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 20.Lu HL, et al. A novel alternatively spliced interleukin-1 receptor accessory protein mIL-1RAcP687. Mol Immunol. 2008;45(5):1374–1384. doi: 10.1016/j.molimm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Smith DE, et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity. 2009;30(6):817–831. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Smith DE, Ibáñez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31(49):18048–18059. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosselin D, Bellavance MA, Rivest S. IL-1RAcPb signaling regulates adaptive mechanisms in neurons that promote their long-term survival following excitotoxic insults. Front Cell Neurosci. 2013;7:9. doi: 10.3389/fncel.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hein AM, et al. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24(2):243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman WJ. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Exp Neurol. 2001;168(1):23–31. doi: 10.1006/exnr.2000.7595. [DOI] [PubMed] [Google Scholar]
- 26.Docagne F, et al. Differential regulation of type I and type II interleukin-1 receptors in focal brain inflammation. Eur J Neurosci. 2005;21(5):1205–1214. doi: 10.1111/j.1460-9568.2005.03965.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith ED, et al. Rapamycin and interleukin-1β impair brain-derived neurotrophic factor-dependent neuron survival by modulating autophagy. J Biol Chem. 2014;289(30):20615–20629. doi: 10.1074/jbc.M114.568659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29(9):1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong L, et al. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosci. 2012;32(49):17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 31.Gardoni F, et al. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1β and NMDA stimulation. J Neuroinflammation. 2011;8(1):14. doi: 10.1186/1742-2094-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhelm BG, et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 2014;344(6187):1023–1028. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29(1):243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 34.Musleh W, Bi X, Tocco G, Yaghoubi S, Baudry M. Glycine-induced long-term potentiation is associated with structural and functional modifications of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA. 1997;94(17):9451–9456. doi: 10.1073/pnas.94.17.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurado S, et al. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron. 2013;77(3):542–558. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305(5692):1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 37.Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci. 2005;8(7):853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- 38.Fortin DA, et al. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci. 2010;30(35):11565–11575. doi: 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loo LS, Tang N, Al-Haddawi M, Dawe GS, Hong W. A role for sorting nexin 27 in AMPA receptor trafficking. Nat Commun. 2014;5:3176. doi: 10.1038/ncomms4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi SH, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 41.Fein JA, et al. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol. 2008;172(6):1683–1692. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park ME, Horch P, Cotman CW. Evaluation of glutamate as a hippocampal neurotransmitter: Glutamate uptake and release from synaptosomes. Brain Res. 1978;142(2):285–299. doi: 10.1016/0006-8993(78)90636-4. [DOI] [PubMed] [Google Scholar]
- 43.Fu Y, Huang ZJ. Differential dynamics and activity-dependent regulation of alpha- and beta-neurexins at developing GABAergic synapses. Proc Natl Acad Sci USA. 2010;107(52):22699–22704. doi: 10.1073/pnas.1011233108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bach ME, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96(9):5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fouquet C, et al. Early detection of age-related memory deficits in individual mice. Neurobiol Aging. 2011;32(10):1881–1895. doi: 10.1016/j.neurobiolaging.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Griffin R, et al. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99(4):1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 47.Bartfai T, et al. A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc Natl Acad Sci USA. 2003;100(13):7971–7976. doi: 10.1073/pnas.0932746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kissner TL, et al. Therapeutic inhibition of pro-inflammatory signaling and toxicity to staphylococcal enterotoxin B by a synthetic dimeric BB-loop mimetic of MyD88. PLoS One. 2012;7(7):e40773. doi: 10.1371/journal.pone.0040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis CN, et al. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc Natl Acad Sci USA. 2006;103(8):2953–2958. doi: 10.1073/pnas.0510802103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113(3):509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 51.Goshen I, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8-10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Spulber S, et al. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. J Neuroimmunol. 2009;208(1-2):46–53. doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Goshen I, et al. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci. 2009;29(11):3395–3403. doi: 10.1523/JNEUROSCI.5352-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalez P, et al. Molecular mechanisms involved in interleukin 1-beta (IL-1β)-induced memory impairment. Modulation by alpha-melanocyte-stimulating hormone (α-MSH) Brain Behav Immun. 2013;34:141–150. doi: 10.1016/j.bbi.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20(18):6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23(5):1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verbitsky M, et al. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn Mem. 2004;11(3):253–260. doi: 10.1101/lm.68204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moraes CA, et al. Activated microglia-induced deficits in excitatory synapses through IL-1β: Implications for cognitive impairment in sepsis. Mol Neurobiol. 2015;52(1):653–663. doi: 10.1007/s12035-014-8868-5. [DOI] [PubMed] [Google Scholar]
- 60.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20(17):6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grant SG. Synaptopathies: Diseases of the synaptome. Curr Opin Neurobiol. 2012;22(3):522–529. doi: 10.1016/j.conb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 63.Goshen I, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 64.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffin WS, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parnet P, Kelley KW, Bluthé RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125(1-2):5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell type-specific interleukin-1beta signaling in the CNS. J Neurosci. 2004;24(29):6482–6488. doi: 10.1523/JNEUROSCI.5712-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi S, Friedman WJ. Inflammatory cytokines IL-1β and TNF-α regulate p75NTR expression in CNS neurons and astrocytes by distinct cell-type-specific signalling mechanisms. ASN Neuro. 2009;1(2):e00010. doi: 10.1042/AN20090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Booth RF, Clark JB. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison DC, et al. The use of quantitative RT-PCR to measure mRNA expression in a rat model of focal ischemia--caspase-3 as a case study. Brain Res Mol Brain Res. 2000;75(1):143–149. doi: 10.1016/s0169-328x(99)00305-8. [DOI] [PubMed] [Google Scholar]
- 71.Taishi P, et al. Brain-specific interleukin-1 receptor accessory protein in sleep regulation. J Appl Physiol. 2012;112(6):1015–1022. doi: 10.1152/japplphysiol.01307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corera AT, Doucet G, Fon EA. Long-term potentiation in isolated dendritic spines. PLoS One. 2009;4(6):e6021. doi: 10.1371/journal.pone.0006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park M, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52(5):817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen RQ, et al. Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology. 2011;36(9):1948–1958. doi: 10.1038/npp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Intlekofer KA, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38(10):2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]