Significance
Nitrification, the sequential aerobic oxidation of ammonia via nitrite to nitrate, is a key process of the biogeochemical nitrogen cycle and catalyzed by two aerobic microbial guilds (nitrifiers): ammonia oxidizers and nitrite-oxidizing bacteria (NOB). NOB are generally considered as metabolically restricted and dependent on ammonia oxidizers. Here, we report that, surprisingly, key NOB of many ecosystems (Nitrospira) convert urea, an important ammonia source in nature, to ammonia and CO2. Thus, Nitrospira supply urease-negative ammonia oxidizers with ammonia and receive nitrite produced by ammonia oxidation in return, leading to a reciprocal feeding interaction of nitrifiers. Moreover, Nitrospira couple formate oxidation with nitrate reduction to remain active in anoxia. Accordingly, Nitrospira are unexpectedly flexible and contribute to nitrogen cycling beyond nitrite oxidation.
Keywords: Nitrospira, nitrification, genome, urease, formate
Abstract
Nitrospira are a diverse group of nitrite-oxidizing bacteria and among the environmentally most widespread nitrifiers. However, they remain scarcely studied and mostly uncultured. Based on genomic and experimental data from Nitrospira moscoviensis representing the ubiquitous Nitrospira lineage II, we identified ecophysiological traits that contribute to the ecological success of Nitrospira. Unexpectedly, N. moscoviensis possesses genes coding for a urease and cleaves urea to ammonia and CO2. Ureolysis was not observed yet in nitrite oxidizers and enables N. moscoviensis to supply ammonia oxidizers lacking urease with ammonia from urea, which is fully nitrified by this consortium through reciprocal feeding. The presence of highly similar urease genes in Nitrospira lenta from activated sludge, in metagenomes from soils and freshwater habitats, and of other ureases in marine nitrite oxidizers, suggests a wide distribution of this extended interaction between ammonia and nitrite oxidizers, which enables nitrite-oxidizing bacteria to indirectly use urea as a source of energy. A soluble formate dehydrogenase lends additional ecophysiological flexibility and allows N. moscoviensis to use formate, with or without concomitant nitrite oxidation, using oxygen, nitrate, or both compounds as terminal electron acceptors. Compared with Nitrospira defluvii from lineage I, N. moscoviensis shares the Nitrospira core metabolism but shows substantial genomic dissimilarity including genes for adaptations to elevated oxygen concentrations. Reciprocal feeding and metabolic versatility, including the participation in different nitrogen cycling processes, likely are key factors for the niche partitioning, the ubiquity, and the high diversity of Nitrospira in natural and engineered ecosystems.
Nitrification, a key aerobic process of the biogeochemical nitrogen (N) cycle, is catalyzed by two guilds of chemolithoautotrophic microorganisms. The ammonia-oxidizing microorganisms (bacteria and archaea; AOM) oxidize ammonia to nitrite, which is subsequently oxidized to nitrate by nitrite-oxidizing bacteria (NOB). Nitrification links aerobic and anaerobic pathways of the N cycle by providing nitrate or nitrite as electron acceptors for dissimilatory nitrate reduction, denitrification, respiratory ammonification, and anaerobic ammonium oxidation (1, 2). The end product of nitrification, nitrate, is an important source of nitrogen for assimilation by many microorganisms and plants. Moreover, nitrification is a key step of biological wastewater treatment but also contributes to N losses from fertilized agricultural soils (3). Being a two-step process that involves two functional groups, nitrification is a prime example of a tight metabolic interaction between free-living microorganisms.
Current insights into the ecology of chemolithoautotrophic NOB suggest that two of the six known NOB genera are restricted to marine ecosystems (Nitrospina and Nitrococcus) (4). The recently identified Nitrolancea was enriched from activated sludge from a wastewater treatment plant (WWTP) (5), whereas Nitrotoga occur in soils and WWTPs (6, 7). Nitrobacter are generally common in terrestrial and limnic habitats. Analyses of Nitrobacter genome sequences provided first insights into the genomic makeup of NOB and revealed a greater metabolic flexibility than anticipated earlier, which included the mixotrophic utilization of various organic substrates (8, 9). However, Nitrobacter require high nitrite concentrations (10, 11), and molecular surveys indicated that Nitrobacter are not the primary NOB in ecosystems with low ambient nitrite levels such as unfertilized soils (12), freshwater habitats (13), and most WWTPs (14).
Among all known NOB, the genus Nitrospira appears to be most widespread in different habitat types and is phylogenetically most diverse. Nitrospira are well adapted to low nitrite concentrations (10, 11) and form at least six phylogenetic lineages (15, 16) that are globally distributed in soils (17, 18), the oceans (19), freshwater habitats (20), hot springs (16), and many other oxic habitats (15). In addition, Nitrospira members are the key NOB in most WWTPs (14, 15). Nitrospira are notoriously difficult to culture under laboratory conditions and, hence, despite their ecological and biotechnological importance, little is known about their ecophysiology. Interestingly, not all members of this genus are restricted to nitrite as their sole source of energy and reductant. Some Nitrospira from marine ecosystems or activated sludge can use simple organic substrates, such as pyruvate, formate, and glycerol, for carbon assimilation and probably also as energy sources in addition to CO2 and nitrite (mixotrophy) (15, 19, 21, 22). Nitrospira moscoviensis even grows by aerobic hydrogen oxidation as an alternative lifestyle outside the N cycle (23). Furthermore, this organism can reduce nitrate with H2 as an electron donor, but under these conditions, growth was not detected (24).
So far, only one study analyzed a fully sequenced Nitrospira genome, which was obtained from N. defluvii (25). This Nitrospira lineage I member had been enriched from a WWTP (26). Here, we analyzed the genome of N. moscovienis representing Nitrospira lineage II, which is the Nitrospira clade most widely distributed in both natural and engineered ecosystems (15). This newly sequenced Nitrospira genome revealed surprising metabolic features that were experimentally confirmed. These findings change the current perception on the interdependence of nitrifiers and demonstrate an unexpected ecophysiological versatility of Nitrospira with contributions to N-cycling processes other than nitrite oxidation.
Results and Discussion
Hydrolysis of Urea.
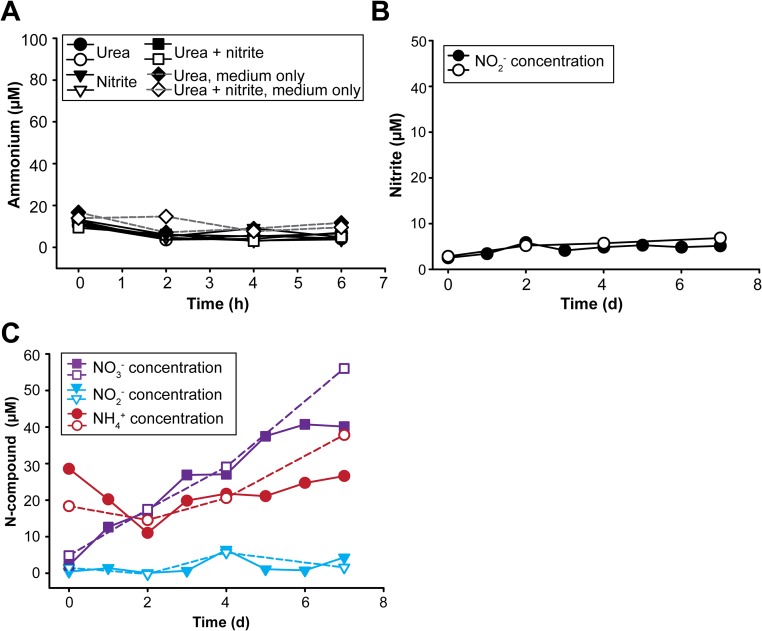
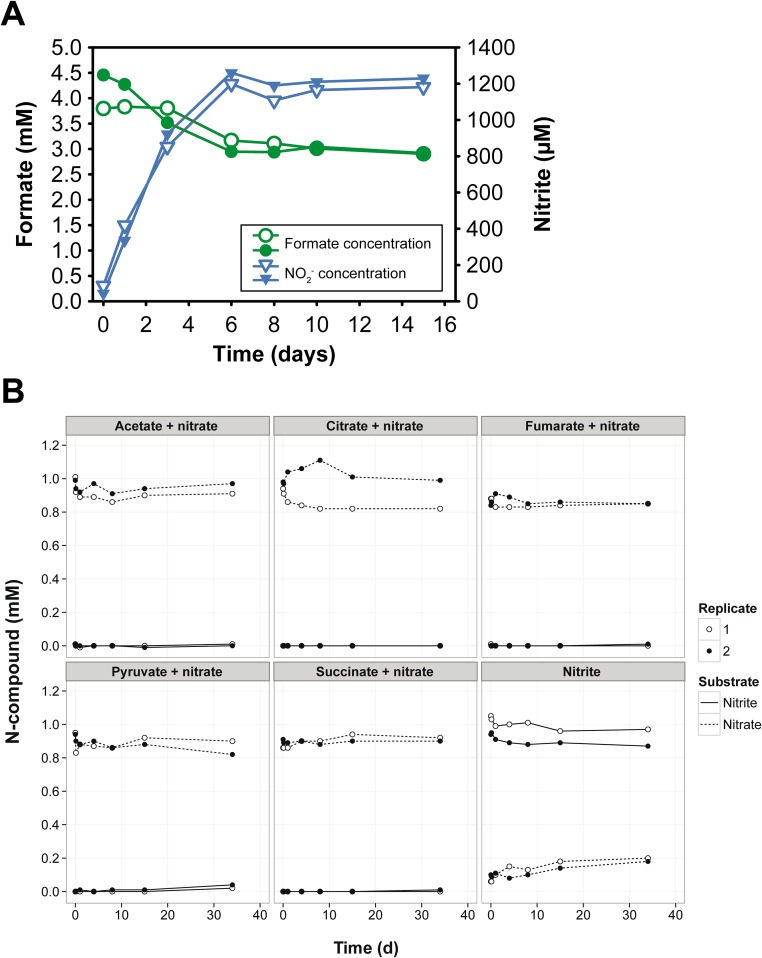
The complete genome of N. moscoviensis comprises 4.59 Mb with 4,863 predicted coding sequences (CDS) (Table S1). Among the most striking features identified in the genome were a functional hydrogenase for nitrite-independent growth (23) and a gene cluster for the utilization of urea. This locus codes for the urea-binding subunit of a urea ABC transporter (NITMOv2_1251), all three subunits of a putative nickel-binding urease (UreABC; NITMOv2_1253, NITMOv2_1255, and NITMOv2_1260), and the accessory proteins UreF, UreG, UreD required for urease maturation (27) (Fig. S1A, Dataset S1, and SI Results and Discussion). Urease (EC 3.5.1.5) catalyzes the ATP-independent hydrolysis of urea resulting in ammonia and carbamate, which spontaneously decomposes into a second molecule of ammonia and bicarbonate (27). In the context of nitrification, urease is an important enzyme that enables some ammonia-oxidizing bacteria and archaea to obtain both ammonia and CO2 from urea. Thus, these urease-positive AOM can use urea as a source of energy, N, and carbon (28). In contrast, the presence of urease-encoding genes in a nitrite-oxidizing bacterium was unexpected because NOB would not be able to use ammonia as energy source and urea degradation by NOB had never been observed. To confirm the ureolytic activity, a pure culture of N. moscoviensis was incubated in liquid mineral medium that contained 1 mM urea. Indeed, within a few hours of incubation, a strong increase of the free ammonium concentration was observed in the culture supernatant, which was independent from the concomitant presence of nitrite and did not occur in the control experiments without N. moscoviensis biomass (Fig. 1). This result suggests that after uptake into the Nitrospira cells by an ABC transporter or passive diffusion (SI Results and Discussion), urea was cleaved by the cytoplasmic urease (Fig. S1B). Ammonia then diffused through the cell membrane into the medium or ammonium was exported by AmtB transporters, which are encoded by three amtB genes in the close vicinity of the urease genes (Fig. S1A). Consistent with the lack of urease genes in the genome of N. defluvii, no ureolytic activity was observed during incubation of this Nitrospira strain in medium containing urea (Fig. S2A).
Table S1.
General genome characteristics of N. moscoviensis and N. defluvii
| Genome feature | N. moscoviensis | N. defluvii |
| Genome size, bp | 4,589,485 | 4,317,083 |
| Average G+C content, % | 62 | 59 |
| No. of CDS | 4,863 | 4,274 |
| Coding density, % | 90.6 | 89.4 |
| CDS with predicted function | 2,391 (56%) | 2,154 (50%) |
| rRNA operon | 1 | 1 |
| tRNA genes | 47 | 46 |
| Species-specific CDS* | 2,161 (44%) | 1,695 (40%) |
| Species-specific CDS with unknown function | 1,547 | 1,216 |
CDS, coding sequences.
CDS with no homolog in the respective other Nitrospira genome were considered to be species-specific. Homologous proteins were defined as ≥30% identical over ≥80% of the amino acid sequence length.
Fig. S1.
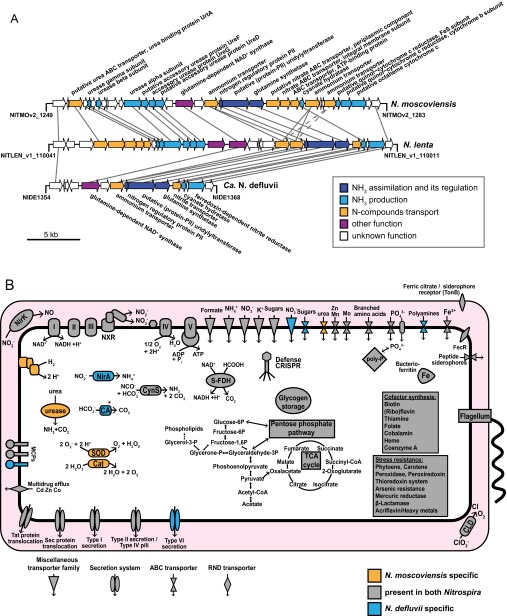
Key genomic and metabolic features of Nitrospira. (A) Schematic representation of the genomic regions in N. moscoviensis and N. lenta that contain the urease genes, the urea ABC transporter, and various other genes involved in the acquisition and metabolism of N compounds. The respective locus in N. defluvii that lacks urease and the urea transporter is shown for comparison. Solid lines connect homologous genes that encode proteins sharing sequence similarities above 50%. Dashed lines connect genes that encode proteins sharing sequence similarities between 30% and 50%. (B) Cell metabolic cartoon constructed from the annotations of the N. moscoviensis and N. defluvii genomes. Core functions, which are shared by both Nitrospira members (gray), and strain-specific features (yellow and blue) are shown. CA, carbonic anhydrase; Cat, catalase; CLD, chlorite dismutase; CRISPR, clustered regularly interspaced short palindromic repeats; CynS, cyanate hydratase (cyanase); MCPs, methyl-accepting chemotaxis proteins; S-FDH, soluble formate dehydrogenase. Enzyme complexes of the electron transport chain are labeled by Roman numerals. The TCA cycle depicts both directions (oxidative and reductive), with the reductive TCA cycle being used by Nitrospira for CO2 fixation. *, N. defluvii possesses a canonical CA, whereas N. moscoviensis has only a putative CA-like protein (NITMOv2_0219) that contains the metal binding sites, but lacks some catalytic residues of canonical CA.
Fig. 1.
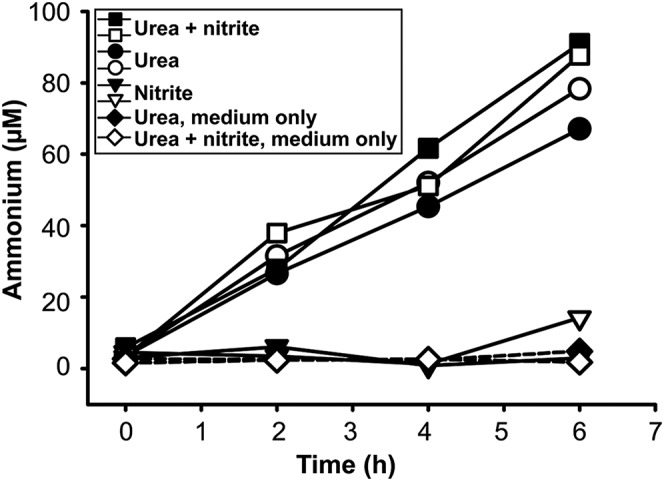
Ureolytic activity of a N. moscoviensis pure culture during incubations with urea or both urea and nitrite. Supply of urea led to the accumulation of ammonium in the culture supernatant. Ammonium formation was not detected in the absence of urea (nitrite-only incubations) and in the control experiments without addition of biomass. The results of two biological replicates are shown for each incubation experiment.
Fig. S2.
Full nitrification of urea by reciprocal feeding. (A) Absence of ureolytic activity in N. defluvii. Incubation of N. defluvii cells in medium containing 1 mM urea or 0.5 mM nitrite or both 1 mM urea and 0.5 mM nitrite. No release of free ammonium was observed in any incubation. Control experiments with cell-free medium containing either 1 mM urea or 1 mM urea and 0.5 mM nitrite confirmed that chemical urea degradation did not affect the results. Two biological replicates are shown for all incubations with N. defluvii. (B) Absence of ureolytic activity in N. europaea. Incubation of N. europaea cells in medium containing 1 mM urea as the sole source of ammonia. No ammonia oxidation (production of nitrite) was observed in the two biological replicates. Aliquots of the same N. europaea biomass were used in the coincubation experiment with N. moscoviensis (see Results and Discussion in the main text). (C) Coincubation of N. moscoviensis and urease-negative N. europaea in presence of 50 µM urea as the source of ammonia. The concentrations of free ammonium, nitrite, and nitrate in the culture supernatant during 7 d of incubation are shown. At the start of the incubation, the medium contained some ammonium, most likely due to carryover with the N. europaea inoculum. Full nitrification occurred in each of the two biological replicates.
Ureolytic Activity of NOB Drives Full Nitrification.
Urea is an important dissolved organic N compound in marine (coastal and open water) and freshwater ecosystems, where it is produced by heterotrophic bacteria and also released by phytoplankton, microfauna, and macrofauna (29). Because commercially synthesized urea plays a major role as plant fertilizer, urea is globally widespread in agricultural soils. Fertilization also contributes to increased urea levels in coastal waters due to riverine input. In addition, a large portion of the N in municipal wastewater occurs as urea. Despite the environmental abundance of urea, not all AOM possess urease (28), and urease-negative AOM are thought to thrive mainly in habitats where free ammonia (NH3) levels are relatively high, such as eutrophic waters and neutral or alkaline soils. They appear to be outcompeted by urease-positive AOM in acid soils where free NH3 is scarce but urea is a source of NH3 (28).
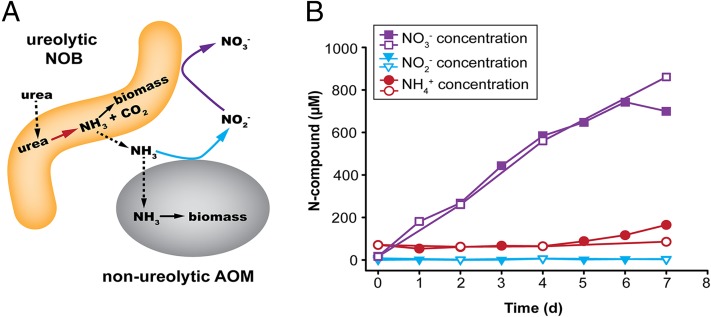
AOM and NOB are generally considered to be mutualistic symbionts because AOM produce nitrite, which is required as substrate and also detoxified by NOB (30). Accordingly, nitrifiers often tightly coaggregate in flocs and biofilms (14), and the abundances of AOA and Nitrospira in soils have been found to correlate (17). This classical scheme dictates that NOB strongly depend on AOM to initiate nitrification. The presence of urease and ureolytic activity in N. moscoviensis changes this picture and opens an interesting perspective on the interactions between NOB and AOM. Ureolytic NOB could actually feed urease-negative AOM by cleaving urea and releasing ammonia. The AOM would subsequently oxidize the ammonia to nitrite, providing the NOB with their actual source of energy (Fig. 2A). The ammonia and CO2 obtained from urea would also be N and carbon sources for assimilation by both partners. Interestingly, the cleavage of urea by NOB represents the initial step that fuels nitrification by such NOB–AOM consortia.
Fig. 2.
Full nitrification by N. moscoviensis and urease-negative AOM through reciprocal feeding. (A) Schematic illustration of the proposed reciprocal feeding interaction between ureolytic NOB such as N. moscoviensis (yellow) and urease-negative AOM such as N. europaea (gray). Solid arrows represent conversions of substrates; dashed arrows the uptake or release of substrates. (B) Concentrations of ammonium, nitrite, and nitrate in a coincubation of N. moscoviensis and urease-negative N. europaea during 7 d of incubation with urea as the sole source of energy and nitrogen. The results of two biological replicates are shown for all incubations.
To test whether this “reciprocal feeding” NOB–AOM interaction actually occurs, we coincubated a pure culture of N. moscoviensis with a pure culture of the urease-negative ammonia-oxidizing bacterium Nitrosomonas europaea, whose sequenced genome lacks any genes for urea utilization (31) and which did not show ureolytic activity in a control experiment (Fig. S2B). The only substrates provided to this coincubation were 1 mM urea as well as O2 and CO2 from air. During 7 d of incubation, nitrate accumulated in the culture supernatant, whereas the concentrations of ammonium and nitrite did not increase (Fig. 2B). The only explanation for this result is full nitrification by reciprocal feeding (Fig. 2A). Full nitrification was also observed in a second coincubation experiment with an initial urea concentration of only 50 µM, which is closer to the micromolar levels of urea found in natural habitats (29) (Fig. S2C).
Intriguingly, genes for urea utilization occur also in NOB other than N. moscoviensis. The recently sequenced draft genome of Nitrospira lenta, a strain which also belongs to Nitrospira lineage II (32), encodes a urea ABC transporter and the urease at a genomic locus that is mostly syntenic with the homologous region in N. moscoviensis (Fig. S1A and Dataset S1). According to the phylogeny of the urease alpha subunit UreC, these two Nitrospira ureases are closely related and clearly distinct from the ureases of all known AOM (Fig. S3). Two Nitrospira-like UreC sequences were also found in an activated sludge metagenome from a municipal WWTP (Aalborg West, Denmark). They were linked to scaffolds that encoded several other proteins with highest sequence similarities to homologs in N. defluvii, strongly suggesting a Nitrospira origin. Together with the UreC sequences from N. moscoviensis and N. lenta, they form a distinct monophyletic clade (Fig. S3). A screening of publicly available metagenomes from various environments retrieved additional UreC sequences that fall into this Nitrospira UreC clade and share high amino acid identity of 85.3–96.3% with UreC from N. moscoviensis, N. lenta, and the sequences from the Danish WWTP. These sequences from soil and freshwater metagenomes (Fig. S3) suggest that Nitrospira with urease occur widespread in different terrestrial and aquatic habitats where urea is available (e.g., due to fertilization). Hence, it is tempting to speculate that reciprocal feeding of Nitrospira and AOM could be a common phenomenon, whose contribution to the total nitrification in different ecosystems remains to be determined.
Fig. S3.
Phylogenetic affiliation of the urease alpha subunits (UreC) from Nitrospira, Nitrospina, and other nitrifiers. A Bayesian 80% consensus amino acid tree is shown. The degree of posterior support of a branch is indicated by a single asterisk for >90% posterior probability (PP), a double asterisk for >99% PP or a triple asterisk for >99.9% PP. For metagenomic UreC sequences, the gene ID is followed by the IMG metagenome ID (for UreC received from IMG) and the description of the source habitat. The scale bar shows 7% estimated sequence divergence.
Single-cell genomes from uncultured Nitrospina-like NOB (accession nos. PRJNA199992 and PRJNA199055) also contain complete sets of urease genes. The genus Nitrospina (4) represents the major known group of marine NOB, and the capability of using urea as N source and for reciprocal feeding with AOM could be beneficial in oceanic habitats, where the availability of urea can be higher than that of ammonium (33). Because the genome of the type strain Nitrospina gracilis (34) lacks urease, this feature is not homogeneously distributed in this genus, as it is not among Nitrospira (see above). The ureases of Nitrospira and Nitrospina are phylogenetically not closely related. The Nitrospina UreC sequences fall into a lineage of ureases from Proteobacteria (Fig. S3), indicating that the ureC gene was subject to lateral gene transfer. All three sequenced Nitrobacter genomes lack urease but encode putative urea carboxylases and allophanate hydrolases, which together might cleave urea by an ATP-dependent mechanism (9). However, these candidate proteins in Nitrobacter are shorter than their functionally characterized homologs in other microorganisms. Similar short variations occur also in the genomes of N. defluvii and N. europaea, both of which are unable to use urea (Fig. S2 A and B), suggesting that these enzymes play a different and yet unknown functional role.
Nitrification by reciprocal feeding may influence the population structure of nitrifiers. AOM lacking urease could survive by tightly interacting with ureolytic NOB if urea is the main source of ammonia. This mechanism could influence the local distribution of urease-negative AOM in microniches shared with ureolytic NOB within soils and biofilms, and it might contribute to a high nitrifier diversity by sustaining populations of urease-negative AOM in otherwise unfavorable habitats. Additionally, it demonstrates that NOB can take critical roles within AOM–NOB consortia beyond nitrite oxidation and shows that the interplay between nitrifiers can be surprisingly complex. Reciprocal feeding interactions represent a compartmentalization of functions, which balances the metabolic and genomic costs among the partners (35) and might thus make a nitrifier consortium bioenergetically more efficient. Additional aspects of complemental AOM–NOB symbioses might include the interspecies transfer of Fe-loaded siderophores and of various organic N and C compounds and cofactors (36). Such complex interactions of nitrifiers may need a tight regulation, which could be achieved by cell-cell communication with diffusible signal molecules (36, 37).
Use of Formate with O2 or Nitrate as Electron Acceptor.
Nitrospira can reach high abundances in deep layers of nitrifying biofilms where low dissolved oxygen concentrations prevail (38, 39). To elucidate whether Nitrospira may survive in such niches by other metabolic activities than aerobic nitrite oxidation, we incubated N. moscoviensis cells under anoxic conditions in the presence of formate as energy source and electron donor and nitrate as terminal electron acceptor. Nitrate was chosen because its reduction by N. moscoviensis to nitrite had already been observed with H2 as electron donor (24). Formate was added because the genomes of N. moscoviensis (Dataset S1) and N. defluvii (25) encode a NAD+-dependent soluble formate dehydrogenase and a transporter from the formate/nitrite transporter family (NITMOv2_3821, NITMOv2_3822, NITMOv2_3823, NITMOv2_3825) (Fig. S1B). Formate is a common product of fermenting organisms, which may occur in the spatial proximity of Nitrospira in hypoxic or anoxic habitats.
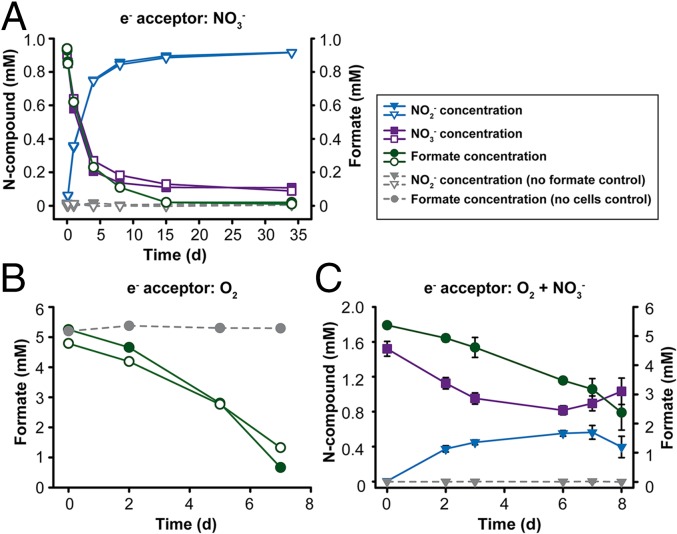
Indeed, the provided nitrate was reduced to nitrite upon addition of 1 mM formate, which was completely consumed during the incubations (Fig. 3A). When formate was provided in excess, its consumption stopped after all nitrate had been reduced to nitrite (Fig. S4A). These results demonstrate the utilization of nitrate instead of oxygen as terminal electron acceptor. The observed nitrate reduction was most likely catalyzed by nitrite oxidoreductase (NXR) (SI Results and Discussion) operating in the reverse direction because no other enzyme for dissimilatory nitrate reduction was found in the genome of N. moscoviensis. All supplied nitrate was nearly stoichiometrically reduced to nitrite (Fig. 3A and Fig. S4A). N. moscoviensis encodes four copper-containing dissimilatory nitrite reductases (NirK), which were apparently not strongly active during these incubations. Because no nitrite was formed in medium containing only nitrate but no formate (Fig. 3A), we can exclude the possibility that N. moscoviensis reduced nitrate with electrons derived from intracellular storage compounds. No nitrate reduction was observed with some other simple organic substrates tested (Fig. S4B and SI Results and Discussion).
Fig. 3.
Formate utilization by a pure culture of N. moscoviensis. (A) Anaerobic consumption of formate with nitrate as terminal electron acceptor. Nitrate was nearly stoichiometrically reduced to nitrite. No nitrite formation from nitrate was observed in the control experiment without formate. The results of two biological replicates are shown for all incubations. (B) Aerobic use of formate with O2 as terminal electron acceptor. For the incubations with N. moscoviensis cells, the results of two biological replicates are shown. Please note that the control experiment with formate but without cells, which confirms the chemical stability of formate, was performed for these incubation conditions only. (C) Aerobic use of formate with both O2 and nitrate as terminal electron acceptors. The oxidation of the formed nitrite became detectable on the seventh day of incubation. Data points show the means of biological replicates (n = 3). Error bars represent SD and are not shown if smaller than symbols. An extended incubation experiment (23 d) confirming the concomitant utilization of formate and nitrite is shown in Fig. S5 D–F.
Fig. S4.
Incubation experiments of N. moscoviensis with organic substrates in anoxia. (A) Anaerobic consumption of formate (initial concentration 4.5 mM) with nitrate (initial concentration 1.2 mM) as terminal electron acceptor. Nitrate was nearly stoichiometrically reduced to nitrite. The consumption of formate, which was provided in excess, ceased when all nitrate had been reduced. The results of two biological replicates are shown. The divergence of the formate concentrations measured on days 0 and 1 was caused by technical problems with formate measurement. The increase in nitrite indicates that formate was consumed by N. moscoviensis in both replicates during this period. (B) Incubations with various organic compounds under anoxic conditions. Nitrate (1 mM) was provided as terminal electron acceptor in absence of O2. The initial concentration of each organic substrate was 1 mM. The consumption of nitrate and production of nitrite, which would indicate the utilization of the respective organic substrate as electron donor, was not observed in any incubation. The concentrations of the organic substrates at the beginning and end of the incubations were identical (not plotted). A control experiment with nitrite (1 mM) and no organic substrate confirmed the absence of nitrite-oxidizing activity under the anoxic conditions applied. The results of two biological replicates are shown for all incubations.
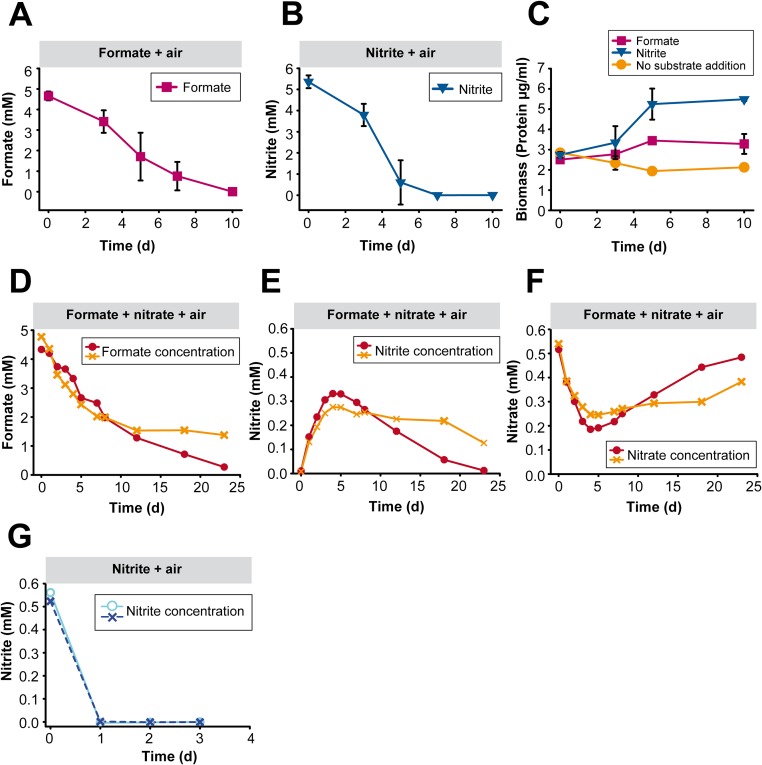
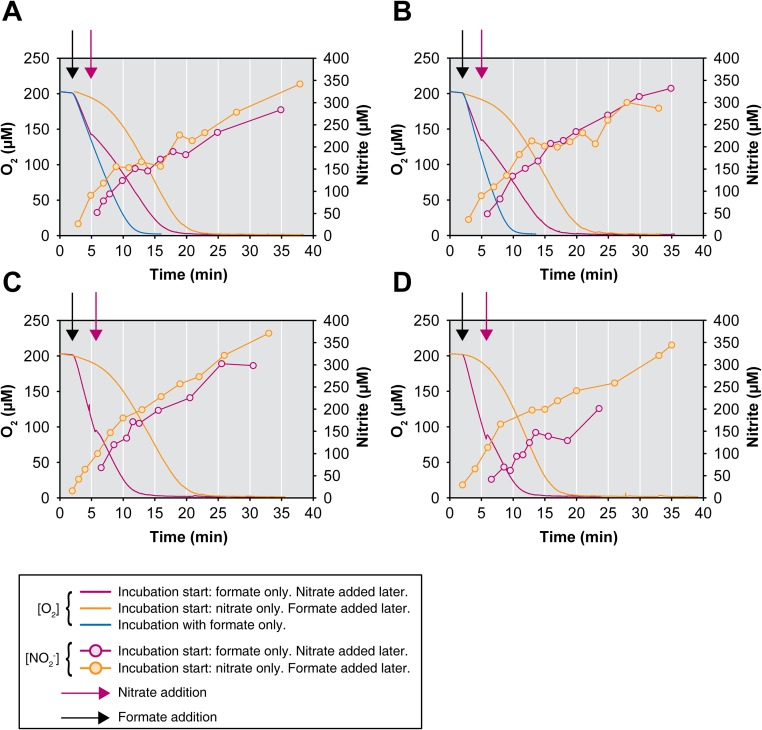
Additional experiments showed that N. moscoviensis also used formate as sole substrate with O2 as terminal electron acceptor (Fig. 3B and Fig. S5A) and grew under these conditions, although growth on formate was weaker than growth on nitrite with O2 as electron acceptor (Fig. S5 B and C). When formate and both electron acceptors nitrate and O2 were supplied, the formate concentration decreased by 1.9 mM, whereas the nitrate concentration decreased only by 0.7 mM during the first 6 d of incubation (Fig. 3C). An explanation could be that the electrons derived from formate were mainly channeled to O2 or used for CO2 fixation. In addition, concurrent reoxidation of the produced nitrite (with O2) may replenish the nitrate pool and, thus, reduce the net consumption of nitrate. Indeed, net nitrite oxidation became detectable after 6 d while formate was still being consumed (Fig. 3C). These data suggested that N. moscoviensis was simultaneously performing three different metabolic reactions: the oxidation of formate with nitrate and O2 as electron acceptors and the oxidation of nitrite with O2 as electron acceptor. This striking physiological flexibility was further investigated in additional experiments. In an extended oxic incubation, formate and nitrate were supplied initially and nitrite was formed by nitrate reduction. The subsequent net decrease of nitrite and increase of nitrate, together with ongoing formate consumption, confirmed that formate and nitrite were used concomitantly as electron donors (Fig. S5 D–F). Net nitrite consumption was slower than in the absence of formate (Fig. S5 E and G). The utilization of O2 as electron acceptor with formate as electron donor was monitored by microrespirometry (Fig. S6). In these experiments, the rate of O2 consumption decreased in the presence of nitrate as an alternative electron acceptor. This decrease of the O2 consumption rate and the concurrent formation of nitrite from nitrate confirmed that N. moscoviensis was using both electron acceptors simultaneously (Fig. S6).
Fig. S5.
Aerobic utilization of formate or nitrite by N. moscoviensis. (A) Aerobic use of formate with O2 as terminal electron acceptor by a pure culture of N. moscoviensis. Data points show the means of biological replicates (n = 3). Error bars represent SD and are not shown if smaller than symbols. This experiment represents an independent replication of the experiment shown in Fig. 3B. (B) Aerobic use of nitrite with O2 as terminal electron acceptor by a pure culture of N. moscoviensis. Data points show the means of biological replicates (n = 3). Error bars represent SD and are not shown if smaller than symbols. This experiment was performed with the same amount of biomass from the same inoculum as the experiment in A. (C) Aerobic growth of N. moscoviensis on formate or nitrite, respectively. Data points show the means of biological replicates (n = 3). Error bars represent SD and are not shown if smaller than symbols. Total biomass protein was measured during the incubations shown in A and B to follow the growth of the cultures in these experiments. In the control experiment, the same amount of N. moscoviensis biomass was incubated in mineral medium without addition of formate or nitrite. Here, total protein decreased likely because of endogenous respiration in the absence of any external electron donor. (D) Long-term incubation of N. moscoviensis with formate and nitrate under oxic conditions. The initial concentrations of formate and nitrate were 5 mM and 0.5 mM, respectively. This graph shows the formate concentration in the culture supernatant. The results of two biological replicates are shown. (E) Nitrite concentrations in the culture supernatant during the incubation experiment shown in D. The initial net increase of the nitrite concentration was caused by nitrate reduction. The following net decrease of nitrite demonstrates the concomitant utilization of nitrite and formate (also see D) from day 6 to the end of the experiment. The results of two biological replicates are shown. (F) Nitrate concentrations in the culture supernatant during the incubation experiment shown in D and E. The initial net decrease and subsequent net increase of the nitrate concentration are consistent with the nitrate-reducing and nitrite-oxidizing activities (D and E) of N. moscoviensis in this experiment. (G) Nitrite oxidation by N. moscoviensis in absence of formate. The rate of nitrite oxidation was considerably higher than in presence of formate (E). Highly similar amounts of N. moscoviensis biomass were used in these incubation experiments (D–G).
Fig. S6.
Utilization of O2 and nitrate as terminal electron acceptors by N. moscoviensis. (A–D) Microrespirometric measurements of O2 consumption with formate (1 mM initial concentration) as electron donor and in presence or absence of nitrate (5 mM initial concentration) are shown. Curves without symbols depict the O2 concentrations in the supernatant of a N. moscoviensis pure culture. Curves with symbols depict the nitrite concentrations in the supernatant. Each graph represents an independent experiment, and all experiments were performed with highly similar amounts of biomass. Black arrows indicate the addition of formate to the cultures. Purple arrows indicate the addition of nitrate to cultures containing only formate. The reduction in the O2 consumption rates in presence of both electron acceptors, and the production of nitrite, show that electrons from formate were distributed to both O2 and nitrate. Please note that experiments with formate in the total absence of nitrate (blue curves) were carried out only twice (A and B).
The aerobic use of formate by N. moscoviensis is consistent with the utilization of 14C-labeled formate by uncultured Nitrospira in oxic-activated sludge, which was recently observed by fluorescence in situ hybridization and microautoradiography (21), and the growth on formate of Nitrospira japonica isolated from activated sludge (40). Formate is aerobically metabolized also by Nitrobacter (41, 42) and Nitrolancea hollandica (5). However, the anaerobic use of an organic compound by NOB, as shown here for a member of the widely distributed lineage II Nitrospira (Fig. 3A), has only been reported for Nitrobacter so far (43, 44). The simultaneous oxidation of an organic substrate and nitrite, together with the parallel use of nitrate and oxygen as electron acceptors, demonstrate a high degree of metabolic versatility that was not anticipated for NOB.
Comparative Genomics Reveals Putative Features for Niche Partitioning of Nitrospira.
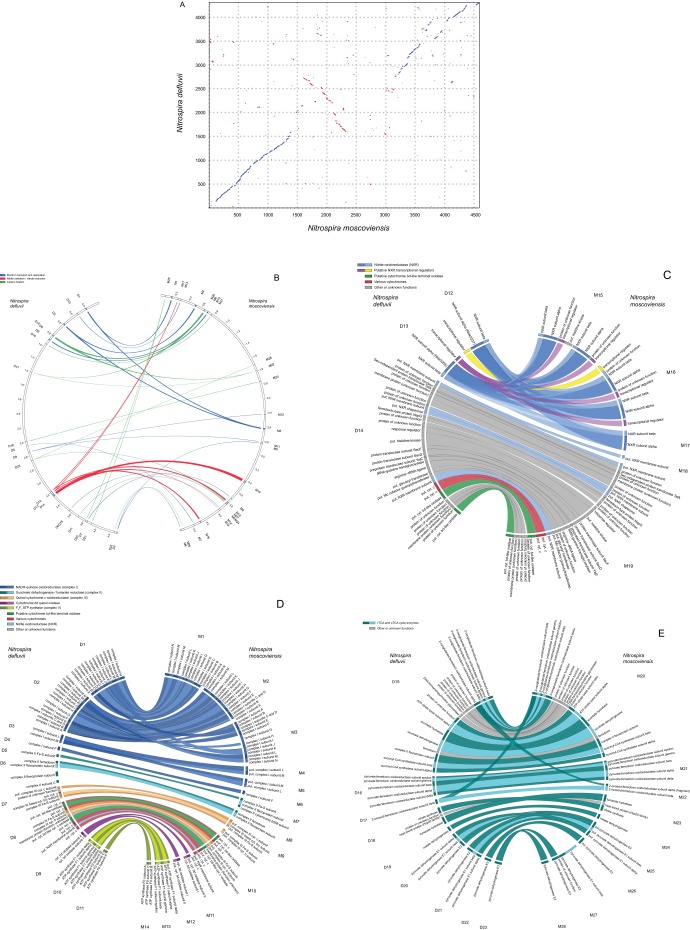
The genome of N. moscoviensis is slightly larger than the previously sequenced genome of N. defluvii (25). Key features of both genomes are summarized in Table S1. Only 56% of all N. moscoviensis CDS have homologs in N. defluvii, and 40–44% of the CDS in each genome code for functions not found in the respective other Nitrospira strain (Table S1). This genomic dissimilarity reflects the affiliation to different Nitrospira lineages and the relatively low 16S rRNA sequence identity of 94.2% between N. moscoviensis and N. defluvii. Nevertheless, the two Nitrospira strains share a highly conserved core metabolism for their chemolithoautotrophic nitrite-oxidizing lifestyle (Fig. S7 and SI Results and Discussion).
Fig. S7.
Whole-genome comparison and core metabolism of N. moscoviensis and Nitrospira defluvii. (A) Whole-genome alignment showing the positions of homologous genes in N. moscoviensis and N. defluvii. Sequence matches with the same orientation are plotted blue, whereas inversions are plotted red. (B) Localization of regions encoding the Nitrospira core metabolism for nitrite oxidation, electron transport, and inorganic carbon fixation in the genomes of N. defluvii (Left) and N. moscoviensis (Right). Each semicircle depicts one full genome. Ribbons connect regions containing homologous core metabolism genes. Tags (D1 to D23 for N. defluvii, M1 to M28 for N. moscoviensis) identify the genomic regions shown in C–E. (C) Highly conserved, syntenic gene arrangements within regions encoding nitrite oxidoreductase (NXR) in the genomes of N. defluvii (Left) and N. moscoviensis (right). Regions are separated by spaces and ribbons connect homologous genes. The tags, which identify the regions, are the same as in B. The order of the regions shown here facilitates the synteny comparison and does not reflect the true genomic localization of these regions, which is shown in B. (D) Highly conserved, syntenic gene arrangements within regions encoding the electron transport chains for nitrite oxidation, reverse electron transport, and the utilization of organic substrates in the genomes of N. defluvii (Left) and N. moscoviensis (Right). Regions are separated by spaces and ribbons connect homologous genes. The tags, which identify the regions, are the same as in B. The order of the regions shown here facilitates the synteny comparison and does not reflect the true genomic localization of these regions, which is shown in B. (E) Highly conserved, syntenic gene arrangements within regions encoding the reductive (rTCA) and oxidative (oTCA) tricarboxylic acid cycles in the genomes of N. defluvii (Left) and N. moscoviensis (Right). The rTCA cycle is the CO2 fixation pathway of Nitrospira. Regions are separated by spaces and ribbons connect homologous genes. The tags, which identify the regions, are the same as in B. The order of the regions shown here facilitates the synteny comparison and does not reflect the true genomic localization of these regions, which is shown in B.
A possibly important, outstanding feature of N. moscoviensis is related to the assimilation of N from nitrite. All cultured Nitrospira (including N. moscoviensis) grow on nitrite as the sole N source for assimilation. In N. defluvii and N. lenta, nitrite reduction to ammonia is most likely catalyzed by a ferredoxin-dependent nitrite reductase, whose gene (nirA) is located in the vicinity of various other genes for N acquisition and assimilation (Fig. S1A and Dataset S1). Surprisingly, N. moscoviensis does not possess nirA (Fig. S1A) or any other known pathway to assimilate N from nitrite. An interesting candidate for this function is an octaheme cytochrome c (OCC) encoded in the same genomic region where the other two Nitrospira genomes contain nirA (Fig. S1A and SI Results and Discussion). Various forms of OCCs have been linked to assimilatory or dissimilatory nitrite reduction in other organisms (2, 45). Intriguingly, N. moscoviensis might link nitrite reduction to ammonia by the OCC with energy conservation by proton translocation across the cell membrane (SI Results and Discussion). This energy gain could partly compensate for the costs of the reverse electron transport, which is needed to provide reductants for assimilatory pathways if nitrite is the sole electron donor. This feature could be an adaptation to highly oligotrophic environments and would distinguish N. moscoviensis from N. defluvii and N. lenta, which use canonical NirA (Fig. S1A).
Consistent with the capability of N. moscoviensis to grow by aerobic H2 oxidation (23), its genome encodes a cytoplasmic group 2a uptake hydrogenase and several proteins that could be involved in electron transfer from the hydrogenase to quinone (23) (NITMOv2_1637 to NITMOv2_1657). These genes are missing in N. defluvii.
Because nitrite oxidation is an aerobic process, Nitrospira must be able to cope with reactive oxygen species (ROS). Interestingly, N. defluvii lacks any genes of superoxide dismutase (SOD), superoxide reductase, and catalase (25), which are widely distributed key enzymes for the defense against ROS. In stark contrast, the genome of N. moscoviensis encodes a canonical SOD and a catalase (Fig. S1B, Dataset S1, and SI Results and Discussion). In addition, N. moscoviensis possesses the putative alternative ROS defense mechanisms as predicted for N. defluvii (25) (SI Results and Discussion). The larger repertoire for ROS defense of N. moscoviensis should confer ecological advantages at elevated oxygen levels. Consistently, the Nitrospira community composition shifted from lineage I (related to N. defluvii) to lineage II Nitrospira (related to N. moscoviensis) in nitrifying chemostats after an increase in the dissolved oxygen concentration (46).
SI Results and Discussion
Urea Transport and Accessory Proteins of Urease in N. moscoviensis.
Urea is a small uncharged molecule that diffuses readily through the lipid bilayers of bacterial membranes (52). Aside from passive diffusion, the transport of urea into bacterial cells is mediated by urea-specific channels such as UreI of Helicobacter (53) or by ATP-dependent ABC transporters such as UrtABCDE in Cyanobacteria (54). ABC transporters for urea consist of a periplasmic substrate-binding protein (UrtA), a dimer of the transmembrane proteins UrtB and UrtC, which form a membrane-crossing pore, and the ATP-binding and -hydrolyzing proteins UrtD and UrtE (54, 55). These ABC transporters show a high affinity for urea (52, 54) and may represent an adaptation to environments with urea concentrations in the micromolar range. Their expression is tightly regulated in dependence on N availability due to the energy demand of this transport system (54, 56). In the genome of N. lenta, the whole gene set (urtABCDE) of the urea ACB transport system is located upstream of the urease genes (Fig. S1A and Dataset S1), suggesting that N. lenta possesses a high-affinity uptake system for urea and, thus, is adapted to habitats where low urea concentrations prevail. In contrast, in N. moscoviensis, only urtA, which encodes the periplasmic urea-binding protein UrtA, is located in close vicinity of the urease genes (Fig. S1A). Whether N. moscoviensis can replace the lacking UrtBCDE proteins with the respective subunits of other ABC transporters encoded in the genome, or whether urea is taken up by passive diffusion only, remains to be determined.
The accessory proteins UreD, UreE, UreF, and UreG are required for the formation of the Ni2+-containing metallocenter in the UreABC apoenzyme during the biosynthesis of urease (27). In addition, the nickel transporter UreH provides Ni2+ (27). The genome of N. moscoviensis contains the ureD, ureF, and ureG genes (Fig. S1A) and ureH (NITMOv2_1657). However, only a 180-nt-long (59 aa) gene fragment of ureE (NITMOv2_1661) was identified, which is unlikely to encode a functional UreE protein because homologs in other organisms are approximately 200 aa in length. Although UreE is required for a functional urease in Helicobacter (57), various other microorganisms lacking ureE genes express active ureases (58). In ureolytic microbes without UreE, this nickel-binding metallochaperone (59) may be substituted by chaperones of other nickel-dependent enzymes (58). Interestingly, in Helicobacter pylori two accessory proteins of nickel-dependent hydrogenase, HypA and HypB, are required for the incorporation of Ni2+ into urease (60). These hydrogenase maturation factors are present in N. moscoviensis, which possesses an active [NiFe] hydrogenase (23). Hence, hydrogenase chaperones might be involved in the assembly of urease and substitute UreE in this organism as well.
Utilization of Organic Substrates by N. moscoviensis.
Aside from formate (see Results and Discussion in the main text), we tested also whether N. moscoviensis can use other simple organic compounds (acetate, fumarate, succinate, citrate, and pyruvate) in combination with nitrate as terminal electron acceptor. Acetate could be provided by fermenting organisms in the spatial proximity of Nitrospira in hypoxic or anoxic habitats, whereas the other compounds are key metabolites that could be released by lysed cells within a biofilm. The genetic repertoire of N. moscoviensis includes the degradation pathways and the respiratory chain needed to use these organic compounds (Fig. S1B). Transmembrane transporters for these substrates were not identified in the genome, but N. moscoviensis encodes permeases of unknown specificities, and we could not exclude the possibility that such transporters may facilitate the uptake of one or more of the tested organics. However, no nitrate reduction was observed during anoxic incubations with any of these substrates (Fig. S4B).
Core Metabolism of Nitrospira for Chemolithoautotrophic Nitrite Oxidation.
A syntenic gene arrangement is conserved in relatively large parts of the N. moscoviensis and N. defluvii genomes (Fig. S7A). Shared genomic features with a highly conserved synteny are the enzymatic repertoire for nitrite oxidation, the electron transport chains for aerobic respiration and reverse electron transport from nitrite to NAD+, and the reductive tricarboxylic acid (rTCA) cycle for CO2 fixation and the oxidative TCA cycle (Fig. S7 B–E). The high degree of similarity in these pathways strongly supports the previous reconstruction of the Nitrospira core metabolism for chemolithoautotrophic nitrite oxidation, which was based on only one sequenced genome (25). The few genetic differences in the core pathways include additional (third) copies of respiratory complexes I and III, a second cytochrome bd oxidase, and five paralogous copies of nitrite oxidoreductase (NXR) subunits NxrA and NxrB in N. moscoviensis, whereas N. defluvii has only two paralogs of these NXR subunits (25) (Fig. S7 C and D).
NXR, the key enzyme for nitrite oxidation, belongs to the complex iron–sulfur molybdoenzyme family with a molybdo-bis(pyranopterin guanine dinucleotide) cofactor-containing catalytic subunit (61). The NXR of Nitrospira is located in the periplasmic space and consists of at least two subunits (NxrA and NxrB). The third (NxrC) subunit may anchor the NXR complex in the cytoplasmic membrane and mediate the transfer of electrons from NXR to the membrane-bound electron transport chain (25). The five paralogs of nxrA and nxrB are clustered in three genomic regions of N. moscoviensis, whereas five putative nxrC genes are located elsewhere in the genome (Fig. S7C). NxrA contains the substrate-binding site with the Mo cofactor (25). Like in N. defluvii, all NxrA paralogs of N. moscoviensis contain an N-terminal twin-arginine motif for export via the twin-arginine protein translocation (Tat) pathway. The presence of this motif is consistent with the periplasmic localization of the active site of NXR in N. moscoviensis (62) and N. defluvii (25). The periplasmic NXR is energetically advantageous and likely explains the strong competitiveness under nitrite-limited conditions of Nitrospira compared with other NOB such as Nitrobacter, whose NXR is located on the cytoplasmic side of the cell membrane (25).
The amino acid similarities among the NXR subunits of N. moscoviensis range from 95.7 to 98.5% for NxrA, from 99.5 to 100% for NxrB, and from 18.6 to 64.1% for the putative NxrC candidates. All five NxrA copies are more similar to one of the two NxrA paralogs (CDS tag Nide3255) (25) in N. defluvii (87.1–87.9%) than to the other one (Nide3237) (83.6–84.2%). Interestingly, the similarity between the two NxrA copies in N. defluvii is only 86.9% and, thus, lower than the similarity between all NxrA copies of N. moscoviensis and one NxrA (Nide3255) of N. defluvii. It is tempting to speculate that the lower similarity between the two NxrA subunits of N. defluvii reflects a functional differentiation, and that all NxrA of N. moscoviensis are functionally more similar to one of the NxrA paralogs (Nide3255) of N. defluvii. Consistently, four of the five nxrA/B gene clusters in N. moscoviensis are preceded by transcriptional regulator genes, which are homologous to a regulator in N. defluvii that occurs directly upstream of the gene encoding NxrA Nide3255 (Fig. S7C). The amino acid similarities between these regulators are relatively high (47–73%). If the genomic localization next to nxrA genes reflects a role of these regulators in the transcriptional control of NXR, then the regulation of these four NXR paralogs in N. moscoviensis may resemble the regulation of Nide3255 in N. defluvii. However, one of these nxr gene clusters in N. moscoviensis also contains a second transcriptional regulator, which is homologous to a regulator upstream of the second NxrA copy of N. defluvii (Nide3237) (Fig. S7C). Hence, in both organisms, at least two different regulation mechanisms for NXR seem to be present that await confirmation and further analysis in future studies.
Each of the five NxrC candidates in N. moscoviensis has a homolog among the four putative NxrC subunits in N. defluvii (25) (Dataset S1), with two of the N. moscoviensis proteins (NITMOv2_3617 and NITMOv2_4208) being homologous to one candidate NxrC in N. defluvii (Nide3271). All NxrC candidates in both Nitrospira genomes have been identified based on sequence similarities to the membrane subunits of other DMSO reductase type II family enzymes (25), but their actual functional roles and the composition of the NXR protein complex in Nitrospira remain to be determined.
Assimilatory Nitrite Reduction by a Putative Octaheme Cytochrome c Nitrite Reductase.
The genome of N. moscoviensis lacks any gene for assimilatory nitrite reductase (NirA). The only gene related to nirA most likely encodes a ferredoxin-dependent sulfite reductase (NITMOV2_0334) and is involved in sulfur assimilation according to its localization close to the genes of sulfate adenylyltransferase.
However, N. moscoviensis contains a gene for an octaheme cytochrome c (OCC) protein, which is located in a region that contains various other genes for N acquisition including the urease (Fig. S1A). Some OCC proteins from other organisms are octaheme nitrite reductases (ONRs) that reduce nitrite to ammonia (63–65). These ONRs may represent an evolutionary link from pentaheme nitrite reductases (Nrf) to the OCC with oxidizing activities, hydroxylamine oxidoreductase (HAO) of aerobic ammonia-oxidizing bacteria and hydrazine oxidoreductase (HZO) of anaerobic ammonium-oxidizing (anammox) organisms (63). The OCC of N. moscoviensis does not belong to this ONR group, but most closely resembles members of another clade (II.2) of the OCC family, which consists mostly of uncharacterized proteins. Interestingly, clade II.2 OCCs seem to be functionally versatile because a protein from this group from Beggiatoa had the activities of ONR and also of HAO and HZO in vitro (65). As a HAO, the enzyme of N. moscoviensis might allow the detoxification of hydroxylamine. Because Nitrospira usually share their habitat with aerobic ammonia-oxidizing bacteria, they may easily encounter hydroxylamine in their direct environment.
Interestingly, a clade II.1 OCC from Nautilia profundicola is involved in the reverse hydroxylamine:ubiquinone reductase module (HURM) pathway that is used to reduce nitrate to ammonia (45). In this pathway, nitrite reduction to hydroxylamine by the OCC is linked to quinol oxidation and proton dislocation across the cell membrane by a cytochrome cM552-like protein (45). N. moscoviensis might use a similar pathway to link nitrite reduction to ammonia with proton translocation. Two genes encoding a Rieske/cytochrome b complex are located upstream of the OCC gene in its genome (Fig. S1A). It is tempting to speculate that this Rieske/cytochrome b complex might generate proton motive force by channeling electrons from the quinol pool to the OCC, similar to the reverse-HURM pathway. Assuming that it has ONR activity, the OCC would then reduce nitrite to ammonia.
Reactive Oxygen Defense.
In contrast to most other aerobic bacteria, N. defluvii lacks SOD and catalase, and its genome does not code for superoxide reductase either (25). The predicted alternative mechanisms for protection against ROS in N. defluvii include ROS detoxification by manganese or polyamines, H2O2 degradation by peroxidases and thioredoxin-dependent peroxiredoxins, binding of free iron by bacterioferritin to reduce the risk of ROS generation, and free radical scavenging by carotenoids (25). In contrast to N. defluvii, N. moscoviensis possesses a canonical SOD and a catalase (Fig. S1B and Dataset S1). The SOD of N. moscoviensis (NITMOv2_2805) binds Fe or Mn based on its overall amino acid sequence similarity to other SODs that require these metal cofactors. The Fe and Mn SODs are difficult to distinguish from each other by sequence analysis, but specific fingerprint residues (66) indicate that the enzyme of N. moscoviensis may be a tetrameric Fe SOD. N. moscoviensis possesses a typical monofunctional, heme-containing catalase that is encoded by two identical gene copies (NITMOv2_0085 and NITMOv2_4696). Either catalase gene belongs to one of two identical copies of a 42-kbp-large Tn7 mobile element. In addition to SOD and catalase, N. moscoviensis possesses the putative ROS defense mechanisms as predicted for N. defluvii (25) except that it lacks a polyamine transporter (Fig. S1B).
Conclusions
The genome of N. moscoviensis revealed a previously unknown type of interaction between NOB and AOM, which could contribute to the ecological success of Nitrospira. Reciprocal feeding with AOM enables Nitrospira possessing urease to indirectly use urea as an energy source, independently from the presence and ureolytic activity of urease-positive AOM. This interaction could facilitate the colonization by Nitrospira of habitats where urea is available and reduce the competition for free nitrite with other NOB, denitrifiers, and anaerobic ammonium oxidizers. Hence, reciprocal feeding should contribute to a high nitrification performance in WWTPs but could partly be responsible for N losses from soils fertilized with urea.
The ecophysiological flexibility, which results from the aerobic and anaerobic use of formate (this study) or H2 (23, 24), may enable Nitrospira to survive periods of nitrite or oxygen deprivation. These metabolisms uncouple the growth of NOB from the nitrification process and could contribute to the unexpected higher abundances of NOB compared with AOM observed in nitrifying activated sludge, biofilm, and freshwater sediment (10, 20, 47, 48). Taken together, Nitrospira play diverse functional roles and even participate in N-cycle processes other than nitrification. For example, nitrate-reducing Nitrospira may provide nitrite as substrate for denitrification or anaerobic ammonium oxidation (anammox) in natural ecosystems and bioreactors. The pronounced genomic differences between N. moscoviensis and N. defluvii, which include urease, ROS defense, and a large number of yet-uncharacterized genes (Table S1), suggest a high degree of functional versatility as basis of ecological niche partitioning within the ubiquitous genus Nitrospira. This versatility likely explains the surprisingly high diversity of Nitrospira that coexist in natural soils (17) and activated sludge (21). Unraveling this complexity will be crucial to deepen our microbiological understanding of nitrification and other N-cycle processes, to optimize wastewater treatment strategies, and to improve the efficiency of N fertilization in agriculture.
Materials and Methods
Genome Sequencing and Annotation.
DNA was isolated from a pure culture of N. moscoviensis (24) as described (34). Paired-end and mate-pair sequencing libraries were prepared and sequenced on an Illumina MiSeq DNA sequencer (SI Materials and Methods). The trimmed reads were assembled by using the CLC genomics workbench v. 6.5.1. The genome annotation platform MicroScope (49) was used for automated prediction and annotation of coding sequences. See SI Materials and Methods for further details.
Metagenomic Screening for Nitrospira-like UreC Sequences and Phylogenetic Analyses.
In total, 3,217 publicly available metagenomes in the IMG database (50) and an additional metagenome form the Aalborg WWTP (MG-RAST ID: 4611649.3) were screened for the presence of Nitrospira-like UreC sequences (SI Materials and Methods). Phylogeny of the metagenomic sequences and UreC proteins of AOB and NOB was reconstructed by using Bali-phy (51) (SI Materials and Methods).
Physiological Experiments.
N. moscoviensis, N. defluvii, and N. europaea were incubated separately in medium with urea as described in SI Materials and Methods. Urea as sole N and energy source was added during coincubations to mixtures of N. moscoviensis and N. europaea. N. moscoviensis was incubated in medium with formate, or formate plus nitrate, under oxic or anoxic conditions. The conversion of substrates was monitored by chemical analyses of aliquots of the medium. See SI Materials and Methods for detailed descriptions.
SI Materials and Methods
Genome Sequencing and Analysis.
DNA was isolated from a pure culture of N. moscoviensis strain NSP M-1 (24) by following the hexadecyltrimethylammonium bromide (CTAB) protocol as described (34). Following extraction, RNA was removed by RNase I digestion (Lucigen).
Paired-end sequencing libraries were prepared by using the Nextera DNA Sample Preparation Kit (Illumina) according to the manufacturer's instructions. Mate-pair sequencing libraries were prepared by using the Nextera Mate-Pair Sample Preparation Kit (Illumina) using the gel-free protocol according to the manufacturer's instructions. The sequencing libraries were sequenced on an Illumina MiSeq DNA sequencer with 2 × 301 bp byusing the MiSeq Reagent Kit v3 (Illumina). The paired-end reads were imported into the CLC genomics workbench software (version 6.5.1, CLCbio; Qiagen) and quality trimmed by requiring a minimum phred score of 20 and a minimum read length of 50 bp. Nextera adapters were removed if found. The trimmed paired-end reads were de novo assembled by using CLC genomics workbench v. 6.5.1 using a kmer of 64 and otherwise default parameters. The de novo assembly was manually inspected by using CLC genomics workbench v. 6.5.1 and the Circos (67) tools implemented in the multimetagenome workflow (68). The initial inspection resulted in cleaning of misassembled contigs and identification of five copies of the NxrAB operon, which proved difficult to assemble because of high similarity between the variants. To assemble the individual NxrAB operons, the nxrA and nxrB genes were separately PCR-amplified with region-specific primer sets, cloned in E. coli, and Sanger-sequenced. The partially assembled NxrAB genes were replaced with their Sanger-sequenced complete counterparts. The mate-pair reads were cleaned by using NextClip v.0.8 (69) with default parameters. Reads from Category A were imported into CLC genomics workbench v. 6.5.1 and mapped to the assembly by using the “map reads to reference” function with 95% similarity over 70% of the read length as cutoff. The mapping was visualized by using the Circos (67) tools implemented in the multimetagenome workflow (68) and used to manually scaffold the initial assembly into a single scaffold. The gaps in the final scaffold were resolved manually by using CLC genomics workbench v.6.5.1 by mapping the trimmed paired-end reads (minimum 2 × 250 bp) to the assembly with 95% similarity over 30% of the read length as cutoff.
The genome annotation platform MicroScope (49) was used for the automated prediction and annotation of CDS. Homologous proteins present in the N. moscoviensis and N. defluvii genomes were identified by using the phyloprofile exploration tool of MicroScope. Only proteins sharing an amino acid sequence identity ≥ 30% over at least 80% of the sequence length were considered as homologs. The annotation of all CDS discussed in this study was refined manually based on the same annotation criteria that were already applied for the annotation of the N. defluvii genome (25). The whole genomes of N. defluvii and N. moscoviensis were aligned by using the PROmer tool of the MUMmer 3.23 software package (70). Matches < 333 aa (1,000 nt) were ignored to reduce the number of spurious matches between partial protein sequences. Syntenic regions were identified by the respective tools of MicroScope and visualized by using Circos (67).
Metagenomic Screening for Nitrospira-Like ureC Sequences and Phylogenetic Analysis of the Urease Alpha Subunit.
A large metagenome from the Aalborg West Wastewater treatment plant in Denmark (MG-RAST ID: 4611649.3) was screened for the presence of Nitrospira-like UreC proteins by first calling genes using prodigal with the metagenome option (71) and then searching the resulting proteins using blastp.
In total, 3,217 publicly available metagenomes in the integrated microbial genomes (IMG) database (50) were screened by using HMMER3 hmmsearch (72) with the hmm model (length = 121) for urease_alpha (PFAM PF00449; ref. 73) and default parameters. Amino acid sequences identified with hmmsearch were required to match the hmm over at least 100 aa positions and screened for similarity (>70%) to the UreC sequence from Nitrospira moscoviensis. Sequences passing these criteria were then clustered at 90% similarity by using Usearch 7.0 (74). Usearch centroids (46 sequences) were used in an exploratory phylogenetic analysis by using five independent chains of 1,100 iterations (600 burnin) with a randomized initial alignment in Bali-phy (51). The UreC protein sequences from N. moscoviensis and N. lenta formed a well-supported cluster (>99% posterior support) with seven centroids derived from metagenomes. All metagenomic sequences that matched any of these seven centroids with >90% amino acid identity were retained for phylogenetic analysis. Additional genomic UreC sequences were identified with blastp by using known ammonium-oxidizing and nitrite-oxidizing bacteria as query and default parameters. The 100 top hits were purged from highly similar sequences and clustered at 90% similarity by using usearch 7.0 and centroids were retained for phylogenetic analysis. Phylogenetic reconstruction was carried out by using five independent chains of 1,100 iterations (600 burn-in, randomized initial alignment) in Bali-phy (51), which simultaneously infers alignment and phylogeny from a set of sequences. The 102 amino acid sequences ranged from 109 aa (C687J26615_103352331 from metagenome 3300002121) to 595 aa (Microcoleus sp. WP_015182704). Posterior tree pools from all five independent runs were combined to determine consensus topology and posterior support for bipartitions. Alignment length ranged from 736 to 817 (mean = 767) in the posterior pool of alignments.
Cultivation of N. moscoviensis and N. europaea.
To obtain biomass for experiments, N. moscoviensis was grown at 37 °C in mineral nitrite medium (23) without agitation and in the dark. This NOB medium had the following composition: 1,000 mL of distilled water; 0.01 g of CaCO3; 0.5 g of NaCl; 0.05 g of MgSO4⋅7H2O; 0.15 g of KH2PO4; 34.4 μg of MnSO4⋅H2O; 50 μg of H3BO3; 70 μg of ZnCl2; 72.6 μg Na2MoO4⋅2H2O; 20 μg of CuCl2⋅2H2O; 24 μg of NiCl2⋅6H2O; 80 μg of CoCl2⋅6H2O; 1 mg of FeSO4⋅7H2O. If not stated otherwise, 0.01 g of NH4Cl was added to the medium as additional N source. After autoclaving, filter-sterilized NaNO2 was added to a final concentration of 1 mM. The nitrite concentration in the medium was regularly checked by using nitrite test stripes (Merkoquant; Merck), and nitrite was replenished when completely consumed. N. europaea was grown at 30 °C on a rotary shaker (100 rpm) in a modified AOB medium described (75). The modified medium composition and preparation protocol were as follows: 900 mL of distilled water; 3.3 g of (NH4)2SO4; 0.41 g of KH2PO4; 0.184 g of MgSO4⋅7H2O; 0.022 g of CaCl2; 0.12 mg of CuSO4⋅7H2O and 0.33 mL of a 30 mM FeSO4⋅7H2O solution in 50 mM Na2EDTA⋅2H2O. After autoclaving, 100 mL of a sterile buffer solution (pH 8.0) containing 6.8 g of KH2PO4 and 0.6 g of NaH2PO4 and 4 mL of a sterile 10% (wt/vol) NaCO3 solution were added separately to adjust the pH to a value of 7.9.
The purity of all nitrifier cultures, including incubated aliquots before and after experiments, was checked by FISH with the EUB338 probe mix (76, 77) that targets most known Bacteria, the Nitrospira lineage II-specific probe Ntspa1151 (78) for N. moscoviensis cultures, the Nitrospira lineage I-specific probe Ntspa1431 (78) for N. defluvii cultures, or the betaproteobacterial AOB-specific probe Nso1225 (79) for N. europaea cultures. For this purpose, aliquots of the biomass were fixed in formaldehyde and FISH was carried out as described (80). Contaminations by other bacteria were not detected in any experiment.
Incubation of N. moscoviensis with Urea.
N. moscoviensis biomass was harvested by centrifugation (8,228 × g, 10 min, 25 °C). The cell pellet was resuspended in 30 mL of ammonium-free NOB medium and 5-mL aliquots of the biomass were added to 300-mL Schott bottles containing 100 mL of ammonium-free NOB medium. To ensure activity of the cells, NaNO2 in the final concentration of 0.35 mM was added to all incubations and the cultures were incubated at 37 °C in the dark. After 2 d, all NaNO2 was consumed and the respective substrates for the different incubation conditions were added to the incubations in the following final concentrations: 0.35 mM NaNO2 and 1 mM urea; only 1 mM urea; and as an activity control, only 0.35 mM NaNO2. All incubation conditions were performed in duplicates. In control experiments, medium containing 0.35 mM NaNO2 and 1 mM urea and medium containing 1 mM urea was incubated without biomass. All incubations were performed for 6 h at 37 °C. Every 2 h, aliquots of the incubations were sampled and centrifuged (17,949 × g, 10 min, 4 °C) to remove cells. In the supernatant, ammonium was measured photometrically by the salicylic acid assay (81).
Incubation of N. defluvii with Urea.
N. defluvii biomass was harvested by centrifugation (6,300 × g, 10 min, 25 °C). The cell pellet was resuspended in ammonium-free NOB medium, and equal aliquots of the biomass were added to 300-mL Schott bottles containing 100 mL of ammonium-free NOB medium. To all incubations, 0.5 mM NaNO2 (final concentration) was added and the cultures were incubated at 30 °C in the dark. After 3 d, all nitrite was consumed. Subsequently, 0.5 mM NaNO2 and 1 mM urea, only 1 mM urea, or only 0.5 mM NaNO2 was added to the respective incubations. All incubations were performed in duplicates. In addition, medium containing 0.5 mM NaNO2 and 1 mM urea and medium containing 1 mM urea was incubated without biomass in control experiments. All incubations were performed at 30 °C for 6 h. Every 2 h, aliquots of all incubations were sampled and centrifuged (17,949 × g for 10 min at 4 °C) to remove cells. In the supernatant, ammonium was measured photometrically by the salicylic acid assay (81).
Incubation of N. europaea with Urea.
N. europaea biomass was harvested by centrifugation (6,300 × g for 20 min at 25 °C) and washed by resuspending the cells in ammonium-free AOB medium. The biomass was centrifuged again, and the cell pellet was resuspended in ammonium-free AOB medium. By using syringes, aliquots (1 mL) of this mixture were added to 300-mL Schott flasks containing 50 mL of ammonium-free AOB medium. These bottles had already been plugged with butyl rubber stoppers, which were fixed with screw caps. The culture was incubated in the presence of 1 mM urea for 7 d at 30 °C in the dark in duplicates. During the incubations, aliquots of the cultures were sampled by using syringes and were centrifuged (14,000 × g for 10 min at 4 °C) to remove cells. In the supernatant, nitrite was measured photometrically by the Griess assay (82).
Coincubation of N. moscoviensis and N. europaea.
N. moscoviensis and N. europaea biomass was harvested by centrifugation (6.300 × g, 20 min, 25 °C), resuspended in AOB medium (see above) that did not contain NH4+, and centrifuged again. After this washing step, the cells were resuspended in ammonium-free AOB medium and mixed in a cell number ratio of 1:1 (details of the cell quantification are provided below). By using syringes, aliquots (1 mL) of this mixture were added to 300-mL Schott flasks containing 50 mL of ammonium-free AOB medium. These bottles had already been plugged with butyl rubber stoppers, which were fixed with screw caps. The mixture was incubated in the presence of 1 mM or 50 µM urea as sole N and energy source for 7 d at 30 °C in duplicates. During the incubations, aliquots of the cultures were sampled by using syringes and centrifuged (14,000 × g, 10 min, 4 °C) to remove cells. In the supernatant, N compounds were measured photometrically by the salicylic acid assay (ammonium) (81) or the Griess assay (nitrite and nitrate) (82). Nitrate was reduced to nitrite before the measurements by addition of vanadium(III) (83), and additional nitrite standards were included to account for inaccuracies in nitrate determination at high nitrite concentrations.
Cell Counts for the Urea Coincubation Experiments with N. moscoviensis and N. europaea.
To achieve a 1:1 ratio of N. moscoviensis and N. europaea in the coincubation experiments, the cell densities in the axenic cultures of each organism were determined. Subsequently, aliquots of each culture containing highly similar amounts of cells were washed and mixed (see above).
For counting, cells were homogeneously suspended by vortexing, and aliquots of each pure culture (5 µL of N. moscoviensis and 25 µL of N. europaea) were diluted separately in 15 mL of 1×PBS. The diluted cell suspensions were filtered separately onto black 0.2-µm polycarbonate GTBP-type membrane filters (Millipore). After washing the cells once in 1×PBS, the cells were stained by incubation for 5 min in 400 µL of a 0.1 µg⋅mL−1 DAPI (4',6-Diamidino-2-phenylindole) solution. Subsequently, the filters were washed again in 1×PBS and air-dried. The stained cells were visualized on the dried filters by epifluorescence microscopy (AXIO-Imager M1; Zeiss) at 1,000× magnification and were counted by using a counting grid (Zeiss) with an area of 0.015 mm2. The cell densities were calculated as follows:
| [S1] |
where C is the cell density, the average cell number per counting grid, V the volume of the filtered culture aliquot, and 15,124.7 the microscope factor (226.87 mm2 as the filter area, which contained cells, divided by the grid area of 0.015 mm2). For each culture, two aliquots of cell suspension were filtered and 10 grid areas per filter were analyzed.
Oxic Incubation of N. moscoviensis with Formate.
For all short-term incubations of N. moscoviensis under oxic conditions with formate, nitrite-grown biomass was harvested by centrifugation (7,232 × g, 20 min, 25 °C), washed in nitrite-free NOB medium, and was centrifuged again. The biomass was again resuspended in nitrite-free NOB medium, and 1-mL aliquots were added by using syringes to 300-mL Schott flasks containing 100 mL of nitrite-free NOB medium. These bottles had already been plugged with butyl rubber stoppers, which were fixed with screw caps. To test whether N. moscoviensis uses formate as the sole substrate, the cells were incubated for 7 d at 37 °C in the presence of sodium formate (initial concentration 5 mM) in duplicates. In a control experiment, nitrite-free NOB medium containing 5 mM sodium formate was incubated without biomass. During all incubations, aliquots were sampled by using syringes, centrifuged (17,949 × g, 10 min, 4 °C), and the formate concentrations in the supernatant were quantified by capillary electrophoresis on a P/ACE MDQ Molecular Characterization System (Beckman Coulter). Anions were separated by using the CEofix Anions 5 kit on an anion exchange column (AS11; 250 × 4 mm; Thermo Scientific Dionex) using a linear KOH gradient (0.1–40 mM in 6 min). To test whether N. moscoviensis would reduce nitrate under oxic conditions with formate as electron donor, cells were incubated for 8 d at 37 °C in the presence of sodium formate (initial concentration 5 mM) and NaNO3 (initial concentration 1.5 mM), or only with 1.5 mM NaNO3 in triplicates. During the incubations, aliquots were sampled by using syringes, centrifuged (17,949 × g, 10 min, 4 °C), and the concentrations of nitrite, nitrate, and formate in the supernatant were measured as described above.
To quantify the growth of N. moscoviensis under oxic conditions with formate or nitrite, respectively, nitrite-grown biomass was harvested by centrifugation (6,300 × g, 20 min, 25 °C) and the cells were washed in nitrite-free NOB medium. Centrifugation and washing of the biomass were repeated until no nitrite and nitrate were detected by NO2− and NO3− test stripes (Merkoquant; Merck) in the discarded supernatant. The biomass was again resuspended in 10 mL of nitrite-free NOB medium, and 1-mL aliquots were distributed to 300-mL Schott flasks containing 50 mL of nitrite-free NOB medium. The biomass aliquots were then incubated with 5 mM formate (final concentration) or 5 mM nitrite (final concentration) in triplicates for 10 d at 37 °C. In a control experiment, N. moscoviensis biomass was incubated in NOB medium without any energy source in triplicates for 10 d at 37 °C. During the incubations, culture aliquots (500 µL) were sampled, centrifuged (17,949 × g, 10 min, 4 °C), and the concentrations of nitrite and formate in the supernatant were measured as described above. In addition, total cell protein was measured by using the BCA protein assay (Thermo Fisher Scientific) according to the manufacturer’s instructions.
For all long-term incubations of N. moscoviensis under oxic conditions with formate and nitrate, nitrite-grown biomass was harvested by centrifugation (6,300 × g, 20 min, 25 °C), and the cell pellet was resuspended in nitrite-free NOB medium. The biomass was added in equal proportions to 300-mL Schott flasks containing 70 mL of nitrite-free NOB medium. To all incubations, 0.5 mM NaNO2 (final concentration) was added and the cultures were incubated at 37 °C in the dark in duplicates. After 3 d, all nitrite was oxidized to nitrate and sodium formate (initial concentration 5 mM) was added to all incubations. For control experiments, to test the nitrite-oxidizing activity in absence of formate, 0.5 mM NaNO2 was added instead of formate. Cells were incubated for 23 d at 37 °C. During all incubations, aliquots were sampled, centrifuged (17,949 × g, 10 min, 4 °C) and the formate, nitrite, and nitrate concentrations in the supernatant were quantified as described above.
Respirometry.
Substrate-dependent O2 consumption rates were measured by using a respiration cell RC-350 (Warner Instruments), equipped with an oxygen sensor (Model 1302) and connected to a picoammeter PA2000 (Unisense). Respiration rates were recorded in SensorTrace Basic (version 3.0.2, Unisense). Measurements were performed by using approximately 20× concentrated N. moscoviensis biomass from actively nitrite-oxidizing pure cultures. Biomass was harvested by centrifugation (1,181 × g, 15 min) and washed twice in NOB medium containing no nitrite or nitrate. For measurements, the cell chamber was filled with 1.5 mL of biomass suspension and closed with the electrode inserted into the general electrode holder EH-100 (Warner Instruments) without inclosing air bubbles. Measurements were performed at 39 °C. Substrates (1 mM Na-formate or 5 mM NaNO3) were added with a 100-µL gas-tight Hamilton syringe (model 1710 RN, Hamilton Laboratory Products) from stock solutions, and samples were taken with the same syringe. Nitrite concentrations were determined photometrically by the Griess assay (82).
Anoxic Incubation of N. moscoviensis with Organic Substrates.
Acetate (C2H3O2Na), formate (CHNaO2), fumarate (C4H2Na2O4), nitrate (NaNO3), and succinate (C4H4Na2O4) stock solutions were prepared in mineral medium in serum bottles and were made anoxic by sequential application of underpressure (with a vacuum pump) and flushing with N2. Subsequently, anoxic stock solutions were autoclaved and stored at 4 °C in the dark. Heat-labile substrate stocks of citrate (C6H5O73Na⋅2H2O), nitrite (NaNO2), and pyruvate (C3H3NaO3) were prepared in mineral medium, sterile filtered, and injected into sterile serum bottles. Solutions were made anoxic as described above under sterile conditions and stored at 4 °C in the dark. Nitrite-free NOB medium was prepared by strictly anoxic techniques as described by Widdel and Bak (84). Aliquots of 10 mL of the anoxic nitrite-free NOB medium were injected into sterile, N2-flushed 30-mL serum bottles, which were closed with butyl rubber stoppers and aluminum crimps. The respective organic substrate and NaNO3, or NaNO3 only, were added to the respective serum bottles to a final concentration of 1 mM each. Additionally, 3 mL of a N2:CO2 (80:20) mixture was added to the headspace of each serum bottle to provide a carbon source. N. moscoviensis biomass was harvested by centrifugation (8,228 × g, 20 min, 25 °C) and washed twice in anoxic nitrite-free NOB medium. After resuspension in the medium, 1 mL of cell suspension was added as inoculum to each serum bottle. The inoculated bottles were incubated at 37 °C in the dark for 34 d. During the incubation, samples were taken by using syringes and were centrifuged (20,817 × g, 20 min, 4 °C). Nitrite, nitrate, and formate concentrations in the supernatant were determined as described above. The concentrations of other organic substrates were determined by capillary electrophoresis as described above for formate.
The anoxic incubation of N. moscoviensis with formate and nitrate was repeated as described above with the following modifications. Aliquots (100 mL) of anoxic nitrite-free NOB medium were injected into sterile, N2-flushed 300-mL Schott flasks, which were closed with butyl rubber stoppers and screw caps. To provide a carbon source, 5 mL of a N2:CO2 (80:20) gas mixture was added to the headspace. After addition of washed (see above) N. moscoviensis biomass, all bottles were incubated for 1 d before the addition of substrates. Formate and nitrate were added to the incubations to final concentrations of 4.5 mM formate and 1.2 mM NaNO3. All incubations were performed at 37 °C for 15 d in the dark. Samples were taken by using syringes and centrifuged (17,949 × g, 10 min, 4 °C). The formate and nitrite concentrations in the supernatant were quantified as described above.
Supplementary Material
Acknowledgments
We thank Boris Nowka and Mario Pogoda for providing biomass of N. lenta and N. moscoviensis, respectively, for genomic analyses; Brigitte Lechthaler for cloning and analyzing the nxrA and nxrB paralogs of N. moscoviensis; Alexander Galushko for help with anoxic experiments and capillary electrophoresis; and the LABGeM team at Genoscope, France, for annotation support within the MicroScope platform. This work was supported by the Vienna Science and Technology Fund Grant LS09-40 (to H.D.), Radboud Excellence Initiative and the Netherlands Organization for Scientific Research Grant 863.14.019 (to S.L.), German Research Foundation Grant SP-667/10-1 (to E.S.), European Research Council Advanced Grant 294343 (to M.W.), and Austrian Science Fund Grant P25231-B21 (to H.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. CP011801 and KR873362–KR873392).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506533112/-/DCSupplemental.
References
- 1.Lam P, et al. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci USA. 2009;106(12):4752–4757. doi: 10.1073/pnas.0812444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraft B, et al. Nitrogen cycling. The environmental controls that govern the end product of bacterial nitrate respiration. Science. 2014;345(6197):676–679. doi: 10.1126/science.1254070. [DOI] [PubMed] [Google Scholar]
- 3.Prosser JI. Soil nitrifiers and nitrification. In: Ward BB, Arp DJ, Klotz MG, editors. Nitrification. Am Soc Microbiol; Washington: 2011. pp. 347–383. [Google Scholar]
- 4.Watson SW, Waterbury JB. Characteristics of two marine nitrite oxidizing bacteria, Nitrospina gracilis nov. gen. nov. sp. and Nitrococcus mobilis nov. gen. nov. sp. Arch Mikrobiol. 1971;77:203–230. [Google Scholar]
- 5.Sorokin DY, et al. Nitrification expanded: Discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012;6(12):2245–2256. doi: 10.1038/ismej.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alawi M, Lipski A, Sanders T, Pfeiffer E-M, Spieck E. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 2007;1(3):256–264. doi: 10.1038/ismej.2007.34. [DOI] [PubMed] [Google Scholar]
- 7.Lücker S, et al. Nitrotoga-like bacteria are previously unrecognized key nitrite oxidizers in full-scale wastewater treatment plants. ISME J. 2015;9(3):708–720. doi: 10.1038/ismej.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starkenburg SR, et al. Genome sequence of the chemolithoautotrophic nitrite-oxidizing bacterium Nitrobacter winogradskyi Nb-255. Appl Environ Microbiol. 2006;72(3):2050–2063. doi: 10.1128/AEM.72.3.2050-2063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starkenburg SR, et al. Complete genome sequence of Nitrobacter hamburgensis X14 and comparative genomic analysis of species within the genus Nitrobacter. Appl Environ Microbiol. 2008;74(9):2852–2863. doi: 10.1128/AEM.02311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R. Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: Quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol. 1999;65(8):3690–3696. doi: 10.1128/aem.65.8.3690-3696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowka B, Daims H, Spieck E. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: Nitrite availability as a key factor in niche differentiation. Appl Environ Microbiol. 2015;81(2):745–753. doi: 10.1128/AEM.02734-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daebeler A, et al. Interactions between Thaumarchaea, Nitrospira and methanotrophs modulate autotrophic nitrification in volcanic grassland soil. ISME J. 2014;8(12):2397–2410. doi: 10.1038/ismej.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovanec TA, Taylor LT, Blakis A, Delong EF. Nitrospira-like bacteria associated with nitrite oxidation in freshwater aquaria. Appl Environ Microbiol. 1998;64(1):258–264. doi: 10.1128/aem.64.1.258-264.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juretschko S, et al. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol. 1998;64(8):3042–3051. doi: 10.1128/aem.64.8.3042-3051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol. 2001;67(11):5273–5284. doi: 10.1128/AEM.67.11.5273-5284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebedeva EV, et al. Isolation and characterization of a moderately thermophilic nitrite-oxidizing bacterium from a geothermal spring. FEMS Microbiol Ecol. 2011;75(2):195–204. doi: 10.1111/j.1574-6941.2010.01006.x. [DOI] [PubMed] [Google Scholar]
- 17.Pester M, et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ Microbiol. 2014;16(10):3055–3071. doi: 10.1111/1462-2920.12300. [DOI] [PubMed] [Google Scholar]
- 18.Freitag TE, Chang L, Clegg CD, Prosser JI. Influence of inorganic nitrogen management regime on the diversity of nitrite-oxidizing bacteria in agricultural grassland soils. Appl Environ Microbiol. 2005;71(12):8323–8334. doi: 10.1128/AEM.71.12.8323-8334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson SW, Bock E, Valois FW, Waterbury JB, Schlosser U. Nitrospira marina gen. nov. sp. nov.: A chemolithotrophic nitrite-oxidizing bacterium. Arch Microbiol. 1986;144:1–7. [Google Scholar]
- 20.Altmann D, Stief P, Amann R, De Beer D, Schramm A. In situ distribution and activity of nitrifying bacteria in freshwater sediment. Environ Microbiol. 2003;5(9):798–803. doi: 10.1046/j.1469-2920.2003.00469.x. [DOI] [PubMed] [Google Scholar]
- 21.Gruber-Dorninger C, et al. Functionally relevant diversity of closely related Nitrospira in activated sludge. ISME J. 2015;9(3):643–655. doi: 10.1038/ismej.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keuter S, Kruse M, Lipski A, Spieck E. Relevance of Nitrospira for nitrite oxidation in a marine recirculation aquaculture system and physiological features of a Nitrospira marina-like isolate. Environ Microbiol. 2011;13(9):2536–2547. doi: 10.1111/j.1462-2920.2011.02525.x. [DOI] [PubMed] [Google Scholar]
- 23.Koch H, et al. Growth of nitrite-oxidizing bacteria by aerobic hydrogen oxidation. Science. 2014;345(6200):1052–1054. doi: 10.1126/science.1256985. [DOI] [PubMed] [Google Scholar]
- 24.Ehrich S, Behrens D, Lebedeva E, Ludwig W, Bock E. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch Microbiol. 1995;164(1):16–23. doi: 10.1007/BF02568729. [DOI] [PubMed] [Google Scholar]
- 25.Lücker S, et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc Natl Acad Sci USA. 2010;107(30):13479–13484. doi: 10.1073/pnas.1003860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spieck E, et al. Selective enrichment and molecular characterization of a previously uncultured Nitrospira-like bacterium from activated sludge. Environ Microbiol. 2006;8(3):405–415. doi: 10.1111/j.1462-2920.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 27.Mobley HLT, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59(3):451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pommerening-Röser A, Koops HP. Environmental pH as an important factor for the distribution of urease positive ammonia-oxidizing bacteria. Microbiol Res. 2005;160(1):27–35. doi: 10.1016/j.micres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Solomon CM, Collier JL, Berg GM, Glibert PM. Role of urea in microbial metabolism in aquatic systems: A biochemical and molecular review. Aquat Microb Ecol. 2010;59(1):67–88. [Google Scholar]
- 30.Stein LY, Arp DJ. Loss of ammonia monooxygenase activity in nitrosomonas europaea upon exposure to nitrite. Appl Environ Microbiol. 1998;64(10):4098–4102. doi: 10.1128/aem.64.10.4098-4102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chain P, et al. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol. 2003;185(9):2759–2773. doi: 10.1128/JB.185.9.2759-2773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowka B, Off S, Daims H, Spieck E. Improved isolation strategies allowed the phenotypic differentiation of two Nitrospira strains from widespread phylogenetic lineages. FEMS Microbiol Ecol. 2015;91(3):fiu031. doi: 10.1093/femsec/fiu031. [DOI] [PubMed] [Google Scholar]
- 33.Alonso-Sáez L, et al. Role for urea in nitrification by polar marine Archaea. Proc Natl Acad Sci USA. 2012;109(44):17989–17994. doi: 10.1073/pnas.1201914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lücker S, Nowka B, Rattei T, Spieck E, Daims H. The genome of Nitrospina gracilis illuminates the metabolism and evolution of the major marine nitrite oxidizer. Front Microbiol. 2013;4:27. doi: 10.3389/fmicb.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa E, Pérez J, Kreft JU. Why is metabolic labour divided in nitrification? Trends Microbiol. 2006;14(5):213–219. doi: 10.1016/j.tim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Arp D, Bottomley PJ. Nitrifiers: More than 100 years from isolation to genome sequences. Microbe. 2006;1:229–234. [Google Scholar]
- 37.Mellbye BL, Bottomley PJ, Sayavedra-Soto LA. The nitrite-oxidizing bacterium Nitrobacter winogradskyi produces N-acyl-homoserine lactone autoinducers. Appl Environ Microbiol. 2015;81(17):5917–5926. doi: 10.1128/AEM.01103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okabe S, Satoh H, Watanabe Y. In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol. 1999;65(7):3182–3191. doi: 10.1128/aem.65.7.3182-3191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almstrand R, Daims H, Persson F, Sörensson F, Hermansson M. New methods for analysis of spatial distribution and coaggregation of microbial populations in complex biofilms. Appl Environ Microbiol. 2013;79(19):5978–5987. doi: 10.1128/AEM.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ushiki N, Fujitani H, Aoi Y, Tsuneda S. Isolation of Nitrospira belonging to sublineage II from a wastewater treatment plant. Microbes Environ. 2013;28(3):346–353. doi: 10.1264/jsme2.ME13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Gool A, Laudelout H. Formate utilization by Nitrobacter winogradskyi. Biochim Biophys Acta. 1966;127(2):295–301. doi: 10.1016/0304-4165(66)90384-9. [DOI] [PubMed] [Google Scholar]
- 42.Bock E. Growth of nitrobacter in the presence of organic matter. II. Chemoorganotrophic growth of Nitrobacter agilis. Arch Microbiol. 1976;108(3):305–312. doi: 10.1007/BF00454857. [DOI] [PubMed] [Google Scholar]
- 43.Bock E, Koops H-P, Möller UC, Rudert M. A new facultatively nitrite oxidizing bacterium, Nitrobacter vulgaris sp. nov. Arch Microbiol. 1990;153:105–110. [Google Scholar]
- 44.Freitag A, Rudert M, Bock E. Growth of Nitrobacter by dissimilatoric nitrate reduction. FEMS Microbiol Lett. 1987;48:105–109. [Google Scholar]
- 45.Hanson TE, Campbell BJ, Kalis KM, Campbell MA, Klotz MG. Nitrate ammonification by Nautilia profundicola AmH: Experimental evidence consistent with a free hydroxylamine intermediate. Front Microbiol. 2013;4:180. doi: 10.3389/fmicb.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park HD, Noguera DR. Nitrospira community composition in nitrifying reactors operated with two different dissolved oxygen levels. J Microbiol Biotechnol. 2008;18(8):1470–1474. [PubMed] [Google Scholar]
- 47.Winkler MKH, Bassin JP, Kleerebezem R, Sorokin DY, van Loosdrecht MCM. Unravelling the reasons for disproportion in the ratio of AOB and NOB in aerobic granular sludge. Appl Microbiol Biotechnol. 2012;94(6):1657–1666. doi: 10.1007/s00253-012-4126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foesel BU, et al. Nitrosomonas Nm143-like ammonia oxidizers and Nitrospira marina-like nitrite oxidizers dominate the nitrifier community in a marine aquaculture biofilm. FEMS Microbiol Ecol. 2008;63(2):192–204. doi: 10.1111/j.1574-6941.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 49.Vallenet D, et al. MicroScope--an integrated microbial resource for the curation and comparative analysis of genomic and metabolic data. Nucleic Acids Res. 2013;41(Database issue, D1):D636–D647. doi: 10.1093/nar/gks1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markowitz VM, et al. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res. 2014;42(Database issue, D1):D560–D567. doi: 10.1093/nar/gkt963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suchard MA, Redelings BD. BAli-Phy: Simultaneous Bayesian inference of alignment and phylogeny. Bioinformatics. 2006;22(16):2047–2048. doi: 10.1093/bioinformatics/btl175. [DOI] [PubMed] [Google Scholar]
- 52.Siewe RM, et al. Urea uptake and urease activity in Corynebacterium glutamicum. Arch Microbiol. 1998;169(5):411–416. doi: 10.1007/s002030050591. [DOI] [PubMed] [Google Scholar]
- 53.Strugatsky D, et al. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2013;493(7431):255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valladares A, Montesinos ML, Herrero A, Flores E. An ABC-type, high-affinity urea permease identified in cyanobacteria. Mol Microbiol. 2002;43(3):703–715. doi: 10.1046/j.1365-2958.2002.02778.x. [DOI] [PubMed] [Google Scholar]
- 55.Eitinger T, Rodionov DA, Grote M, Schneider E. Canonical and ECF-type ATP-binding cassette importers in prokaryotes: Diversity in modular organization and cellular functions. FEMS Microbiol Rev. 2011;35(1):3–67. doi: 10.1111/j.1574-6976.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 56.Beckers G, Bendt AK, Krämer R, Burkovski A. Molecular identification of the urea uptake system and transcriptional analysis of urea transporter- and urease-encoding genes in Corynebacterium glutamicum. J Bacteriol. 2004;186(22):7645–7652. doi: 10.1128/JB.186.22.7645-7652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benoit SL, Mehta N, Weinberg MV, Maier C, Maier RJ. Interaction between the Helicobacter pylori accessory proteins HypA and UreE is needed for urease maturation. Microbiology. 2007;153(Pt 5):1474–1482. doi: 10.1099/mic.0.2006/003228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambelli B, Musiani F, Benini S, Ciurli S. Chemistry of Ni2+ in urease: Sensing, trafficking, and catalysis. Acc Chem Res. 2011;44(7):520–530. doi: 10.1021/ar200041k. [DOI] [PubMed] [Google Scholar]
- 59.Musiani F, Zambelli B, Stola M, Ciurli S. Nickel trafficking: Insights into the fold and function of UreE, a urease metallochaperone. J Inorg Biochem. 2004;98(5):803–813. doi: 10.1016/j.jinorgbio.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Olson JW, Mehta NS, Maier RJ. Requirement of nickel metabolism proteins HypA and HypB for full activity of both hydrogenase and urease in Helicobacter pylori. Mol Microbiol. 2001;39(1):176–182. doi: 10.1046/j.1365-2958.2001.02244.x. [DOI] [PubMed] [Google Scholar]
- 61.Rothery RA, Workun GJ, Weiner JH. The prokaryotic complex iron-sulfur molybdoenzyme family. Biochim Biophys Acta. 2008;1778(9):1897–1929. doi: 10.1016/j.bbamem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Spieck E, Ehrich S, Aamand J, Bock E. Isolation and immunocytochemical location of the nitrite-oxidizing system in nitrospira moscoviensis. Arch Microbiol. 1998;169(3):225–230. doi: 10.1007/s002030050565. [DOI] [PubMed] [Google Scholar]
- 63.Klotz MG, et al. Evolution of an octahaem cytochrome c protein family that is key to aerobic and anaerobic ammonia oxidation by bacteria. Environ Microbiol. 2008;10(11):3150–3163. doi: 10.1111/j.1462-2920.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 64.Tikhonova TV, et al. Molecular and catalytic properties of a novel cytochrome c nitrite reductase from nitrate-reducing haloalkaliphilic sulfur-oxidizing bacterium Thioalkalivibrio nitratireducens. Biochim Biophys Acta. 2006;1764(4):715–723. doi: 10.1016/j.bbapap.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 65.MacGregor BJ, et al. Why orange Guaymas Basin Beggiatoa spp. are orange: Single-filament-genome-enabled identification of an abundant octaheme cytochrome with hydroxylamine oxidase, hydrazine oxidase, and nitrite reductase activities. Appl Environ Microbiol. 2013;79(4):1183–1190. doi: 10.1128/AEM.02538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwasigroch JM, Wintjens R, Gilis D, Rooman M. SODa: An Mn/Fe superoxide dismutase prediction and design server. BMC Bioinformatics. 2008;9:257. doi: 10.1186/1471-2105-9-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krzywinski M, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albertsen M, et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol. 2013;31(6):533–538. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- 69.Leggett RM, Clavijo BJ, Clissold L, Clark MD, Caccamo M. NextClip: An analysis and read preparation tool for Nextera Long Mate Pair libraries. Bioinformatics. 2014;30(4):566–568. doi: 10.1093/bioinformatics/btt702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5(2):R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyatt D, et al. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eddy SR. Accelerated Profile HMM Searches. PLOS Comput Biol. 2011;7(10):e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finn RD, et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42(Database issue, D1):D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 75.Hyman MR, Arp DJ. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992;267(3):1534–1545. [PubMed] [Google Scholar]
- 76.Amann RI, et al. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daims H, Brühl A, Amann R, Schleifer K-H, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: Development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22(3):434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 78.Maixner F, et al. Nitrite concentration influences the population structure of Nitrospira-like bacteria. Environ Microbiol. 2006;8(8):1487–1495. doi: 10.1111/j.1462-2920.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 79.Mobarry BK, Wagner M, Urbain V, Rittmann BE, Stahl DA. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol. 1996;62(6):2156–2162. doi: 10.1128/aem.62.6.2156-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daims H, Stoecker K, Wagner M. 2005. Fluorescence in situ hybridisation for the detection of prokaryotes. Molecular Microbial Ecology, eds Osborn AM, Smith CJ (Bios-Garland, Abingdon, UK), pp 213–239.
- 81.Kandeler E, Gerber H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils. 1988;6(1):68–72. [Google Scholar]
- 82.Griess-Romijn van Eck E. Physiological and chemical tests for drinking water. NEN. 1966;1056:IV-2. [Google Scholar]
- 83.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 84.Widdel F, Bak F. 1992. Gram-negative mesophilic sulfate-reducing bacteria. The Prokaryotes, eds Balows A, Trüper HG, Dworking M, Harder W, Schleifer K-H (Springer, New York), 2nd Ed, pp 3352–3378.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.