Abstract
Purpose
Cortisol is frequently assayed as a stress-responsive biomarker which changes over the course of minutes to meet the demands of an individual’s social context. Salivary cortisol is often utilized as a non-invasive sampling methodology which possesses important health implications. A critical barrier to psychobiological research involving salivary cortisol is a time-delay of days to months before cortisol results are obtained via immunoassay, long after the individual is no longer proximate to the social context in which they provided the sample. The current study was designed to address this critical barrier through creation of a lateral flow technology (LFT) cortisol device capable of measuring salivary cortisol within minutes of sample collection. LFT is frequently used within commercial point-of-care settings to obtain rapid answers to the presence/absence of a biomarker. The present study extends LFT into the research domain by presenting performance characteristics of a quantitative LFT which measures salivary cortisol within 20 minutes of sample collection.
Methods
Saliva samples on N=29 adults (15 males) were obtained in the morning and afternoon using Passive Drool and then the Super•SAL™ Extra Collection Device (hereafter Super•SAL™) and later assayed with LFT and a commercially available enzyme-immunoassay.
Findings
Results show LFT correlated well with these collection methods (R=.872 with Super•SAL™; R=.739 with Passive Drool, p-values<.0001) and at comparable levels to correspondence of Super•SAL™ with Passive Drool (R=.798, p<.0001) which were measured with the same assay.
Implications
These results open up an exciting new possibility to integrate this technological advance into stress research, including knowing and potentially changing the individual’s social context in a time-sensitive manner. Methodological improvements such as this have the possibility of refining conceptual models of stress reactivity and regulation.
Keywords: Cortisol, Immunoassay, Lateral Flow Technology, Stress Regulation, Collection Methods
Introduction
Stress is a leading cause of morbidity and mortality in the US1. A small set of biomarkers provide information about chronic and acute stressors.2 Cortisol is putatively the most frequently investigated stress biomarker3 because cortisol is linked with many physiological processes such as neural development and cell death4, immune function5, learning and memory6, sleep7, metabolism and fat distribution8, growth and development9, reproduction10, and aging11. The powerful role of cortisol in shaping health outcomes is illustrated by its clinical value in treatments ranging from mild rashes in over-the-counter creams to life-saving efforts12.
Cortisol is the endproduct of the hypothalamic-pituitary-adrenal (HPA) axis. After appraisal of the social context in limbic and paralimbic neural circuitries13,14, the hypothalamus releases corticotropic releasing hormone which initiates a hormonal cascade that culminates with adrenocorticotropic hormone (ACTH) stimulating the release of cortisol from the adrenal cortex and into the blood15. After ~15min of the onset of a stressor, cortisol levels peak16–18. As a lipid soluble hormone, cortisol easily crosses through cellular membranes which allows it to travel directly to cell nuclei to change gene expression19,20 especially in the brain where it is responsible for terminating the stress response4 via negative feedback.21,22 Cortisol also acts throughout the body where it influences physiology over seconds, minutes, hours and days23. Salivary measurement of small steroids like cortisol take advantage of the fact that free-cortisol is lipid-soluble; this biologically-active fraction of total cortisol passes through the acinar cells to enter saliva via passive diffusion in proportion to cortisol’s entrance into cell nuclei24.
Measuring cortisol in saliva has opened up a window of opportunity to conduct stress-related research involving many repeated measures25 or applications with vulnerable populations26,27 or in unique settings28,29. Salivary measurement has even demonstrated unique diagnostic and treatment information about cortisol related diseases such as Addison’s syndrome or Cushing’s disease which previously required much more invasive measurements throughout treatment30–33. First generation salivary cortisol assays relied on radiolabeled cortisol antibodies in radioimmunoassays which were highly sensitive and specific. This sensitivity was enhanced with the next generation of assays that quantified cortisol levels via optical density measurements in commercially available enzyme- or luminescence-immunoassays which required much lower sample volumes to be effective. These particular methods rely on the degree of color change of bound horseradish peroxidase to estimate hormone concentrations relative to a standard curve. The range of sensitivities using these assays provided a limit of detection in the pg/mL range, a necessary pre-requisite for salivary steroid hormone detection where concentrations are low.
A critical barrier in the stress field is that current immunoassay methodologies do not provide reportable results for cortisol for days to months after collection. Very recent studies are beginning to explore the feasibility of point-of-care measurement of salivary cortisol34–37, typically utilizing Lateral Flow Test (LFT) assays to deliver qualitative or quantitive test results within minutes of sample collection38. The present study describes the first in a new generation of assays for measuring salivary hormones like cortisol using an LFT device [IDENTIFYING INFORMATION SUPPRESSED]. The first US patent using the term “ Lateral Flow” was filed in 1988 and awarded in 199039 although several companies had patented elements of that concept earlier. The first successful commercial use of an LFT was the EPT urine human chorionic gonadotropin hormone dipstick pregnancy test. LFT has been widely used in industry since the late 1980s and early 1990s, primarily targeting commercial needs for Point of Care assays40. In similar fashion to EIA and LIA, LFT relies on the detection of an emitted signal [in this case fluorescence] to provide a fully quantitative cortisol readout but, unlike EIA and LIA or even technologies like Mass Spectrometry or Microarrays, LFTs have not been widely used in the academic community. The reasons for this may be related to education, intellectual property considerations, and a lack of LFT suppliers that cater to the specific needs and recommendations of researchers.
The Cortisol LFT technology is a unique proprietary format that improves upon several related patented technologies41–43. We designed this LFT specifically for the research community in order to provide major advantages over currently available methodologies for saliva: (1) the assay takes minutes from sample collection to end results; (2) the assay and reading unit [Litebox Image Analysis Module™, LIAM™] are portable, allowing real time cortisol assessment to be performed in a variety of point-of-care and non traditional settings; (3) the assay retains many of the advantages of available methodologies (e.g., high sensitivity and quantitative output), so few sacrifices in assay quality are required for real-time, point-of-care cortisol measurement. The present study describes the methodology and performance characteristics of real-time LFT cortisol including comparison of this technology with another commercially available non-LFT assay. The goal was to determine correspondence and, by extension, viability of obtaining real-time cortisol scores within the near future.
Methods
Participants
All procedures were approved by the Institutional Review Board at the IDENTIFYING INFORMATION SUPPRESSED and all participants provided informed consent. Participants were excluded if they were taking oral steroids, or had eaten or consumed alcohol within 1 hour, or were over age 36 years. After exclusion, the final sample size was N=29.
Procedures
Participants provided four saliva samples, two in the morning prior to 11:30 AM (M= 8:54 AM, range=6:11–11:27 AM) and two in the afternoon/evening after 1 PM (M=4:46 PM, range=1:29–11:11 PM). These rough times were selected in order to obtain high and low cortisol levels anticipated by the diurnal rhythm of the hormone. A total of 3 participants provided AM or PM samples only, leaving a total N=55 samples. Order of sampling was not counter-balanced so as to avoid carry-over effects. First, participants provided an unstimulated saliva sample via Passive Drool by expectorating directly into a 2 mL polypropylene cryovial (M=3.43min, SD=3.07min for collection times). Immediately following the Passive Drool sample and questionnaire (M=10.53min later, SD=15.97), participants provided a second saliva sample using the Super•SAL™ Extra Collection Device (IDENTIFYING INFORMATION SUPPRESSED), hereafter termed Super•SAL™. This device serves as the first stage of LFT cortisol collection as it contains an absorbent pad which collects up to 2 mL of saliva (M=2.63min, SD=1.52min for collection times). The absorbent pad filters many extraneous substances from saliva which is necessary with LFT as these types of assays are sensitive to viscosity and sample quality. Once the sample volume adequacy indicator on Super•SAL™ indicated ample volume had been collected (by changing from light green to blue), participants removed the collection device, inserted it into the compression tube and expressed the saliva into a cryovial using force on the handle. The compression tube contains a proprietary filtration medium, which removes additional extraneous materials and unwanted particulates that can compromise the LFT. In this way, the Super•SAL™ provided purified saliva for the LFT as well as a confirmation specimen for later testing. Samples were immediately frozen at −80°C and shipped on dry ice to IDENTIFYING INFORMATION SUPPRESSED for assay by LFT. Residual samples were then shipped on dry ice to (IDENTIFYING INFORMATION SUPPRESSED) for independent confirmation using EIA methodology.
Methods
IBL Cortisol Assay
Both Passive Drool and Super•SAL™ specimens were assayed using a solid phase enzyme-linked immunosorbant assay using reagents with the same lot and expiration date purchased from IBL International (Hamburg, Germany, www.IBL-International.com). The IBL kit uses 50 uL of saliva with an expected range of 0.015–5ug/dL cortisol and reported correspondence with IBL cortisol luminescence assay of R=.96. Intra- and inter-assay CVs are, on average, 4.9 and 8.2%, respectively.
LFT Cortisol System
Saliva from Super•SAL™ was assayed using the LFT; Passive Drool was not utilized given viscosity concerns (see above). Given the novelty of this assay, non-proprietary details are provided. The basic platform is a positive read small molecule competitive quantitative LTF strip with several components. The first major component is a test housing which incorporates the two LFT strips. A sample pad located directly below the housing sample well acts as a reservoir to distribute saliva to a conjugate pad. The conjugate pad then accepts the saliva as a fluid medium for hydration of the dried conjugate. The VerOFy® LFT point-of-care cortisol system then employs two Europium Fluorescent particle based conjugates that react with free and conjugate bound cortisol in the saliva sample (hereafter termed fluid as the properties of saliva have been purposely altered to accommodate the LFT). A nitrocellulose membrane then accepts reacted-conjugate from the conjugate pad. The nitrocellulose membrane then binds the reaction products to the two capture areas which each contain two capture bands that are immobilized onto the nitrocellulose membrane. The first pair of bands consists of immobilized BSA-Cortisol. The second pair of bands is a combination of binding molecules that scavenge any conjugate that does not bind to the BSA-Cortisol bands. An absorption pad then accepts fluid from the nitrocellulose membrane, providing the capillary engine to continuously pull fluid through the strip.
The second major component is the Litebox Image Analysis Module (LIAM™) which is a fluorescent LFT cassette reader with onboard capability to analyze and transfer cortisol data via bluetooth connection. After the LFT strips have been exposed to sample, the VerOFy® test housing is inserted into the LIAM™ reader as the test housing is specifically engineered to expose the nitrocellulose membrane on the LFT strip to exciting UV light in a precise orientation to ensure reproducible image analysis. The LIAM™ uploads test information, including lot-to-lot calibration curve parameters by reading a QR code on the housing. The LIAM™ is also equipped with a digital temperature sensor that allows for kinetic temperature corrections to be made within the operating range of 18–30°C as LFT is known to be sensitive to temperature. Ultimately, the LIAM™ will generate an algorithm to perform the quantitation to report cortisol scores in µg/mL units with data transferred to a computer or smart-phone. At present, results are reported as T/R ratio: A ‘test/reference’ ratio in which low ratio values indicate cortisol near zero and higher ratio scores indicate greater binding in the secondary capture zone relative to the primary capture zone on the strip. That is, binding of cortisol to the primary zone is displaced and captured by the secondary band when cortisol is high (see Figure 1).
Figure 1.
Illustration of the LIAM™ reader and test housing where the VerOFy® LFT strips are integrated, as well as the Super•SAL™ Extra Collection Device.
Statistical Analyses
First, we examined performance specifications of the assay using t-tests and the coefficient of variation (CV). Correspondence across assay type was calculated as Pearson’s bivariate correlations and then Fisher R-to-Z transformation to determine whether the magnitude of one correlation was significantly larger than another. Lastly, a series of linear regressions relied on the R2 to elucidate how much variance in LFT or Passive Drool, respectively, were accounted for by the other assays. We then added collection time or high-vs-low concentration, respectively, to the linear regression to determine if these explained or changed the correspondence across assays.
Results
Performance Specifications
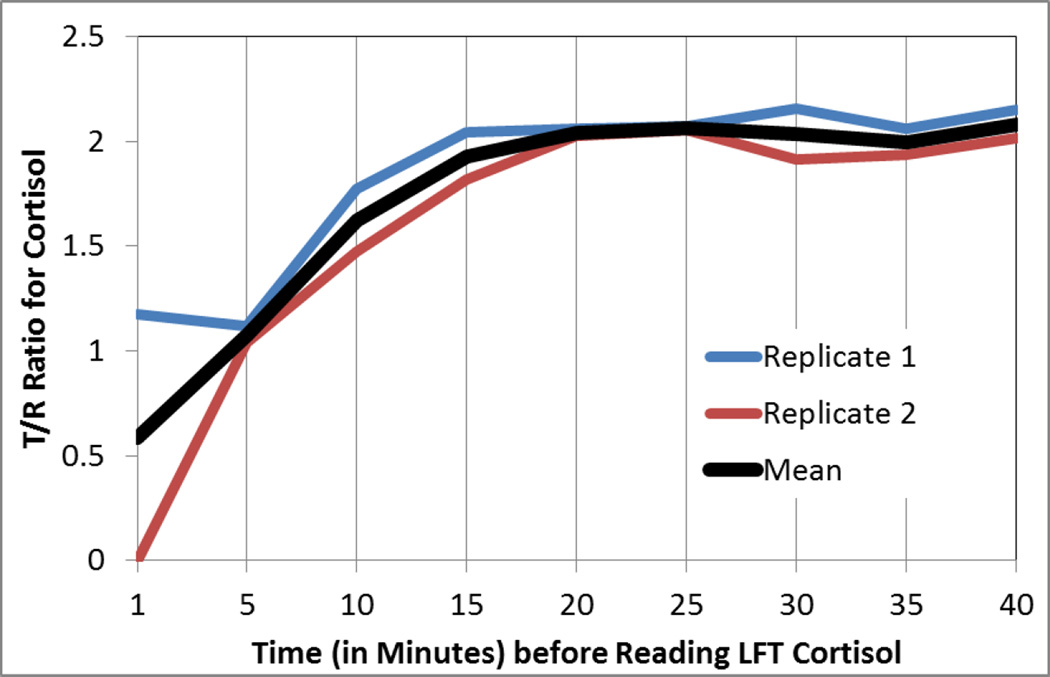
Time-Course
To determine the time-course of the Lateral Flow Test, that is the optimal time to read the cortisol scores after sample collection, saliva samples were run through our collection and purification system and read at 5 minute intervals. Figure 2 shows the rise in cortisol levels as the sample flows down across the nitrocellulose membrane and binds to cortisol. Stability appeared to be achieved at 20 minutes post collection. To probe further, a series of t-tests were calculated in which samples assayed before a time-threshold were compared with samples assayed after a time-threshold. Samples assayed at 10min, t(7)=4.7, p<.002, and 15min, t(7)=2.8, p<.025, were significantly lower than the samples assayed later. The sample assayed at 20 min, t(7)=1.91, p=.10, and 25min, t(7)=1.35, p=.22, and 30min, t(7)=.97, p=.36 were not significantly different from one another, suggesting stability in concentration was achieved around 20 minutes. Hereafter, all LFT is reported according to a read-time of 20 minutes.
Figure 2.
Timecourse of LFT cortisol suggests the optimal read time is 20 minutes post-collection, but stable reads are achieved earlier.
Variation
Performance specifications for LFT cortisol were good. Out of the possible 55 LFT devices, 4 failed QC specifications (7.2%) based on area of the strip or image brightness.
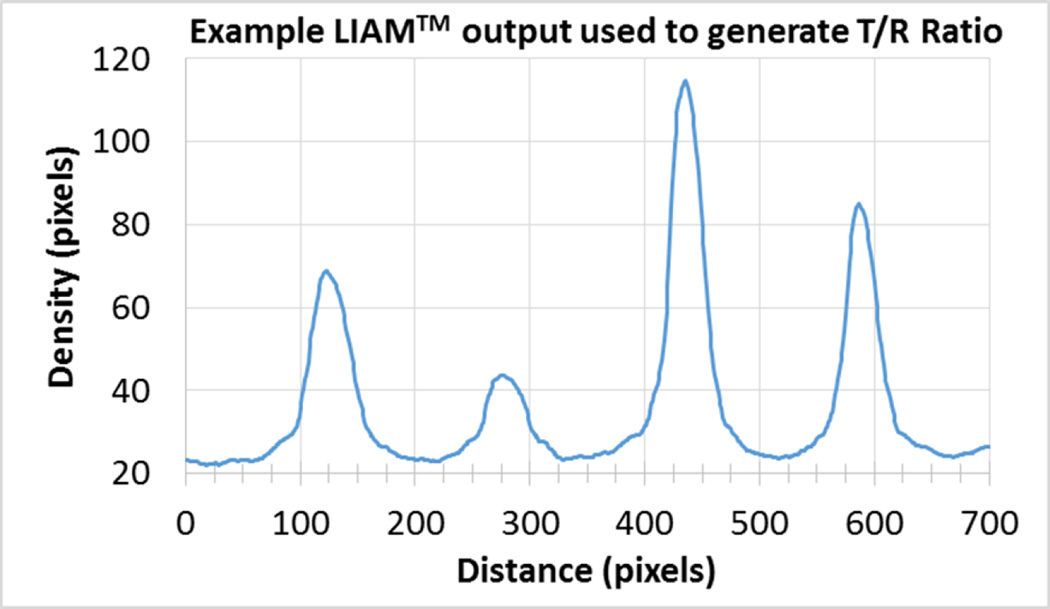
Strips were selected a priori for quality control based on total area of the strip and image brightness. All strips were developed with a saliva pool purified with Super•SAL™ and run N=5 times using strips from the same lot (CORT140528CB-2). The inter-assay coefficient of variation was excellent, with 6.99% variation across the five replicates. The inter-assay CV for low QC strips was not acceptable given the low area of the strip and poor light quality (49.15%), demonstrating the importance of high QC. Figure 3 illustrates the reading for one replicate run to illustrate the calculation of the T/R ratio.
Figure 3.
Example LIAM™ output used to generate T/R Ratio. Like other assays, initial results are reported in optical density and then later converted to concentration values.
To determine the lower limit of detection, the precision of read was measured using a purified saliva pool containing low cortisol in T/R units based on N=5 bright readings. A calculated %CV was determined to be 7% (i.e., SD of .032), the lowest level of cortisol that can be distinguished from zero is .513 T/R (2SD from zero T/R). Using our current algorithm as a reference, this corresponds to 0.91 ng/mL (i.e., 2.51 nmol/L), which is well within the expected range of salivary cortisol using the IBL EIA kit.
Participant Characteristics
For the human saliva component, participants included 32 adults (15 males) between the ages of 18 and 36 years (M=27.5, SD=4.704). Participant ethnicities included Caucasian (65.6%), Hispanic (18.8%), African American (3.1%), Asian or Pacific Islander (3.1%), and Other (6.3%) ethnicities. For all of the saliva samples collected, only one sample out of 51 (2%) fell below the LLD of 0.91 ng.mL (i.e., 2.51 nmol/L).
Correspondence with Enzyme-Immunoassay
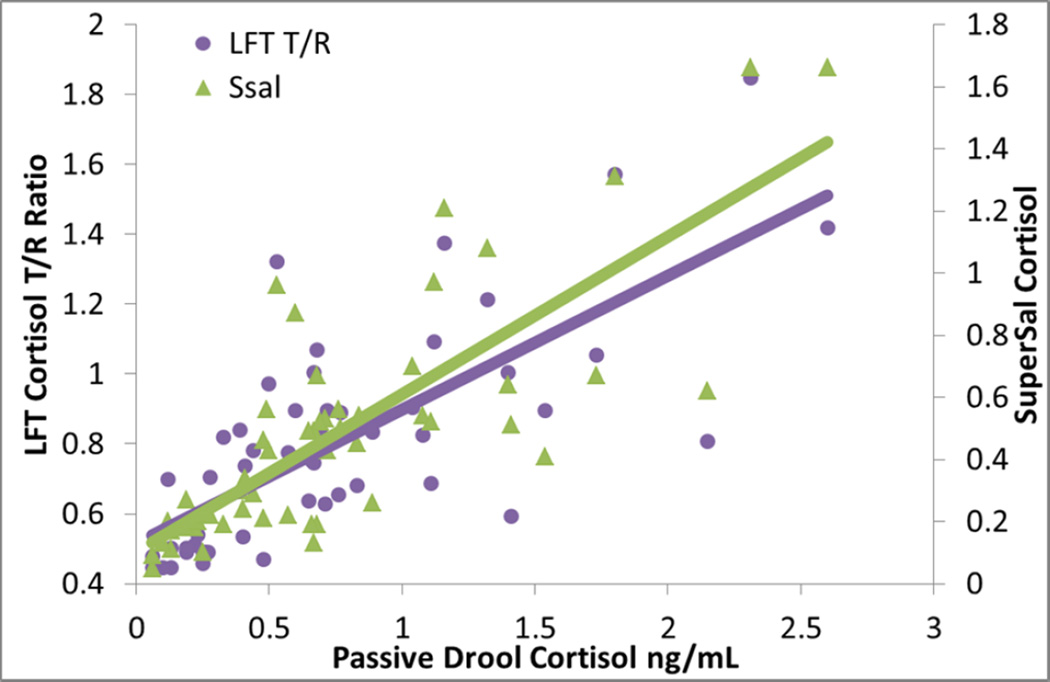
Correspondence across assay types is based on N=55 for the immunoassays and N=51 for LFT cortisol. Bivariate correlations of LFT with other methods were highly significant (R=.872 with Super•SAL™; R=.739 with Passive Drool, p-values<.0001). Using Fisher R-toZ transformation, we found that LFT showed a trend for a better correlation with Super•SAL™ than Passive Drool (Z=1.93, p=.054). The inter-correlation of Passive Drool with Super•SAL™ was highly significant (R=.798 with Super•SAL™, p<.0001). Using Fisher’s r-to-z transformation, we determined that the correlations of Passive Drool with Super•SAL™ or LFT were comparable in magnitude (Z=.24, p=.47). Figure 4 illustrates these inter correlations.
Figure 4.
Inter-correlations between techniques for measuring cortisol are high. Both Passive Drool and Super•SAL™ cortisol are obtained with a commercially available assay from IBL; LFT cortisol was obtained within 20 minutes of assay start.
A series of linear regressions were then run in which Passive Drool and then Super•SAL™ predicted LFT cortisol to further determine whether Super•SAL™ or Passive Drool was best related to LFT. Passive Drool significantly predicted LFT cortisol, β=.74, p<.0001, explaining 55% of the variance in LFT cortisol, F(1, 49)=58.9, p<.0001. When Super•SAL™ was added a predictor, an additional 22% of the variance in LFT cortisol was explained, F(1,48)=44.49, p<.0001, suggesting Super•SAL™ is related to LFT cortisol beyond the correlation with Passive Drool. More specifically, while Super•SAL™ predicted LFT cortisol independently of Passive Drool, β=.79, p<.0001, Passive Drool no longer contributed independently to the prediction of LFT cortisol, β= .10, p=.40. Conversely, we found that Super•SAL™ alone explained 76% of the variance in LFT cortisol, F(1,49)=155.82, p<.0001, but Passive Drool did not add further in the prediction of LFT cortisol, explaining 0.4% additional variance in LFT cortisol, F(1,48)=.73, p=.4. These findings suggest that LFT cortisol is related to other methods of measuring cortisol, and may be best related to Super•SAL™ cortisol which shares a collection device methodology.
Given that Passive Drool may be considered the “gold standard”, we then determined which method was best correlated with Passive Drool Cortisol. We conducted a parallel series of linear regressions to see whether Super•SAL™ or LFT best correlated with Passive Drool. Beyond the 55% of the variance in Passive Drool cortisol explained by LFT cortisol, Super•SAL™ explained an additional 11% of the variance in Passive Drool, F(1,48)=15.29, p<.0001, and only Super•SAL™ remained an independent predictor of Passive Drool, β=.68, p<.0001 for Super•SAL™ and β=.15, p=.4 for LFT. LFT cortisol did not explain the variance in Passive Drool cortisol beyond Super•SAL™, R2=.5%, F(1,48)=.73, p=.4. Findings suggest Passive Drool cortisol is significantly related to LFT cortisol, but Super•SAL™ explains additional variance in cortisol beyond the LFT methodology perhaps related to the fact that both Passive Drool and Super•SAL™ were assayed with the same IBL assay. Taken together, all three methodologies are highly inter-correlated, but Super•SAL™ may be uniquely related to the other technologies, likely through shared collection devices (i.e., LFT) or assay techniques (i.e., Passive Drool).
To determine whether inter-correlations significantly differed between AM and PM collection times (correspondence of LFT with Super•SAL™ was R=.857 in AM and R=.719 in PM; correspondence of LFT with Passive Drool was R=.567 in AM and R=.729 in PM, p-values <.05), we conducted a series of linear regressions in which the main effect of assay type and time (AM or PM) were included in step 1, and the interaction between assay type and time of day was added in the step 2. With Passive Drool as the outcome, we did not find that the inter-correlation with LFT differed by time of day, F(1,47)=.54, p=.47, nor did we find that the inter-correlation with Super•SAL™ differed by time of day, F(1,51)=1.0, p=.32. Similarly, we did not find that the inter-correlation of LFT with Super•SAL™ differed by time of day, F(1,47)=.03, p=.87. Findings suggest that the inter-correlations across assay type did not systematically change across the day.
Parallel analyses contrasted specifically low vs high cortisol concentrations across each assay type using a median split. We did not find that the inter-correlation of Passive Drool with LFT, F(1,47)=.29, p=.59, or Passive Drool with Super•SAL™, F(1,51)=2.24, p=.14, or LFT with Super•SAL™, F(1,47)=.73, p=.4, differed by low vs high concentrations, respectively. In summary, these three assay types are highly inter-correlated and the magnitude of their association does not systematically vary by time of day or concentration.
Discussion
The biomarker cortisol is frequently investigated in psychobiological research, with hundreds of studies across the past few decades illustrating its utility. A critical barrier in the stress field is that current immunoassay methodologies do not provide reportable results for cortisol for days to months after collection. Failure to obtain time-sensitive information is especially problematic for this hormone as cortisol constantly changes to allow the individual to efficiently encode and filter salient environmental cues44. Using LFT and a portable LIAM™ we were able to assay cortisol reliably within 20 minutes with strong assay performance specifications and correspondence with other methodologies.
Real-time cortisol measurement has the potential to advance the field of stress physiology. Real-time technology with other psychobiological measures – for example blood pressure, heart rate, or skin conductance– illustrate the tremendous impact that real-time results can have on the stress-field including permitting biofeedback studies46, clinical trials to improve relaxation47 and enhancing cardiac diagnosis and clinical outcomes48. We set out to confront the time-sensitivity barrier with the HPA axis as these systems can be dissociated49. We believe the field is poised to consider novel HPA biomarkers given the powerful impact of very recent advances in cortisol technology (e.g., hair cortisol45).
Obtaining salivary cortisol scores within 20 minutes of sample collection is advantageous as this time-course matches well with the time-course of peak cortisol reactivity following an acute stressor and precedes the time in which behavioral effects of cortisol are anticipated23 or before negative feedback emerges50. Practically speaking, this technology may be useful for ensuring that the individual is no longer stressed by laboratory arrival before initiating a targeted stressor. More interestingly, this technology may open up the possibility of identifying stress responders while still within the laboratory. Successful laboratory stressors frequently trigger a stress response in ~50–70% of participants13,17. Even for robust laboratory challenges, it is problematic when a large percentage of individuals are non-responders. Some individuals may appear as non-responders when they appraise the challenge, but successfully regulate responsivity prior to crossing the relatively high stress threshold of the HPA axis51. Conversely, it may be maladaptive for some individuals to appear as non-responders52. In this case, failure to show an HPA stress response signifies that the individual was hyporesponsive to stress, with a low capacity to mount a stress response even in situations that call for it. This lack of malleability in the HPA axis is problematic because such individuals would not be able to recalibrate their physiological functioning to meet the demands of a changing environment, increasing the individuals risk for stress-related diseases53 and problems54. Early identification of non-responders within 20 minutes is an important step toward distinguishing between these types of non-responders and further advancing understanding psychopathology risk.
The time-course of 20 minutes delay is typical of LFT38, with shorter read-times for qualitative results and longer read-times for quantitative results36. We recognize that shorter delays may be desirable while still maintaining quantitative results as for the VerOFy® system. Future experiments will determine whether earlier readings on the LIAM™ produce cortisol scores which can be reliably extrapolated to inform the individual’s final cortisol reading. It is possible at present that each sample could be read multiple times beginning as early as 5 minutes post collection and then finalized by 20 minutes post collection. In the research setting, for example, this possibility would allow stressors to be modified during the challenge after a non-responder is identified so that the experimenter could enhance the acute challenge. A handful of studies have explored how modifications to social evaluative threat51,55–59 or uncontrollability57,60,61 can be experimentally adjusted to influence rates of cortisol responsivity. The next step is to implement such modifications with information about the individual’s current physiology prior to initiation of a modification.
Correspondence of LFT cortisol with Passive Drool and Super•SAL™ cortisol scores obtained with the IBL commercial assay was good with correlations near or above R=.80. Future analyses will determine whether similar correspondence is obtained with Mass Spectrometry as well as other commercially available assays. Much of the degradation in correspondence was introduced prior to LFT but this was necessary given that LFT is sensitive to sample viscosity and particulates. With traditional assays, particulates in saliva are reduced by the first freeze-thaw cycle, centrifugation, and (in some cases) dilution or extraction. With the LFT, none of these initial steps are required so as to avoid the time delay in protocols that these steps necessitate (ranging from 10 minutes to 2 days). Indeed, costly lab equipment such as ultracold freezers or centrifuges are not needed at all which opens up a possibility of sample collection and assay in a range of settings without access to refrigeration or reliable shipping62,63. The current methodology requires the portable fluorescence LIAM™ reader (whose cost is on par with an inexpensive laptop computer) to obtain results but no further large equipment. This technology thus reduces cost by eliminating the need for costly lab equipment, shipping and storage. Moreover, cortisol is currently analyzed at substantial cost per sample, regardless of its utility. This practical burden is large given that stress protocols fail in 30 to 50% of participants13,17,64. Real time analytical information about this stress biomarker has the ability to exert a powerful impact on the stress field. Although final costs have not yet been determined, we anticipate that the cost of VerOFy® cortisol will be significantly cheaper than current laboratory-based methods which are typically $12–$25 dollars for external laboratories.
There are several limitations to the present study. First, we recognize that correspondence of LFT with traditional assays is not perfect and further validation may be desirable on a study-by-study basis. Therefore, the collection device was designed to allow for a confirmation specimen to be obtained and shipped to a laboratory, if desired. Secondly, we recognize the inherent variability in results and ongoing calibration of the algorithm to convert LTF cortisol to standardized units, and consequently recommend the device is used for research purposes at this time. Indeed, we believe that a major advantage of this technology is that it is being created through collaboration between researchers and LFT experts so as to create a device which best accommodates the needs and desires of the research community. This diverges from the traditional applications of LFT. Nonetheless, we acknowledge that at present the technology is not yet likely to be applicable to diagnostic or treatment adherence domains, such as for Addison’s syndrome or Cushing’s disease, where laboratory- or clinic-based approaches are more widely accepted32,65. Thirdly, time-course data requires having the portable LIAM™ on-site. This was not necessary to obtain correspondence data in the current study; nevertheless, planned studies will obtain cortisol data in real-time across different sites. Finally, future directions include extension of this technology to other biomarkers frequently investigated in saliva.
Regardless of these limitations, we believe that measurement of this time-varying stress biomarker can be enhanced through targeted exploration of its time-sensitive nature. The advantage of obtaining cortisol values within 20 minutes of saliva sample collection represents an exciting new methodological direction with the potential to contribute to conceptually-impactful research directions.
Acknowledgments
This research was supported by a phase I (R43AT006634) and phase II (R44 AT006634) Small Business Innovative Research Award to PDS. The first author collected all samples and conducted all statistical analyses to ensure the analyses were independent of the Oasis team. RLB, MJL, CRC and PDS are owners or employees of Oasis Diagnostics®, the company who is developing the VerOFy® technology. The authors wish to acknowledge the Stress Physiology Investigative Team (SPIT lab) for all their help as well as the other employees at Oasis Diagnostics® and Middleton Research.
Footnotes
The first author has no conflicts of interest to declare: EAS does not have a financial stake in the product and sits as a volunteer on the board of advisors of Oasis Diagnostics®.
References
- 1.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 2.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Lupien SJ, Ouellet-Morin I, Hupbach A, et al. Beyond the stress concept: Allostatic Load--A developmental biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. 2nd ed. Vol. 2. Hoboken, NJ: John Wiley & Sons; 2006. pp. 578–628. [Google Scholar]
- 4.Uno H, Eisele S, Sakai A, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28(4):336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 5.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heffelfinger AK, Newcomer JW. Glucocorticoid effects on memory function over the human life span. Dev Psychopathol. 2001;13(3):491–513. doi: 10.1017/s0954579401003054. [DOI] [PubMed] [Google Scholar]
- 7.McMillen IC, Mulvogue HM, Kok JS, Deayton JM, Nowak R, Adamson TM. Circadian rhythms in sleep and wakefulness and in salivary melatonin and cortisol concentrations in mothers of term and preterm infants. Sleep. 1993;16(7):624–631. doi: 10.1093/sleep/16.7.624. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145(6):2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: predictions to disinhibited social approach and diurnal cortisol activity. Dev Psychopathol. 2011;23(3):859–871. doi: 10.1017/S0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 2000;5(2):105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- 11.Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1990;19(2):126–146. doi: 10.2165/00003088-199019020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Pruessner JC, Dedovic K, Pruessner M, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23(7):663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 16.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 17.Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 18.Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. 2nd ed. Vol. 2. Hoboken, NJ: John Wiley & Sons; 2006. pp. 533–577. [Google Scholar]
- 19.De Kloet ER. Hormones and the stressed brain. Ann N Y Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- 20.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 21.Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150(1):83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 22.Spijker AT, van Rossum EF. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology. 2012;95(3):179–186. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- 23.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 24.Granger DA, Kivlighan KT, Fortunato C, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92(4):583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2012;54(5):493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunnar M, Connors J, Isensee J. Lack of stability in neonatal adrenocortical reactivity because of rapid habituation of the adrenocortical response. Dev Psychobiol. 1989;22(3):221–233. doi: 10.1002/dev.420220304. [DOI] [PubMed] [Google Scholar]
- 27.Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011;23(4):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larina IM, Bystritskaya AF, Smirnova TM. Psychophysiological monitoring under conditions of real and simulated microgravity. Hum Physiol. 1999;25(5):574–579. [PubMed] [Google Scholar]
- 29.Chatterton RT, Jr, Vogelsong KM, Lu YC, Hudgens GA. Hormonal responses to psychological stress in men preparing for skydiving. J Clin Endocrinol Metab. 1997;82(8):2503–2509. doi: 10.1210/jcem.82.8.4133. [DOI] [PubMed] [Google Scholar]
- 30.Ross IL, Levitt NS, Van der Walt JS, et al. Salivary cortisol day curves in Addison's disease in patients on hydrocortisone replacement. Horm Metab Res. 2013;45(1):62–68. doi: 10.1055/s-0032-1321855. [DOI] [PubMed] [Google Scholar]
- 31.Smans L, Lentjes E, Hermus A, Zelissen P. Salivary cortisol day curves in assessing glucocorticoid replacement therapy in Addison's disease. Hormones (Athens) 2013;12(1):93–100. doi: 10.1007/BF03401290. [DOI] [PubMed] [Google Scholar]
- 32.Raff H. Utility of salivary cortisol measurements in Cushing's syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3647–3655. doi: 10.1210/jc.2009-1166. [DOI] [PubMed] [Google Scholar]
- 33.Restituto P, Galofre JC, Gil MJ, et al. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clin Biochem. 2008;41(9):688–692. doi: 10.1016/j.clinbiochem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Kaushik A, Vasudev A, Arya SK, Pasha SK, Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens Bioelectron. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 35.Nara S, Tripathi V, Singh H, Shrivastav TG. Colloidal gold probe based rapid immunochromatographic strip assay for cortisol. Anal Chim Acta. 2010;682(1–2):66–71. doi: 10.1016/j.aca.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 36.Zangheri M, Cevenini L, Anfossi L, et al. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens Bioelectron. 2015;64:63–68. doi: 10.1016/j.bios.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 37.Choi S, Kim S, Yang J-S, Lee J-H, Joo C, Jung H-I. Real-time measurement of human salivary cortisol for the assessment of psychological stress using a smartphone. Sensing and Bio-Sensing Research. 2014;2(0):8–11. [Google Scholar]
- 38.Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 39.Eisenger RW, Khalil MH, Katz DH, Sargeant RB Inventors. Lateral Flow, non-bibulous membrane assay protocols. 1990 [Google Scholar]
- 40.Wong R, Tse H. Lateral Flow Immunoassay. Humana Press; 2009. [Google Scholar]
- 41.Bauer JS, Hyatt TP, Wang H, Buck RL Inventors. Positive Detection lateral Flow apparatus and method for small and large analytes. 2009 [Google Scholar]
- 42.Buck RL Inventor. Lateral Flow Test Strip with Migrating Label. 2009 [Google Scholar]
- 43.Boehringer H, Rowley G, Pronovost AD Inventors. Quantitative Lateral Flow assays and devices. 2005 [Google Scholar]
- 44.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharpley CF, McFarlane JR, Slominski A. Stress-linked cortisol concentrations in hair: what we know and what we need to know. Rev Neurosci. 2012;23(1):111–121. doi: 10.1515/RNS.2011.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGrady A. Effects of group relaxation training and thermal biofeedback on blood pressure and related physiological and psychological variables in essential hypertension. Biofeedback Self Regul. 1994;19(1):51–66. doi: 10.1007/BF01720670. [DOI] [PubMed] [Google Scholar]
- 47.van Santen M, Bolwijn P, Verstappen F, et al. A randomized clinical trial comparing fitness and biofeedback training versus basic treatment in patients with fibromyalgia. J Rheumatol. 2002;29(3):575–581. [PubMed] [Google Scholar]
- 48.Rokos IC, French WJ, Mattu A, et al. Appropriate cardiac cath lab activation: optimizing electrocardiogram interpretation and clinical decision-making for acute ST-elevation myocardial infarction. Am Heart J. 2010;160(6):995–1003. doi: 10.1016/j.ahj.2010.08.011. 1003 e1001–1008. [DOI] [PubMed] [Google Scholar]
- 49.Andrews J, Pruessner JC. The combined propranolol/TSST paradigm--a new method for psychoneuroendocrinology. PLoS One. 2013;8(2):e57567. doi: 10.1371/journal.pone.0057567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jahn AL, Fox AS, Abercrombie HC, et al. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychiatry. 2010;67(2):175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosch JA, de Geus EJ, Carroll D, et al. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med. 2009;71(8):877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrowski K, Herold U, Joraschky P, Wittchen HU, Kirschbaum C. A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Raubenheimer PJ, Young EA, Andrew R, Seckl JR. The role of corticosterone in human hypothalamic-pituitary-adrenal axis feedback. Clin Endocrinol (Oxf) 2006;65(1):22–26. doi: 10.1111/j.1365-2265.2006.02540.x. [DOI] [PubMed] [Google Scholar]
- 54.Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behav Sci Law. 2009;27(2):137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the 'Trier Social Stress Test'. Psychoneuroendocrinology. 2009;34(7):1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Wiemers US, Schoofs D, Wolf OT. A friendly version of the trier social stress test does not activate the HPA axis in healthy men and women. Stress. 2013;16(2):254–260. doi: 10.3109/10253890.2012.714427. [DOI] [PubMed] [Google Scholar]
- 57.Westenberg PM, Bokhorst CL, Miers AC, et al. A prepared speech in front of a pre-recorded audience: subjective, physiological, and neuroendocrine responses to the Leiden Public Speaking Task. Biol Psychol. 2009;82(2):116–124. doi: 10.1016/j.biopsycho.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. J Pers Soc Psychol. 2010;98(1):47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- 59.Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychol. 2008;27(1):116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- 60.Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 61.Creswell JD, Welch WT, Taylor SE, Sherman DK, Gruenewald TL, Mann T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychol Sci. 2005;16(11):846–851. doi: 10.1111/j.1467-9280.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- 62.Worthman CM, Panter-Brick C. Homeless street children in Nepal: use of allostatic load to assess the burden of childhood adversity. Dev Psychopathol. 2008;20(1):233–255. doi: 10.1017/S0954579408000114. [DOI] [PubMed] [Google Scholar]
- 63.Decker SA. Low salivary cortisol and elevated depressive affect among rural men in Botswana: reliability and validity of laboratory results. J Physiol Anthropol. 2006;25(1):91–101. doi: 10.2114/jpa2.25.91. [DOI] [PubMed] [Google Scholar]
- 64.Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ceccato F, Barbot M, Zilio M, et al. Performance of salivary cortisol in the diagnosis of Cushing's syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol. 2013;169(1):31–36. doi: 10.1530/EJE-13-0159. [DOI] [PubMed] [Google Scholar]