Abstract
Objectives
Traumatic brain injury has a high morbidity and mortality in both civilian and military populations. Blast and other mechanisms of traumatic brain injury damage the brain by causing neurons to disconnect and atrophy. Such traumatic axonal injury can lead to persistently vegetative and minimally conscious states, for which limited treatment options exist, including physical, occupational, speech and cognitive therapies.
More than 60,000 patients have received vagus nerve stimulation for epilepsy and depression. In addition to decreased seizure frequency and severity, patients report enhanced mood, reduced daytime sleepiness independent of seizure control, increased slow wave sleep, and improved cognition, memory, and quality of life.
Early stimulation of the vagus nerve accelerates the rate and extent of behavioral and cognitive recovery after fluid percussion brain injury in rats.
Methods
We recently obtained FDA approval for a pilot prospective randomized crossover trial to demonstrate objective improvement in clinical outcome by placement of a vagus nerve stimulator in patients who are recovering from severe traumatic brain injury. Our hypothesis is that stimulation of the vagus nerve results in increased cerebral blood flow and metabolism in the forebrain, thalamus and reticular formation, which promotes arousal and improved consciousness, thereby improving outcome after traumatic brain injury resulting in minimally conscious or persistent vegetative states.
Discussion
If this study demonstrates that vagus nerve stimulation can safely and positively impact outcome, then a larger randomized prospective crossover trial will be proposed.
Keywords: Epilepsy, minimally conscious state, traumatic brain injury, vagus nerve stimulation, vegetative status, clinical trial
Introduction
Traumatic brain injury (TBI) affects over 1 million American people, resulting in heterogeneous neurological outcomes that in severe cases entail high morbidity and mortality.1 Recent progress in intensive care potentially decreases mortality for severe TBI cases, but survivors may be left with impaired consciousness resulting in vegetative or minimally conscious states (VS or MCS). VS demonstrate the complete absence of behavioral evidence for self or environmental awareness, with spontaneous eye opening along with evidence of sleep-wake cycles on EEG.2, 3 By contrast, MCS is defined as a condition of severely altered consciousness in which minimal, clearly discernible, but inconsistent behavioral evidence of self or environmental awareness on a reproducible or sustained basis is demonstrated.2 Recovery of consciousness after 12 months is unlikely in adults and children with traumatic brain injuries, and even less likely to occur if there is an anoxic component to the injury.4
The main neuropathological abnormality in VS patients is thought to be subcortical damage, resulting from damage to the white matter of the cerebral hemispheres and/or the thalamus leaving disconnected intact cortex unable to function.5 It has been suggested that functional neuroimaging methods are more sensitive than anatomic methods in distinguishing patients in VS from those in MCS, because the former can evaluate corticothalamic function,6 preserved residual cognitive function, and some degree of conscious awareness in minimally conscious patients unable to follow commands or communicate reliably.7, 8 Patients with MCS demonstrate more continuous improvement and have significantly more favorable outcomes by 1 year post injury than those diagnosed with VS.9 Thus, length of time that MCS patients are in MCS may not predict their outcome.10 In addition, there are no time intervals for possible permanency of the VS.11 At present there is no efficacious therapy for VS or MCS patients.
Vagus Nerve Stimulation
The vagus nerve has special visceral efferent fibers that project to the nucleus ambiguus from the pharyngeal and laryngeal muscles. Its general visceral efferent fibers arising from the dorsal motor nucleus innervate the heart, lungs, and viscera. The vagus also has sensory afferent projections, notably from the concha of the ear. The vagus’ visceral afferents travel via the tractus solitarius to the thalamus, amygdala and forebrain, and via the medullary reticular formation to other cortical areas. 12-14 In these vagus nerve related brain circuitries, there are excitatory and inhibitory neurotransmitters, including NE, serotonin (5-HT), γ-aminobutyric acid (GABA), and glutamate.14, 15 The vagus nerve contains myelinated A- and B- and unmyelinated C-fibers, conveying sensory information from viscera.15
The vagus’ diffuse projections mediate a number of visceral reflexes including the baroreceptive response, coughing, vomiting and swallowing. Projections to the hypothalamus affect appetite, blood pressure and volume homeostasis.
In 1938, Percival Bailey stimulated the severed proximal end of the vagus nerve in cats resulting in cortical EEG (electroencephalogram) changes.16 Fifty years later neurophysiologist Jacob Zabara developed his vagal nerve stimulator that reduced strychnine-induced seizures in dogs17. His work was duplicated in other species including monkeys18 and rats.19, 20 Pilot studies with the human VNS for epilepsy began in November 1988.21 Seven of the first nine patients implanted saw a reduction in seizure frequency and two patients were seizure-free at one year.21, 22
The Food and Drug Administration approved the VNS for the treatment of epilepsy in 1997. Clinical trials with VNS for epilepsy have been performed as crossover trials with the stimulator on or off and as randomized prospective trials with low versus high levels of stimulation.23 The FDA approved VNS for treatment resistant depression in 2005.24 Currently over 60,000 patients have been implanted with VNS.25, 26
VNS directly and indirectly modulates subcortical and cortical brain function via chronic intermittent repeated electrical stimulation of the vagus nerve. Although only cleared by the FDA for medically refractory epilepsy and depression, it is under investigation for a wide variety of disorders, including Alzheimer’s disease, migraine, multiple sclerosis, and eating disorders.27 Recent studies in Alzheimer’s patients demonstrate that many either improve or do not decline after device implantation.28 Transcutaneous VNS may attenuate postoperative cognitive dysfunction in elderly patients,29 and VNS may improve cognitive impairment and quality of life in cerebral palsy patients via seizure suppression and interictal discharge reduction.30
Kumaria and others have proposed VNS as a treatment for TBI.31 VNS has improved behavioral and cognitive recovery after fluid percussion brain injury in rats.32, 33
Seizures on EEG are represented by abnormal synchronization of neuronal activity. Desynchronization, then, should diminish seizure activity. VNS was first hypothesized to work via desynchronization of the EEG13 and this has recently been supported by electrophysiologic data34. Michael Chase and colleagues demonstrated in cats that the frequency dependent effect of VNS on EEG was due to activation of particular fibers at varying frequencies.13 VNS-induced seizure suppression is attributed to activation of vagal A- and B- fibers, 35 although C-fibers were originally thought to be responsible for its effects.19 VNS at frequencies above 70 Hertz and intensities greater than 3 Volts were shown to be desynchronizing. If the intensity was dropped to less than 3 Volts, only myelinated fibers were stimulated, and the EEG would be synchronized. Lower frequencies (20 to 50 Hertz) and higher voltages were also shown to be desynchronizing. Desynchronization was shown to be the result of stimulation of vagus fibers that conduct at 1 to 15 m/s.36 It has also been suggested that VNS can modulate cortical synchrony and excitability via the action of acetylcholine on muscarinic receptors, indicating sensory processing and neural plasticity in anesthetized rat auditory cortex.25
Early studies suggested that, unlike in animals, VNS did not affect human EEG regardless of level of alertness.37 On longer follow-up, however, at a mean of 16.8 months after implantation, EEG changes were seen after VNS, with desynchronization dominating over synchronization and diminished inter-ictal spiking.38 Long term VNS treatment has reduced interictal epileptiform discharges (IEDs) in adult patients with epilepsy.39, 40 In children, a decrease in the number of IEDs has also been seen as early as three months after stimulator implantation.41 Despite understanding some of the properties of stimulation that are most likely to result in EEG desynchronization, we still do not have a clear understanding of the exact mechanism of VNS.
Altered neurotransmitter levels and cerebral blood flow
Vagus projections via the tractus solitarius and medullary reticular formation may be responsible for the anti-epileptic impact and other effects of VNS. In rat studies, the hippocampus and striatum have altered metabolism after VNS.42 VNS also results in an increase in the endogenous stem cell proliferation in the adult rat dentate gyrus of hippocampus that could be mediated in part by changes in hippocampal noradrenergic activity.43 NE loss in brain increases neuronal damage following focally induced limbic status epilepticus, and the protective effect of brain NE has already been demonstrated in epilepsy and other neurological disorders, including learning and memory.44 Also in rats, neuronal activity in the locus ceruleus is directly modified by VNS,45 which increases extracellular norepinephrine concentrations in the rat cortex, hippocampus, and amygdala,46, 47 that may enhance recognition memory in human subjects.48 It has been suggested that VNS initially enhances the firing activity and pattern of norepinephrine neurons of the locus ceruleus, and subsequently those of 5-HT neurons of the dorsal raphe nuclei via tractus solitarius, contributing to the efficacy of VNS for depression.49
VNS led to an increase in the levels of the inhibitory transmitter GABA in partial epilepsy patients. 50 VNS may also modulate the cortical excitability of brain areas associated with epileptogenesis via GABAA receptor plasticity.51
In addition, acute VNS induced specific expression of nuclear fos gene that is an immediate early gene and a marker for neuronal activation in several forebrain structures, including the amygdala, cingulate cortex, hypothalamus, the brainstem, locus ceruleus, noradrenergic nuclei, and thalamus.52 Chronic VNS significantly increased staining of ΔFosB (a marker of chronic neuronal activation) bilaterally in tractus solitarius, locus ceruleus, parabrachial nucleus, dorsal raphe nuclei, and in many cortical and limbic areas of brain including those involved in mood and cognition53, 54. The VNS effects were more widespread than those caused by the antidepressants.54 Locus ceruleus neurons impact Fos protein expression, suggesting a plastic mechanism for diminishing epileptogenesis.55 These structures activated by the vagus may play an important role in the control of epilepsy, recognition memory, and control of arousal47, 56, 57.
Projections to the frontal cortex and cingulate gyrus are highlighted on positron emission studies of patients receiving the vagus nerve stimulator for depression.58 Decreased excitatory and increased inhibitory neurotransmission are observed after both acute and chronic VNS.46, 50
VNS also leads to changes in cerebral blood flow as measured by positron emission testing (PET). Both high and low levels of stimulation increased cerebral blood flow in humans to the bilateral thalami, hypothalami and insular cortices.59-61 Chronic stimulation induces similar cerebral blood flow changes in humans.62 Chronic VNS for treatment of patients with resistant depression also increases regional cerebral blood flow in the dorsolateral prefrontal cortex.63 It is thought to alter limbic circuitry to reduce epileptogenesis and enhance mood.60 It is likely that VNS results in increased cerebral blood flow and metabolism in the forebrain, thalamus and reticular formation,60 which promotes arousal and improved consciousness,64 thereby improving outcome after TBI resulting in VS or MCS.
VNS for epilepsy
Of the 0.3% of the general population that are active epileptics, two-thirds are controlled by medical therapy, and one-third are refractory even after 18 months of aggressive therapy with two standard anti-epileptic agents.65 If such patients do not have a focally resectable lesion resulting in their epilepsy, or continue to seize after resection of such a lesion66, they are possibly candidates for alternative surgical therapy, such as stimulation.
VNS for epilepsy demonstrates increasing benefit after a greater period of time. Five-year follow-up of 26 patients revealed that while seizure frequency decreased from baseline by 28% at one year, it further dropped to 72% less than baseline at five years,67 indicating a persistent and cumulative effect of VNS on seizures.23 A 12-year experience also supports that VNS is safe and effective long term treatment for epilepsy.68 A recent meta-analysis of VNS outcomes including 74 clinical studies with 3321 patients with intractable epilepsy revealed VNS is effective in reducing seizure frequency by 50% in approximately 50% of patients, with a delayed benefit more than 1 year after surgery26. One study of children demonstrated potential reduction in seizure severity and improvement in well-being without areduction in seizure frequency69.
VNS seizure patients report enhanced mood,70, 71 reduced daytime sleepiness and increase alertness72 independent of seizure control, increased slow wave sleep,73 improved cognition,74 memory,75 and quality of life.71, 76 Children with VNS are noted to have enhanced verbal communication, school performance,77 quality of life, and cost savings.78 Grill, et al. described 2 nonverbal children (8 and 9 years) who began to speak within months of VNS implantation for the treatment of refractory epilepsy.79 A slight improvement in alertness and communicative skills was also observed in young patients with Dravet syndrome by VNS.80
The most common device-related complications were infection or lead breakage.81 The sudden unexplained death in epilepsy rate in VNS patients drops from 5.5 per 1000 over the first two years, a rate comparable to that of other epilepsy cohorts, to 1.7 per 1000 thereafter.82 Hoarseness, other vocal changes, coughing during stimulation, and exacerbation of sleep apnea83, 84 are the most frequent complaints of patients undergoing VNS.85 These symptoms are thought to be due to proximity of the stimulator to the recurrent laryngeal nerve.86
VNS is FDA cleared for MRI scanning; a transmit and receive head coil is used with a 1.5 Tesla scanner, and more recent data suggests 3.0 Tesla scanners are also safe87. MRIs have been performed with the stimulator turned on to evaluate the impact of the stimulation.88-94 In one study, the patient had a seizure on the table and diffusion weighted imaging demonstrated transient ischemia at the focus, which returned to normal at seizure completion.95
Traumatic Brain Injury (TBI) and Impaired Consciousness
Diffuse axonal injury (DAI), the most frequent cause of persistently VS and MCS96 after brain injury, occurs as a result of rapid acceleration-deceleration injury of the axons, sometimes in conjunction with delayed axonal disconnections. It can be defined as the occurrence of diffuse damage to axons in the cerebral hemispheres, corpus callosum, and brain stem with three different grades.97 Impaired consciousness is postulated to be due to damage to forebrain arousal inputs from thalamic and midbrain structures.98-100 In rats, neuronal disconnection appears to occur due to breakdown of the subaxolemmal cytoskeletal network.101, 102 Diffuse injury causes axonal degeneration near the neuron cell body leading to neuronal atrophy rather than apoptosis.103, 104 Such atrophy has been quantified by structural imaging in human TBI;105 the brain appears to melt away while cranial fluid-filled spaces enlarge on serial scans in the severely injured patient.100
The majority of patients who will recover from a post-traumatic vegetative state improve within the first six months, and recover to severe disability or become minimally conscious.4 Current treatment after the acute phase of TBI consists of cognitive, physical, occupational and speech therapy in conjunction with neuropsychological care and optimal management of concurrent medical problems. A randomized trial of cognitive versus home therapy in military TBI patients revealed no benefit for the former.106 There is a tremendous need for development of therapies with a direct ability to improve function in the post-TBI.
Diagnostic and prognostic value of functional neuroimaging in patients with VS or MCS
The rate of misdiagnosis between VS and MCS is as high as 40%,107, 108 due to insufficiently sensitive standardized neurobehavioral assessment scales. It is expected that fMRI might supplement conventional neurological examination in the classification and prognosis of those patients. The differentiation between VS and MCS could better predict the patient prognosis and even influence clinical management because of the critical neruopathological differences between these two groups of patients,3 indicating that MCS and VS are actually distinct physiological entities with different brain integrative capacities.
VS patients potentially demonstrate a residual neural encoding of basic sound attributes without further high order processing of functional integration, whereas MCS patients have a more elaborate level of processing, and more cortico-cortical function connectivity as compared with VS patients.109, 110 Self-related auditory stimuli can induce more extended neural activity and cerebral processing in the anterior cingulate cortex in patients with MCS.111 Those data indicate key structures in the cortical language network remain functional in MCS, despite the absence of consistent command following enabling reliable communication.
The patients’ own name by a familiar voice (personally meaningful information) can also activate the cerebral cortex in both VS and MCS patients.112 Some VS and MCS patients demonstrated speech comprehension which correlated with their subsequent behavioral recovery 6 months later,113 A percentage of VS or MCS patients can willfully two-way communicate and modulate their brain function via mental imagery, inconsistent with a clinical behavioral response,114 indicating their higher intact cognitive function. These findings indicate the potential role of functional neuroimaging in the diagnostic and prognostic evaluation of these patients.
More preserved functional default network connectivity was demonstrated in MCS patients than VS patients, proportionate to the extent of their impaired consciousness.115 Some MCS patients may have a severe deficit of resting cerebral activity sufficient to preserve cerebral networks necessary for recognition and interaction, but not to drive these networks spontaneously,116 which provides the anatomic and physiological foundation for electric stimulation.
VS patients demonstrated more widespread regional thalamic abnormalities than MCS patients, and these differences potentially explain their clinical profile117 as a result of the critical role of the thalamus in regulation of arousal and human consciousness.64 Functional restoration of thalamocortical disconnection (prefrontal and anterior cingulate cortices) in the VS patient paralleled recovery of consciousness.118 Via partially preserved conscious processing that is not detected by behavioral assessment, some VS or MCS patients have the capacity to learn trace conditioning, which is a good predictor of recovery.119 fMRI revealed a VS patient preserved some degree of conscious awareness,8 and axonal regrowth has been observed in late recovery from MCS.120 In addition, the depth and breadth of preserved cognitive function in VS or MCS patients can be detected by a hierarchy of cognitive tasks.7 Those findings suggest that a correlation between structural lesions and functional damage may predict individual patient prognosis.121
Augmenting arousal with deep brain stimulation (DBS)
Direct stimulation of brain centers that project to mesial forebrain cortex has been used in an attempt to arouse patients with impaired consciousness. DBS is a neurosurgical procedure in which small holes are drilled in the skull so that electrodes can be passed through the cortex and underlying white matter into thalamic nuclei or basal ganglia, and then connected to an impulse generator in the chest wall. The majority of patients receiving DBS have had Parkinson’s disease, essential tremor, depression or epilepsy. Attempts to treat VS with DBS have had mixed results. A 1993 French study of 25 patients with bilateral DBS into the centromedian and parafascicular nuclei reported that half were unchanged from baseline, and the remainder had increasing levels of awareness but remained vegetatively disabled.122 Ten year follow-up of 12 post-traumatic VS and MCS patients who received either centromedian and parafascicular nuclei or mesencephalic reticular formation stimulation in a Japanese study demonstrated that the few MCS patients who improved were able to return to functional lives.123 More recently, Schiff et al targeted the anterior intralaminar thalamic nuclei with DBS in a MCS patient six years after brain injury and demonstrated improved arousal, motor, and communication abilities.124
Augmenting arousal with VNS
DBS entails drilling holes in the skull and passing electrodes through about eight centimeters of brain into deep nuclei, and thus carries the risk of causing cerebral hemorrhage, among other complications. A less invasive alternative for promotion of arousal in the brain is stimulation of the vagus nerve in the neck, which activates numerous areas including the locus ceruleus, resulting in increased cerebral norepinephrine.46 Afferent and efferent pathways coursing through the thalamus, are associated with conscious sensibilities and activities.64 The medullary reticular formation is associated with the reticular activating system, which is important in arousal.
VNS also decreases seizure activity and IEDs in the brain.125, 126 Such epileptiform activity is frequently present, though not always diagnosed, in the injured brain.127 Enhanced mood and cognition, particularly at low levels of stimulation (0.5 mA,)48 are also observed.
Early stimulation of the vagus nerve accelerates the rate and extent of behavioral and cognitive recovery after fluid percussion brain injury in rats, and provides indirect evidence that the brain’s norepinephrine system may have a positive effect on the recovery of function following TBI.32, 33 Interestingly, VNS appears to mediate some of these positive effects by attenuation of cerebral edema ipsilateral to the side of the injury.128 A second mechanism by which stimulation appears to exert positive effects is through a reduction in injury to GABAergic inhibitory neurons within the cerebral cortex and possibly the hippocampal formation following rat TBI, which facilitates the recovery of behavioral function.129 GABAA receptor-mediated neuronal inhibition can be also enhanced by VNS,51 which may contribute to the clinical efficacy of VNS.
TBI triggers a cerebral inflammatory response.130 VNS potentially attenuates the systemic inflammatory response via inhibition of tumor necrosis factor (TNF) synthesis, preventing the shock development during endotoxemia.131 In contrast, vagotomy significantly increased TNF synthesis and promoted rats to develop lethal shock.131 The nicotinic acetylcholine receptor α7 subunit on immune cells surrounding the vicinity of cholinergic axon terminals is required for VNS modulated acetylcholine inhibition of inflammatory response.132 These receptors transmit the cholinergic signal into inhibition of TNF release and inflammation. Acetylcholine-producing memory T cells in the spleen are required for inhibition of cytokine production via macrophages by VNS, and consequently these T cells can relay neural signals in a vagus nerve circuit that controls innate immune responses.133 Thus, vagal function has been considered as the cholinergic anti-inflammatory pathway.134
VNS prevents an increase in TNF in mice following TBI via upregulation of the gastric peptide hormone ghrelin.135 Intriguingly, ghrelin receptor expression was detected in tissues from the rat dorsal motor nucleus of the vagus and ghrelin promotes neural proliferation in those nuclei.136 Acute VNS can also significantly reduce intestinal TNF levels and prevent intestinal permeability in mice following TBI.137 Intestinal inflammation also reduced neuron proliferation from the dorsal motor nucleus of the vagus in rats which can also be attenuated by ghrelin.138
Accumulating evidence strongly supports the idea that inflammation and the immune response play a critical role in the pathophysiology of epilepsy.139-143 For example, pro-inflammatory mediators like cytokines prominently in glia and to a lesser extent in neurons might play an important role in the development of epileptogenesis. The functional interactions between cytokines and classic neurotransmitters such as glutamate and GABA favor the establishment of chronic neuronal heperexcitability and injury, and network reorganization, thus promoting seizures and synaptic plasticity.139, 144, 145 Cytokine induction in patients with refractory epilepsy was altered by long-term VNS, indicating an immunomodulatory effect of VNS.146 VNS can modulate the immune system via rebalancing of pro-inflammatory and anti-inflammatory cytokines in patients with refractory epilepsy.147
Brain injury and inflammation also results in disruption of the blood brain barrier (BBB).139, 141 BBB impairment is intimately associated with epilepsy, followed by neuronal loss and impaired functions.148-152 The extravasated albumin via increased BBB permeability into the brain’s extracellular space binds to astrocytic transforming growth factor (TGF) β receptors and induces astrocytic transformation and dysfunction, a cascade of events initiated by increased BBB permeability that leads to neuronal excitotoxicity and loss, and eventually epilepsy.152 This compromised BBB can be induced by TBI, and can last several days to weeks or even years after the acute event.153 Unresolved BBB damage following TBI results in cerebral edema, inflammation, epilepsy, cognitive disabilities, neurodegenerative pathologies such as Alzheimers disease, or death.153 VNS can attenuate BBB disruption after TBI in mice154 and modulate the inflammatory response in epilepsy patients.147 Transcutaneous VNS has also been proposed to attenuate postoperative cognitive dysfunction in elderly patients by decreasing inflammatory response.29
A Pilot Prospective Randomized Crossover Clinical Trial
The purpose of this pilot study is to demonstrate objective improvement in clinical outcome by placement of a vagus nerve stimulator in patients with impaired consciousness who are recovering from severe traumatic brain injury. Our hypothesis is that stimulation of the vagus nerve results in increased cerebral blood flow and metabolism in the forebrain, thalamus and reticular formation60, which promotes arousal and improved consciousness64, thereby improving outcome after traumatic brain injury resulting in minimally conscious or persistent vegetative states. Our primary outcome measure is clinical improvement on two validated brain injury outcome assessment scales. A secondary outcome measure is demonstration of increased activity in the forebrain and thalamic regions as assessed by functional magnetic resonance imaging. If this study demonstrates that vagus nerve stimulation can safely and positively impact outcome, then a larger randomized prospective crossover trial will be proposed.
Twelve subjects will participate in a prospective randomized crossover pilot study for eighteen (18) months after device implantation. Subjects will cross over between no stimulation (device off) and stimulation (device on) every three months for six periods yielding a total trial time of 18 months. The trial will be unilaterally blinded in that subjects and researchers assessing outcome measures will not know if the devices are on or off. Subjects will be randomized at study initiation to on or off for the first three months and then switched to the opposite setting every three months for the duration of the study (18 months). There will NOT be a control group of patients without impaired consciousness or brain injury.
Inclusion criteria include age between 18 and 60, and having sustained a moderate to severe traumatic brain injury (Disability Rating Scale155 score of 18 to 29, Table 1) more than 4 months from starting the study, with or without concurrent seizure activity. While patients who have had craniotomies may be enrolled in the study, those with hydrocephalus or active intracranial pressure elevation will be excluded as treatment of their ongoing neurosurgical disease will confound evaluation of their outcome. Exclusion criteria include prior vagotomy, retained metal contraindicating an MRI, concurrent active severe medical problems that render surgery to place the device unsafe, a history of sleep apnea, myocardial infarction or arrest, cardiac conduction abnormalities, and conditions which could prevent the patient from surviving the duration of the study. Pregnancy or intent to become pregnant during the course of the study will also result in exclusion from the study. Patients with pre-existing central nervous system disease or associated comorbidities that may not allow for 18 month follow-up will also be excluded. Stable orthopedic or other traumatic body injuries are not a contraindication.
Table 1.
Disability Rating Scale (abridged)
| Modalities Tested: |
Eye opening
|
Communication ability
|
Motor response
|
Feeding (cognitive ability only)
|
Toileting (cognitive ability only)
|
Grooming (cognitive ability only)
|
Level of Functioning
|
Employability
|
A rehabilitation medicine physician will evaluate each patient prior to implantation of the vagus nerve stimulator. In addition to standardized assessments in speech, physical and occupational therapy, patients will be assessed with the FIM™ instrument and Functional Assessment Measure (FIM+FAM). (Table 2) and the JFK Coma Recovery Scale (Table 3)156. Brain MRI will be performed to assess the extent of traumatic injury at enrollment. Resting and activational functional magnetic resonance imaging will provide an assessment of pre-operative cerebral activity and EEG will be used to assess baseline pre-operative neuronal electrophysiologic activity and eliminate the possibility that subclinical seizures contribute to the patient’s poor neurologic status.
Table 2.
FIM™ instrument and Functional Assessment Measure (FIM+FAM)
| Modalities Tested: |
Self Care Items:
|
Sphincter Control:
|
Mobility Items:
|
Locomotion:
|
Communication Items:
|
Psychosocial Adjustment:
|
Cognitive function:
|
Table 3.
JFK Coma Recovery Scale (abridged)
Modalities Tested:
|
Patients enrolled in the study will receive concurrent standard of care treatment through the Veterans Administration Hospital System prior to enrollment, for the duration of treatment, and following withdrawal from the study. Such care will include optimal pharmacologic management, including cognitive effectors such as bromocriptine or methylphenidate, which must be initiated prior to enrollment rather than during the study period. Anticonvulsants will be administered if indicated and may be altered as medically necessary during the study period. Physical, occupational, speech, and cognitive therapy will be continued during the study.
Surgical implantation of the vagus nerve stimulator will be performed under general anesthesia using standard techniques157. The device components will be provided by Cyberonics and consist of a titanium-housed 5.2 by 5.2 by 0.7 centimeter pulse generator with a lithium carbon monofluoride battery, and a 43 centimeter lead wire with two platinum/iridium helical electrodes and a tethering anchor. The battery life is estimated at six years, but is affected by stimulation parameters.
Vagus nerve stimulators are implanted on the left side since studies in dogs have demonstrated that stimulation of the right vagus slowed heart rate more than stimulation of the left158. A three to four centimeter horizontal incision is made in a left mid-neck crease extending from the medial aspect of the sternocleidomastoid muscle to the midline. A separate subclavicular incision is made for the generator. We bluntly generate a subcutaneous pocket the size of the generator and then perform tunneling for the stimulator lead from the cervical to the infraclavicular incision, taking care to pass over the clavicle. The lead is pulled through with the tunneling device and attached to the generator with a hex screwdriver. The generator is then tucked into the infraclavicular pocket to avoid movement during the duration of the procedure, but leaving sufficient slack on the lead to allow easy manipulation and placement.
Attention is then returned to the cervical incision where the vagus nerve is found nestled in between the common carotid artery and internal jugular vein just medial to the sternocleidomastoid muscle. The two vagus nerve stimulator helical electrodes, and a third anchor tether coil are then applied in a cranial to caudal fashion using vascular forceps. After wound closure, but prior to removal of drapes and reversal of anesthesia, lead impedance is checked with a 14 second one milliampere, 20 Hertz pulse to ensure that the helical electrodes are in good contact with the nerve and the lead is inserted into the generator correctly. Bradycardia on testing would suggest that the leads were placed too cranial along the course of the nerve and are impinging on cardiac branchpoints159.
Patients will be able to resume rehabilitation one day after device implantation. Since the optimal stimulation parameters to arouse patients from impaired consciousness are unknown, just before beginning the randomized trial, we will perform a supervised stimulation parameter titration trial. The device will be turned on and set to deliver 30 second pulses every five minutes at the lowest current of 0.5 milliamperes. The frequency will be set at 10 Hz and the current will be increased at 0.5 mA intervals to 2.5 mA. If no impact is seen the frequency will be increased at 10 Hz intervals and the trial repeated with increasing current until maximal settings of 30 Hz and 2.5 mA are reached. The patient will be closely observed for one hour after each parameter change both for changes in level of awareness and for distress. If there are any signs of discomfort including grimacing, tachycardia, diaphoresis, fidgeting or more classical sympathetic or parasympathetic overdrive symptoms the stimulation parameters will be titrated back down.
Assuming the titration trial does not reveal settings that have an immediate impact on consciousness, we will initiate stimulation at 0.5 milliamperes, 0.5 millisecond pulse-width, 30 Hertz for 30 seconds, then off for 5 minutes, as has been optimized from cognition studies of epilepsy patients48, 160. Randomization to stimulation or no stimulation will begin two weeks after the implantation surgery. Patients who are clinically unchanged after three months of stimulation will have the current increased by 0.5 milliamperes for every three months of stimulation that they fail to improve, to a maximum level of 2.5 milliamperes over 24 months (figure). If patients appear uncomfortable, first frequency, then on-off times and finally current will be titrated down to minimize discomfort.
figure.
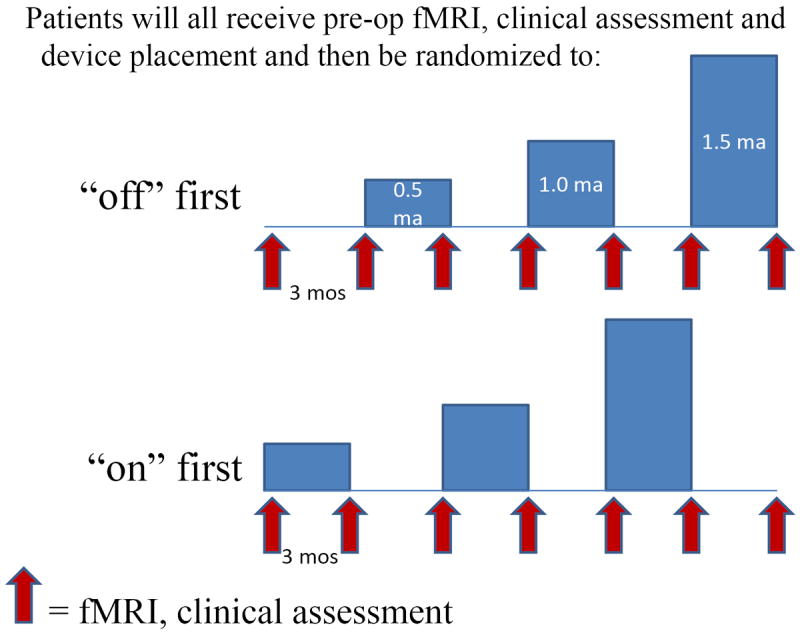
The study design is that after device implantation patients will be randomized to either of two groups. They will then cycle through three month periods where the device is alternately turned on and off at gradually increasing stimulation parameters. Assessments will be performed at periodic intervals as indicated.
Patients implanted with VNS for this study will be continuously monitored for alterations in clinical status. This monitoring will consist of daily checking vital signs (heart rate, blood pressure, temperature and peripheral oxygen saturation) as well as mental status. If patients have alterations in any of the above, they will be evaluated by medical personnel and if necessary, referred to the principal investigator to ensure that no adverse event has occurred that would necessitate altering stimulation.
Although not a formal measure of outcome assessment, EEG will be utilized if necessary to ensure that patients are not having subclinical seizure activity, as frequently occurs in brain injured patients. Patients noted to have interictal spikes will be administered antieplieptics as clinically indicated, and have the stimulation parameters of their vagus nerve stimulator titrated for optimal seizure control if necessary.
As a safety measure in case the patient has a medical emergency, patients are discharged from the hospital with a magnet which prevents any stimulation from occurring when held over the generator.
Patients and their caretakers will also be advised to stay away from functioning microwave ovens as these can be associated with heating of the device. They will further be advised not to touch or attempt to manipulate the device under the skin.
Outcomes will be assessed by a rehabilitation medicine physician blinded to device status at three month intervals with the FIM™ instrument and Functional Assessment Measure (FIM+FAM, Table 2) which has been found reliable and validated by numerous studies in severely brain injured patients 161-164. The JFK Coma Recovery Scale (Table 3) which has been independently validated as an outcome measure in brain injured patients with impaired consciousness 156, 165 will also be used to evaluate outcome. Patients will be followed for a minimum of 18 months after enrollment unless they or their legal surrogates choose to leave the study.
Functional MRI with resting and activational parameters will be performed pre-operatively and at three month intervals. We will utilize a transmit and receive head coil with the patient placed head first into the scanner. We will utilize an average head SAR of 1 to 2.24 W/kg for a 70 kg patient and dB/dt < 10T/s as described previously87. The temperature probe overlying the electrode will be placed directly over the skin incision used for electrode placement at the medial aspect of the sternocleidomastoid. The actual distance to the electrode is therefore approximately a few centimeters, but dependent on the thickness of patient’s skin, subcutaneous fat, and neck musculature. The probes placed in the patient’s ipsilateral axilla and on the scalp will vary in distance depending on the patient’s body habitus. Based on our previous surgical experience demonstrating that any damage to or manipulation of the vagus nerve intraoperatively immediately tends to affect heart rate, we would expect to see changes in heart rate if diathermy of the nerve occurs, and thus we will continuously monitor heart rate.
All patients will have 3 temperature probes (overlying the device, ipsilateral axilla and cranial) and heart rate monitors in place with continuous monitoring throughout the duration of the MRI scan to ensure that diathermy does not occur. An increase in temperature of one degree centigrade on any part of the patient’s body, or a change in heart rate of greater than 20 beats per minute will result in cancellation of the MRI study.
The major caveats limiting data interpretation are that every injured brain will have unique pathology, and most will exhibit varying rates of spontaneous recovery166. Determining how much recovery is occurring spontaneously, and how much is due to stimulation is particularly difficult in the first six months after injury when it is more likely for patients to have dramatic improvements. The FIM+FAM scale has previously been able to show significant levels of improvement after an intervention with 18 treated TBI patients versus untreated controls167. If we see a trend towards benefit with stimulation, but without statistical significance, then we would propose a follow-up trial with a greater number of patients and longer experimental period (e.g. patients would receive stimulation or not, for six month periods rather than three months before crossing over.) We will work with a statistician to calculate the number of patients we need to enroll in order to achieve a significant result.
Failure to demonstrate any effect of vagus nerve stimulation could be due to several factors including genuine lack of efficacy, incorrect stimulation parameters or insensitive outcome measures. Regarding the latter possibility, the FIM+FAM scale has been found to be among the most sensitive and reliable outcome measures after TBI162, but other scales such as the Coop/Wonka charts, Neurobehavioral Rating Scale, Frenchay Activity Index, and the Sickness Impact Profile168, 169 have also been validated for use in TBI populations164. Neuropsychiatric rehabilitation personnel, caregivers and others who are observing the patient will be asked what changes they have noticed in the patient when stimulation is initiated, and scales tailored to look for these particular modalities will be added to the assessment protocols enacted every three months.
If a patient progresses through the 18-month trial without improvement at stimulation parameters up to 2.0 mA, surrogate consent, Institutional Review Board (IRB) approval, and additional funding will be sought to continue titration up to 3.5 mA and 130 Hertz, which are the maximal stimulator settings160. If funding is not obtainable at that time, subjects or their surrogates will still be given the option to continue stimulation and titration with only IRB approval. Subjects or their surrogates will also have the option to have the device turned off or explanted.
Patients who recover from their minimally conscious state during the course of the study, and are competent to make their own decisions, will be given the option to continue with the study as planned, or withdraw from the study according to their personal wishes. If they elect to withdraw from the study, they may continue with stimulation, have the device turned off, or explanted.
Conclusions
Compelling evidence exists to suggest that vagus nerve stimulation may improve outcomes after severe brain injury. The clinical trial for which we have obtained FDA IDE clearance will be the first step in determining the efficacy of VNS for improvement of consciousness after severe brain injury.
Table 4.
Stimulation parameters
| Initial | Range through study | |
|---|---|---|
| On time | 30 sec | |
| Off time | 5 minutes | |
| Output current | 0.5 mamp 0.5 to 2.5 mamp | |
| Frequency | 10 Hz | 10 to 30 Hz |
| Pulse Width | 500 usec | |
| Magnet current | 0.5 mamp | |
| Magnet duration | 60 sec | |
| Magnet pulse width | 500 usec | |
Acknowledgments
FUNDING
CS was supported by the AOA Carolyn L. Kuckein Medical Student Summer Research Fellowship.
US was supported by 1I01RX000319-01
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. The Journal of head trauma rehabilitation. 2006 Nov-Dec;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002 Feb 12;58(3):349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 3.Giacino J, Whyte J. The vegetative and minimally conscious states: current knowledge and remaining questions. The Journal of head trauma rehabilitation. 2005 Jan-Feb;20(1):30–50. doi: 10.1097/00001199-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Medical aspects of the persistent vegetative state (2). The Multi-Society Task Force on PVS. The New England journal of medicine. 1994 Jun 2;330(22):1572–1579. doi: 10.1056/NEJM199406023302206. [DOI] [PubMed] [Google Scholar]
- 5.Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain : a journal of neurology. 2000 Jul;123(Pt 7):1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- 6.Goldfine AM, Schiff ND. Consciousness: its neurobiology and the major classes of impairment. Neurologic clinics. 2011 Nov;29(4):723–737. doi: 10.1016/j.ncl.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman MR, Rodd JM, Davis MH, et al. Do vegetative patients retain aspects of language comprehension? Evidence from fMRI. Brain : a journal of neurology. 2007 Oct;130(Pt 10):2494–2507. doi: 10.1093/brain/awm170. [DOI] [PubMed] [Google Scholar]
- 8.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006 Sep 8;313(5792):1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 9.Giacino JT, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. The Journal of head trauma rehabilitation. 1997;12(4):36–51. [Google Scholar]
- 10.Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Archives of physical medicine and rehabilitation. 2005 Apr;86(4):746–754. doi: 10.1016/j.apmr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Laureys S, Boly M. What is it like to be vegetative or minimally conscious? Current opinion in neurology. 2007 Dec;20(6):609–613. doi: 10.1097/WCO.0b013e3282f1d6dd. [DOI] [PubMed] [Google Scholar]
- 12.Vonck K, Van Laere K, Dedeurwaerdere S, Caemaert J, De Reuck J, Boon P. The mechanism of action of vagus nerve stimulation for refractory epilepsy: the current status. J Clin Neurophysiol. 2001 Sep;18(5):394–401. doi: 10.1097/00004691-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31(Suppl 2):S1–6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- 14.Fornai F, Ruffoli R, Giorgi FS, Paparelli A. The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. The European journal of neuroscience. 2011 Jun;33(12):2169–2178. doi: 10.1111/j.1460-9568.2011.07707.x. [DOI] [PubMed] [Google Scholar]
- 15.Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. The chemical neuroanatomy of vagus nerve stimulation. Journal of chemical neuroanatomy. 2011 Dec;42(4):288–296. doi: 10.1016/j.jchemneu.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Bailey P, Bremer F. Sensory Cortical Representation of the Vagus Nerve. J Neurophysiology. 1938;1:4405–4412. [Google Scholar]
- 17.Zabara J. Neuroinhibition of xylazine induced emesis. Pharmacol Toxicol. 1988 Aug;63(2):70–74. doi: 10.1111/j.1600-0773.1988.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 18.Lockard JS, Congdon WC, DuCharme LL. Feasibility and safety of vagal stimulation in monkey model. Epilepsia. 1990;31(Suppl 2):S20–26. doi: 10.1111/j.1528-1157.1990.tb05844.x. [DOI] [PubMed] [Google Scholar]
- 19.Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1990;31(Suppl 2):S7–19. doi: 10.1111/j.1528-1157.1990.tb05852.x. [DOI] [PubMed] [Google Scholar]
- 20.Woodbury JW, Woodbury DM. Vagal stimulation reduces the severity of maximal electroshock seizures in intact rats: use of a cuff electrode for stimulating and recording. Pacing Clin Electrophysiol. 1991 Jan;14(1):94–107. doi: 10.1111/j.1540-8159.1991.tb04053.x. [DOI] [PubMed] [Google Scholar]
- 21.Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):S40–43. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 22.Uthman BM, Wilder BJ, Hammond EJ, Reid SA. Efficacy and safety of vagus nerve stimulation in patients with complex partial seizures. Epilepsia. 1990;31(Suppl 2):S44–50. doi: 10.1111/j.1528-1157.1990.tb05849.x. [DOI] [PubMed] [Google Scholar]
- 23.DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000 Sep;41(9):1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 24.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience and biobehavioral reviews. 2005 May;29(3):493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011 Aug 25;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011 Dec;115(6):1248–1255. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 27.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010 Apr;27(2):130–138. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 28.Merrill CA, Jonsson MA, Minthon L, et al. Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J Clin Psychiatry. 2006 Aug;67(8):1171–1178. doi: 10.4088/jcp.v67n0801. [DOI] [PubMed] [Google Scholar]
- 29.Xiong J, Xue FS, Liu JH, et al. Transcutaneous vagus nerve stimulation may attenuate postoperative cognitive dysfunction in elderly patients. Medical hypotheses. 2009 Dec;73(6):938–941. doi: 10.1016/j.mehy.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Jaseja H. Vagal nerve stimulation: exploring its efficacy and success for an improved prognosis and quality of life in cerebral palsy patients. Clinical neurology and neurosurgery. 2008 Sep;110(8):755–762. doi: 10.1016/j.clineuro.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Kumaria A, Tolias CM. Is there a role for vagus nerve stimulation therapy as a treatment of traumatic brain injury? British journal of neurosurgery. 2012 Jun;26(3):316–320. doi: 10.3109/02688697.2012.663517. [DOI] [PubMed] [Google Scholar]
- 32.Smith DC, Modglin AA, Roosevelt RW, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. Journal of neurotrauma. 2005 Dec;22(12):1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DC, Tan AA, Duke A, et al. Recovery of function after vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. Journal of neurotrauma. 2006 Oct;23(10):1549–1560. doi: 10.1089/neu.2006.23.1549. [DOI] [PubMed] [Google Scholar]
- 34.Jaseja H. EEG-desynchronization as the major mechanism of anti-epileptic action of vagal nerve stimulation in patients with intractable seizures: clinical neurophysiological evidence. Medical hypotheses. 2010 May;74(5):855–856. doi: 10.1016/j.mehy.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Krahl SE, Senanayake SS, Handforth A. Destruction of peripheral C-fibers does not alter subsequent vagus nerve stimulation-induced seizure suppression in rats. Epilepsia. 2001 May;42(5):586–589. doi: 10.1046/j.1528-1157.2001.09700.x. [DOI] [PubMed] [Google Scholar]
- 36.Chase MH, Nakamura Y, Clemente CD, Sterman MB. Afferent vagal stimulation: neurographic correlates of induced EEG synchronization and desynchronization. Brain Res. 1967 Jun;5(2):236–249. doi: 10.1016/0006-8993(67)90089-3. [DOI] [PubMed] [Google Scholar]
- 37.Hammond EJ, Uthman BM, Reid SA, Wilder BJ, Ramsay RE. Vagus nerve stimulation in humans: neurophysiological studies and electrophysiological monitoring. Epilepsia. 1990;31(Suppl 2):S51–59. doi: 10.1111/j.1528-1157.1990.tb05850.x. [DOI] [PubMed] [Google Scholar]
- 38.Koo B. EEG changes with vagus nerve stimulation. J Clin Neurophysiol. 2001 Sep;18(5):434–441. doi: 10.1097/00004691-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Kuba R, Nesvadba D, Brazdil M, Oslejskova H, Ryzi M, Rektor I. Effect of chronic vagal nerve stimulation on interictal epileptiform discharges. Seizure. 2010 Jul;19(6):352–355. doi: 10.1016/j.seizure.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Chen X, Lin Z, et al. Long-term effect of vagus nerve stimulation on interictal epileptiform discharges in refractory epilepsy. Journal of the neurological sciences. 2009 Sep 15;284(1-2):96–102. doi: 10.1016/j.jns.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Hallbook T, Lundgren J, Blennow G, Stromblad LG, Rosen I. Long term effects on epileptiform activity with vagus nerve stimulation in children. Seizure. 2005 Dec;14(8):527–533. doi: 10.1016/j.seizure.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Dedeurwaerdere S, Cornelissen B, Van Laere K, et al. Small animal positron emission tomography during vagus nerve stimulation in rats: a pilot study. Epilepsy Res. 2005 Dec;67(3):133–141. doi: 10.1016/j.eplepsyres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Experimental neurology. 2008 Dec;214(2):259–265. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Giorgi FS, Pizzanelli C, Biagioni F, Murri L, Fornai F. The role of norepinephrine in epilepsy: from the bench to the bedside. Neuroscience and biobehavioral reviews. 2004 Sep;28(5):507–524. doi: 10.1016/j.neubiorev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett. 2005 May 13;379(3):174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 46.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006 Nov 13;1119(1):124–132. doi: 10.1016/j.brainres.2006.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behavioral neuroscience. 2004 Feb;118(1):79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 48.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nature neuroscience. 1999 Jan;2(1):94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 49.Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. Journal of psychiatry & neuroscience : JPN. 2009 Jul;34(4):272–280. [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995 Mar;20(3):221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 51.Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABA(A) receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Res. 2003 Jun-Jul;55(1-2):59–70. doi: 10.1016/s0920-1211(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 52.Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995 Sep;22(1):53–62. doi: 10.1016/0920-1211(95)00035-9. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008 Jul;33(8):1884–1895. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 54.Furmaga H, Sadhu M, Frazer A. Comparison of DeltaFosB immunoreactivity induced by vagal nerve stimulation with that caused by pharmacologically diverse antidepressants. The Journal of pharmacology and experimental therapeutics. 2012 May;341(2):317–325. doi: 10.1124/jpet.111.188953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giorgi FS, Blandini F, Cantafora E, et al. Activation of brain metabolism and fos during limbic seizures: the role of locus coeruleus. Neurobiology of disease. 2008 Jun;30(3):388–399. doi: 10.1016/j.nbd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. The Journal of comparative neurology. 2002 Jun 17;448(1):53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- 57.Schiff ND, Fins JJ. Deep brain stimulation and cognition: moving from animal to patient. Current opinion in neurology. 2007 Dec;20(6):638–642. doi: 10.1097/WCO.0b013e3282f1c6e4. [DOI] [PubMed] [Google Scholar]
- 58.Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res. 2006 Mar 31;146(2):179–184. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Henry TR, Bakay RA, Pennell PB, Epstein CM, Votaw JR. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. prolonged effects at high and low levels of stimulation. Epilepsia. 2004 Sep;45(9):1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- 60.Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998 Sep;39(9):983–990. doi: 10.1111/j.1528-1157.1998.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 61.Henry TR, Votaw JR, Pennell PB, et al. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999 Apr 12;52(6):1166–1173. doi: 10.1212/wnl.52.6.1166. [DOI] [PubMed] [Google Scholar]
- 62.Van Laere K, Vonck K, Boon P, Versijpt J, Dierckx R. Perfusion SPECT changes after acute and chronic vagus nerve stimulation in relation to prestimulus condition and long-term clinical efficacy. J Nucl Med. 2002 Jun;43(6):733–744. [PubMed] [Google Scholar]
- 63.Kosel M, Brockmann H, Frick C, Zobel A, Schlaepfer TE. Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Res. 2011 Mar 31;191(3):153–159. doi: 10.1016/j.pscychresns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Annals of the New York Academy of Sciences. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 65.Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England journal of medicine. 2000 Feb 3;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 66.Benbadis SR, Tatum WO, Vale FL. When drugs don’t work: an algorithmic approach to medically intractable epilepsy. Neurology. 2000 Dec 26;55(12):1780–1784. doi: 10.1212/wnl.55.12.1780. [DOI] [PubMed] [Google Scholar]
- 67.Spanaki MV, Allen LS, Mueller WM, Morris GL., 3rd Vagus nerve stimulation therapy: 5-year or greater outcome at a university-based epilepsy center. Seizure. 2004 Dec;13(8):587–590. doi: 10.1016/j.seizure.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Uthman BM, Reichl AM, Dean JC, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004 Sep 28;63(6):1124–1126. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]
- 69.Klinkenberg S, Aalbers MW, Vles JS, et al. Vagus nerve stimulation in children with intractable epilepsy: a randomized controlled trial. Developmental medicine and child neurology. 2012 Apr 28; doi: 10.1111/j.1469-8749.2012.04305.x. [DOI] [PubMed] [Google Scholar]
- 70.Hallbook T, Lundgren J, Stjernqvist K, Blennow G, Stromblad LG, Rosen I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behaviour and mood. Seizure. 2005 Oct;14(7):504–513. doi: 10.1016/j.seizure.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Klinkenberg S, Majoie HJ, van der Heijden MM, Rijkers K, Leenen L, Aldenkamp AP. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clinical neurology and neurosurgery. 2012 May;114(4):336–340. doi: 10.1016/j.clineuro.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Malow BA, Edwards J, Marzec M, Sagher O, Ross D, Fromes G. Vagus nerve stimulation reduces daytime sleepiness in epilepsy patients. Neurology. 2001 Sep 11;57(5):879–884. doi: 10.1212/wnl.57.5.879. [DOI] [PubMed] [Google Scholar]
- 73.Hallbook T, Lundgren J, Kohler S, Blennow G, Stromblad LG, Rosen I. Beneficial effects on sleep of vagus nerve stimulation in children with therapy resistant epilepsy. Eur J Paediatr Neurol. 2005;9(6):399–407. doi: 10.1016/j.ejpn.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Dodrill CB, Morris GL. Effects of Vagal Nerve Stimulation on Cognition and Quality of Life in Epilepsy. Epilepsy Behav. 2001 Feb;2(1):46–53. doi: 10.1006/ebeh.2000.0148. [DOI] [PubMed] [Google Scholar]
- 75.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of vagus nerve stimulation on memory. Cogn Behav Neurol. 2006 Sep;19(3):119–122. doi: 10.1097/01.wnn.0000213908.34278.7d. [DOI] [PubMed] [Google Scholar]
- 76.Sirven JI, Sperling M, Naritoku D, et al. Vagus nerve stimulation therapy for epilepsy in older adults. Neurology. 2000 Mar 14;54(5):1179–1182. doi: 10.1212/wnl.54.5.1179. [DOI] [PubMed] [Google Scholar]
- 77.Helmers SL, Wheless JW, Frost M, et al. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol. 2001 Nov;16(11):843–848. doi: 10.1177/08830738010160111101. [DOI] [PubMed] [Google Scholar]
- 78.Helmers SL, Duh MS, Guerin A, et al. Clinical outcomes, quality of life, and costs associated with implantation of vagus nerve stimulation therapy in pediatric patients with drug-resistant epilepsy. Eur J Paediatr Neurol. 2012 Jan 17; doi: 10.1016/j.ejpn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Grill MF, Ng YT. Dramatic first words spoken in 2 children after vagus nerve stimulation. Seminars in pediatric neurology. 2010 Mar;17(1):54–57. doi: 10.1016/j.spen.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Zamponi N, Passamonti C, Cappanera S, Petrelli C. Clinical course of young patients with Dravet syndrome after vagal nerve stimulation. Eur J Paediatr Neurol. 2011 Jan;15(1):8–14. doi: 10.1016/j.ejpn.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Patel NC, Edwards MS. Vagal nerve stimulator pocket infections. The Pediatric infectious disease journal. 2004 Jul;23(7):681–683. doi: 10.1097/01.inf.0000131632.25375.c7. [DOI] [PubMed] [Google Scholar]
- 82.Annegers JF, Coan SP, Hauser WA, Leestma J. Epilepsy, vagal nerve stimulation by the NCP system, all-cause mortality, and sudden, unexpected, unexplained death. Epilepsia. 2000 May;41(5):549–553. doi: 10.1111/j.1528-1157.2000.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 83.Malow BA, Edwards J, Marzec M, Sagher O, Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000 Nov 28;55(10):1450–1454. doi: 10.1212/wnl.55.10.1450. [DOI] [PubMed] [Google Scholar]
- 84.Marzec M, Edwards J, Sagher O, Fromes G, Malow BA. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003 Jul;44(7):930–935. doi: 10.1046/j.1528-1157.2003.56202.x. [DOI] [PubMed] [Google Scholar]
- 85.Ardesch JJ, Buschman HP, Wagener-Schimmel LJ, van der Aa HE, Hageman G. Vagus nerve stimulation for medically refractory epilepsy: A long-term follow-up study. Seizure. 2007 Oct;16(7):579–585. doi: 10.1016/j.seizure.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Shaw GY, Sechtem P, Searl J, Dowdy ES. Predictors of laryngeal complications in patients implanted with the Cyberonics vagal nerve stimulator. The Annals of otology, rhinology, and laryngology. 2006 Apr;115(4):260–267. doi: 10.1177/000348940611500403. [DOI] [PubMed] [Google Scholar]
- 87.Gorny KR, Bernstein MA, Watson RE., Jr 3 Tesla MRI of patients with a vagus nerve stimulator: initial experience using a T/R head coil under controlled conditions. Journal of magnetic resonance imaging : JMRI. 2010 Feb;31(2):475–481. doi: 10.1002/jmri.22037. [DOI] [PubMed] [Google Scholar]
- 88.Benbadis SR, Nyhenhuis J, Tatum WO, Murtagh FR, Gieron M, Vale FL. MRI of the brain is safe in patients implanted with the vagus nerve stimulator. Seizure. 2001 Oct;10(7):512–515. doi: 10.1053/seiz.2001.0540. [DOI] [PubMed] [Google Scholar]
- 89.Mu Q, Bohning DE, Nahas Z, et al. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatry. 2004 Apr 15;55(8):816–825. doi: 10.1016/j.biopsych.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 90.Chae JH, Nahas Z, Lomarev M, et al. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003 Nov-Dec;37(6):443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 91.Narayanan JT, Watts R, Haddad N, Labar DR, Li PM, Filippi CG. Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia. 2002 Dec;43(12):1509–1514. doi: 10.1046/j.1528-1157.2002.16102.x. [DOI] [PubMed] [Google Scholar]
- 92.Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, George MS. Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol. 2001 Aug;36(8):470–479. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002 Jul-Aug;36(4):219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 94.Sucholeiki R, Alsaadi TM, Morris GL, 3rd, Ulmer JL, Biswal B, Mueller WM. fMRI in patients implanted with a vagal nerve stimulator. Seizure. 2002 Apr;11(3):157–162. doi: 10.1053/seiz.2001.0601. [DOI] [PubMed] [Google Scholar]
- 95.Tatum WO, Malek A, Recio M, Orlowski J, Murtagh R. Diffusion-weighted imaging and status epilepticus during vagus nerve stimulation. Epilepsy Behav. 2004 Jun;5(3):411–415. doi: 10.1016/j.yebeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Graham DI, Adams JH, Murray LS, Jennett B. Neuropathology of the vegetative state after head injury. Neuropsychological rehabilitation. 2005 Jul-Sep;15(3-4):198–213. doi: 10.1080/09602010443000452. [DOI] [PubMed] [Google Scholar]
- 97.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989 Jul;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 98.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain research. 2002 Sep;39(2-3):107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 99.Steriade M, Glenn LL. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. Journal of neurophysiology. 1982 Aug;48(2):352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- 100.Bigler ED, Ryser DK, Gandhi P, Kimball J, Wilde EA. Day-of-injury computerized tomography, rehabilitation status, and development of cerebral atrophy in persons with traumatic brain injury. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2006 Oct;85(10):793–806. doi: 10.1097/01.phm.0000237873.26250.e1. [DOI] [PubMed] [Google Scholar]
- 101.Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta neurochirurgica. 2006 Feb;148(2):181–193. doi: 10.1007/s00701-005-0674-4. discussion 193-184. [DOI] [PubMed] [Google Scholar]
- 102.Stone JR, Okonkwo DO, Dialo AO, et al. Impaired axonal transport and altered axolemmal permeability occur in distinct populations of damaged axons following traumatic brain injury. Experimental neurology. 2004 Nov;190(1):59–69. doi: 10.1016/j.expneurol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 103.Lifshitz J, Kelley BJ, Povlishock JT. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. Journal of neuropathology and experimental neurology. 2007 Mar;66(3):218–229. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- 104.Singleton RH, Zhu J, Stone JR, Povlishock JT. Traumatically induced axotomy adjacent to the soma does not result in acute neuronal death. J Neurosci. 2002 Feb 1;22(3):791–802. doi: 10.1523/JNEUROSCI.22-03-00791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trivedi MA, Ward MA, Hess TM, et al. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: relationship with duration of coma. Journal of neurotrauma. 2007 May;24(5):766–771. doi: 10.1089/neu.2006.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salazar AM, Warden DL, Schwab K, et al. Cognitive rehabilitation for traumatic brain injury: A randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. Jama. 2000 Jun 21;283(23):3075–3081. doi: 10.1001/jama.283.23.3075. [DOI] [PubMed] [Google Scholar]
- 107.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC neurology. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ. 1996 Jul 6;313(7048):13–16. doi: 10.1136/bmj.313.7048.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boly M, Faymonville ME, Peigneux P, et al. Auditory processing in severely brain injured patients: differences between the minimally conscious state and the persistent vegetative state. Archives of neurology. 2004 Feb;61(2):233–238. doi: 10.1001/archneur.61.2.233. [DOI] [PubMed] [Google Scholar]
- 110.Laureys S, Faymonville ME, Degueldre C, et al. Auditory processing in the vegetative state. Brain : a journal of neurology. 2000 Aug;123(Pt 8):1589–1601. doi: 10.1093/brain/123.8.1589. [DOI] [PubMed] [Google Scholar]
- 111.Qin P, Di H, Liu Y, et al. Anterior cingulate activity and the self in disorders of consciousness. Human brain mapping. 2010 Dec;31(12):1993–2002. doi: 10.1002/hbm.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Di HB, Yu SM, Weng XC, et al. Cerebral response to patient’s own name in the vegetative and minimally conscious states. Neurology. 2007 Mar 20;68(12):895–899. doi: 10.1212/01.wnl.0000258544.79024.d0. [DOI] [PubMed] [Google Scholar]
- 113.Coleman MR, Davis MH, Rodd JM, et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain : a journal of neurology. 2009 Sep;132(Pt 9):2541–2552. doi: 10.1093/brain/awp183. [DOI] [PubMed] [Google Scholar]
- 114.Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. The New England journal of medicine. 2010 Feb 18;362(7):579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 115.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain : a journal of neurology. 2010 Jan;133(Pt 1):161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schiff ND, Rodriguez-Moreno D, Kamal A, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology. 2005 Feb 8;64(3):514–523. doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- 117.Fernandez-Espejo D, Junque C, Bernabeu M, Roig-Rovira T, Vendrell P, Mercader JM. Reductions of thalamic volume and regional shape changes in the vegetative and the minimally conscious states. Journal of neurotrauma. 2010 Jul;27(7):1187–1193. doi: 10.1089/neu.2010.1297. [DOI] [PubMed] [Google Scholar]
- 118.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000 May 20;355(9217):1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 119.Bekinschtein TA, Shalom DE, Forcato C, et al. Classical conditioning in the vegetative and minimally conscious state. Nature neuroscience. 2009 Oct;12(10):1343–1349. doi: 10.1038/nn.2391. [DOI] [PubMed] [Google Scholar]
- 120.Voss HU, Uluc AM, Dyke JP, et al. Possible axonal regrowth in late recovery from the minimally conscious state. The Journal of clinical investigation. 2006 Jul;116(7):2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boly M. Measuring the fading consciousness in the human brain. Current opinion in neurology. 2011 Aug;24(4):394–400. doi: 10.1097/WCO.0b013e328347da94. [DOI] [PubMed] [Google Scholar]
- 122.Cohadon F, Richer E. Deep cerebral stimulation in patients with post-traumatic vegetative state. 25 cases. Neuro-Chirurgie. 1993;39(5):281–292. [PubMed] [Google Scholar]
- 123.Yamamoto T, Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychological rehabilitation. 2005 Jul-Sep;15(3-4):406–413. doi: 10.1080/09602010443000353. [DOI] [PubMed] [Google Scholar]
- 124.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007 Aug 2;448(7153):600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 125.Kuba R, Guzaninova M, Brazdil M, Novak Z, Chrastina J, Rektor I. Effect of vagal nerve stimulation on interictal epileptiform discharges: a scalp EEG study. Epilepsia. 2002 Oct;43(10):1181–1188. doi: 10.1046/j.1528-1157.2002.08202.x. [DOI] [PubMed] [Google Scholar]
- 126.Santiago-Rodriguez E, Alonso-Vanegas M, Cardenas-Morales L, Harmony T, Bernardino M, Fernandez-Bouzas A. Effects of two different cycles of vagus nerve stimulation on interictal epileptiform discharges. Seizure. 2006 Dec;15(8):615–620. doi: 10.1016/j.seizure.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 127.Ronne-Engstrom E, Winkler T. Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta neurologica Scandinavica. 2006 Jul;114(1):47–53. doi: 10.1111/j.1600-0404.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 128.Clough RW, Neese SL, Sherill LK, et al. Cortical edema in moderate fluid percussion brain injury is attenuated by vagus nerve stimulation. Neuroscience. 2007 Jun 29;147(2):286–293. doi: 10.1016/j.neuroscience.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 129.Neese SL, Sherill LK, Tan AA, et al. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: An immunocytochemical study. Brain Res. 2007 Jan 12;1128(1):157–163. doi: 10.1016/j.brainres.2006.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001 Sep;16(3):165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 131.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000 May 25;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 132.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003 Jan 23;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 133.Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011 Oct 7;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain, behavior, and immunity. 2005 Nov;19(6):493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 135.Bansal V, Ryu SY, Lopez N, et al. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation. 2012 Feb;35(1):214–220. doi: 10.1007/s10753-011-9307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang W, Lin TR, Hu Y, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. The Journal of physiology. 2004 Sep 15;559(Pt 3):729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bansal V, Costantini T, Ryu SY, et al. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. The Journal of trauma. 2010 May;68(5):1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ammori JB, Zhang WZ, Li JY, Chai BX, Mulholland MW. Effects of ghrelin on neuronal survival in cells derived from dorsal motor nucleus of the vagus. Surgery. 2008 Aug;144(2):159–167. doi: 10.1016/j.surg.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2012 Apr 13; doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune responses in epilepsy. Glia. 2012 Aug;60(8):1258–1268. doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- 141.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nature reviews Neurology. 2011 Jan;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li G, Bauer S, Nowak M, et al. Cytokines and epilepsy. Seizure. 2011 Apr;20(3):249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 143.Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Experimental neurology. 2011 Oct 1; doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 144.Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett. 2011 Jun 27;497(3):223–230. doi: 10.1016/j.neulet.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 145.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain, behavior, and immunity. 2008 Aug;22(6):797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 146.De Herdt V, Bogaert S, Bracke KR, et al. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. Journal of neuroimmunology. 2009 Sep 29;214(1-2):104–108. doi: 10.1016/j.jneuroim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 147.Majoie HJ, Rijkers K, Berfelo MW, et al. Vagus nerve stimulation in refractory epilepsy: effects on pro- and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation. 2011;18(1):52–56. doi: 10.1159/000315530. [DOI] [PubMed] [Google Scholar]
- 148.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004 Sep 8;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain : a journal of neurology. 2007 Feb;130(Pt 2):521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 150.Marchi N, Teng Q, Ghosh C, et al. Blood-brain barrier damage, but not parenchymal white blood cells, is a hallmark of seizure activity. Brain Res. 2010 Sep 24;1353:176–186. doi: 10.1016/j.brainres.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006 Nov;47(11):1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 152.Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009 Aug;85(2-3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature reviews Neurology. 2010 Jul;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lopez NE, Krzyzaniak MJ, Costantini TW, et al. Vagal nerve stimulation decreases blood-brain barrier disruption after traumatic brain injury. The journal of trauma and acute care surgery. 2012 Jun;72(6):1562–1566. doi: 10.1097/TA.0b013e3182569875. [DOI] [PubMed] [Google Scholar]
- 155.Rappaport M, Herrero-Backe C, Rappaport ML, Winterfield KM. Head injury outcome up to ten years later. Archives of physical medicine and rehabilitation. 1989 Dec;70(13):885–892. [PubMed] [Google Scholar]
- 156.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Archives of physical medicine and rehabilitation. 2004 Dec;85(12):2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 157.Reid SA. Surgical technique for implantation of the neurocybernetic prosthesis. Epilepsia. 1990;31(Suppl 2):S38–39. doi: 10.1111/j.1528-1157.1990.tb05847.x. [DOI] [PubMed] [Google Scholar]
- 158.Randall WC, Ardell JL, Becker DM. Differential responses accompanying sequential stimulation and ablation of vagal branches to dog heart. Am J Physiol. 1985 Jul;249(1 Pt 2):H133–140. doi: 10.1152/ajpheart.1985.249.1.H133. [DOI] [PubMed] [Google Scholar]
- 159.Tatum WO, Moore DB, Stecker MM, et al. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 1999 Apr 12;52(6):1267–1269. doi: 10.1212/wnl.52.6.1267. [DOI] [PubMed] [Google Scholar]
- 160.Physician’s Manual NeuroCybernetic Prosthesis Programming Software Model 250 Version 4.6. Cyberonics, Inc.: 2002. [Google Scholar]
- 161.Hawley CA, Taylor R, Hellawell DJ, Pentland B. Use of the functional assessment measure (FIM+FAM) in head injury rehabilitation: a psychometric analysis. Journal of neurology, neurosurgery, and psychiatry. 1999 Dec;67(6):749–754. doi: 10.1136/jnnp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seel RT, Wright G, Wallace T, Newman S, Dennis L. The Utility of the FIM+FAM for Assessing Traumatic Brain Injury Day Program Outcomes. The Journal of head trauma rehabilitation. 2007 Sep-Oct;22(5):267–277. doi: 10.1097/01.HTR.0000290971.56130.c8. [DOI] [PubMed] [Google Scholar]
- 163.Turner-Stokes L. Standardized outcome assessment in brain injury rehabilitation for younger adults. Disability and rehabilitation. 2002 May 10;24(7):383–389. doi: 10.1080/096382801101550. [DOI] [PubMed] [Google Scholar]
- 164.van Baalen B, Odding E, van Woensel MP, Roebroeck ME. Reliability and sensitivity to change of measurement instruments used in a traumatic brain injury population. Clinical rehabilitation. 2006 Aug;20(8):686–700. doi: 10.1191/0269215506cre982oa. [DOI] [PubMed] [Google Scholar]
- 165.Giacino JT, Kezmarsky MA, DeLuca J, Cicerone KD. Monitoring rate of recovery to predict outcome in minimally responsive patients. Archives of physical medicine and rehabilitation. 1991 Oct;72(11):897–901. doi: 10.1016/0003-9993(91)90008-7. [DOI] [PubMed] [Google Scholar]
- 166.Gray DS, Burnham RS. Preliminary outcome analysis of a long-term rehabilitation program for severe acquired brain injury. Archives of physical medicine and rehabilitation. 2000 Nov;81(11):1447–1456. doi: 10.1053/apmr.2000.16343. [DOI] [PubMed] [Google Scholar]
- 167.Walker W, Seel R, Gibellato M, et al. The effects of Donepezil on traumatic brain injury acute rehabilitation outcomes. Brain Inj. 2004 Aug;18(8):739–750. doi: 10.1080/02699050310001646224. [DOI] [PubMed] [Google Scholar]
- 168.Pagulayan KF, Hoffman JM, Temkin NR, Machamer JE, Dikmen SS. Functional limitations and depression after traumatic brain injury: examination of the temporal relationship. Archives of physical medicine and rehabilitation. 2008 Oct;89(10):1887–1892. doi: 10.1016/j.apmr.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 169.Pagulayan KF, Temkin NR, Machamer J, Dikmen SS. A longitudinal study of health-related quality of life after traumatic brain injury. Archives of physical medicine and rehabilitation. 2006 May;87(5):611–618. doi: 10.1016/j.apmr.2006.01.018. [DOI] [PubMed] [Google Scholar]


