Abstract
Background
Previous analyses identified specific geographic areas in Philadelphia (hotspots) associated with negative outcomes along the HIV care continuum. We examined individual and community factors associated with residing in these hotspots.
Methods
Retrospective cohort of 1,404 persons newly diagnosed with HIV in 2008–2009 followed for 24 months after linkage to care. Multivariable regression examined associations between individual (age, sex, race/ethnicity, HIV transmission risk, and insurance status) and community (economic deprivation, distance to care, access to public transit, and access to pharmacy services) factors and the outcomes: residence in a hotspot associated with poor retention in care and residence in a hotspot associated with poor viral suppression.
Results
24.4% and 13.7% of persons resided in hotspots associated with poor retention and poor viral suppression, respectively. For persons residing in poor retention hotspots, 28.3% were retained in care compared to 40.4% of those residing outside hotspots (p<0.05). Similarly, for persons residing in poor viral suppression hotspots, 51.4% achieved viral suppression compared to 75.3% of those outside hotspots (p<.0.05). Factors significantly associated with residence in a poor retention hotspots included: female sex, lower economic deprivation, greater access to public transit, shorter distance to medical care, and longer distance to pharmacies. Factors significantly associated with residence in a poor viral suppression hotspots included; female sex, higher economic deprivation, and shorter distance to pharmacies.
Conclusions
Individual and community-level associations with geographic hotspots may inform both content and delivery strategies for interventions designed to improve retention in care and viral suppression.
Keywords: GIS, Spatial patterns, care linkage, retention, viral suppression
INTRODUCTION
The HIV care continuum identifies distinct points for intervention, with the ultimate goal of improving health outcomes for people with HIV infection and reducing HIV transmission in the community.1 Prior studies indicate that linkage to care, retention in care, and viral suppression are influenced by a variety of individual, health system, and community-level factors.2–10 While linkage to care represents the entry point for HIV disease management, retention in care and sustained viral suppression are necessary to achieve the individual and public health benefits of HIV treatment.3,11 As monitoring of HIV infection increasingly focuses on the HIV care continuum11–14, analyses of public health surveillance data may reveal geographic patterns that vary at each step.15,16 Moreover, a better understanding of the structural and community-level factors that influence or impede completion of HIV care continuum steps is needed17–23.
In prior work, we identified geographic areas in Philadelphia, PA with significantly higher concentrations of individuals with poor retention in care and poor viral suppression15. In total, 14 census tracts were associated with poor retention in care (3.7% of all census tracts in Philadelphia) and 12 census tracts were associated with poor viral suppression (3.2% of all census tracts in Philadelphia). Interestingly, the geographic areas identified for poor retention in care and poor viral suppression were unique, with no geographic overlap. This suggested that distinct community-level factors might be responsible for these poor outcomes. The current analyses build on prior work and aim to identify individual and community-level factors associated with residing in hotspots of poor retention in care and residing in hotspots of poor viral suppression in hopes of informing the development of interventions to improve the final steps of the HIV care continuum.
METHODS
Data Source & Study Population
Data were extracted from the City of Philadelphia’s Enhanced HIV/AIDS Reporting System (eHARS), a database containing information on all HIV cases reported to the Philadelphia Department of Public Health (PDPH) AIDS Activities Coordinating Office Surveillance Unit. Philadelphia has mandatory name-based case reporting of all new HIV infections. Additionally, local mandates require reporting of all CD4 cell counts <350/mL (or CD4 percent <25%) and all HIV-1 RNA levels to the PDPH. Thus, eHARS contains records and laboratory results of all people living with HIV (PLWH) who were diagnosed with HIV in Philadelphia, were a resident of Philadelphia at any time after their HIV diagnosis, and all PLWH who received care in Philadelphia after their HIV diagnosis.
The eHARS database contains information collected through medical record abstraction including identifiers, such as name, address, date of birth, and address at diagnosis, as well as laboratory results which are received electronically and imported into the database. Death data from the Pennsylvania Bureau of Vital Statistics, Social Security Death Master Index, and the National Death Index are routinely matched to eHARS data to identify deceased persons and document cause of death when available. The eHARS data are routinely monitored to identify duplicate cases and undergo quality control and verification to ensure that abstracted data are correctly assigned to unique case records.
Because the current analyses rely on exact identification of case locations and focus on patients successfully linked to care, individuals were included if they had a: (1) HIV diagnosis date in 2008 or 2009, (2) Philadelphia address at time of diagnosis, and (3) were successfully linked to care, defined as documentation of 1 or more CD4 or viral load test results after the date of diagnosis. Those with invalid or insufficient address data, along with persons with only a correctional facility address at time of diagnosis, were excluded from analyses (N=157). Cases were followed for 24 months after linkage to care.
The time period of 2008 to 2009 was selected to further define factors associated with poor retention in care and poor viral suppression and to allow for a 24-month interval for observation, as described below.
Outcome Variables
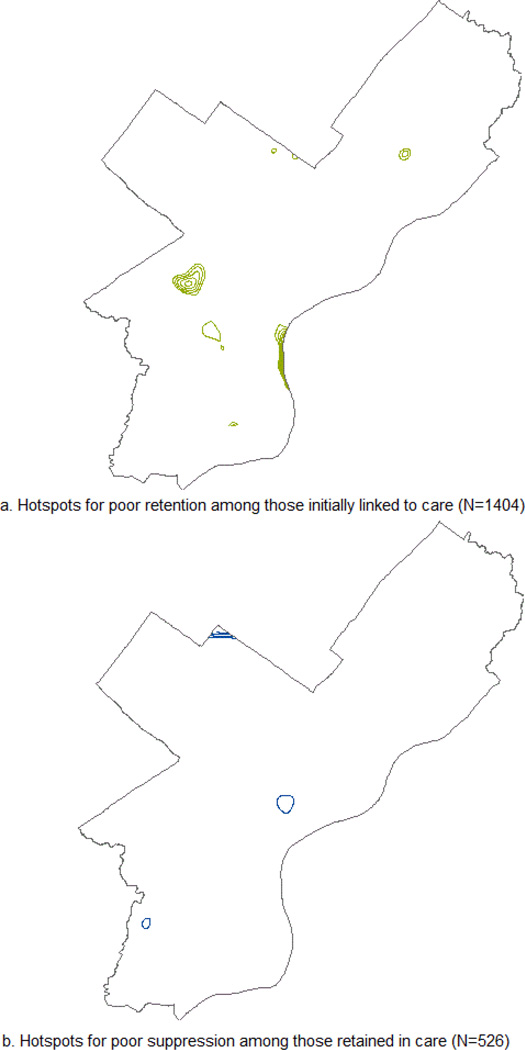
Previous analyses of this cohort identified geographic areas of the city where patients with negative outcomes (not retained in care, not virally suppressed) exhibited significant clustering compared to the cohort as a whole.15 For these analyses, we used these previously identified areas to classify cases dichotomously as either in or near (within 5,000 feet) a significant hot-spot, or not in or near a significant hot-spot. Hot-spots were identified by calculating the local version of the cross K function, which uses a marked-point process to compare sub-groups and is described elsewhere.15 Figure 1 provides maps showing the locations of these hotspots.
FIGURE 1.
Hotspots for poor HIV treatment retention and viral supression among individuals diagnosed in 2008 and 2009
Retention in care was defined using the National Quality Forum Medical Visit Frequency Measure24. This measure defines retention in care as completing at least 1 medical visit with a provider with prescribing privileges in each 6-month interval of the 24-month measurement period, with a minimum of 60 days between medical visits. Date of first linkage to care defined the start of the 24-month measurement period. We used laboratory reports of CD4 counts and/or viral load testing as a proxy for HIV medical care visits. Previous studies have shown high correlation between laboratory test and medical visit data and retention in care.25 Viral suppression was classified as evidence of HIV-1 RNA <200 copies per milliliter closest to the end of the 24-month measurement period +/− 120 days. For the viral suppression analysis, we only included persons who met the retention in care definition so that we could look at predictors of viral suppression that are independent of retention in care.
Predictor Variables
Individual-Level Factors
For each person, we defined age, sex at birth, race/ethnicity, HIV transmission risk, and insurance status at the time of HIV diagnosis. Date of HIV diagnosis was defined as the date of collection of confirmatory test results. Age was categorized into three groups: <25, 25–44, and ≥45 years old. Race/ethnicity categories were divided into four groups: non-Hispanic white, non-Hispanic Black, Hispanic, and other. Transmission risk was grouped into heterosexual, men who had sex with men (MSM), injection drug use (IDU), and other/unknown. Patients who had IDU in combination with another risk factor (e.g., MSM, heterosexual transmission) were classified as IDU. Insurance status was classified as Medicaid, Medicare, private, no insurance, and other or unknown.
Community-Level Factors
Travel distance to medical care was assessed using the spatial locations of cases and HIV care providers. Cases were geocoded using the street address at time of HIV diagnosis. Medical care sites where initial linkage to care occurred were geocoded using the facility address. Distances were calculated (in miles) through network analysis using street-level data to calculate the distance required to travel from point A (residence) to point B (medical care) under normal driving conditions.
All pharmacies currently operating in Philadelphia were geocoded based on street address. The locations were assigned to census tracts using the 2010 decennial census tracts and summarized to calculate the total number of pharmacies in each tract. Pharmacy densities were calculated as the rate of pharmacy locations (per 1,000 population), using the 2010 population data from the U.S. Census Bureau. In addition, network analyses were conducted to determine the five closest pharmacies for each case using street-level data as the network dataset. Distances were calculated (in miles) using the “closest facility” function of the ArcGIS Network Analyst. Travel distance from point A (residence) to point B (pharmacy) was determined under normal driving conditions for the five closest pharmacies to each case, and an average distance was calculated for inclusion in the model.
Access to public transit was assessed by including data from a transit network dataset created from the General Transit Feed System (GTFS) provided by the Southeastern Pennsylvania Transit Authority (SEPTA) and tools developed by Environmental Systems Research Institute (ESRI)26. The SEPTA GTFS data includes all transit lines (buses, subways, and light rails) that serve Philadelphia, including the spatial locations of lines and stops, as well as schedule information. For this analysis we assigned census tracts to all SEPTA stops within Philadelphia, and summarized the data to calculate the rate (per 1,000 population) of transit access in each tract.
Economic deprivation was assessed using a measure calculated from several components of the American Community Survey released by the U.S. Census Bureau. Data elements included in the index are: 1) percent employed in low-wage occupation; 2) percent households in poverty; 3) percent households receiving food stamps; 4) percent female-headed households with dependent children; and 5) percent less than high school education. The percents of these five data elements were summed and averaged to created the deprivation score. For purposes of interpretation, a higher score indicates greater economic deprivation.
Statistical Analyses
Univariate statistics were used to describe the dataset. Multivariate logistic regression models were used to assess relationships between individual and community-level predictors and the two outcomes. Models were fitted using a forward stepwise logistic regression, with variables entered into the model based on a 0.05 significance level of the univariate chi-square. Sex at birth, economic deprivation, public transit coverage, distance to medical care, and the average distance to the closest five pharmacies were included in model 1 (retention in care). Sex at birth, economic deprivation, and the average distance to the closest five pharmacies were included in model 2 (viral suppression). Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) are presented. Relationships were considered statistically significant at P <0.05. Analyses were conducted using SAS 9.2.
RESULTS
Demographics of the cohort are presented in Table 1. A total of 1,404 persons were diagnosed with HIV in 2008–2009, had a valid Philadelphia address at diagnosis, and successfully linked to HIV medical care. The population was predominantly male (69.6%), Black race/ethnicity (61.0%), between the ages of 25 and 44 at time of HIV diagnosis (47.2%) and had either MSM (38.3%) or heterosexual (40.5%) as their HIV risk factor. The mean deprivation score of the cohort was 19.33 indicating a higher degree of economic deprivation in our sample than the City of Philadelphia as a whole (15.66). The mean pharmacy density was 0.35 pharmacies per 1,000 population, and mean public transit route density was 5.55 routes per 1,000 population. The mean distance to the facility of care was 3.65 miles, while the mean distance to the five closest pharmacies was 0.56 miles.
Table 1.
Sample characteristics for those linked to care and multivariate regression results by residence in a hotspot for poor retention in care
| Predictors and Values | Total | In a Hotspot for Poor Retention in Care |
Not in a Hotspot for Poor Retention in Care |
Multivariable Regression Model |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Label | Level | N | % | N | % | N | % | AOR | 95% CI |
| Age at diagnosis | < 25 | 341 | 24.29 | 78 | 22.74 | 263 | 24.79 | 1 | |
| 25–44 | 663 | 47.22 | 166 | 48.40 | 497 | 46.84 | |||
| 45+ | 400 | 28.49 | 99 | 28.86 | 301 | 28.37 | |||
| Sex at Birth | Male | 977 | 69.59 | 267 | 77.84 | 710 | 66.92 | Ref | |
| Female | 427 | 30.41 | 76 | 22.16 | 351 | 33.08 | 0.633 | 0.454–0.884 | |
| Race | Hispanic | 260 | 18.52 | 35 | 10.20 | 225 | 21.21 | 1 | |
| Other/UNK | 60 | 4.27 | 21 | 6.12 | 39 | 3.68 | |||
| Black | 857 | 61.04 | 209 | 60.93 | 648 | 61.07 | |||
| White | 227 | 16.17 | 78 | 22.74 | 149 | 14.04 | |||
| Transmission Risk | MSM | 538 | 38.32 | 164 | 47.81 | 374 | 35.25 | 1 | |
| IDU | 127 | 9.05 | 17 | 4.96 | 110 | 10.37 | |||
| Heterosexual | 569 | 40.53 | 125 | 36.44 | 444 | 41.85 | |||
| Other/NIR | 170 | 12.11 | 37 | 10.79 | 133 | 12.54 | |||
| Insurance | Medicaid | 420 | 29.91 | 73 | 21.28 | 347 | 32.70 | 1 | |
| Medicare | 131 | 9.33 | 35 | 10.20 | 96 | 9.05 | |||
| None | 230 | 16.38 | 56 | 16.33 | 174 | 16.40 | |||
| Other/UNK | 321 | 22.86 | 85 | 24.78 | 236 | 22.24 | |||
| Private | 302 | 21.51 | 94 | 27.41 | 208 | 19.60 | |||
| Economic Deprivation Index | Mean (MED) | 19.33 | (19.64) | 15.3 | (14.75) | 20.63 | (20.78) | 0.920 | 0.903–0.937 |
| Pharmacy Density (rate per 1,000 population) | Mean (MED) | 0.35 | (0.24) | 0.38 | (0.25) | 0.34 | (0.24) | 1 | |
| Public Transit Route Density (rate per 1,000 population) | Mean (MED) | 5.55 | (4.92) | 6.33 | (5.86) | 5.30 | (4.70) | 1.041 | 1.000–1.085 |
| Distance to Care (in miles) | Mean (MED) | 3.65 | (2.88) | 2.92 | (2.27) | 3.88 | (3.08) | 0.847 | 0.797–0.899 |
| Average Distance to 5 closest pharmacies (in miles) | Mean (MED) | 0.56 | (0.55) | 0.56 | (0.57) | 0.56 | (0.55) | 2.4123 | 1.144–5.086 |
Variable was not significant in univariate analysis and was not entered into the regression model for poor retention.
Economic Deprivation was calculated from the American Community Survey data elements of percent employed in low-wage occupation, percent households in poverty, percent households receiving food stamps, percent female-headed households with dependent children, and percent less than high school education.
Distance to care was assessed using the spatial locations of cases and HIV care providers using the street address at time of HIV diagnosis and the address of the medical care sites where initial linkage to care.
Overall, among this cohort of patients linked to care, 37.5% were retained in care and 27.0% achieved viral suppression. In total, 24.4% of the sample resided in a poor retention hotspot and 13.7% in a poor viral suppression hotspot. Outcomes varied significantly by residence in a hotspot. For persons residing in a poor retention hotspot, 28.3% of cases were retained in care compared to 40.4% among those residing outside a poor retention hotspot (p<0.05). For those residing in a hotspot of poor viral suppression, 51.4% of those retained in care achieved viral suppression compared to 75.3% respectively for persons who did not reside in a poor retention hotspot (p<.0.05).
Associations with Residing in a Poor Retention Hotspot
As shown in Table 1, females were less likely to reside in geographic areas associated with poor retention in care (AOR = 0.63; 95% CI 0.45–0.88). Economic deprivation was inversely associated with residence in these areas (AOR = 0.92; 95% CI 0.90–0.94), indicating that as deprivation increased the probability of residing in a hotspot associated with poor retention decreased. The probability of residing in a hotspot for poor retention in care increased as the proportion of transit lines serving that area increased (AOR = 1.04; 95% CI 1.00–1.09). In addition, residence in a hotspot for poor retention in care was associated with longer distance to the five nearest pharmacies (AOR = 2.41; 95% CI 1.14–5.09), specifically as distance to a pharmacy increased the probability of residing in a hotspot increased. Travel distance to the location of HIV medical care was inversely associated with residence in a hotspot (AOR = 0.85; 95% CI 0.80–0.90), indicating that the probability of residing in a poor retention hotspot decreased as the distance traveled for care increased. Age, race/ethnicity, HIV transmission risk and insurance status were not significant in the univariate analyses and were therefore not included in the model.
Associations with Residing in Poor Viral Suppression Hotspots
As shown in Table 2, females were more likely to reside in hotspots associated with poor viral suppression (AOR = 1.74; 95% CI 1.00–3.01). Economic deprivation was significantly associated with residence in areas of poor viral suppression (AOR = 1.09; 95% CI 1.05–1.12), indicating that as deprivation increased the probability of residing in one of these areas also increased. In contrast to the poor retention model, the average travel distance to the five closest pharmacies was inversely associated with residence in poor viral suppression hotspots (AOR = 0.12; 95% CI 0.02–0.70), indicating that the probability of residing in a hotspot decreased as distance to the 5 closest pharmacies increased. Age, sex, race/ethnicity, HIV transmission risk and insurance status were not significant in the univariate analyses and were therefore not included in the model.
Table 2.
Sample characteristics and multivariate regression results by residence in a hotspot for poor viral suppression
| Predictors and Values | Total | In a Hotspot for Poor Viral Suppression |
Not in a Hotspot for Poor Viral Suppression |
Multivariable Regression Model |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Label | Level | N | % | N | % | N | % | AOR | 95% CI |
| Age at diagnosis | < 25 | 99 | 18.82 | 14 | 19.44 | 85 | 18.72 | 1 | |
| 25–44 | 262 | 49.80 | 31 | 43.06 | 231 | 50.88 | |||
| 45+ | 165 | 31.36 | 27 | 37.50 | 138 | 30.40 | |||
| Sex at Birth | Male | 354 | 67.30 | 39 | 54.17 | 315 | 69.38 | Ref | |
| Female | 172 | 32.69 | 33 | 45.83 | 139 | 30.62 | 1.735 | 1.000–3.010 | |
| Race | Hispanic | 93 | 17.68 | 20 | 27.78 | 73 | 16.08 | 1 | |
| Other/UNK | 20 | 03.80 | 1 | 1.39 | 19 | 4.19 | |||
| Black | 308 | 58.55 | 39 | 54.17 | 269 | 59.25 | |||
| White | 105 | 19.96 | 12 | 16.67 | 93 | 20.48 | |||
| Transmission Risk | MSM | 191 | 36.31 | 23 | 31.94 | 168 | 37.00 | 1 | |
| IDU | 45 | 08.55 | 9 | 12.50 | 36 | 7.93 | |||
| Heterosexual | 207 | 39.35 | 25 | 34.72 | 182 | 40.09 | |||
| Other/NIR | 83 | 15.77 | 15 | 20.83 | 68 | 14.98 | |||
| Insurance | Medicaid | 156 | 29.65 | 25 | 34.72 | 131 | 28.85 | 1 | |
| Medicare | 45 | 8.55 | 6 | 8.33 | 39 | 8.59 | |||
| None | 87 | 16.53 | 5 | 6.94 | 82 | 18.06 | |||
| Other/UNK | 121 | 23.00 | 17 | 23.61 | 104 | 22.91 | |||
| Private | 117 | 22.24 | 19 | 26.39 | 98 | 21.59 | |||
| Economic Deprivation Index | Mean (MED) | 19.33 | (19.64) | (23.49) | (23.69) | (18.57) | (19.63) | 1.086 | 1.048–1.124 |
| Pharmacy Density (rate per 1,000 population) | Mean (MED) | 0.35 | (0.24) | 0.32 | (0.29) | 0.33 | (0.24) | 1 | |
| Public Transit Route Density (rate per 1,000 population) | Mean (MED) | 5.55 | (4.92) | 4.87 | (4.59) | 5.47 | (4.93) | 1 | |
| Distance to Care (in miles) | Mean (MED) | 3.65 | (2.88) | 3.50 | (3.06) | 3.97 | (3.08) | 1 | |
| Average Distance to 5 closest pharmacies (in miles) | Mean (MED) | 0.56 | (0.55) | 0.52 | (0.49) | 0.56 | (0.55) | 0.124 | 0.022–0.694 |
Variable was not significant in univariate analysis and was not entered into the regression model for poor retention.
Economic Deprivation was calculated from the American Community Survey data elements of percent employed in low-wage occupation, percent households in poverty, percent households receiving food stamps, percent female-headed households with dependent children, and percent less than high school education.
Distance to care was assessed using the spatial locations of cases and HIV care providers using the street address at time of HIV diagnosis and the address of the medical care sites where initial linkage to care.
DISCUSSION
In previous analyses, we identified hotspots of poor outcomes along the HIV care continuum for persons diagnosed with HIV in Philadelphia in 2008 and 2009. We found that for individuals who resided in a hotspot, 54.3% of individuals diagnosed with HIV were linked to care, 24.0% retained in care, and 9.1% virally suppressed. In comparison, 64.6%, 33.0%, and 26.3% of individuals living outside of a hotspot were linked to care, retained in care, and virally suppressed, respectively. In multivariable regression models controlling for patient factors, residence in a hotspot was the only variable significantly associated with each of these outcomes. In this subsequent analysis of the same cohort, we found that community-level factors were more likely than individual-level characteristics to be associated with geographic clusters of both poor retention and poor viral suppression.
Interestingly, we identified different associations with residence in a hotspot for poor retention in care compared to residence in a hotspot of poor viral suppression - less economic depression versus greater economic depression, and longer distance to nearest pharmacies versus shorter distance to pharmacies respectively. These findings suggest that although retention in care and viral suppression are related, a unique set of factors influence each outcome. It is frequently assumed that persons not retained in HIV care are not on ART and therefore have detectable viral loads. However, recent studies indicate that persons on stable ART regimens complete fewer HIV medical visits than their counterparts, and thus may not meet standard definitions of retention in care27,28. Our analyses did not exclude persons who were virally suppressed from the retention in care model. Thus it is possible that our retention in care hotspot analysis may have captured a subset of individuals with well-controlled HIV infection despite less frequent medical visits. This may explain why persons residing in hotspots for poor retention in care were less likely to live in neighborhoods that were more economically depressed, had higher public transit coverage, and travelled shorter distance to HIV medical care. Qualitative data are needed to better understand the socioeconomic, clinical, and healthcare utilization characteristics of these individuals
Hotspots with poor viral suppression were more likely to be economically deprived and have a shorter average distance to the five nearest pharmacies. While personal income was not available in our dataset, economic deprivation of the census tract of residence was used as a proxy. The association between residence in a poor viral suppression hotspot and economic deprivation was consistent with prior literature, which describes poverty as a common factor associated with lower access to HIV care and treatment adherence.29–32 Residence in an area of higher economic deprivation may be a marker for personal economic deprivation, as well as other factors known to interfere with treatment adherence such as housing instability and food insecurity.33–35 Importantly, women were more likely to reside in hotspots for poor viral suppression than men. A number of prior studies have noted that women are less likely to achieve virally suppression.36–38 Our data suggests that after controlling for several community-level and individual-level characteristics, gender disparities remain. Further research is needed to better understand the influence of structural, behavioral, and biological factors on viral suppression among women.
In our analysis, residence in a hotspot for poor viral suppression was actually associated with a shorter distance to the nearest five pharmacies compared to residence in other areas. Previous studies have evaluated the relationship between distance to HIV medical care and access to healthcare noting that nearly half of PLWH travelled more than three miles farther than the closest HIV care provider2,39. These findings indicate that selection of a medical provider is complex and that factors other than proximity influence this choice. Similar to the process of choosing a healthcare provider, selecting a pharmacy is influenced by multiple factors (e.g. preference of a particular company over another, hours of operation, proximity to other businesses, services offered such as onsite immunizations or pill box refills).40 A recent study evaluating the relationship between socio-economic status and access to prescription medications in New York City found that pharmacies in poor communities had a 24% increase in odds of medications being out of stock for each 10 percentage-point increase in the number of households in poverty. Moreover, while the overall density of pharmacies in poor communities was greater than low-poverty communities, poor communities had (1) a higher density of small, independent pharmacies with limited stock and shorter hours of operation and (2) a lower density of large, chain pharmacies compared to low-poverty communities40. As a result, for PLWH living in economically deprived neighborhoods proximity to a pharmacy may not directly translate to access to ART and viral suppression. Access to medications is critical to the management of HIV infection and achievement of viral suppression. Additional data are needed that will specifically address why patients choose a pharmacy and how they access their pharmacy (i.e. type of transportation versus mail-order). Answers to these questions, analyzed in conjunction with observed travel distances, may better explain patients’ care seeking behavior and help determine future interventions.
Our study is limited in that data are derived from a relatively small number of PLWH linked to care within a single city with a high prevalence of HIV infection and economic deprivation. Thus, our findings may not generalize to other cities. Also, we used HIV surveillance dataset; as such we could not assess ART coverage, may have incompletely accounted for migration out of Philadelphia among the patients in the cohort, and used laboratory data as a proxy for medical visits. In addition, not all persons diagnosed with HIV during 2008 and 2009 would have been eligible for ART based on national treatment guidelines. This may explain the lower-than-expected viral suppression rate. To evaluate community factors (economic deprivation, treatment and pharmacy accessibility) we created indices that have not been widely tested for validity or reliability in this population. While we used reasonable strategies for assigning values to geographic areas, these indices need to demonstrate utility in additional analyses. Similarly, because we used existing data to describe personal characteristics at the time of diagnosis, we were unable to account for many important behavioral characteristics and their change over time. While this is an important limitation, our interest was in determining the value of existing data in understanding barriers to care and informing interventions. Finally, we were unable to account for changes in residence over time. This may reduce the precision of our associations between community-level factors and residence in hotspots for poor retention in care and poor viral suppression.
The analyses reported here represent a new approach to understanding individual and community-level barriers to retention in HIV care and viral suppression. Our findings suggest that geographically targeted interventions may be warranted and that different strategies will be needed to address the various steps along the HIV care continuum. Further research is needed to identify variables that describe salient community characteristics, improve the precision of measurement, and guide the development of content for interventions to improve retention in care and viral suppression.
Acknowledgments
Source of Funding:
Supported in part by a cooperative agreement with the Centers for Disease Control and Prevention (PS13-130202, #U62/003959). Preparation of this manuscript was supported in part by the following grants: P30-AI-45008, P30-AI-087714, K23-MH097647;. The sponsors had no involvement in the research or resulting publication.
BRY has provided consultancy to and received grants to his institution from Gilead Sciences. KAB participates in the Speaker’s Bureau for Gilead Sciences and received grants to her institution from Gilead Science.
Footnotes
Conflicts of Interest
Conflicts: The other authors have no conflicts of interest to disclose.
- Second National CFAR/APC HIV Continuum of Care Working Group Meeting; Washington, DC; Feb. 3–4, 2014
- 9th International Conference on HIV Treatment and Prevention Adherence; June 8–10, 2014, Miami.
Contributor Information
Michael G. Eberhart, AIDS Activities Coordinating Office, Philadelphia Department of Public Health, Michael.Eberhart@phila.gov.
Baligh R. Yehia, Department of Medicine, Division of Infectious Diseases, Perelman School of Medicine, University of Pennsylvania Philadelphia Veterans Affairs Center for Health Equity Research and Promotion, byehia@upenn.edu.
Amy Hillier, University of Pennsylvania School of Design, ahillier@design.upenn.edu.
Chelsea D. Voytek, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, chelseav@mail.med.upenn.edu.
Danielle J. Fiore, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, dtobin@mail.med.upenn.edu.
Michael Blank, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, mblank2@mail.med.upenn.edu.
Ian Frank, Department of Medicine, Division of Infectious Diseases, Perelman School of Medicine, University of Pennsylvania, franki@mail.med.upenn.edu.
David S. Metzger, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Treatment Research Institute, dsm@mail.med.upenn.edu.
Kathleen A. Brady, AIDS Activities Coordinating Office, Philadelphia Department of Public Health, Department of Medicine, Division of Infectious Diseases, Perelman School of Medicine, University of Pennsylvania, Kathleen.A.Brady@phila.gov.
REFERENCES
- 1.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Mar 15;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eberhart MG, Yehia BR, Hillier A, et al. Behind the Cascade: Analyzing Spatial Patterns Along the HIV Care Continuum. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2013 Nov;64:S42–S51. doi: 10.1097/QAI.0b013e3182a90112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westergaard RP, Hess T, Astemborski J, Mehta SH, Kirk GD. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. Aids. 2013 Oct 23;27(16):2559–2566. doi: 10.1097/QAD.0b013e328363bff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koblin BA, Andrasik M, Austin J. Preparing for the unexpected: the pivotal role of social and behavioral sciences in trials of biomedical HIV prevention interventions. Journal of acquired immune deficiency syndromes. 2013 Jul;63(Suppl 2):S183–S186. doi: 10.1097/QAI.0b013e31829a3a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehia BR, Fleishman JA, Metlay JP, Moore RD, Gebo KA. Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. Jama. 2012 Jul 25;308(4):339–342. doi: 10.1001/jama.2012.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yehia BR, Kangovi S, Frank I. Patients in transition: avoiding detours on the road to HIV treatment success. Aids. 2013 Jun 19;27(10):1529–1533. doi: 10.1097/QAD.0b013e328360104e. [DOI] [PubMed] [Google Scholar]
- 7.Yehia BR, Schranz AJ, Momplaisir F, et al. Outcomes of HIV-infected patients receiving care at multiple clinics. AIDS and behavior. 2014 Aug;18(8):1511–1522. doi: 10.1007/s10461-013-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yehia BR, French B, Fleishman JA, et al. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. Journal of acquired immune deficiency syndromes. 2014 Mar 1;65(3):333–339. doi: 10.1097/QAI.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehia BR, Ketner E, Momplaisir F, et al. Location of HIV Diagnosis Impacts Linkage to Medical Care. Journal of acquired immune deficiency syndromes. 2014 Dec 2; doi: 10.1097/QAI.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehia BR, Rebeiro P, Althoff KN, et al. The Impact of Age on Retention in Care and Viral Suppression. Journal of acquired immune deficiency syndromes. 2014 Dec 31; doi: 10.1097/QAI.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall HI, Tang T, Westfall AO, Mugavero MJ. HIV care visits and time to viral suppression, 19 U.S. jurisdictions, and implications for treatment, prevention and the national HIV/AIDS strategy. PloS one. 2013;8(12):e84318. doi: 10.1371/journal.pone.0084318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner EM, Young B. The HIV care cascade through time. The Lancet infectious diseases. 2014 Jan;14(1):5–6. doi: 10.1016/S1473-3099(13)70272-X. [DOI] [PubMed] [Google Scholar]
- 13.Mahle Gray K, Tang T, Shouse L, Li J, Mermin J, Hall HI. Using the HIV surveillance system to monitor the National HIV/AIDS Strategy. American journal of public health. 2013 Jan;103(1):141–147. doi: 10.2105/AJPH.2012.300859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford TN, Sanderson WT, Thornton A. Impact of poor retention in HIV medical care on time to viral load suppression. Journal of the International Association of Providers of AIDS Care. 2014 May-Jun;13(3):242–249. doi: 10.1177/2325957413491431. [DOI] [PubMed] [Google Scholar]
- 15.Eberhart MG, Yehia BR, Hillier A, et al. Behind the Cascade: Analyzing Spatial Patterns Along the HIV Care Continuum. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;64:S42–S51. doi: 10.1097/QAI.0b013e3182a90112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Underwood C, Hendrickson Z, Van Lith LM, Lengwe Kunda JE, Mallalieu EC. Role of community-level factors across the treatment cascade: a critical review. Journal of acquired immune deficiency syndromes. 2014 Aug 15;66(Suppl 3):S311–S318. doi: 10.1097/QAI.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 17.Beer L, Oster AM, Mattson CL, Skarbinski J for the Medical Monitoring P. Disparities in HIV transmission risk among HIV-infected black and white MSM, Medical Monitoring Project, 2009. Aids. 2013 Aug 12; doi: 10.1097/QAD.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rourke SB, Bekele T, Tucker R, et al. Housing characteristics and their influence on health-related quality of life in persons living with HIV in Ontario, Canada: results from the positive spaces, healthy places study. AIDS and behavior. 2012 Nov;16(8):2361–2373. doi: 10.1007/s10461-012-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauman LJ, Braunstein S, Calderon Y, et al. Barriers and facilitators of linkage to HIV primary care in New York City. Journal of acquired immune deficiency syndromes. 2013 Nov 1;64(Suppl 1):S20–S26. doi: 10.1097/QAI.0b013e3182a99c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckman TG, Somlai AM, Peters J, et al. Barriers to care among persons living with HIV/AIDS in urban and rural areas. AIDS care. 1998;10(3):365–375. doi: 10.1080/713612410. [DOI] [PubMed] [Google Scholar]
- 21.Whitman S, Silva A, Shah A, Ansell D. Diversity and disparity: GIS and small-area analysis in six Chicago neighborhoods. Journal of medical systems. 2004;28(4):397–411. doi: 10.1023/b:joms.0000032854.99522.0d. [DOI] [PubMed] [Google Scholar]
- 22.Continelli T, McGinnis S, Holmes T. The effect of local primary care physician supply on the utilization of preventive health services in the United States. Health & place. 2010;16(5):942–951. doi: 10.1016/j.healthplace.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Keesee MS, Natale AP, Curiel HF. HIV positive Hispanic/Latinos who delay HIV care: analysis of multilevel care engagement barriers. Social work in health care. 2012;51(5):457–478. doi: 10.1080/00981389.2012.662208. [DOI] [PubMed] [Google Scholar]
- 24.Forum NQ. Medical Visit Frequency. [Accessed May 6, 2013];2013 http://www.qualityforum.org/Home.aspx. [Google Scholar]
- 25.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. Journal of acquired immune deficiency syndromes. 2013 Mar 1;62(3):356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ArcGIS - ArcToolbox - Add GTFS to Network Dataset [computer program]. Version 10.1. Redlands, CA: Morang, Melinda & Stevens, Patrick; ESRI; [Google Scholar]
- 27.Horberg MHL, Towner W, Gambatese R, Klein D, Antoniskis D, Kadlecik P, Kovach D, Remmers C, Silverberg M. HIV spectrum of engagement cascade in a large integrated care system by gender, age, methodologies. 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. 2013. [Google Scholar]
- 28.Baligh R, Yehia AJS, Agwu Allison L, Berry Stephen A, Metlay Joshua P, Gebo Kelly A. The HIV Treatment Cascade: Is There More to the Story?. 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston. 2014. [Google Scholar]
- 29.Vaughan AS, Rosenberg E, Shouse RL, Sullivan PS. Connecting race and place: a county-level analysis of White, Black, and Hispanic HIV prevalence, poverty, and level of urbanization. American journal of public health. 2014 Jul;104(7):e77–e84. doi: 10.2105/AJPH.2014.301997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shacham E, Lian M, Onen NF, Donovan M, Overton ET. Are neighborhood conditions associated with HIV management? HIV medicine. 2013 Nov;14(10):624–632. doi: 10.1111/hiv.12067. [DOI] [PubMed] [Google Scholar]
- 31.Riley ED, Gandhi M, Hare C, Cohen J, Hwang S. Poverty, unstable housing, and HIV infection among women living in the United States. Current HIV/AIDS reports. 2007 Dec;4(4):181–186. doi: 10.1007/s11904-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 32.McMahon J, Wanke C, Terrin N, Skinner S, Knox T. Poverty, hunger, education, and residential status impact survival in HIV. AIDS and behavior. 2011 Oct;15(7):1503–1511. doi: 10.1007/s10461-010-9759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalichman SC, Grebler T. Stress and poverty predictors of treatment adherence among people with low-literacy living with HIV/AIDS. Psychosomatic medicine. 2010 Oct;72(8):810–816. doi: 10.1097/PSY.0b013e3181f01be3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalichman SC, Hernandez D, Cherry C, Kalichman MO, Washington C, Grebler T. Food insecurity and other poverty indicators among people living with HIV/AIDS: effects on treatment and health outcomes. Journal of community health. 2014 Dec;39(6):1133–1139. doi: 10.1007/s10900-014-9868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldenburg CE, Perez-Brumer AG, Reisner SL. Poverty matters: contextualizing the syndemic condition of psychological factors and newly diagnosed HIV infection in the United States. Aids. 2014 Nov 28;28(18):2763–2769. doi: 10.1097/QAD.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cescon A, Patterson S, Chan K, et al. Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study. PloS one. 2013;8(12):e83649. doi: 10.1371/journal.pone.0083649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. Journal of acquired immune deficiency syndromes. 2014 Apr 15;65(5):587–596. doi: 10.1097/QAI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFall AM, Dowdy DW, Zelaya CE, et al. Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity. Journal of acquired immune deficiency syndromes. 2013 Nov 1;64(3):289–298. doi: 10.1097/QAI.0b013e3182a095e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eberhart MG, Voytek CD, Hillier A, Metzger DS, Blank MB, Brady KA. Travel Distance to HIV Medical Care: A Geographic Analysis of Weighted Survey Data from the Medical Monitoring Project in Philadelphia, PA. AIDS and behavior. 2014 Apr;18(4):776–782. doi: 10.1007/s10461-013-0597-7. [DOI] [PubMed] [Google Scholar]
- 40.Amstislavski P, Matthews A, Sheffield S, Maroko AR, Weedon J. Medication deserts: survey of neighborhood disparities in availability of prescription medications. Int J Health Geogr. 2012 Nov 9;:11. doi: 10.1186/1476-072X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]