Abstract
Desmoplastic melanoma (DM) is a variant of melanoma, which typically affects chronically sun-damaged skin of elderly patients. Pure DM displays a low density of fusiform melanocytes in a collagen-rich matrix. In mixed DM, tumor cell density is higher, and parts of the tumor lack abundant stromal fibrosis. Both pure and mixed DM usually express S100 protein homogeneously. We report herein an unusual bi-phenotypic tumor characterized by the association of a pure DM with an undifferentiated solid spindle cell nodule. It occurred on the scalp of a 66 year-old man. A biopsy of the undifferentiated spindle cell nodule was initially interpreted at a commercial laboratory as atypical fibroxanthoma. The pure DM was seen only in the excisional specimen. All cells of the pure DM stained for S100 protein and SOX10. The adjacent solid sarcomatoid spindle cell nodule lacked expression of S100 protein, SOX10, as well as melan-A, gp100 and microphthalmia transcription factor in more than 95% of its tumor cells. While focal expression of melanocyte differentiation antigens in the solid tumor component made us favor a combined DM with sarcomatoid de-differentiation, we also considered the possibility of a collision scenario, i.e., a pleomorphic dermal sarcoma incidentally colliding with a DM. To further assess a possible relationship of the sarcomatoid nodule with the DM, we performed next-generation sequencing analysis on each component separately. The analysis revealed shared chromosomal copy number changes and a high number of common mutations, thereby supporting the concept of a DM with a de-differentiated sarcomatoid component. An interesting finding is the presence of mutations of the neurofibromin gene in both tumor components.
Keywords: desmoplastic melanoma, sarcoma, biphenotypic tumor, next-generation sequencing
INTRODUCTION
Desmoplastic melanoma (DM) is a rare sclerosing variant of melanoma (1), which typically affects chronically sun-damaged skin of elderly patients (2, 3). The tumor cells of DM are usually fusiform and amelanotic. Immunohistochemically, they often fail to label for antibodies to melan-A/MART-1, microphthalmia-associated transcription factor (MITF) or gp100 (4), but are reactive for S100 protein and SOX10 (5-7). In its clinical course DM differs from other melanomas by a higher propensity for persistent growth/local recurrence (3). DM has been classified into pure and mixed variants (8, 9). Patients with pure pauci-cellular DM are unlikely to have regional lymph node involvement by the tumor and have a more favorable prognosis compared to patients with mixed tumors (10).
Mixed DMs are characterized by areas of high cell density, often manifesting as compact fascicular or sheet-like growth without or only minimal intervening fibrous stroma (8, 9). Biologically one may regard the transition from pure to mixed DM as equivalent to a low grade sarcoma undergoing progression to a higher grade. Most mixed DMs retain full expression of the neural crest antigens S100 protein and SOX10 (11). However, there are exceptions (11, 12). Herein we report a scenario of a mixed DM with an undifferentiated sarcomatoid appearance.
MATERIALS AND METHODS
Immunohistochemical Analysis
Five micron thick sections were taken from formalin-fixed and paraffin-embedded tissue. An automated immunohistochemistry system (Ventana BenchMark XT, Ventana Medical Systems, Inc., Tucson, AZ) was used (standard avidin-biotin procedure). The antibodies tested included S100 protein, SOX10, A103, microphthalmia-associated transcription factor (MITF), HMB-45, CK5/6, 4A4/p63, 34BE12, desmin, and smooth muscle actin. They were used according to the manufacturer’s instructions (Ventana Medical Systems, Inc., Tucson, AZ).
Micro-dissection and DNA extraction
The desmoplastic and solid sarcomatoid tumor components were manually scraped off from sections of archival paraffin-embedded tissue into sterile Eppendorf tubes. For the desmoplastic tumor component a section was used, which did not contain the solid tumor component. For the solid tumor component, only tissue from the nodule (visible without microscope) was scraped off. No adjacent tissue was used. Microscopically uninvolved skin was used as normal background control. DNA was extracted and purified with a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Next-generation sequencing
All exons of 230 cancer-associated genes in the DM, solid tumor and the normal tissue were captured using custom-designed oligonucleotide probes (Agilent SureSelect) and sequenced as paired-end 75-base pair reads to an average read depth of >500-fold on an Illumina HiSeq 2000 sequencer. Reads were aligned to the reference human genome hg19 using the Burrows-Wheeler Alignment tool (13) and post-processed using the Genome Analysis Toolkit (GATK) according to GATK best practices (14). Somatic alterations (single base substitutions, small insertions and deletions, and copy number alterations) were identified according to their presence in the tumor genome and absence from the corresponding normal genome. Single-nucleotide variants were called using muTect (15) and retained if the variant allele frequency in the tumor was >5 times that in the matched normal. Insertions and deletions of bases (indels) were called using the SomaticIndelDetector tool in GATK. All candidate mutations and indels were reviewed manually using the Integrative Genomics Viewer. Increases and decreases in sequence coverage (tumor:normal) were used to infer amplifications and deletions, respectively.
CASE REPORT
Clinical Findings
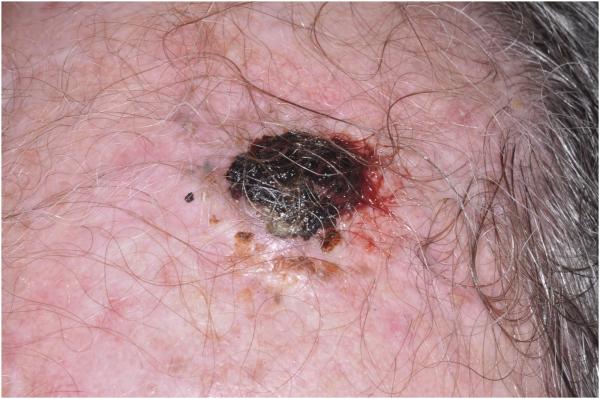
The patient was a 66-year-old male who presented with a crusted hemorrhagic nodule on the scalp (Fig. 1). There was no personal or family history of melanoma, but the patient had several prior non-melanoma skin cancers.
Figure 1.
Tumor with hemorrhagic crust on the scalp of a 66 year-old man.
Histopathological Findings
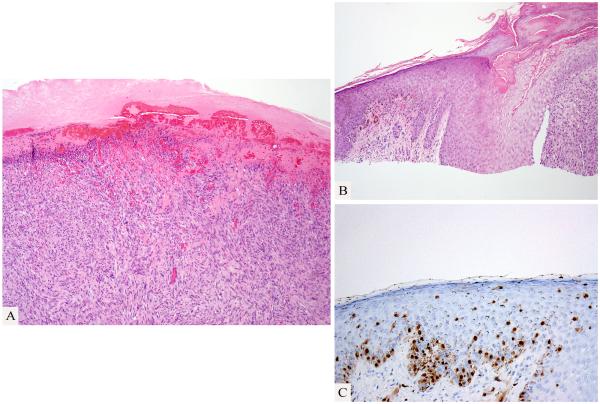
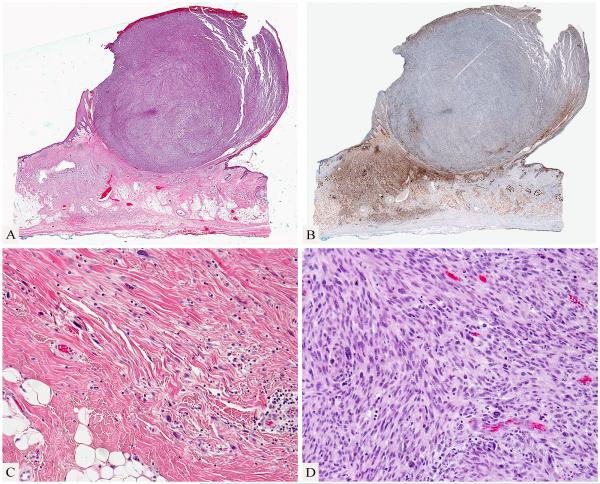
An initial shave biopsy of the tumor showed an ulcerated pleomorphic spindle cell tumor (Fig. 2), which was reported to be immunohistochemically negative for melanocytic and epithelial markers. A diagnosis of “atypical fibroxanthoma” was rendered at a commercial dermatopathology laboratory. When the biopsy findings were reviewed at a cancer center a focal atypical pigmented melanocytic proliferation was detected in the epidermis and dermis (Fig. 2) adjacent to the amelanotic spindle cell proliferation, which led to the suspicion of a possible dedifferentiated melanoma. Review of the surgical excision revealed a biphasic tumor, with a large polypoid and densely cellular pleomorphic spindle cell nodule with many mitotic figures and a pauci-cellular fibrosing spindle cell proliferation in the dermis and subcutis (Fig. 3). A few small lymphocytic aggregates were found in association with the tumor.
Figure 2.
Shave biopsy from the edge of the tumor. Ulcerated spindle cell proliferation (A). Adjacent pigmented melanocytic proliferation (B; Hematoxylin and eosin stain; C: Immmunohistochemistry for microphthalmia-associated transcription factor).
Figure 3.
A spindle cell nodule associated with desmoplastic melanoma. Spindle cell nodule protruding above the skin surface and adjacent fibrosing process in dermis and subcutis (A). Desmoplastic melanoma is highlighted by an immunostain for S100 protein while the cellular spindle cell nodule is negative for S100 protein (B). The desmoplastic melanoma shows a pauci-cellular infiltrate of hyperchromatic spindle cells in a fibrous stroma (C). The large nodule is composed of densely cellular pleomorphic spindle cells (D).
Immunohistochemically, all tumor cells were negative for CK5/6, 34BE12, p63, smooth muscle actin and desmin. The pauci-cellular dermal and subcutaneous fibrosing spindle cell proliferation was strongly and homogeneously immunoreactive for S100 protein (Fig. 3) and SOX10. Although the vast majority of pleomorphic tumor cells in the polypoid nodule were negative for S100 protein and Sox 10, a few cells were positive. Rare few small clusters of tumor cells within the cellular nodule and isolated spindle cells were also noted to be immunoreactive for melan-A and/or gp100.
Molecular Findings
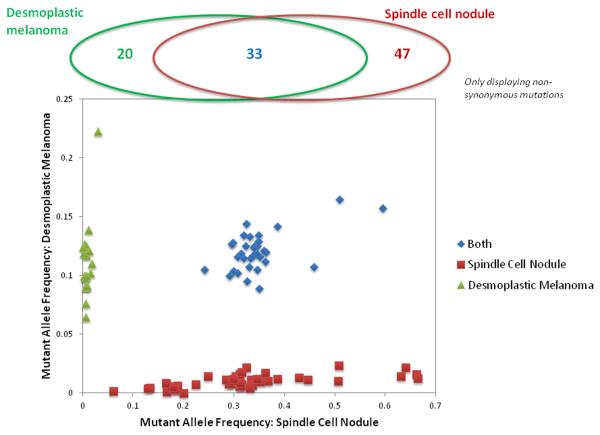
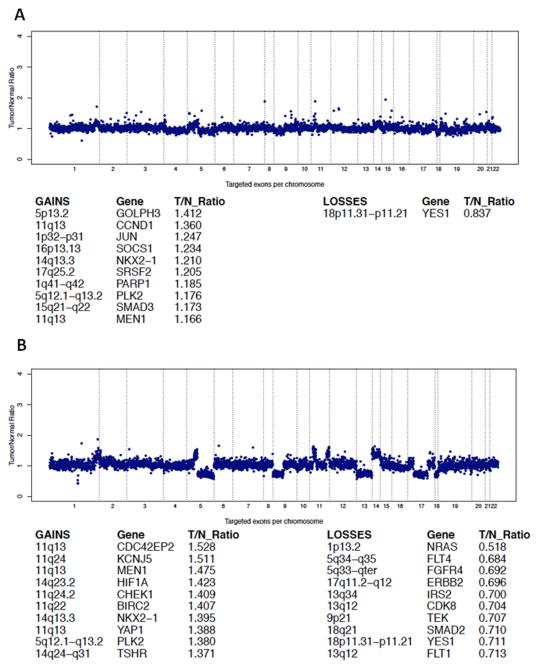
Next-generation sequencing revealed multiple identical mutations of cancer-associated genes shared by the tumor cells of both the undifferentiated sarcomatoid tumor as well as the DM component (Fig. 4, Table 1). There were 33 mutations shared by both the DM and sarcomatoid components. A number of additional non-shared mutations were identified: twenty in the DM and 47 in the sarcomatoid nodule (Table 1). Next-generation sequencing analysis permitted also assessment of gene or chromosome copy number gains or losses. Both tumor components shared copy number gains at 11q, 14q, and 5q and losses in 18p (Fig. 5). At 11q13, the amplified genes included CCND1 in the desmoplastic melanoma (Fig. 5A) and MEN1, CDC42EP2, CCND1, and YAP1 in the spindle cell nodule (Fig. 5B).
Figure 4.
Mutant allele frequencies observed in the desmoplastic melanoma and the spindle cell nodule. Mutations are colored according to their presence in one or both samples.
Table 1.
Next-generation sequencing analysis. Common and distinct mutations in the pure desmoplastic melanoma and de-differentiated sarcomatoid nodule.
| Mutations in both tumor components |
Mutations in spindle cell nodule |
Mutations in desmoplastic melanoma |
|||
|---|---|---|---|---|---|
|
| |||||
| Gene | Protein | Gene | Protein | Gene | Protein |
|
| |||||
| ARID1A | p.G800E | PRKAA2 | p.M93I | ARID1A | p.P1563S |
| NOTCH2 | p.M1242I | ERBB4 | p.R1155Q | PRKAA2 | p.R239C |
| NTRK1 | p.G743E | ERBB4 | p.E1147K | EPHB3 | p.W840 |
| PBRM1 | p.P1174fs | ERBB4 | p.P925L | FGFR3 | p.E140K |
| PBRM1 | p.R1173C | MYD88 | p.Y180C | MAP3K1 | p.S816F |
| EPHA6 | p.S948F | EPHA5 | p.S261F | ROS1 | p.S648F |
| EPHB1 | p.P227S | TET2 | p.P1962L | MAGI2 | p.P42S |
| EPHA5 | p.R238Q | FBXW7 | p.R35C | EPHB4 | p.A636V |
| PNRC1 | p.Y28fs | TERT | p.A1040T | SUFU | p.R123C |
| TNFAIP3 | p.R702T | IL7R | p.D166N | FGFR2 | p.M722I |
| MAGI2 | p.G1156E | NOTCH4 | p.G1266R | FGFR2 | p.V691M |
| MAGI2 | p.E1066K | EPHA7 | p.R360K | CBL | p.P417L |
| PIK3CG | p.S449F | ROS1 | p.R364K | CBL | p.F418I |
| PREX2 | p.R117C | PREX2 | p.R297S | BRCA2 | p.H1731Y |
| PTPRD | p.E406K | PREX2 | p.R1449_splice | BRCA2 | p.L2654F |
| CDKN2A | p.E26 | ATM | p.V1944I | BUB1B | p.P90L |
| HRAS | p.P179L | MLL | p.P1767S | NF1 | p.571_572WE>K |
| PIK3C2G | p.P9S | CBL | p.F194L | NF1 | p.G1166S |
| MLL2 | p.R5179C | CBL | p.E366_splice | SMARCA4 | p.P1344S |
| MLL2 | p.S849L | CBL | p.P707L | KDM6A | p.P921L |
| TBK1 | p.F566fs | GLI1 | p.P1028L | ||
| HSP90AA1 | p.L220F | HNF1A | p.E66D | ||
| PKM2 | p.E304K | DICER1 | p.P1086S | ||
| IDH2 | p.G144R | NTRK3 | p.R630_splice | ||
| TSC2 | p.G1367S | IGF1R | p.S393F | ||
| TP53 | p.R248Q | IGF1R | p.P614S | ||
| TP53 | p.P219L | GRIN2A | p.D742N | ||
| TP53 | p.V147A | GRIN2A | p.H96Y | ||
| NF1 | p.R2179C | CYLD | p.S560F | ||
| RPTOR | p.G801_splice | NF1 | p.L507fs | ||
| NOTCH3 | p.D2103N | NF1 | p.Q531 | ||
| PTPRT | p.E520K | NF1 | p.R1204W | ||
| EP300 | p.R604Q | SMARCA4 | p.A317V | ||
| PIK3CD | p.P311L | NOTCH3 | p.P2153S | ||
| ARID1A | p.P1569L | NOTCH3 | p.P2036L | ||
| CIC | p.P660S | ||||
| ASXL1 | p.P1334L | ||||
| PTPRT | p.R1067C | ||||
| PTPRT | p.G829E | ||||
| PTPRT | p.E630Q | ||||
| ERG | p.A355T | ||||
| ERG | p.P313S | ||||
| WAS | p.P448S | ||||
| AR | p.L145R | ||||
| ATRX | p.V1604M | ||||
Bold: in Catalogue of Somatic Mutations in Cancer (COSMIC) database
Figure 5.
Copy number changes in the desmoplastic melanoma (A) and the spindle cell nodule (B).
DISCUSSION
We report herein a biphasic malignant tumor, of which one component was a DM while the other cell population had an un-differentiated sarcomatoid appearance. The light microscopic findings (DM and melanoma in situ immediately adjacent to sarcomatoid tumor; focal labeling of rare tumor cells in the sarcomatoid nodule for melanocyte makers) strongly suggested that the tumors were related. Next-generation sequencing revealed that both tumor components shared a common mutational heritage and thereby supported our interpretation of a mixed DM with sarcomatoid de-differentiation (as opposed to a collision of a DM with a pleomorphic sarcoma). Since a few rare cells were found in the sarcomatoid nodule, which were immunoreactive for melanocyte markers, one may question the purity of the sarcomatoid tumor sample. However, given the low number of the immuno-positive cells (less than 2% of the sarcomatoid tumor cell population) and the variant allele frequencies (Fig. 4), it is unlikely that the few immunoreactive cells solely explained the shared mutations in the two components.
Sarcomatous de-differentiation of a melanoma is rare. Kacerovska et al. (16) reported a DM associated with a myofibroblastic sarcoma. The patient presented with a right groin mass two years after diagnosis of a DM on the right heel with lymph node metastasis. Similar to our case, the right groin mass showed a biphasic cellular composition: one immunoreactive for S100 protein and negative for smooth muscle actin; the other with a reverse immunophenotype. While one is tempted to interpret the case as a DM with sarcomatoid de-differentiation the possibility of an unusual collision scenario could not be ruled out.
New technology can provide additional evidence useful for such a diagnostic issue. Tumor genomic profiling via next-generation sequencing has become an essential part of both cancer research and diagnostic medicine. Some of the applications include establishing the origin of synchronous metastases in patients with multiple primary tumors (17), characterization of unknown primary tumors (18), and identification of targets for treatment (19). The utility of next-generation sequencing for the distinction of a biphasic tumor from a collision incident has recently been demonstrated in a case of shared mutations, including mutations in the patched gene (PTCH1), in both the epithelial and mesenchymal tumor cell populations of a basal cell carcinosarcoma (20). The current case provides another example of the utility of this methodology for selected diagnostic problems. Similarly, it could be used to distinguish a collision event from a biphasic tumor in other settings, such as that of an atypical fibroxanthoma –like proliferation occurring adjacent to a squamous cell carcinoma (21, 22). Sequence analysis also revealed interesting information of the melanoma-associated mutations. It also permitted assessment of gene or chromosome copy number gains or losses, which attests to the potential of this technology to substitute for traditional cytogenetic methods.
In conventional melanoma, one of the most important pathways in tumor progression is the RAS-RAF-MAPK pathway (23). However, little is known about the mutation profile of DM, but we know that BRAF mutations are very rare in this variant of melanoma (24). In the mixed DM reported herein, both S100 protein-positive and negative (sarcomatoid) tumor cell populations shared mutations in HRAS and other melanoma-associated genes, such as cyclin-dependent kinase inhibitor 2A (CDKN2A). While BRAF and NRAS mutation are common in melanomas (25), HRAS mutations are rarely detected (26) and are more commonly associated with Spitz tumors (27) or deep penetrating nevi(28). It is of particular interest that several mutations in the neurofibromin (NF1) gene were detected in both components, since prior cytogenetic studies revealed allelic losses of NF1 in two thirds of DM (29). Since DM has so far not been associated with a distinct mutation, this observation merits further investigation as a potential important molecular feature of DM. Other genes and pathways previously reported in DM include a polymorphism of RET, encoding a receptor tyrosine kinase whose ligand is glial cell line-derived neurotrophic factor (GDNF) (11). In addition, gene expression profiling has shown multiple altered pathways in DMs, including decrease in the expression of genes involved in melanin pigment synthesis and increased expression of neurotrophic factors (30). In a recent study, pure and mixed DMs were compared and increased CD117 staining was demonstrated in the mixed variant (11). The authors suggested that changes in KIT protein expression may be involved in tumor progression. In the present case, a number of other mutations previously not reported in DM were detected (Table 1). The role of these mutations in tumor progression of DM needs further investigation in future studies.
In addition to mutations, the DM and the spindle cell nodule shared a few loci with amplifications and deletions (Fig. 5). Among these was gain of 11q13, where both tumor components showed amplification of CCND1. Furthermore, the spindle cell nodule harbored gains of additional genes in the same locus (MEN1, CDC42EP2, and YAP1). This finding is of interest and potentially relevant for tumor progression, as prior studies have also linked 11q13/CCND1 gain with higher risk of melanoma metastasis and tumor progression (31). It is of interest that a copy number increase of 11p was noted in both tumor components. Isolated copy gains have previously been associated with sclerosing Spitz nevi with HRAS mutations (27, 32). However, the context is different in our case. The increase in 11p is accompanied by other chromosomal aberrations.
Finally, tumor genome profiling may reveal therapeutic targets (19). While metastatic disease was not detected in our patient and therefore systemic therapy was not required, the tumor components harbored mutations in potentially targetable pathways including the phosphatidylinositide 3-kinase (PI3 kinase) pathway (PIK3CD, PIK3CG, and PIK3C2G genes). Several PI3 kinase inhibitors are in clinical trials including those targeting the protein product of PIK3CD mutated in the present case (33).
In summary, we report a DM with sarcomatous de-differentiation. The case illustrates a diagnostic pitfall (sampling of the of undifferentiated melanoma component led to an erroneous diagnosis of atypical fibroxanthoma) and documents the value of next-generation sequencing in establishing a shared mutational heritage of biphasic tumors as well as shared copy number changes. Our findings also expand the spectrum of mixed DM by documenting that it can form a de-differentiated sarcomatoid tumor component.
Footnotes
Disclosures: The authors did not receive any funding for this work and have no conflicts of interest to declare.
REFERENCES
- 1.Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma) Cancer. 1971;28:914–936. doi: 10.1002/1097-0142(1971)28:4<914::aid-cncr2820280415>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825–833. doi: 10.1016/j.jaad.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lens MB, Newton-Bishop JA, Boon AP. Desmoplastic malignant melanoma: a systematic review. Br J Dermatol. 2005;152:673–678. doi: 10.1111/j.1365-2133.2005.06462.x. [DOI] [PubMed] [Google Scholar]
- 4.Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321–330. doi: 10.1016/j.cll.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Palla B, Su A, Binder S, et al. SOX10 expression distinguishes desmoplastic melanoma from its histologic mimics. Am J Dermatopathol. 2013;35:576–581. doi: 10.1097/DAD.0b013e31827a0b98. [DOI] [PubMed] [Google Scholar]
- 6.Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008;35:1014–1019. doi: 10.1111/j.1600-0560.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Herberth FI, Karamchandani J, Kim J, et al. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2010;37:944–952. doi: 10.1111/j.1600-0560.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 8.Busam KJ, Mujumdar U, Hummer AJ, et al. Cutaneous desmoplastic melanoma: reappraisal of morphologic heterogeneity and prognostic factors. Am J Surg Pathol. 2004;28:1518–1525. doi: 10.1097/01.pas.0000141391.91677.a4. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins WG, Busam KJ, Ben-Porat L, et al. Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol. 2005;12:207–213. doi: 10.1245/ASO.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 10.George E, McClain SE, Slingluff CL, et al. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol. 2009;36:425–432. doi: 10.1111/j.1600-0560.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller DD, Emley A, Yang S, et al. Mixed versus pure variants of desmoplastic melanoma: a genetic and immunohistochemical appraisal. Mod Pathol. 2012;25:505–515. doi: 10.1038/modpathol.2011.196. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed A, Gonzalez RS, Lawson D, et al. SOX10 Expression in Malignant Melanoma, Carcinoma, and Normal Tissues. Appl Immunohistochem Mol Morphol. 2013;21:506–510. doi: 10.1097/PAI.0b013e318279bc0a. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kacerovska D, Michal M, Kutzner H, et al. Metastatic desmoplastic malignant melanoma associated with low-grade myofibroblastic sarcoma. Am J Dermatopathol. 2009;31:490–494. doi: 10.1097/DAD.0b013e31819afdaa. [DOI] [PubMed] [Google Scholar]
- 17.De Mattos-Arruda L, Bidard FC, Won HH, et al. Establishing the origin of metastatic deposits in the setting of multiple primary malignancies: The role of massively parallel sequencing. Mol Oncol. 2013 doi: 10.1016/j.molonc.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tothill RW, Li J, Mileshkin L, et al. Massively-parallel sequencing assists the diagnosis and guided treatment of cancers of unknown primary. J Pathol. 2013 doi: 10.1002/path.4251. [DOI] [PubMed] [Google Scholar]
- 19.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiuru M, McDermott G, Coit D, Berger M, Busam K. Basal Cell Carcinosarcoma With PTCH1 Mutations in Both Epithelial and Sarcomatoid Primary Tumor Components and in the Sarcomatoid Metastasis. American Journal of Surgical Pathology. 2013;38:138–142. doi: 10.1097/PAS.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 21.Arsenovic N, Sen S, Naik V, et al. Trichilemmal cyst with carcinoma in situ within an atypical fibroxanthoma. Am J Dermatopathol. 2009;31:587–590. doi: 10.1097/DAD.0b013e3181a0d235. [DOI] [PubMed] [Google Scholar]
- 22.Hyatt AM, Mutasim DF, Spicknall KE. Collision of atypical fibroxanthoma and acantholytic squamous cell carcinoma in situ. Am J Dermatopathol. 2012;34:563–564. doi: 10.1097/DAD.0b013e31822e63c8. [DOI] [PubMed] [Google Scholar]
- 23.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 24.Davison JM, Rosenbaum E, Barrett TL, et al. Absence of V599E BRAF mutations in desmoplastic melanomas. Cancer. 2005;103:788–792. doi: 10.1002/cncr.20861. [DOI] [PubMed] [Google Scholar]
- 25.Reifenberger J, Knobbe CB, Sterzinger AA, et al. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int J Cancer. 2004;109:377–384. doi: 10.1002/ijc.11722. [DOI] [PubMed] [Google Scholar]
- 26.Kunz M, Dannemann M, Kelso J. High-throughput sequencing of the melanoma genome. Exp Dermatol. 2013;22:10–17. doi: 10.1111/exd.12054. [DOI] [PubMed] [Google Scholar]
- 27.Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000;157:967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bender RP, McGinniss MJ, Esmay P, et al. Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi. Mod Pathol. 2013;26:1320–1328. doi: 10.1038/modpathol.2013.77. [DOI] [PubMed] [Google Scholar]
- 29.Gutzmer R, Herbst RA, Mommert S, et al. Allelic loss at the neurofibromatosis type 1 (NF1) gene locus is frequent in desmoplastic neurotropic melanoma. Hum Genet. 2000;107:357–361. doi: 10.1007/s004390000374. [DOI] [PubMed] [Google Scholar]
- 30.Busam KJ. Cutaneous desmoplastic melanoma. Adv Anat Pathol. 2005;12:92–102. doi: 10.1097/01.pap.0000155071.86944.a6. [DOI] [PubMed] [Google Scholar]
- 31.Gerami P, Jewell SS, Pouryazdanparast P, et al. Copy number gains in 11q13 and 8q24 [corrected] are highly linked to prognosis in cutaneous malignant melanoma. J Mol Diagn. 2011;13:352–358. doi: 10.1016/j.jmoldx.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastian BC, Wesselmann U, Pinkel D, et al. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J Invest Dermatol. 1999;113:1065–1069. doi: 10.1046/j.1523-1747.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- 33.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N Engl J Med. 2014 doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]