Abstract
Cognitive decline is a common health problem among breast cancer patients and understanding trajectories of cognitive change following among breast cancer survivors is an important public health goal. We conducted a longitudinal study to investigate the cognitive function changes from 18 month to 3 years after breast cancer diagnosis among participants of the Shanghai Breast cancer survivor study, a population-based cohort study of breast cancer survivors. In our study, we completed cognitive function evaluation for 1,300 breast cancer survivors at the 18th month’s survey and 1,059 at 36th month’s survey, respectively, using a battery of cognitive function measurements. We found the scores in attention and executive function, immediate memory and delayed memory significantly improved from 18 to 36 months after breast cancer diagnosis. The improvements appeared in breast cancer survivors receiving treatments (i.e., surgery, radiotherapy, tamoxifen, or chemotherapy combined with or without tamoxifen), but not in those who received neither chemotherapy nor tamoxifen treatment. The results indicate that cognitive functions, particularly immediate verbal episodic memory, and delayed memory significantly improved among breast cancer survivors from 18 to 36 months after cancer diagnosis. In general, comorbidity was inversely associated with the improvements.
Keywords: Breast cancer, Cognitive function, Prognosis, Survival
Introduction
Advances in therapies have led to dramatic improvements in the survival rates of breast cancer survivors [1]. As a result, the 5- and 10-year relative survival rates for breast cancer are 86 and 78 %, respectively, among US women [2]. In our recent study of Chinese women with breast cancer living in Shanghai, 5-year survival rates were 88.5 % [3]. A variety of health problems associated with cancer diagnosis and its treatments, such as cognitive dysfunction, have attracted growing in attention in the research and clinical management of breast cancer survivors [4].
Cognitive dysfunction is common among breast cancer survivors [5-8] and is a serious concern for individuals both during active treatment and, thereafter, as it has the potential to substantially disrupt decision-making abilities and career, family, and social functioning more generally [6, 9]. This cognitive dysfunction, widely known as “chemobrain” represents a significant public health problem with far reaching implications [10-13]. One well-designed study found that 61 % of patients may have “chemobrain” after chemotherapy, with 50 % of patients experiencing persistent symptoms for 1 year or longer [5]. Although the exact mechanisms are not clear, possible contributors to “chemobrain” may include indirect toxicity and oxidative damage, direct injury to neurons, sex hormone changes, and inflammation associated with cancer therapies, such as radiation, chemotherapy, and hormonal therapy [14-18]. Of note, some recent studies have found signs of cognitive function improvement shortly after completing of chemotherapy [7, 19] suggesting “chemo brain” may be recoverable. However, no study has conducted to examine the trajectory of cognitive recovery long after completion of cancer treatment.
With the number of breast cancer survivors increasing, even as the duration of survival increases, understanding the cognitive function changes with time is critical for developing preventive and interventional strategies for cognitive dysfunction in breast cancer survivors. We conducted a longitudinal study to investigate the cognitive function changes from 18 months to 3 years after breast cancer diagnosis among participants of the Shanghai Breast Cancer Survivor Study (SBCSS).
Methods
Participants
The study was approved by the IRB of all the institutes involving in the study. The subjects included in this report were participants in the SBCSS, which is a population-based breast cancer survivor cohort of women who were permanent residents of Shanghai, China, and diagnosed with primary breast cancer between March 2002 and April 2006. A total of 5,042 women with newly diagnosed breast cancer and between ages 20 and 75 were recruited approximately 6 months after cancer diagnosis. Women with In situ breast cancer (accounted for only 3 % of overall breast cancer in Shanghai) were excluded from this study.
When we started to add the cognitive component in our breast cancer survival study, about two-thirds of participants completed their 18th month’s follow-up survey. As a result, a total of 1,605 SBCSS participants, who were diagnosed of breast cancer between December 2004 and April 2006 and were alive at the 18th month’s follow-up, were approached for the cognitive assessment during their 18th month’s follow-up survey. We excluded 48 survivors from the study because they had a prior history of stroke. The remaining 1,557 breast cancer patients participated in this study.
We compared characteristics between 1,557 eligible participants with the participants in whole cohort (5,042 participants) and found that social demographics, age at cancer diagnosis, and clinical features are similar between these two study populations.
Among the 1,557 eligible participants, 1,300 (83.5 %) completed the cognitive function evaluation at the 18th month’s follow-up survey. These cognitive function study participants were invited to participate in the 2nd evaluation at the 36th month’s post-diagnosis survey. A total of 1,059 survivors completed the 2nd cognitive function evaluation with a response rate of 81.5 %. The reasons of non-response were refusal (216 cases, 13.9 %), moving (13 cases, 0.8 %), and other reasons (28 cases, 1.8 %) for the first evaluation, and refusal (164 cases, 12.6 %), moving (8 cases, 0.6 %), death (41 cases, 3.2 %), and other reason (28 cases, 2.2 %) for the 2nd evaluation (Fig. 1).
Fig. 1.
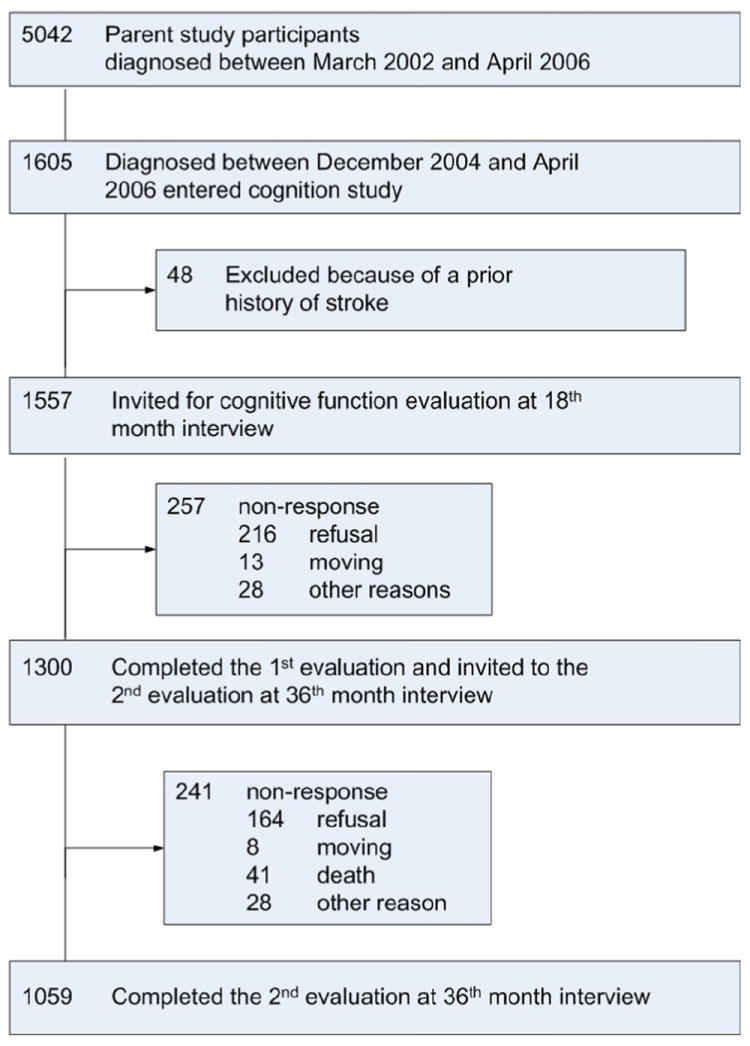
Consort diagram of Shanghai women breast cancer cohort study cognition substudy
Data collection
At enrollment, approximately 6 months after cancer diagnosis, a face-to-face interview was administered for each eligible breast cancer case using a structured questionnaire to gather information on demographics, cancer diagnosis, menopausal statue and syndrome, comorbidity, surgery, chemotherapy, radiotherapy, tamoxifen, and other hormonal treatment, as well as Chinese traditional medicine. Among patients who ever used tamoxifen, long-term tamoxifen users were those who were still using tamoxifen at their 36th month’s visit, and short-term tamoxifen users were those who stopped using tamoxifen at the 36th month’s visit. More details of clinical and lifestyle factors collection and verification were described in the papers published previously [20, 21]. Medical charts were reviewed to obtain information on tumor characteristics, include TNM stage, ER and PR status and verify cancer treatment information.
In-person interviews were administered again at 18th and 36th months after cancer diagnosis, respectively, to collect information on disease recurrence and survival status, treatment, and to capture changes in health status, including comorbidity, menopausal status, and syndrome. We asked each participant about the presence of menopausal symptoms including hot flashes, night sweats, depressed mood, vaginal dryness, and dry skin or skin dryness/itching since diagnosis and during adjuvant treatment for breast cancer at baseline interview.
Cognitive function assessment
Cognitive function was assessed using a battery comprising three widely used tools, all with robust psychometric properties: (1) a measure of immediate and delayed verbal episodic memory, the Logical Memory Subtest from the Chinese Version of the Wechsler Memory Scale [22]; (2) a measure of language/executive function (Chinese Version of the Category Fluency Test) [23]; and (3) a measure of attention/executive function (Chinese Version of the Stroop Test) [24].
Previously, we have conducted a study in Shanghai to evaluate the diagnostic validity of a short battery of cognitive tests for mild cognitive impairment and Alzheimer’s disease. We selected 50 Alzheimer’s disease patients (NINCDS/ADRDA criteria) and 50 mild cognitive impairment (Petersen criteria) patients who came to Huashan Hospital, Fudan University, Shanghai, China for a neurologic work-up for dementia. We also selected 50 healthy community-dwelling volunteers matched for sex and age. The initial screen included the Chinese version of the Mini-Mental State Examination. A clinical evaluation and informant-based instruments were subsequently administered. The Clinical Dementia Rating Scale was used to assess dementia severity.
We found that the logic memory subtest, category fluency test, and Stroop test were able to significantly discriminate Alzheimer’s disease from mild cognitive impairment, Alzheimer’s disease versus controls and mild cognitive impairment versus controls (P < 0.05). Age, education, and scores from the logic memory subtest, category fluency, and Stroop tests were used in multiple logistic regression models and a composite score of these variables generated. The largest area under the receiver-operator characteristic (ROC) curve was 1.00 [95 % confidence interval (95 % CI) 0.95–1.00] for Alzheimer’s disease versus normal and 0.88 (95 % CI 0.79–0.94) for mild cognitive impairment versus controls. This validated battery was used in the current study.
The study interviewers, supervisors, and project director were formally trained to conduct cognitive function tests by a neurologist at the Shanghai Huashan Hospital, Fudan University [25].
Statistical methods
Demographic variables and selected characteristics were compared between subjects eligible for cognition component study and subjects who completed the examinations by the Student t test for continuous variables and Chi square test for dichotomous variables. Relations between age at diagnosis and scores of cognition components were measured using linear regression model. Scores of cognition components were compared by demographic variables and selected characteristics using ANOVA. Paired t tests were used to compare the cognition functions measured at the 18th and 36th month’s visits. Statistical data analyses were performed with SAS 9.2 software (SAS Institute, Cary, NC). All of the reported P values were two-tailed, and statistical significance was set at P ≤ 0.05.
Results
In Table 1, we compared the demographic variables and selected characteristics between 1,557 eligible participants, 1,300 breast cancer survivors who completed cognitive function examination at the 18th month’s visit and 1,059 breast cancer survivors who completed cognitive function examination at 36th month’s visit. We found that there were no significant differences between three groups on age, income, education achievements, menopausal status, menopausal syndromes at the time of being tested on cognitive function, TNM stages, ER and PR status, cancer treatments, and comorbidity of the breast cancer survivors.
Table 1.
Comparison of baseline characteristics between participants eligible for the cognitive study and those who completed the study
| Characteristic | 18th month’s visit
|
36th month’s visit
|
|||
|---|---|---|---|---|---|
| Participants eligible for cognition test (n = 1,557) | Participants finished cognition test (n = 1,300) | Pa | Participants finished cognition test (n = 1,059) | Pb | |
| Age at diagnosis (years) | |||||
| Median | 51.2 | 51.3 | 51.5 | ||
| Q1–Q3 | 47.0–59.2 | 47.0–59.4 | 0.839 | 47.1–59.6 | 0.631 |
| Income (¥/mon/capita), % | |||||
| <1000 | 47.0 | 46.6 | 47.1 | ||
| 1000–2000 | 37.9 | 38.9 | 38.4 | ||
| >2000 | 15.1 | 14.5 | 0.832 | 14.5 | 0.970 |
| Education, % | |||||
| Elementary school or below | 44.0 | 43.9 | 44.3 | ||
| Middle or high school | 38.1 | 39.2 | 39.2 | ||
| College or above | 17.9 | 16.9 | 0.752 | 16.5 | 0.960 |
| Menopause status, % | |||||
| Pre-menopausal | 47.5 | 47.5 | 46.6 | ||
| Post-menopausal | 52.5 | 52.5 | 0.995 | 53.4 | 0.666 |
| Menopausal syndromes, % | |||||
| Yes | 83.0 | 82.6 | 82.9 | ||
| No | 17.0 | 17.4 | 0.797 | 17.1 | 0.851 |
| Charlsoncomorbidity index, % | |||||
| 0 | 80.4 | 80.2 | 80.3 | ||
| ≥1 | 19.6 | 19.8 | 0.904 | 19.7 | 0.984 |
| TNM grade for cancer, % | |||||
| I | 37.4 | 37.9 | 38.5 | ||
| IIA | 34.5 | 34.3 | 34.7 | ||
| IIB | 13.8 | 13.9 | 13.9 | ||
| III and above | 9.5 | 9.0 | 8.3 | ||
| Unknown | 4.9 | 4.9 | 0.994 | 4.5 | 0.970 |
| ER, % | |||||
| Negative | 34.4 | 33.9 | 33.2 | ||
| Positive | 64.9 | 65.5 | 66.3 | ||
| Not known | 0.7 | 0.6 | 0.914 | 0.5 | 0.836 |
| PR, % | |||||
| Negative | 43.8 | 43.1 | 43.3 | ||
| Positive | 55.5 | 56.3 | 56.2 | ||
| Not known | 0.7 | 0.6 | 0.879 | 0.5 | 0.892 |
| Surgery, % | |||||
| Yes | 99.6 | 99.5 | 99.5 | ||
| No | 0.4 | 0.5 | 0.962 | 0.5 | 0.970 |
| Radiology, % | |||||
| Yes | 33.0 | 33.1 | 32.8 | ||
| No | 67.0 | 66.9 | 0.971 | 67.2 | 0.873 |
| Chemotherapy, % | |||||
| Yes | 92.7 | 92.7 | 92.9 | ||
| No | 7.3 | 7.3 | 0.959 | 71 | 0.833 |
| Tamoxifen, % | |||||
| Yes | 46.7 | 46.3 | 47.4 | ||
| No | 53.3 | 53.7 | 0.837 | 52.6 | 0.611 |
Characteristics compared between participants eligible for the cognition study and those who completed the the18th month’s cognitive assessment
Characteristics compared between participants who completed the 18th month’s cognitive assessment and those who completed the 36th month’s cognitive assessment
We compared the cognitive functions conducted at the 18th month’s visit by demographic variables and characteristics (Table 2). We found the scores of logical memory subtest test (both immediate and delayed memory), category fluency test and Stroop test were all consistently inversely correlated with age, whereas higher cognitive function scores were associated with higher income and educational achievements. After adjusting for age at diagnosis, income, and education, post-menopausal breast cancer survivors had higher scores in all of the tests than pre-menopausal women. We also found the women with earlier stage at diagnosis and use of chemotherapy had higher scores in the fluency and Stroop tests. We did not find significant differences in these cognitive measures between tamoxifen users and non-users at the 18th month.
Table 2.
Cognitive scores at the 18th month’s visit by baseline life style factors and demographics, 2003–2009
| Characteristic | Immediate memory
|
Delayed memory
|
Fluency
|
Stroop
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score
|
Score
|
Score
|
Score
|
|||||||||
| Meana | SEb | Pc | Meana | SEb | Pc | Meana | SEb | Pc | Meana | SEb | Pc | |
| Age (years), β | −0.083d | 0.013 | <0.00e | −0.092d | 0.013 | <0.001e | – | |||||
| 0.224d | 0.023 | <0.001e | – | |||||||||
| 0.964d | 0.053 | <0.001e | ||||||||||
| Monthly income (¥/person) | ||||||||||||
| <1000 | 9.44 | 0.18 | 8.71 | 0.18 | 40.62 | 0.33 | 73.46 | 0.72 | ||||
| 1000–2000 | 10.43 | 0.19 | 9.55 | 0.19 | 42.30 | 0.35 | 78.09 | 0.76 | ||||
| >2000 | 11.08 | 0.33 | <0.001 | 10.22 | 0.33 | <0.001 | 42.88 | 0.61 | <0.001 | 78.54 | 1.34 | <0.001 |
| Education | ||||||||||||
| Elementary school or below | 8.65 | 0.19 | 7.87 | 0.19 | 39.23 | 0.34 | 69.31 | 0.75 | ||||
| Middle or high school | 10.60 | 0.19 | 9.80 | 0.19 | 42.86 | 0.35 | 79.78 | 0.77 | ||||
| College or above | 12.49 | 0.31 | <0.001 | 11.55 | 0.31 | <0.001 | 44.81 | 0.57 | <0.001 | 84.56 | 1.25 | <0.001 |
| Menopausal status | ||||||||||||
| Pre- | 9.44 | 0.21 | 8.58 | 0.21 | 40.89 | 0.39 | 72.75 | 0.86 | ||||
| Post- | 10.63 | 0.20 | <0.001 | 9.87 | 0.20 | <0.001 | 42.25 | 0.37 | 0.030 | 78.94 | 0.80 | <0.001 |
| Menopausal syndromes (at 18 m) | ||||||||||||
| No | 9.86 | 0.18 | 9.04 | 0.18 | 41.01 | 0.33 | 73.76 | 0.72 | ||||
| Yes | 10.02 | 0.21 | 0.564 | 9.05 | 0.21 | 0.978 | 41.15 | 0.40 | 0.789 | 74.10 | 0.87 | 0.767 |
| Charlson comorbidity index | ||||||||||||
| 0 | 10.03 | 0.13 | 9.24 | 0.13 | 41.68 | 0.24 | 76.41 | 0.50 | ||||
| ≥1 | 10.21 | 0.27 | 0.580 | 9.33 | 0.27 | 0.777 | 41.27 | 0.50 | 0.466 | 74.31 | 1.11 | 0.092 |
| TNM stage | ||||||||||||
| I | 10.24 | 0.19 | 9.56 | 0.19 | 41.99 | 0.35 | 77.77 | 0.77 | ||||
| IIA | 9.97 | 0.20 | 9.02 | 0.20 | 41.14 | 0.37 | 75.17 | 0.81 | ||||
| IIB | 10.12 | 0.31 | 9.34 | 0.31 | 42.56 | 0.57 | 75.86 | 1.27 | ||||
| III and IV | 9.70 | 0.40 | 9.83 | 0.39 | 39.97 | 0.72 | 73.92 | 1.58 | ||||
| Unknown | 9.90 | 0.54 | 0.728 | 9.10 | 0.53 | 0.264 | 42.10 | 0.97 | 0.026 | 72.25 | 2.15 | 0.025 |
| ER status | ||||||||||||
| Negative | 9.85 | 0.20 | 8.99 | 0.20 | 41.06 | 0.37 | 76.18 | 0.81 | ||||
| Positive | 10.18 | 0.15 | 9.41 | 0.15 | 41.88 | 0.27 | 75.94 | 0.59 | ||||
| Not known | 9.82 | 1.49 | 0.416 | 8.20 | 1.48 | 0.181 | 41.54 | 2.73 | 0.199 | 72.35 | 6.04 | 0.811 |
| PR status | ||||||||||||
| Negative | 10.11 | 0.18 | 9.29 | 0.18 | 41.38 | 0.33 | 76.09 | 0.72 | ||||
| Positive | 10.03 | 0.16 | 9.24 | 0.16 | 41.77 | 0.29 | 75.97 | 0.63 | ||||
| Not known | 9.82 | 1.49 | 0.932 | 8.20 | 1.48 | 0.760 | 41.54 | 2.74 | 0.666 | 72.36 | 6.04 | 0.826 |
| Surgery | ||||||||||||
| No | 12.75 | 1.72 | 11.73 | 1.71 | 38.64 | 3.16 | 73.42 | 6.98 | ||||
| Yes | 10.06 | 0.12 | 0.119 | 9.25 | 0.12 | 0.149 | 41.61 | 0.22 | 0.348 | 76.01 | 0.47 | 0.711 |
| Radiology | ||||||||||||
| No | 10.11 | 0.15 | 9.32 | 0.14 | 41.73 | 0.27 | 76.15 | 0.59 | ||||
| Yes | 9.99 | 0.21 | 0.651 | 9.13 | 0.21 | 0.439 | 41.34 | 0.38 | 0.406 | 75.68 | 0.84 | 0.649 |
| Chemotherapy | ||||||||||||
| No | 9.80 | 0.46 | 9.04 | 0.46 | 39.74 | 0.83 | 72.09 | 1.84 | ||||
| Yes | 10.09 | 0.12 | 0.547 | 9.28 | 0.12 | 0.626 | 41.75 | 0.22 | 0.022 | 76.31 | 0.49 | 0.028 |
| Tamoxifen | ||||||||||||
| No | 10.06 | 0.16 | 9.19 | 0.16 | 41.26 | 0.29 | 75.60 | 0.65 | ||||
| Yes | 10.10 | 0.17 | 0.849 | 9.36 | 0.17 | 0.467 | 42.03 | 0.32 | 0.072 | 76.61 | 0.70 | 0.289 |
| Use tamoxifen after 6th month | ||||||||||||
| No | 9.87 | 0.60 | 8.59 | 0.60 | 40.77 | 1.09 | 76.17 | 2.43 | ||||
| Yes | 10.06 | 0.18 | 0.774 | 9.36 | 0.18 | 0.220 | 42.01 | 0.33 | 0.282 | 76.08 | 0.72 | 0.972 |
Mean scores of cognitive assessment
Standard errors
P values adjusted for age at diagnosis, education and income at baseline
Regression coefficients between age and cognitive score
P values adjusted for education and income at baseline
We examined the cognitive function changes between the 18th and 36th month’s visits for those who finished both cognitive assessments (Table 3). Compared to the assessment conducted at the18th month’s visit, 56.58 % of women had increased scores on the immediate memory test, 49.77 % on the verbal fluency test, 56.12 % on the Stroop test, and 58.08 % on the delayed memory test at the 36th month’s visit. On average, the scores of immediate memory test improved by 1.32 points (95 % CI 1.10–1.54), average scores of Stroop test improved by 1.35 points (95 % CI 0.68–2.02), and average scores of the delayed memory test increased 1.58 points (95 % CI 1.37–1.80).
Table 3.
Cognitive function change from the 18 to the 36th month’s assessment stratified by different treatment
| Number of participants | Mean difference | SEa | P-value | P-valueb | ||||
|---|---|---|---|---|---|---|---|---|
| All participants | ||||||||
| Immediate memory | 1030 | 1.32 | 0.11 | <0.001 | <0.001 | |||
| Delayed memory | 1006 | 1.58 | 0.11 | <0.001 | <0.001 | |||
| Fluency | 1059 | 0.37 | 0.20 | 0.089 | 0.259 | |||
| Stroop test | 1059 | 1.35 | 0.34 | <0.001 | 0.055 | |||
| Patients received surgery | ||||||||
| Immediate memory | 1025 | 1.32 | 0.11 | <0.001 | <0.001 | |||
| Delayed memory | 1001 | 1.59 | 0.11 | <0.001 | <0.001 | |||
| Fluency | 1054 | 0.37 | 0.20 | 0.098 | 0.270 | |||
| Stroop test | 1054 | 1.38 | 0.34 | <0.001 | 0.052 | |||
| Patients received chemotherapy | ||||||||
| Immediate memory | 957 | 1.35 | 0.12 | <0.001 | <0.001 | |||
| Delayed memory | 935 | 1.61 | 0.11 | <0.001 | <0.001 | |||
| Fluency | 984 | 0.40 | 0.21 | 0.080 | 0.248 | |||
| Stroop test | 984 | 1.46 | 0.34 | <0.001 | 0.043 | |||
| Patients received radiotherapy | ||||||||
| Immediate memory | 340 | 1.53 | 0.20 | <0.001 | <0.001 | |||
| Delayed memory | 334 | 1.81 | 0.20 | <0.001 | <0.001 | |||
| Fluency | 347 | 0.33 | 0.36 | 0.365 | 0.570 | |||
| Stroop test | 347 | 1.56 | 0.60 | <0.001 | 0.186 | |||
| Patients received tamoxifen | ||||||||
| Immediate memory | 490 | 1.27 | 0.16 | <0.001 | <0.001 | |||
| Delayed memory | 481 | 1.36 | 0.15 | <0.001 | <0.001 | |||
| Fluency | 501 | 0.24 | 0.29 | 0.485 | 0.624 | |||
| Stroop test | 501 | 1.23 | 0.53 | 0.005 | 0.222 | |||
| Long-term tamoxifen user | ||||||||
| Immediate memory | 401 | 1.32 | 0.18 | <0.001 | <0.001 | |||
| Delayed memory | 393 | 1.40 | 0.17 | <0.001 | <0.001 | |||
| Fluency | 410 | 0.12 | 0.32 | 0.791 | 0.813 | |||
| Stroop test | 410 | 1.21 | 0.60 | 0.016 | 0.264 | |||
| Short-term tamoxifen user | ||||||||
| Immediate memory | 89 | 1.08 | 0.35 | 0.003 | 0.086 | |||
| Delayed memory | 88 | 1.19 | 0.35 | <0.001 | 0.062 | |||
| Fluency | 91 | 0.74 | 0.63 | 0.286 | 0.508 | |||
| Stroop test | 91 | 1.35 | 1.07 | 0.161 | 0.596 | |||
| Patients received both tamoxifen and chemotherapy | ||||||||
| Immediate memory | 434 | 1.30 | 0.17 | <0.001 | <0.001 | |||
| Delayed memory | 426 | 1.34 | 0.16 | <0.001 | <0.001 | |||
| Fluency | 444 | 0.23 | 0.31 | 0.562 | 0.659 | |||
| Stroop test | 444 | 1.32 | 0.52 | 0.004 | 0.205 | |||
| Patients received chemotherapy but no tamoxifen treatment | ||||||||
| Immediate memory | 522 | 1.40 | 0.16 | <0.001 | <0.001 | |||
| Delayed memory | 508 | 1.83 | 0.16 | <0.001 | <0.001 | |||
| Fluency | 539 | 0.53 | 0.28 | 0.074 | 0.249 | |||
| Stroop test | 539 | 1.58 | 0.46 | <0.001 | 0.112 | |||
| Patients received tamoxifen but no chemotherapy | ||||||||
| Immediate memory | 56 | 1.09 | 0.50 | 0.020 | 0.151 | |||
| Delayed memory | 55 | 1.56 | 0.45 | 0.001 | 0.035 | |||
| Fluency | 57 | 0.32 | 0.85 | 0.691 | 0.821 | |||
| Stroop test | 57 | 0.56 | 2.28 | 0.860 | 0.873 | |||
| Patients receive neither chemotherapy nor tamoxifen treatment | ||||||||
| Immediate memory | 17 | 0.47 | 0.42 | 0.235 | 0.661 | |||
| Delayed memory | 16 | 0.13 | 0.87 | 0.781 | 0.905 | |||
| Fluency | 18 | −0.72 | 1.73 | 0.668 | 0.789 | |||
| Stroop test | 18 | −2.17 | 1.77 | 0.265 | 0.701 | |||
Standard errors
Adjusted for age at diagnosis, education, income, menopausal status, depression, menopausal syndrome, TNM status, and event of relapse
Regardless of treatments (e.g., surgery, chemotherapy, radiotherapy, tamoxifen, or both chemotherapy and tamoxifen), cognitive functions including immediate memory, delayed memory, and/or Stroop tests significantly improved from 18 to 36 month after cancer diagnosis. Likewise, the long-term tamoxifen user showed the same improvement patterns. However, for the short-term tamoxifen user, scores on the Stroop test did not significantly improve. Although sample size was substantially reduced among those who received tamoxifen but not chemotherapy, scores of immediate memory and delayed memory test significantly improved. On the other hand, breast cancer survivors who received neither chemotherapy nor tamoxifen showed no significant improvement in any of the tests. After further adjustment for age, education, income, menopausal status, depression, menopausal syndrome, TNM status, and event of relapse, the improvement pattern showed that immediate memory and delayed memory improvement were significant, but most of the significant improvement in Stroop test turned to be non-significant except the group of patients received chemotherapy. The improvements among those with tamoxifen use less than 3 years were not significant. Also, the improvement for immediate memory among those who used tamoxifen, but did not get chemotherapy was not significant after adjustment. Thus, the improvements were significant only among those who used chemotherapy or used tamoxifen more than 3 years.
Further, we conducted analyses to examine whether demographics and disease characteristics were related to the changes in cognitive function score (Table 4). After adjustment for age, education, income, menopausal status, depression, menopausal syndrome, TNM status, and event of relapse, we found that age was inversely related to the improvement of immediate memory, Fluency, and Stroop test scores but without statistics significance, and women with collage education had greater improvement in Fluency test scores comparing with women with less education. We also found, comorbidity was associated with less improvement of immediate memory.
Table 4.
Cognitive function change from the 18 to the 36th month’s assessment stratified by baseline characteristics
| Characteristic | Immediate memory (n = 1,030)
|
Delayed memory (n = 1,006)
|
Fluency (n = 1,059)
|
Stroop (n = 1,059)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | SEa | Pb | Mean difference | SEa | Pb | Mean difference | SEa | Pb | Mean difference | SEa | Pb | |
| Age (years), β | −0.002c | 0.018 | 0.908 | −0.001c | 0.020 | 0.945 | −0.024c | 0.032 | 0.443 | −0.098c | 0.055 | 0.076 |
| Monthly income (¥/person) | ||||||||||||
| <1000 | 1.29 | 0.17 | 1.49 | 0.17 | 0.17 | 0.30 | 1.10 | 0.52 | ||||
| 1000–2000 | 1.29 | 0.18 | 1.69 | 0.18 | 0.38 | 0.32 | 1.99 | 0.56 | ||||
| >2000 | 1.49 | 0.32 | 0.842 | 1.57 | 0.31 | 0.727 | 1.00 | 0.57 | 0.475 | 0.47 | 0.99 | 0.295 |
| Education | ||||||||||||
| Elementary school or below | 1.44 | 0.18 | 1.72 | 0.18 | 0.86 | 0.31 | 1.92 | 0.54 | ||||
| Middle or high school | 1.34 | 0.18 | 1.56 | 0.18 | −0.53 | 0.32 | 1.37 | 0.56 | ||||
| College or above | 0.97 | 0.30 | 0.420 | 1.28 | 0.29 | 0.459 | 1.21 | 0.53 | 0.002 | −0.23 | 0.93 | 0.160 |
| Menopausal status | ||||||||||||
| Pre | 1.52 | 0.21 | 1.69 | 0.21 | 0.93 | 0.37 | 0.95 | 0.64 | ||||
| Post | 1.14 | 0.19 | 0.250 | 1.48 | 0.19 | 0.533 | −0.11 | 0.34 | 0.077 | 1.70 | 0.58 | 0.461 |
| Charlson comorbidity index | ||||||||||||
| 0 | 1.43 | 0.13 | 1.68 | 0.12 | 0.56 | 0.22 | 1.44 | 0.39 | ||||
| ≥1 | 0.86 | 0.26 | 0.049 | 1.16 | 0.26 | 0.070 | −0.39 | 0.47 | 0.073 | 0.98 | 0.81 | 0.611 |
| TNM grade for cancer | ||||||||||||
| I | 1.22 | 0.18 | 1.37 | 0.18 | 0.17 | 0.32 | 1.60 | 0.56 | ||||
| IIA | 1.34 | 0.19 | 1.65 | 0.19 | 0.81 | 0.34 | 0.63 | 0.58 | ||||
| IIB | 1.69 | 0.30 | 2.01 | 0.30 | 0.12 | 0.54 | 1.10 | 0.93 | ||||
| III and IV | 0.95 | 0.40 | 1.10 | 0.39 | 0.47 | 0.72 | 2.21 | 1.24 | ||||
| Unknown | 1.52 | 0.55 | 0.565 | 2.47 | 0.53 | 0.089 | −0.69 | 0.93 | 0.455 | 3.93 | 1.61 | 0.307 |
| ER | ||||||||||||
| Negative | 1.27 | 0.19 | 1.65 | 0.19 | 0.38 | 0.35 | 1.67 | 0.60 | ||||
| Positive | 1.34 | 0.14 | 1.54 | 0.13 | 0.39 | 0.24 | 1.23 | 0.42 | ||||
| Not known | 2.45 | 1.61 | 0.751 | 2.43 | 1.58 | 0.774 | −2.58 | 2.91 | 0.595 | −3.88 | 5.03 | 0.483 |
| PR | ||||||||||||
| Negative | 1.14 | 0.17 | 1.43 | 0.17 | 0.26 | 0.30 | 1.29 | 0.52 | ||||
| Positive | 1.44 | 0.15 | 1.68 | 0.15 | 0.49 | 0.27 | 1.44 | 0.46 | ||||
| Not known | 2.35 | 1.61 | 0.322 | 2.42 | 1.58 | 0.467 | −2.58 | 2.91 | 0.510 | −3.89 | 5.03 | 0.568 |
| Surgery | ||||||||||||
| No | 0.81 | 1.62 | −0.81 | 1.59 | 2.22 | 2.93 | −3.55 | 5.08 | ||||
| Yes | 1.32 | 0.11 | 0.754 | 1.59 | 0.11 | 0.132 | 0.36 | 0.20 | 0.528 | 1.37 | 0.34 | 0.333 |
| Radiology | ||||||||||||
| No | 1.22 | 0.14 | 1.48 | 0.14 | 0.47 | 0.25 | 1.39 | 0.43 | ||||
| Yes | 1.52 | 0.20 | 0.229 | 1.78 | 0.20 | 0.223 | 0.17 | 0.36 | 0.503 | 1.28 | 0.63 | 0.885 |
| Chemotherapy | ||||||||||||
| No | 1.16 | 0.44 | 1.30 | 0.44 | 0.65 | 0.79 | 0.68 | 1.36 | ||||
| Yes | 1.33 | 0.12 | 0.701 | 1.60 | 0.11 | 0.506 | 0.35 | 0.21 | 0.719 | 1.40 | 0.36 | 0.610 |
| Tamoxifen | ||||||||||||
| No | 1.38 | 0.15 | 1.77 | 0.15 | 0.49 | 0.28 | 1.37 | 0.48 | ||||
| Yes | 1.27 | 0.16 | 0.632 | 1.37 | 0.16 | 0.075 | 0.23 | 0.29 | 0.524 | 1.33 | 0.50 | 0.955 |
| Chinese traditional medicine | ||||||||||||
| No | 1.16 | 0.25 | 1.45 | 0.24 | 1.01 | 0.44 | 1.18 | 0.75 | ||||
| Yes | 1.36 | 0.12 | 0.469 | 1.61 | 0.12 | 0.555 | 0.21 | 0.22 | 0.101 | 1.40 | 0.39 | 0.799 |
Standard errors
Adjusted for age at diagnosis, education, income, menopausal status, depression, menopausal syndrome, TNM status, and event of relapse
Regression coefficients between age at diagnosis and score difference
Discussion
We found that cognitive functions, particularly short memory, attention, and executive function (tested by the Stroop test) and delayed memory significantly improved among breast cancer survivors from 18 to 36 months after cancer diagnosis. Improvements in immediate memory, delayed memory, and attention/executive were seen among survivors ever treated with surgery, radiotherapy, tamoxifen, or chemotherapy combined with or without tamoxifen. On the other hand, there were no significant improvements among those who received neither chemotherapy nor tamoxifen. We found that older age was related to less improvement in immediate memory, verbal fluency, and attention/executive. Lower educational achievement was associated with less improvement in verbal fluency test. Comorbidity seemed to be associated with less improvement in immediate memory and verbal fluency. To our best knowledge, this is the first study to investigate the long-term cognitive changes among breast cancer survivors.
Many previous studies conducted in general aging populations suggesting that age is the strongest factor associated with cognitive function and cognitive decline [26-28]. Our finding is also consistent with that from previous studies conducted among breast cancer survivors in which older age was associated with both cognitive function at baseline and cognitive function change [29-32]. A previous study found breast cancer patients who underwent both chemotherapy and hormonal therapy experienced the most severe and persistent decline in cognitive function [33], but the decline improved right after the cessation of treatment [34]. In our study, we conducted the first cognitive function assessment at 18 months after cancer diagnosis by then most of women should have completed their cancer treatment. Because we did not have cognitive function assessment before cancer diagnosis, we could not evaluate the cognitive function decline related cancer treatment. On the other hand, we found cognitive function improvement between 18 and 36 months after cancer diagnosis, suggesting the cognitive function recovery lasted to 36 months after diagnosis. The improvements appeared among those who received treatments (i.e., surgery, radiotherapy, tamoxifen, or chemotherapy combined with or without tamoxifen), but not among those who received neither chemotherapy nor tamoxifen treatment.
The SBCSS is a population-based cohort study. We added the cognitive function component in the study after about half of participants completed the 18th month’s follow-up visit. Thus, we were only able to add the component to a subset of subjects. However, we found there are no significant differences in demographic variables and selected characteristics between eligible subjects and those who participated in the cognitive function component study. Thus, selection bias is unlikely. In our study, we were unable to compare the cognitive functions between before treatment and after treatment. The temporal sequence was not clear in the analysis of the associations between demographic variables and selected characteristics and cognitive function at 18 months after diagnosis. However, we longitudinally investigated the associations of these factors with cognitive changes between the 18th and 36th month’s visit. To evaluate the effect of treatments (including chemotherapy and radiotherapy) on cognitive functions is not our focus. Instead, our study focused to understand how cognitive function evolves in a long run after cancer treatment and what factors may affect these changes among long-term breast cancer survivors.
One concern is that psychomotor speed, which is commonly impaired in breast cancer survivors, cannot be evaluated by the battery we used. Thus, future studies are needed to examine the changes in psychomotor speed among breast cancer survivors. Another weakness of the study is that we did not conduct IQ assessment. Thus, we were not able to control for IQ as a potential confounding factor. Although there were 18 months between the two tests, it is still possible that practice effects contribute partially to the cognitive improvements we observed. Further studies are necessary to confirm our results.
In summary, cognitive functions, particularly short-term, attention and executive function, and long-term memory significantly improved among breast cancer survivors from the 18th- to the 36th-month after cancer diagnosis. The improvements appeared in those who received treatments, but not among those who did not receive any treatment. Future studies are warranted to not only replicate the findings, explore the unidentified predictive factors, but also understand the potential mechanism.
Acknowledgments
The authors thank the breast cancer patients who participated in the study and the research staff of the Shanghai Breast Cancer Survivor Study for their dedication and contributions to the study. The parent study was supported by grants from the US Department of Defense Breast Cancer Research Program (Grant DAMD 17-02-1-0607), the National Cancer Institute (Grant R01 CA118229) and The Shanghai Public Health Talented Professional Overseas Training Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of interest The authors indicated no potential conflicts of interest.
Contributor Information
Ying Zheng, Department of Cancer Prevention and Control, Shanghai Municipal Center for Disease Control and Prevention, Shanghai 200336, China.
Jianfeng Luo, Department of Health Statistics and Social Medicine, School of Public Health, Fudan University, Shanghai 200032, China.
Pingping Bao, Department of Cancer Prevention and Control, Shanghai Municipal Center for Disease Control and Prevention, Shanghai 200336, China.
Hui Cai, Division of Epidemiology, Department of Medicine, Vanderbilt University School of Medicine, 2525 West End Ave, Suite 300, Nashville, TN 37203-1738, USA.
Zhen Hong, Department of Neurology, Shanghai Hua Shan Hospital, Fudan University, Shanghai 200051, China.
Ding Ding, Department of Neurology, Shanghai Hua Shan Hospital, Fudan University, Shanghai 200051, China.
James C. Jackson, Division of Critical Care, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37203-1738, USA
Xiao-Ou Shu, Division of Epidemiology, Department of Medicine, Vanderbilt University School of Medicine, 2525 West End Ave, Suite 300, Nashville, TN 37203-1738, USA.
Qi Dai, Email: qi.dai@vanderbilt.edu, Division of Epidemiology, Department of Medicine, Vanderbilt University School of Medicine, 2525 West End Ave, Suite 300, Nashville, TN 37203-1738, USA.
References
- 1.Boyle P, Levin B, editors. World Cancer Report. International Agency for Research on Cancer; Lyon: 2008. [Google Scholar]
- 2.Brenner H. Long-term survival rates of cancer survivors achieved by the end of the 20th century: a period analysis. Lancet. 2002;360(1131–1135):2002. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 3.Shu XO, Zheng Y, Cai H, GuK Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wefel JS, Meyers CA. Cancer as a risk factor for dementia: a house built on shifting sand. J Natl Cancer Inst. 2005;97:788–789. doi: 10.1093/jnci/dji167. [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 6.Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-oncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer–preliminary results of an observational longitudinal study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Bender CM, Paraska KK, Sereika SM, Ryan CM, Berga SL. Cognitive function and reproductive hormones in adjuvant therapy for breast cancer: a critical review. J Pain Symptom Manage. 2001;21:407–424. doi: 10.1016/s0885-3924(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 10.Asher A. Cognitive Dysfunction Among Cancer Survivors. Am J Phys Med Rehabil. 2011;90:S16–S26. doi: 10.1097/PHM.0b013e31820be463. [DOI] [PubMed] [Google Scholar]
- 11.Hurria A, Somlo G, Ahles T. Renaming ‘Chemobrain’. Cancer Invest. 2007;25:373–377. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 12.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 13.Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, Kalofonos HP. Either called ‘Chemobrain’ or ‘Chemofog’, the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J Pain Symptom Manage. 2011;41:126–139. doi: 10.1016/j.jpainsymman.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 14.McAllister TW, Ahles TA, Saykin AJ, Ferguson RJ, McDonald BC, Lewis LD, Flashman LA, Rhodes CH. Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psychiatry Rep. 2004;6:364–371. doi: 10.1007/s11920-004-0023-y. [DOI] [PubMed] [Google Scholar]
- 15.Barton D, Loprinzi C. Novel approaches to preventing chemotherapy-induced cognitive dysfunction in breast cancer: the art of the possible. Clin Breast Cancer. 2002;3:S121–S127. doi: 10.3816/cbc.2002.s.023. [DOI] [PubMed] [Google Scholar]
- 16.Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, Klaunig JE, Champion VL, Unverzagt FW, Saykin AJ. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137:493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- 18.Avisar A, River Y, Schiff E, Bar-Sela G, Steiner M, Ben-Arye E. Chemotherapy-related cognitive impairment: does integrating complementary medicine have something to add? review of the literature. Breast Cancer Res Treat. 2012;136:1–7. doi: 10.1007/s10549-012-2211-5. [DOI] [PubMed] [Google Scholar]
- 19.Mar Helen G, Houédé-Tchen Fan Nadine, Yi Qi-Long, Chemerynsky I, Downie FP, Sabate K, Tannock IF. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 20.Chen Xiaoli, Wei Lu, Ying Zheng GuK, Chen Z, Zheng W, Shu XO. Exercise, tea consumption, and depression among breast cancer survivors. J Clin Oncol. 2010;28:991–998. doi: 10.1200/JCO.2009.23.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epplein M, Zheng Y, Zheng W, Chen Z, Gu K, Penson D, Lu W, Shu XO. Quality of Life after Breast Cancer Diagnosis and Survival. J Clin Oncol. 2011;29:406–412. doi: 10.1200/JCO.2010.30.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmon DP, Jin H, Zhang M, Grantc I, Yud E. Neuro-psychological assessment of Chinese elderly in the Shanghai Dementia survey. Clin Neuropsychol. 1995;9:159–168. [Google Scholar]
- 23.Chan AS, Poon MW. Performance of 7- to 95-year-old individuals in a Chinese version of the category fluency test. J Int Neuropsychol Soc. 1999;5:525–533. doi: 10.1017/s135561779956606x. [DOI] [PubMed] [Google Scholar]
- 24.Lee TM, Chan CC. Stroop interference in Chinese and English. J Clin Exp Neuropsychol. 2000;22:465–471. doi: 10.1076/1380-3395(200008)22:4;1-0;FT465. [DOI] [PubMed] [Google Scholar]
- 25.He J, Iosif AM, Lee DY, Martinez O, Ding D, Carmichael O, Mortimer JA, Zhao QH, Chu SG, Guo QH, et al. Brain morphology and cerebrovascular risk in mild cognitive impairment and dementia: sCOBHI-P study. Arch Neurol. 2010;67:1231–1237. doi: 10.1001/archneurol.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bano D, Agostini M, Melino G, Nicotera P. Ageing, neuronal connectivity and brain disorders: an unsolved ripple effect. Mol Neurobiol. 2011;43:124–130. doi: 10.1007/s12035-011-8164-6. [DOI] [PubMed] [Google Scholar]
- 27.Cullum S, Huppert FA, McGee M, Dening T, Ahmed A, Paykel ES, Brayne C. Decline across different domains of cognitive function in normal ageing: results of a longitudinal population-based study using CAMCOG. Int J Geriatr Psychiatry. 2000;15:853–862. doi: 10.1002/1099-1166(200009)15:9<853::aid-gps211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Badgio PC, Worden BL. Cognitive functioning and aging in women. J Women Aging. 2007;19:13–30. doi: 10.1300/J074v19n01_02. [DOI] [PubMed] [Google Scholar]
- 29.Klepin H, Mohile S, Hurria A. Geriatric assessment in older survivors with breast cancer. J Natl Compr Cancer Netw. 2009;7:226–236. doi: 10.6004/jnccn.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitsiades N, Correa D, Gross CP, Hurria A, Slovin SF. Cognitive effects of hormonal therapy in older adults. Semin Oncol. 2008;35:569–581. doi: 10.1053/j.seminoncol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Rossi A, Colantuoni G, Maione P, Ferrara C, Airoma G, Barzelloni ML, Castaldo V, Gridelli C. Chemotherapy of breast cancer in the elderly. Curr Med Chem. 2005;12:297–310. doi: 10.2174/0929867053363261. [DOI] [PubMed] [Google Scholar]
- 32.Bourbonniere M, Kagan SH. Nursing intervention and older adults who have cancer: specific science and evidence based practice. Nurs Clin North Am. 2004;39:529–543. doi: 10.1016/j.cnur.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Vodermaier A. Breast cancer treatment and cognitive function: the current state of evidence, underlying mechanisms and potential treatments. Womens Health. 2009;5:503–516. doi: 10.2217/whe.09.36. [DOI] [PubMed] [Google Scholar]
- 34.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]


