Abstract
Wnt/β-catenin signaling plays indispensable roles in both embryonic development and adult homeostasis. Abnormal regulation of this pathway is implicated in many types of cancer. Consequently, substantial efforts have made to develop therapeutic agents as anticancer drugs by specifically targeting the Wnt/β-catenin pathway. Here we systematically review the potential therapeutic agents that have been developed to date for inhibition of the Wnt/β-catenin cascade as well as current status of clinical trials of some of these agents.
Keywords: Wnt/β-catenin signaling, β-catenin, cancer, small molecules, biologic agents, therapeutic, safety and clinic
Introduction
Wnt/β-catenin signaling is an evolutionarily conserved pathway and it plays indispensable roles in both embryonic development and adult homeostasis. The Wnt/β-catenin network was first reported in 1982 with identification of mouse proto-oncogene int1 (also known as Wnt1). Later the homolog of int1, Wingless (Wg) from Drosophila melanogaster, was identified to promote wing development in fruit flies [1,2]. Since then, accumulative studies have reported essential roles of the Wnt/β-catenin signaling in development of various organs and tissues such as the brain, spinal cord, kidney, heart, liver, lungs, limbs and eyes [3,4]. In addition, the Wnt/β-catenin signaling also plays crucial roles in adult homeostasis such as regeneration of skin, gut, hair and bone marrow [5-8].
Aberrant Wnt/β-catenin signaling can lead to developmental malformations and is associated with many types of disease including fibrosis, gastric and colorectal tumors, melanomas and hepatocellular carcinomas [9-15]. Given the critical roles of Wnt/β-catenin pathway in cancer, substantial efforts have been made to develop therapeutic approaches to target this pathway. In this article, we review recent efforts to develop therapeutic agents, with a focus on small molecules, for targeting the Wnt/β-catenin pathway.
The Wnt/β-catenin signaling pathway
β-catenin is the central mediator of the Wnt/β-catenin signaling and it is dynamically distributed in multiple subcellular locations (Figure 1). For instance, β-catenin in adherens junctions is involved in cell-cell contacts, and its levels in cytoplasm are tightly regulated whereas in nucleus, β-catenin is implicated in transcriptional regulation and chromatin modification [16,17]. In quiescent cells, the cytoplasmic β-catenin is bounds to a large protein assembly called ‘destruction complex’ which consists of several proteins including Axin, adenomatous polyposis coli (APC), the Ser/Thr kinases glycogen synthase kinase 3β (GSK3β) and casein kinase Iα (CKIα) [3,18]. In the destruction complex, β-catenin is initially phosphorylated by CKIα at Ser45 followed by further phosphorylation at Ser33, Ser37 and Thr41 (in human) by GSK3β [19]. Finally the phosphorylated β-catenin is marked with ubiquitin mediated by β-transducin-repeat-containing protein (β-TrCP) for subsequent proteasome-dependent degradation [3,18].
Figure 1.
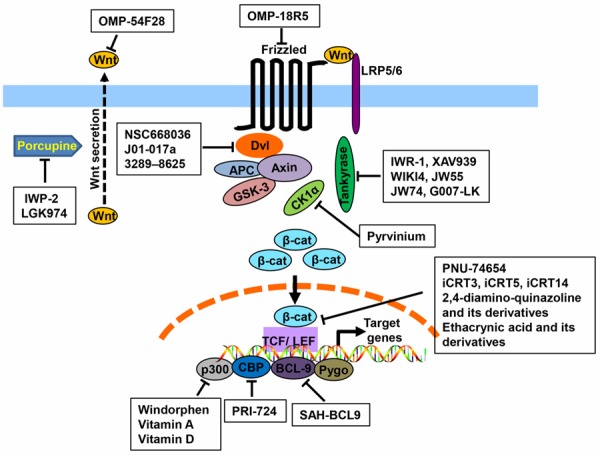
Schematic representation of the Wnt/β-catenin signaling pathway and the oncology-indication drug candidates discussed in the paper. The molecular targets of both biological agents and small molecule drug candidates are shown.
Activation of the Wnt/b-catenin signaling is initiated by Wnt ligand stimulation (Figure 1). When Wnt ligands bind to the Frizzled receptor and the co-receptor lipoprotein receptor-related protein 5/6 (LRP5/6), the Dishevelled (Dvl) sequesters Axin and GSK3β from cytoplasm to the membrane, resulting in decomposition of the destruction complex. As a consequence, less β-catenin is phosphorylated for degradation and unphosphorylated (active) β-catenin accumulates and translocates to the nucleus. Nuclear β-catenin then interacts with transcription factors, the T-cell factor (TCF)/lymphocyte enhancer factor (LEF), and co-activators to regulate the transcription of various target genes [3,18]. These associated co-activators include B-cell lymphoma 9 (BCL9), Pygopus (Pygo), CREB-binding protein (CBP) and its homologue protein p300.
The Wnt/β-catenin signaling pathway and cancer
Abnormal regulation of the Wnt/β-catenin signaling is implicated in various types of cancer. In 1991, genetic mutations in the tumor suppressor APC were first found to be associated with colorectal cancer [20-22]. These mutations typically resulted in truncated APC proteins that are incapable of binding β-catenin and Axin, leading to aberrant activation of the Wnt/β-catenin signaling [23,24]. Later, mutations in another core component of the destruction complex, Axin, have been reported to display a predisposition for colorectal cancer [25,26]. Meanwhile genetic mutations of β-catenin that abnormally activate the Wnt/β-catenin signaling were also observed in colorectal cancer [27]. These mutations prevent phosphorylation of the serine and threonine residues of β-catenin targeted by GSK3β or CKIα [27]. Importantly, effects of mutations in the Wnt/β-catenin cascade are not limited to colorectal cancers only. For instance, messenger RNA splicing and missense mutations in the β-catenin gene were described in melanoma progression [28,29] and other solid tumors such as liver cancer [30], thyroid tumors [31] and ovarian neoplasms [32].
Besides genetic mutations of the Wnt/β-catenin cascade, abnormal expression of the signaling proteins by epigenetic alteration is also involved in various types of cancer. For instance, the reduced activity or absence of extracellular Wnt antagonist, the secreted Frizzled-related proteins (SFRPs) has been reported in colorectal, breast, prostate, lung cancers [33-37]. Furthermore, increased expression of Wnt ligands and Dvl has been demonstrated to be associated with many types of cancer as well [38-42].
Potential therapeutic agents targeting the Wnt/β-catenin cascade
Given the critical roles of abnormal activation of the Wnt/β-catenin signaling in various types of cancer, substantial efforts have been made to develop therapeutic agents including biological agents and small molecule agents to target this pathway.
Biological agents
Several biological agents have been studied in cancer by specifically targeting either aberrantly overexpressed Wnt receptors or the Wnt ligands (Figure 1). For instance, inhibition of Wnt/β-catenin signaling by adenovirus-mediated expression of Dickkopf-1 (Dkk-1), a potent secreted Wnt antagonist that interacts with the LRP5/6 co-receptors, has been shown to suppress epithelium proliferation in small intestine and colon [43,44]. In addition, it has also been shown that Mesd, another LRP5/6 co-receptor inhibitor, effectively inhibits prostate cancer PC-3 cell proliferation in vitro and markedly decreases growth of breast cancer in the mouse mammary tumor virus-Wnt-1 transgenic mice [45,46]. In addition, OMP-54F28 (also known as FZD8-Fc or F8CRDhFc), a proprietary fusion protein comprised of the cysteine-rich domain (CRD) of frizzled family receptor 8 (Fzd8) fused to the human immunoglobulin Fc domain, was shown to bind to all Wnt ligands to block the Wnt/β-catenin signaling [47]. The clinic Phase I trial of OMP-54F28 is underway.
Moreover, large antibodies and small peptides have been developed to target the Wnt/β-catenin signaling as well. For instance, previous studies have demonstrated that monoclonal antibodies-neutralizing Wnt3a can suppress prostate tumor growth in a mouse model [48], and antibodies targeting Frizzled receptors are effective in various preclinical models including those of breast, colon and liver cancer [49,50]. Another monoclonal antibody which targets Frizzled receptors, OMP-185, was shown to inhibit tumor growth in mouse xenograft models, reduce tumor-initiating cell frequency and display synergistic activity with chemotherapeutic agents [51]. Other than the large antibodies, small peptides represent another potential therapeutic approach to inhibit the Wnt/β-catenin signaling for cancer therapy. For instance, a hydrocarbon-stapled peptide has been reported to inhibit Wnt/β-catenin signaling by directly targeting β-catenin and interfering with its interaction with TCF proteins [52]. In addition, a stabilized alpha helix of BCL9 (SAH-BCL9) has been developed. The SAH-BCL9 peptide was shown to selectively suppress Wnt signaling by dissociating β-catenin/BCL9 complex, and exhibit antitumor effects in mouse xenograft models of colorectal carcinoma and Interleukin-6-dependent multiple myeloma [53].
Small molecule inhibitors of the Wnt/β-catenin pathway
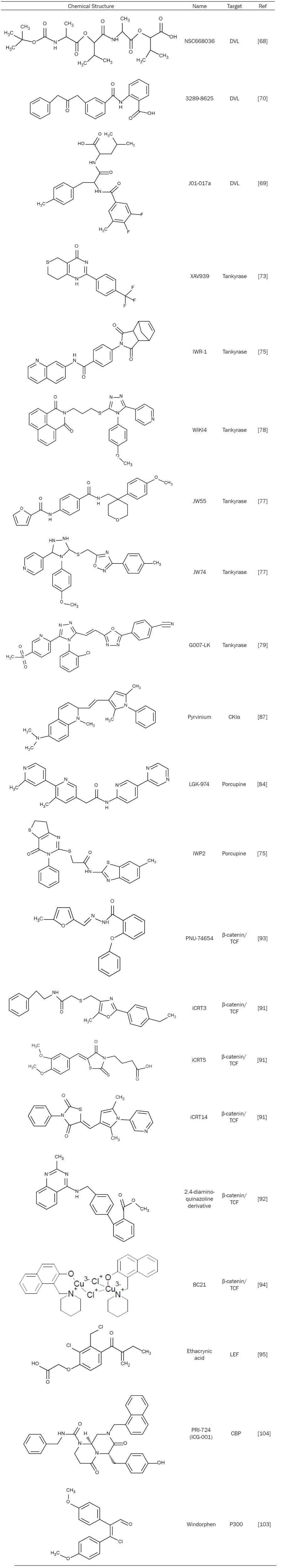
Compared to biological agents, small molecule drugs exhibit several advantages including lower cost with greater ease of manufacturing, oral bioavailability and ability to penetrate into cells for intracellular targets. Therefore, tremendous research has been performed in the past to develop specific small molecules to target the Wnt/β-catenin pathway. Here we classify the small molecule inhibitors of the Wnt/β-catenin signaling into three groups: small molecules that target cytoplasmic proteins, small molecules that target transcriptional factors and small molecules that target the co-activators (Figure 1 and Table 1).
Table 1.
Selected small molecules that inhibit the Wnt/β-catenin signaling
|
Small molecules that target cytoplasmic proteins of the Wnt/β-catenin cascade
Dvl
Three Dishevelled isoforms, Dvl1, Dvl2 and Dvl3, have been identified in mammalian species [54-58]. All Dvl proteins contain three functional domains: an N-terminal DIX (Dishevelled/Axin) domain, a central PDZ (Postsynaptic density 95, Discs Large, Zonula occludens-1) domain and a C-terminal DEP domain (Dvl Egl-10, Pleckstrin). The DIX domain is responsible for the polymerization of Dvl in the Wnt signalosome [59,60] whereas the DEP domain is suggested to be essential for the DVL binding to membrane lipids during planar epithelial polarization [61-63]. The PDZ domain is a modular protein interaction domain, and it directly interacts with the cytosolic C-terminal tail of the Frizzled to transduce signals from the Frizzled receptor to the downstream signaling cascade [64]. However, this interaction between the Dvl PDZ domain and the Frizzled can be suppressed when the Dvl PDZ domain is bound by the Dapper proteins that promote Dvl lysosomes-mediated degradation [65-67].
The special role of the Dvl PDZ domain in the Wnt pathway makes it an ideal pharmaceutical target. NSC668036, a small molecule Wnt inhibitor by targeting Dvl PDZ domain, was identified from National Cancer Institute small-molecule library by using structure-based virtual ligand screening [68]. Based on NSC668036 study, the same research group further developed a more potent small molecule inhibitor of the Dvl PDZ domain, named J01-017a, under guidance of the 3-dimensional quantitative structure-activity relationship analysis [69]. In addition, another small molecule inhibitor of the Dvl PDZ domain, compound 3289-8625, has been identified through structure-based ligand screening and NMR spectroscopy [70]. All three compounds (NSC 668036, J01-07a and 3289-8625) block the Wnt/β-catenin signaling by interacting with the groove of Dvl PDZ domain where the inhibitory Dapper proteins bind.
Tankyrase inhibitors
Tankyrase is a subgroup of Poly (ADP-ribose) polymerases (PARPs) family. Two isoforms of Tankyrase, Tankyrase 1 (PARP5a) and Tankyrase 2 (PARP5b), have been identified to be involved in the Wnt/β-catenin signaling [71,72]. Both Tankyrase isoforms interact with and poly(ADPribosyl)ate Axin to stimulate Axin degradation through the ubiquitin-proteasome pathway [73,74].
A few small molecule Tankyrase inhibitors have been developed to inhibit the Wnt/β-catenin signaling (Table 1). In 2009, Chen et al. reported the first Tankyrase inhibitor, IWR-1, in screening a diverse synthetic chemical library by using a Wnt luciferase reporter assay [75]. It was shown that IWR-1 stabilized Axin for inhibition of the Wnt/β-catenin signaling by targeting Tankyrases. In the same year, Huang and colleagues identified another Tankyrase inhibitor, XAV939, which functions in a similar way to IWR-1 [73]. Recently, several additional potent and selective tankyrase inhibitors have been reported including WIKI4, JW55, JW74 and G007-LK [76-79].
Porcupine inhibitors
Porcupine is a membrane-bound O-acyltransferase (MBOAT) specific to Wnt post-translational acylation, which is required for subsequent Wnt secretion [80]. Loss of Porcupine leads to inhibition of Wnt ligand-driven signaling activities in knockout mouse models [81,82]. In humans, loss-of-function mutations in the Porcupine gene lead to focal dermal hypoplasia whose phenotype is consistent with inactivation of the Wnt signaling pathway during embryogenesis and development [29].
Targeting Porcupine for Wnt inhibition may represent a new therapeutic strategy for cancer therapy. In 2009, Chen et al. identified the first Porcupine inhibitor IWP-2 which disrupts Wnt signaling by preventing Porcupine-dependent lipidation of Wnt proteins [75]. Later the same group further developed a number of novel Porcupine inhibitors with diverse chemical structures [83]. In addition, Liu and colleagues recently discovered a new class of specific small-molecule Porcupine inhibitor LGK974 after screening approximately 2.4 million compounds by using Wnt-secreting cells (a stable L-cell line overexpressing Wnt3A) which were co-cultured with the Wnt luciferase reporter cells [84]. LGK974 has been shown to potently inhibit Wnt signaling in vitro, and is efficacious in multiple tumor models including murine and rat mechanistic breast cancer models and a human head and neck squamous cell carcinoma model [84].
CK1 inhibitors
In the destruction complex, β-catenin is initially phosphorylated by CKIα at Ser45. This CKIα-mediated phosphorylation is a critical step for ubiquitin-dependent degradation of β-catenin [19]. By screening libraries of FDA-approved drugs, Pyrvinium was identified with the ability to activate CK1α, leading to enhanced degradation of β-catenin [85]. Later, Pyrvinium was further shown to improve cardiac remodeling in a mouse myocardial infarction model [86]. However, the concept that Pyrvinium inhibits Wnt/β-catenin signaling by activation of CK1α was challenged by a recent study that ruled out any direct stimulatory effect of Pyrvinium on CK1α, implying that Pyrvinium may inhibit the Wnt/β-catenin signaling through other mechanisms [87].
Another CK1 kinase isoform, CK1ε, is involved in phosphorylation of E-cadherin upon Wnt binding to Frizzled-LRP5/6 receptor, leading to the release of β-catenin from its complex with E-cadherin and subsequent increase of the cellular threshold of free β-catenin [88]. Recently Cheong and colleagues have identified a small molecule, PF670462, which can potently suppress the Wnt/β-catenin signaling by inhibiting CK1ε activity [89].
Small molecules that target transcriptional factors of the Wnt/β-catenin cascade
As previously descripted, nuclear β-catenin needs to interact with transcription factor TCF/LEF to regulate the target gene transcription. Thus, disrupting the interaction berween β-catenin and TCF/LEF in the nucleus represents another therapeutic avenue to block the Wnt/β-catenin signaling pathway.
In a high-throughput enzyme-linked immunosorbent assay (ELISA) screen of a natural product library, eight compounds were identified to exhibit dose-dependent inhibition of the β-catenin/TCF complex formation [90]. Subsequently six of the compounds were shown to block complex formation in the gel retardation assay, inhibit TCF reporter gene activity and down-regulate target gene expression. Three of these six compounds can display efficacy in inhibiting axis duplication induced by β-catenin in the Xenopus [90]. Additionally after screened around 15,000 compounds, Gonsalves and colleagues have identified three new small molecules, iCRT3, iCRT5 and iCRT14, which can disrupt the β-catenin/TCF interaction, down-regulate the Wnt target gene expression and kill colorectal cancer cells [91]. In another high-throughput screen, Chen and colleagues discovered a novel compound, 2,4-diamino-quinazoline, which disrupts the interaction between β-catenin with TCF4. A further structure-activity relationship study yielded a number of new analogues including compound 16k which exhibited good cellular potency, solubility, metabolic stability and oral bioavailability [92].
Other than high-throughput screening, computation and structure-based approaches have also been successfully utilized in developing small molecules to inhibit the β-catenin/TCF interaction. For instance, drug-like TCF-competitive small molecule, PNU-74654, were discovered after a 17,700 compounds subset of the Pharmacia corporate collection was docked to the hot spot of the TCF-binding surface on β-catenin by using the Flo-QXP program [93]. In another crystal structure-based virtual screen of about 1990 small-molecules, BC21 was identified to bind to the armadillo repeat structure of β-catenin and reduce β-catenin/TCF reporter activity [94]. Additionally, the diuretic agent ethacrynic acid (EA) was identified to display an inhibitory effect on the Wnt/β-catenin signaling in a cell-based Wnt reporter assay. Immune co-precipitation experiments demonstrated that EA could directly bind to LEF-1 protein and destabilize the β-catenin/LEF-1 complex [95]. Several more potent EA derivatives were developed and shown to block the Wnt/β-catenin signaling and decrease survival of chronic lymphocytic leukemia cells [96].
Small molecules that target the co-activators of the Wnt/β-catenin cascade
A number of transcriptional co-activators in nucleus are required for Wnt target gene expression. These include CBP, p300, Pygo, BCL-9 and many other basal transcriptional machinery components. CBP and p300 share up to 93% identity at the amino acid level, and it has long been thought that they are functional redundant [3,97]. However, recent collective studies have demonstrated that CBP and p300 have definitive and distinct roles both in vitro and in vivo [98-100]. Particularly, a new model about unique roles of CBP and p300 in the Wnt/β-catenin signaling cascade for stem cells has been developed: CBP induces a transcriptional program for proliferation and the maintenance of stem cell potency, whereas p300 leads to a transcriptional program for stem cell differentiation [101,102]. We and others in the field have recently identified small molecules that selectively target CBP and p300 for inhibition of the Wnt/ β-catenin signaling [103,104].
CBP inhibitors
Emami and colleagues have identified the small molecule PRI-724 (also named as ICG-001) that down-regulates the Wnt/β-catenin signaling by specifically binding to CBP [104]. PRI-724 was shown to selectively induce apoptosis in colon carcinoma cells but not in normal colon cells, and exhibit antitumor activity in the mouse xenograft models of colon cancer [104]. Interestingly, PRI-724 binds specifically to the co-activator CBP, but not to the closely related homologue p300.
p300 inhibitors
In a zebrafish-based phenotype screen, we recently discovered a compound named Windorphen that selectively targets p300 histone acetyltransferase for Wnt signal inhibition. Windorphen displays remarkable specificity toward p300, and selectively kills cancer cells that harbor Wnt-activating mutations, supporting the therapeutic potential of Windorphen [103]. In addition, the mechanism study of vitamin A and vitamin D suggested that they may target p300 to inhibit Wnt signal for cancer therapy of the acute promyelocytic leukaemia chemoprevention [105].
Safety of targeting the Wnt/β-catenin signaling
Despite the crucial role of aberrant activation of the Wnt/β-catenin signaling in cancer, cautions must be taken when targeting this pathway for cancer therapy because the Wnt/β-catenin pathway is also indispensable in developmental processes and adult tissue homeostasis. A recent review by Kahn provides a detailed discussion of safety concerns targeting of the Wnt pathways [106]. Here, we would like to highlight certain safety issues from the aspect of regenerative biology.
The Wnt signaling plays a central role in stem cell proliferation and pluripotency. For instance, in the intestinal tract, stem cells residing at the most bottom part of the crypt stochastically self-renew and produce the transit-amplifying progenitor cells for intestinal homeostasis [107,108]. Both genetic disruption of Wnt pathway and ectopic expression of the Wnt antagonist Dkk-1 lead to rapid loss of transient-amplifying cells and crypt structures [6,43,109-111]. Conversely, aberrant activation of the Wnt pathway results in an increase of intestinal stem cell numbers and robust proliferation in the crypt [6,112]. Given the critical role of Wnt signaling in intestinal tract stem cells, targeting this pathway for cancer therapy may cause safety issue. Indeed, disruption of the Wnt/β-catenin signaling by adenovirus-mediated expression of Dkk-1 in mice has been shown to suppress epithelium proliferation in small intestine and colon, accompanied by progressive architectural degeneration with the loss of crypts, villi, and glandular structure by 7 days [153]. In addition, the Wnt signaling pathway is an important regulator of hematopoietic stem cells (HSCs). Overexpress of β-catenin promotes the proliferation and inhibit the differentiation of HSCs, conversely ectopic expression of Axin, or a frizzled ligand-binding domain to suppress the Wnt pathway leads to the inhibition of HSC growth in vitro and reduced reconstitution in vivo [113]. Therefore, general inhibition of the Wnt/β-catenin signaling for cancer therapy may potentially cause damage to normal stem cell function required for normal tissue hemostasis and thus, a careful assessment of drug safety is required.
Encouragingly, recent studies have indicated that the negative effects caused by blocking the Wnt/β-catenin signaling on normal tissue hemostasis could be reversible. Chen and colleagues have shown that zebrafish treated with the Wnt inhibitor IWR-1 fail to regenerate caudal fin tissue as expected. However, nine days post-removal of IWR-1, fish that were treated with IWR-1 displayed fin tissue regrowth, suggesting the stem cells required for caudal fin regeneration are able to resume normal function [75]. In another study, Tian and colleagues have demonstrated that acute loss of the intestinal lgr5+ stem cells in the intestine did not disrupt cellular homeostasis. Furthermore, it was followed by recovery and the renewal of the Wnt-regulated stem cell population, suggesting that the Wnt-regulated stem cell population can be re-populated by a quiescent stem cell refractory to Wnt perturbations [114,115]. Therefore, side effects associated with disrupting the Wnt/β-catenin pathway could be minimized if proper dosing and scheduling of Wnt inhibitors are considered.
Therapeutic agents in clinic study
Biological therapeutic agents
A number of biologic therapeutic agents targeting the Wnt pathway have entered clinical trials (Table 2). The clinic Phase I trial of one of these agents, OMP18R5 (also known as Vantictumab, a fully humanized monoclonal antibody that targets frizzled receptor), has recently been completed by OncoMed Pharmaceuticals [116]. 18 patients with solid tumors were treated in 5 dose-escalation cohorts (0.5 and 1 mg/kg weekly; 0.5 mg/kg every 2 weeks; 1 and 2.5 mg/kg every 2 weeks). The most common related adverse events included Grade 1 and 2 fatigue, vomiting, abdominal pain, constipation, diarrhea and nausea. The only potentially drug-related adverse events were dose-limiting toxicities of Grade 3 diarrhea and vomiting in 1 patient treated with 1 mg/kg every week. One patient receiving 0.5 mg/kg every week had a bone fracture on day 110, and three cases exhibited prolonged stable disease in patients with neuroendocrine tumors [116]. The open-label Phase 1 dose escalation study of OMP-18R5 in patients with solid tumors continues and is expected to be completed by June 2016. Another OncoMed agent OMP-54F28, a proprietary fusion protein with Fzd8 that binds to all Wnt ligands, was co-developed with Bayer was initiated recently for solid tumor treatment.
Table 2.
Current clinical trials of the potential therapeutic agents that target the Wnt/β-catenin pathway
| Name | Company | Target | Agent Type | Disease | Clinical Phase |
|---|---|---|---|---|---|
| OMP18R5 (vantictumab) | OncoMed Pharmaceuticals | frizzled | Biologic agents | Solid tumors | Open-label Phase 1 dose escalation study |
| OMP-54F28 | OncoMed Pharmaceuticals/ Bayer | Wnt | Biologic agents | Solid tumors | Phase I |
| LGK974 | Novartis Pharmaceuticals | Porcupine | Small Molecule | Melanoma, breast cancer and pancreatic adenocarcinoma | Phase I |
| CWP232291 | JW Pharmaceutical | β-catenin | Small Molecule | acute myeloid leukemia | Phase I |
| PRI-724 | Prism/Eisai pharmaceuticals | β-catenin/CBP | Small Molecule | advanced myeloid malignancies | Open-Label Phase 1 dose escalation study |
Small molecule therapeutic agents
Other than biological agents, clinical trials of small molecule agents have also been conducted in recent years too (Table 2). For instance, Novartis Pharmaceuticals initiated a Phase I trial of the small molecule Porcupine inhibitor LGK974 for multiple malignancies (melanoma, breast cancer and pancreatic adenocarcinoma) that are associated with aberrant Wnt signaling. It is expected that the Phase I trial to obtain a maximum tolerated dose of LGK974 will be completed in January 2017. In addition, JW Pharmaceutical recently initiated a Phase I clinical study of CWP232291, a small molecule prodrug targeting β-catenin for degradation, in acute myeloid leukemia patients. The clinical trial is expected to be completed at the end of 2015, and no results have been disclosed publicly yet. As described previously, small molecule PRI-724 can block interaction of CBP with β-catenin for Wnt signaling inhibition [104]. The initial results of the Phase I clinic trial of PRI-724 has been disclosed publically [117]. Overall, PRI-724 was given to 18 patients as a continuous infusion for 7 days, and the drug exhibited an acceptable toxicity profile with only one dose-limiting toxicity of grade 3 reversible hyperbilirubinaemia. An Open-Label dose-escalation phase I/II study of PRI-724 for patients with advanced myeloid malignancies is still ongoing. Additionally, clinical trials of combination of PRI-724 with other therapeutic agents are underway. For instance, a Phase I trial was initiated to treat patient with colorectal cancer by administering PRI-724 in combination with a modified regimen of FOLFOX6 (mFOLFOX 6). Furthermore, a current Phase I trial is testing continuous intravenous doses of PRI-724, in combination with Gemcitabine, to treat patients with advanced or metastatic pancreatic adenocarcinoma.
Concluding remarks
The Wnt/β-catenin signaling is a highly evolutionarily conserved key pathway, and aberrant activation of this pathway is implicated in a broad range of diseases including cancer. Despite extensive studies of the Wnt/β-catenin signaling in almost three decades, targeting this pathway as a therapeutic strategy is still at its infancy. It still remains unclear which targets in the pathway may offer an ideal therapeutic lead for drug discovery. Particularly we shall bear in mind that the Wnt/β-catenin signaling also plays crucial roles in tissue homeostasis and repair, thus successfully targeting aberrant Wnt/β-catenin signaling in cancer will require a fine balancing act. In addition, the fact that several known targets in the Wnt/β-catenin pathway are also implied in other pathways applies another layer of complexity. Despite these complications, our understanding of this pathway in both normal physiology and pathophysiology continues to improve, and significant excitement has been generated to develop therapeutic agents targeting the Wnt/β-catenin signaling for disease treatment. In recent years, a number of small molecule and biologic agents that target the Wnt/β-catenin signaling have entered clinical trials. In the coming years, it shall be clear whether these potential therapeutic agents targeting Wnt/β-catenin pathway will be efficacious to cancer.
Acknowledgements
This work was supported by the seed fund of College of Veterinary Medicine at Western University of Health Sciences, Faculty Development Grant from Chinese American Faculty Association of Southern California (CAFA) and the ReproCELL’s Innovative Research Grant. The authors would like to acknowledge ChemAxon (http://www.chemaxon.com) for providing an academic license to their software.
Disclosure of conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 2.Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987;6:1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 4.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 5.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 6.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth MJ, Mak KK, Yang Y, Bodine DM. beta-Catenin expression in the bone marrow microenvironment is required for long-term maintenance of primitive hematopoietic cells. Stem Cells. 2009;27:1109–1119. doi: 10.1002/stem.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 9.Dees C, Distler JH. Canonical Wnt signalling as a key regulator of fibrogenesis - implications for targeted therapies? Exp Dermatol. 2013;22:710–713. doi: 10.1111/exd.12255. [DOI] [PubMed] [Google Scholar]
- 10.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 13.Berwick DC, Harvey K. The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. Biochem Soc Trans. 2012;40:1123–1128. doi: 10.1042/BST20120122. [DOI] [PubMed] [Google Scholar]
- 14.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 16.Fagotto F. Looking beyond the Wnt pathway for the deep nature of beta-catenin. EMBO Rep. 2013;14:422–433. doi: 10.1038/embor.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller RK, Hong JY, Munoz WA, McCrea PD. Beta-catenin versus the other armadillo catenins: assessing our current view of canonical Wnt signaling. Prog Mol Biol Transl Sci. 2013;116:387–407. doi: 10.1016/B978-0-12-394311-8.00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald BT, Tamai K, He X. Wnt/betacatenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of betacatenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 20.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 21.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 22.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 23.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 24.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 26.Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 28.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of betacatenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 29.Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends in Mol Med. 2012;18:483–493. doi: 10.1016/j.molmed.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Han ZG. Functional Genomic Studies: Insights into the Pathogenesis of Liver Cancer. Annual Rev Genomics Hum Genet. 2012;13:171–205. doi: 10.1146/annurev-genom-090711-163752. [DOI] [PubMed] [Google Scholar]
- 31.Sastre-Perona A, Santisteban P. Role of the wnt pathway in thyroid cancer. Front Endocrinol (Lausanne) 2012;3:31. doi: 10.3389/fendo.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatcliffe TA, Monk BJ, Planutis K, Holcombe RF. Wnt signaling in ovarian tumorigenesis. Int J Gynecol Cancer. 2008;18:954–962. doi: 10.1111/j.1525-1438.2007.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldwell GM, Jones C, Gensberg K, Jan S, Hardy RG, Byrd P, Chughtai S, Wallis Y, Matthews GM, Morton DG. The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res. 2004;64:883–888. doi: 10.1158/0008-5472.can-03-1346. [DOI] [PubMed] [Google Scholar]
- 34.Lee AY, He B, You L, Dadfarmay S, Xu ZD, Mazieres J, Mikami I, McCormick F, Jablons DM. Expression of the secreted frizzled-related protein gene family is downregulated in human mesothelioma. Oncogene. 2004;23:6672–6676. doi: 10.1038/sj.onc.1207881. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 36.Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Sekido Y. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- 37.Zou H, Molina JR, Harrington JJ, Osborn NK, Klatt KK, Romero Y, Burgart LJ, Ahlquist DA. Aberrant methylation of secreted frizzledrelated protein genes in esophageal adenocarcinoma and Barrett’s esophagus. Int J Cancer. 2005;116:584–591. doi: 10.1002/ijc.21045. [DOI] [PubMed] [Google Scholar]
- 38.Rhee CS, Sen M, Lu DS, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- 39.Milovanovic T, Planutis K, Nguyen A, Marsh JL, Lin F, Hope C, Holcombe RF. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol. 2004;25:1337–1342. [PubMed] [Google Scholar]
- 40.Okino K, Nagai H, Hatta M, Nagahata T, Yoneyama K, Ohta Y, Jin E, Kawanami O, Araki T, Emi M. Up-regulation and overproduction of DVL-1, the human counterpart of the Drosophila dishevelled gene, in cervical squamous cell carcinoma. Oncol Rep. 2003;10:1219–1223. doi: 10.3892/or.10.5.1219. [DOI] [PubMed] [Google Scholar]
- 41.Uematsu K, Kanazawa S, You L, He B, Xu Z, Li K, Peterlin BM, McCormick F, Jablons DM. Wnt pathway activation in mesothelioma: evidence of Dishevelled overexpression and transcriptional activity of beta-catenin. Cancer Res. 2003;63:4547–4551. [PubMed] [Google Scholar]
- 42.Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 43.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 45.Liu CC, Prior J, Piwnica-Worms D, Bu GJ. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu WY, Liu CC, Thottassery JV, Bu GJ, Li YH. Mesd Is a Universal Inhibitor of Wnt Coreceptors LRP5 and LRP6 and Blocks Wnt/beta-Catenin Signaling in Cancer Cells. Biochemistry. 2010;49:4635–4643. doi: 10.1021/bi1001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Placencio V, Iturregui JM, Uwamariya C, Sharif-Afshar AR, Koyama T, Hayward SW, Bhowmick NA. Prostate tumor progression is mediated by a paracrine TGF-beta/Wnt3a signaling axis. Oncogene. 2008;27:7118–7130. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. doi: 10.1186/1476-4598-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pode-Shakked N, Harari-Steinberg O, Haberman-Ziv Y, Rom-Gross E, Bahar S, Omer D, Metsuyanim S, Buzhor E, Jacob-Hirsch J, Goldstein RS, Mark-Danieli M, Dekel B. Resistance or sensitivity of Wilms’ tumor to anti-FZD7 antibody highlights the Wnt pathway as a possible therapeutic target. Oncogene. 2011;30:1664–1680. doi: 10.1038/onc.2010.549. [DOI] [PubMed] [Google Scholar]
- 51.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grossmann TN, Yeh JTH, Bowman BR, Chu Q, Moellering RE, Verdine GL. Inhibition of oncogenic Wnt signaling through direct targeting of beta-catenin. Proc Natl Acad Sci U S A. 2012;109:17942–17947. doi: 10.1073/pnas.1208396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao JJ, Mani M, Lemieux M, Carrasco DE, Ryan J, Horst D, Fulciniti M, Munshi NC, Xu W, Kung AL, Shivdasani RA, Walensky LD, Carrasco DR. Targeted disruption of the BCL9/betacatenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4:148ra117. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 55.Semenov MV, Snyder M. Human dishevelled genes constitute a DHR-containing multigene family. Genomics. 1997;42:302–310. doi: 10.1006/geno.1997.4713. [DOI] [PubMed] [Google Scholar]
- 56.Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: Isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- 57.Pizzuti A, Amati F, Calabrese G, Mari A, Colosimo A, Silani V, Giardino L, Ratti A, Penso D, Calza L, Palka G, Scarlato G, Novelli G, Dallapiccola B. cDNA characterization and chromosomal mapping of two human homologues of the Drosophila dishevelled polarity gene. Hum Mol Genet. 1996;5:953–958. doi: 10.1093/hmg/5.7.953. [DOI] [PubMed] [Google Scholar]
- 58.Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Dev Dyn. 1996;207:253–262. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 59.Wharton KA Jr. Runnin’ with the Dvl: Proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 61.Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA, Noutsou M, Rudiger SG, Schwamborn K, Schambony A, Maurice MM. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci U S A. 2012;109:E812–820. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao YM, Lee HJ, Wu AL, Fang YM, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization (vol 11, pg 286, 2009) Nat Cell Biol. 2009;11:508–508. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Punchihewa C, Ferreira AM, Cassell R, Rodrigues P, Fujii N. Sequence requirement and subtype specificity in the high-affinity interaction between human frizzled and dishevelled proteins. Protein Sci. 2009;18:994–1002. doi: 10.1002/pro.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Gao X, Wen J, Ning Y, Chen YG. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem. 2006;281:8607–8612. doi: 10.1074/jbc.M600274200. [DOI] [PubMed] [Google Scholar]
- 66.Chen H, Liu L, Ma B, Ma TM, Hou JJ, Xie GM, Wu W, Yang FQ, Chen YG. Protein kinase A-mediated 14-3-3 association impedes human Dapper1 to promote dishevelled degradation. J Biol Chem. 2011;286:14870–14880. doi: 10.1074/jbc.M110.211607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheyette BN, Waxman JS, Miller JR, Takemaru KI, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelledassociated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Developmental Cell. 2002;2:449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 68.Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- 69.Shan J, Zhang X, Bao J, Cassell R, Zheng JJ. Synthesis of potent dishevelled PDZ domain inhibitors guided by virtual screening and NMR studies. Chem Biol Drug Des. 2012;79:376–383. doi: 10.1111/j.1747-0285.2011.01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem. 2009;284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsiao SJ, Smith S. Tankyrase function at telomeres, spindle poles, and beyond. Biochimie. 2008;90:83–92. doi: 10.1016/j.biochi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 73.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Liu SM, Mickanin C, Feng Y, Charlat O, Michaud GA, Schirle M, Shi XY, Hild M, Bauer A, Myer VE, Finan PM, Porter JA, Huang SMA, Cong F. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol. 2011;13:623–U292. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]
- 75.Chen BZ, Dodge ME, Tang W, Lu JM, Ma ZQ, Fan CW, Wei SG, Hao WN, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wntdependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehtio L, Chi NW, Krauss S. Tankyrases as drug targets. FEBS J. 2013;280:3576–3593. doi: 10.1111/febs.12320. [DOI] [PubMed] [Google Scholar]
- 77.Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D, Gradl D, Paulsen JE, Machonova O, Dembinski JL, Dinh H, Krauss S. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71:197–205. doi: 10.1158/0008-5472.CAN-10-1282. [DOI] [PubMed] [Google Scholar]
- 78.James RG, Davidson KC, Bosch KA, Biechele TL, Robin NC, Taylor RJ, Major MB, Camp ND, Fowler K, Martins TJ, Moon RT. WIKI4, a novel inhibitor of tankyrase and Wnt/sscatenin signaling. PLoS One. 2012;7:e50457. doi: 10.1371/journal.pone.0050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voronkov A, Holsworth DD, Waaler J, Wilson SR, Ekblad B, Perdreau-Dahl H, Dinh H, Drewes G, Hopf C, Morth JP, Krauss S. Structural basis and SAR for G007-LK, a lead stage 1,2,4-triazole based specific tankyrase 1/2 inhibitor. J Med Chem. 2013;56:3012–3023. doi: 10.1021/jm4000566. [DOI] [PubMed] [Google Scholar]
- 80.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 82.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dodge ME, Moon J, Tuladhar R, Lu J, Jacob LS, Zhang LS, Shi H, Wang X, Moro E, Mongera A, Argenton F, Karner CM, Carroll TJ, Chen C, Amatruda JF, Lum L. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. J Biol Chem. 2012;287:23246–23254. doi: 10.1074/jbc.M112.372029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Pan SF, Hsieh MH, Ng N, Sun FX, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo GR, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che JW, Carey TE, Vanasse G, Harris JL. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM 3rd, Lee E. Smallmolecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venerando A, Girardi C, Ruzzene M, Pinna LA. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB downregulation and GSK3 activation. Biochem J. 2013;452:131–137. doi: 10.1042/BJ20121140. [DOI] [PubMed] [Google Scholar]
- 88.Del Valle-Perez B, Arques O, Vinyoles M, de Herreros AG, Dunach M. Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol Cell Biol. 2011;31:2877–2888. doi: 10.1128/MCB.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheong JK, Nguyen TH, Wang H, Tan P, Voorhoeve PM, Lee SH, Virshup DM. IC261 induces cell cycle arrest and apoptosis of human cancer cells via CK1delta/varepsilon and Wnt/beta-catenin independent inhibition of mitotic spindle formation. Oncogene. 2011;30:2558–2569. doi: 10.1038/onc.2010.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 91.Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three smallmolecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci U S A. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen ZC, Venkatesan AM, Dehnhardt CM, Dos Santos O, Delos Santos E, Ayral-Kaloustian S, Chen L, Geng Y, Arndt KT, Lucas J, Chaudhary I, Mansour TS. 2,4-Diamino-quinazolines as inhibitors of beta-catenin/Tcf-4 pathway: Potential treatment for colorectal cancer. Bioorg & Med Chem Lett. 2009;19:4980–4983. doi: 10.1016/j.bmcl.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 93.Trosset JY, Dalvit C, Knapp S, Fasolini M, Veronesi M, Mantegani S, Gianellini LM, Catana C, Sundstrom M, Stouten PF, Moll JK. Inhibition of protein-protein interactions: the discovery of druglike beta-catenin inhibitors by combining virtual and biophysical screening. Proteins. 2006;64:60–67. doi: 10.1002/prot.20955. [DOI] [PubMed] [Google Scholar]
- 94.Tian W, Han X, Yan M, Xu Y, Duggineni S, Lin N, Luo G, Li YM, Huang Z, An J. Structurebased discovery of a novel inhibitor targeting the beta-catenin/Tcf4 interaction. Biochemistry. 2012;51:724–731. doi: 10.1021/bi201428h. [DOI] [PubMed] [Google Scholar]
- 95.Lu D, Liu JX, Endo T, Zhou H, Yao S, Willert K, Schmidt-Wolf IG, Kipps TJ, Carson DA. Ethacrynic acid exhibits selective toxicity to chronic lymphocytic leukemia cells by inhibition of the Wnt/beta-catenin pathway. PLoS One. 2009;4:e8294. doi: 10.1371/journal.pone.0008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin GY, Lu DS, Yao SY, Wu CCN, Liu JX, Carson DA, Cottam HB. Amide derivatives of ethacrynic acid: Synthesis and evaluation as antagonists of Wnt/beta-catenin signaling and CLL cell survival. Bioorg & Med Chem Lett. 2009;19:606–609. doi: 10.1016/j.bmcl.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMillan M, Kahn M. Investigating Wnt signaling: a chemogenomic safari. Drug Discov Today. 2005;10:1467–1474. doi: 10.1016/S1359-6446(05)03613-5. [DOI] [PubMed] [Google Scholar]
- 98.Kung AL, Rebel VI, Bronson RT, Ch’ng LE, Sieff CA, Livingston DM, Yao TP. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14:272–277. [PMC free article] [PubMed] [Google Scholar]
- 99.Yamauchi T, Oike Y, Kamon J, Waki H, Komeda K, Tsuchida A, Date Y, Li MX, Miki H, Akanuma Y, Nagai R, Kimura S, Saheki T, Nakazato M, Naitoh T, Yamamura K, Kadowaki T. Increased insulin sensitivity despite lipodystrophy in Crebbp heterozygous mice. Nat Genet. 2002;30:221–226. doi: 10.1038/ng829. [DOI] [PubMed] [Google Scholar]
- 100.Roth JF, Shikama N, Henzen C, Desbaillets I, Lutz W, Marino S, Wittwer J, Schorle H, Gassmann M, Eckner R. Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. EMBO J. 2003;22:5186–5196. doi: 10.1093/emboj/cdg473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teo JL, Ma H, Nguyen C, Lam C, Kahn M. Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci U S A. 2005;102:12171–12176. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kahn M. Symmetric division versus asymmetric division: a tale of two coactivators. Future Med Chem. 2011;3:1745–1763. doi: 10.4155/fmc.11.126. [DOI] [PubMed] [Google Scholar]
- 103.Hao J, Ao A, Zhou L, Murphy CK, Frist AY, Keel JJ, Thorne CA, Kim K, Lee E, Hong CC. Selective small molecule targeting betacatenin function discovered by in vivo chemical genetic screen. Cell Rep. 2013;4:898–904. doi: 10.1016/j.celrep.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] . Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 106.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 108.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H. A Critical Role for the Wnt Effector Tcf4 in Adult Intestinal Homeostatic Self-Renewal. Mol Cell Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 111.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-Catenin is essential for intestinal Homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 113.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 114.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan KS, Chia LA, Li XN, Ootani A, Su J, Lee JY, Su N, Luo YL, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith DC, Rosen LS, Chugh R, Goldman JW, Xu L, Kapoun A, Brachmann RK, Dupont J, Stagg RJ, Tolcher AW, Papadopoulos KP. First-inhuman evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. Journal of Clinical Oncology. 2013:31. [Google Scholar]
- 117.El-Khoueiry AB, Ning Y, Yang DY, Cole S, Kahn M, Zoghbi M, Berg J, Fujimori M, Inada T, Kouji H, Lenz HJ. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. Journal of Clinical Oncology. 2013:31. [Google Scholar]


