Abstract
Transarterial chemoembolization (TACE) is commonly used for the treatment of locally advanced hepatocellular carcinoma (HCC) by its dual effects of chemotherapy and ischemic hypoxia. However, one of the side effects of TACE is the introduction of hypoxic condition, which in turn activates hypoxia-induced survival pathways and enhances VEGF-induced neovascularization by stabilizing HIF-1α expression. Herein, the preclinical therapeutic efficacy of the combined treatment of everolimus, a novel mTOR inhibitor and TACE for the treatment of HCC was investigated. The MHCC-97L cells were used for the study of the effect of combined treatment on cell proliferation and cellular apoptosis. HUVEC cells were used for the study on tube formation under different treatments. Inhibitions on the Akt/mTOR pathways were also studied. Finally, the effect on tumor growth was further study using an in vivo orthotopic model. The results demonstrated that everolimus enhanced the therapeutic efficacy of TACE in inhibiting cell proliferation, promoting apoptosis and inhibiting tube formation of endothelial cells by blocking the Akt/mTOR signaling pathway in vitro and inhibiting tumor growth and neoangiogenesis in vivo. Based on this preclinical study, the potential of combining everolimus with TACE was guaranteed which suggested the use of the combination therapy in the clinical treatment of advanced HCC patients.
Keywords: Everolimus, hepatocellular carcinoma, hypoxia, mTOR signaling pathway, transarterial chemoembolization, tumor growth
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide [1]. Surgery for HCC including partial liver resection and liver transplantation remains the most effective treatment for HCC. However, only a minority of HCC patients are eligible to the surgical treatment and poor prognosis with high recurrence rate is always observed [2-4]. In addition, adjuvant systemic chemotherapy has not been shown to be beneficial in HCC patients due to the chemoresistance of HCC cells and poor tolerance and side effects of anti-tumor drugs to cirrhotic patients [2,5]. Therefore, it is worthwhile to develop more efficient treatment for unresectable HCC patients.
Transarterial chemoembolization (TACE) is commonly used for the treatment of unresectable HCC. It is done by injecting an embolic particles coated with chemotherapeutic agents such as cisplatin into the hepatic artery. It then works by blocking the blood supply to the tumor as well as delivering chemotherapeutic drug directly to the tumor. TACE was found to prolong the overall survival of patients with unresectable HCC compared with conservative treatment in two randomized trials [6,7]. However, some studies showed that TACE cannot completely eliminate the risk of a recurrence [8,9]. In addition, blocking the blood supply induces hypoxic condition, which in turn stabilizes the hypoxia inducible factor 1α (HIF-1α) protein. HIF-1α activates the transcription of genes that are involved in angiogenesis, cell survival, and cell migration and invasion which are crucial to cancer growth and recurrence [10]. Therefore, there is still room for improvement to enhance the tumor eliminating effect of TACE in order to prevent recurrence in HCC.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase and member of the phosphoinositide 3-kinase (PI3K)-related kinase family. It integrates and transmits signals from a diverse array of signaling pathways to regulate cell survival and growth [11]. mTOR exists in two complexes, mTORC1 and mTORC2, which controls cell growth and cell survival, respectively. Several reports have indicated that mTOR is a positive regulator of hypoxia inducible factor 1 (HIF-1) expression and activity [12], and the inhibition of HIF-mediated gene expression is considered to be related to the anti-tumor activity of mTOR inhibitors [13]. In addition, HIF-1 activates vascular endothelial growth factor (VEGF) gene expression [14]. Therefore, the Akt/mTOR signaling pathway is also important in regulating neovascularization which is a crucial factor for the success of TACE treatment [15].
Everolimus is an mTOR inhibitor which is currently used as an immunosuppressant [16-18] and as a molecular targeting drug in cancers such as renal cell carcinoma and breast cancer. Our previous study has demonstrated that everolimus suppressed HCC tumor growth and sensitized tumor cells to cisplatin-induced cytotoxicity [19]. Therefore, we hypothesized that everolimus enhances the therapeutic efficacy of TACE by inhibiting the mTOR mediated HIF-1 expression and sensitizing the cisplatin effect on HCC tumor cells. The combination effect of everolimus and cisplatin under hypoxic condition was first studied by in vitro experiments. We then mimicked the TACE treatment using a mouse orthotopic HCC model by hepatic artery ligation (HAL) and intraprotal vein injection of cisplatin. The effect of everolimus on this in vivo model was then investigated. Therefore, with this study, the pre-clinical enhancing effect of everolimus on TACE treatment can be demonstrated which provides the basis for future clinical practice of everolimus on TACE treatment.
Materials and methods
Drugs and reagents
Everolimus was kindly provided by Novartis Pharmaceuticals Corporation. All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified below.
Cell culture and treatment
MHCC97L cells (Liver Cancer Institute, Fudan University) were maintained in DMEM (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies) and 1% penicillin/streptomycin (Life Technologies) at 37°C in a humidified 5% CO2 atmosphere. MHCC97L cells were stably transfected with luciferase expressing construct for the ease of detection in the in vivo study. Human umbilical vein endothelial cells (HUVEC; Life Technologies) were grown in a complete endothelial growth medium (Life Technologies) and were used between passages 4-6.
For the in vitro study, cells were treated as 1) Control group: in normoxic condition (20% O2, 5% CO2 and 75% N2) without any drug treatment, 2) Hypoxia group: in hypoxic condition (1% O2, 5% CO2 and 94% N2) maintained in an OxyCycler C42 hypoxia chamber (Biospherix, St. Lacona, NY), 3) Hypoxia + C group: in hypoxic condition and 6 μM cisplatin, 4) Hypoxia + E group: in hypoxic condition and 10 nM everolimus, and 5) Hypoxia + C + E group: in hypoxic condition, 6 μM cisplatin and 10 nM everolimus.
Cell proliferation assay
The treatment effect on MHCC97L cells was first examined by MTT assay as previously described [20]. Briefly, cells were plated in 96-well culture plates for 24 hours and media were replaced with the treatment media with increasing concentrations of everolimus (0 to 100 nM) under normoxia, hypoxia or hypoxia with 6 μM cisplatin. After 72 hours, viability was assessed with the replacement of treatment media with MTT solution (1 mg/ml) (Life Technologies) and the average readings of the absorbance at 570 nm from 3 replicates of each treatment were determined.
Apoptosis assay
After MHCC97L cells were treated for 48 hours, cells were harvested, washed with ice-cold PBS, and stained with annexin V-FITC antibody (BD Biosciences Pharmingen). The percentage of apoptotic cells (annexin V positive cells) were determined by flow cytometry using the Cytomics FC500 Analyzer (Beckman-Coulter, CA). Unstained cells were used as a negative control.
Tube formation assay
The tube formation ability of the HUVEC cells was determined by the Endothelial Cell Tube Formation Assay (Life technologies) according to the manufacturer’s instructions. The bottoms of a 96-well plate were first coated with Geltrex™ Reduced Growth Factor Basement Membrane Matrix and 5 × 104 HUVEC cells were seeded on the surface of the gel and after treatment for 6 h, cells were stained with Calcein AM (Life technologies) and assessed under a fluorescence microscope (400 × magnification).
Multiplex bead assay
After MHCC97L cells were treated for 24 hours, cells were harvested and washed in ice-cold PBS. The expression levels of phosphorylated mTOR (Ser2448), p70S6K (Thr412), RPS6 (Ser235/Ser236), Akt (Ser473), TSC2 (Ser939), GSK3α (Ser21), GSK3β (Ser9), and p53 (Ser15) were quantified using a bead-based MILLIPLEX® multiplex assay (Millipore, Billerica, MA) according to the manufacturer’s instructions. Briefly, cells were lysed in MILLIPLEX MAP lysis buffer and diluted with an equal volume of MILLIPLEX MAP cell assay buffer. Capture antibody beads were diluted in MILLIPLEX MAP cell assay buffer and added to a Beadlike filter plate. 25 μl of the diluted cell lysate was then transferred to each well of the filter plate and incubated for 2 h at room temperature with shaking. After incubation, the beads were washed twice with cell assay buffer, and biotinylated detection antibodies diluted in cell assay buffer were added and incubated for 1 h at room temperature with shaking. After filtering, MILLIPLEX MAP streptavidin-phycoerythrin solution was added and incubated for 30 min at room temperature with shaking. Finally, cell assay buffer was added after filtering, and signals were read using the Bio-Plex Luminex 200 system (Bio-Rad, Hercules, CA). The fluorescence signal of each phosphorylated protein was normalized with that of GAPDH.
Immunoblotting
After treatment, cells were lysed by ice-cold RIPA buffer containing 150 mM NaCl, 1 mM EDTA, 1% (v/v) NP-40, 0.25% (w/v) sodium deoxycholate, 1 mM PMSF, and protease inhibitor cocktail (Roche Diagnostics, Penzbery, Germany) in 50 mM Tris-HCl buffer, pH 7.4. Equal amount of protein was loaded onto a 10% SDS-polyacrylamide gel under reducing condition and transferred to PVDF membrane (Amersham Bioscience, Piscataway, NJ). Blots were probed with HIF-1α (Novus Biologicals, Littleton, CO), VEGF (Santa Cruz Biotechnology) and β actin (Sigma) overnight at 4°C. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (DAKO) and visualized using an enhanced chemiluminescence detection system (ECL Plus) (Amersham) according to the manufacturer’s instructions.
In vivo orthotopic mouse HCC model
All the animal experiments were approved by the Committee on the Use of Live Animals in Teaching and Research of The University of Hong Kong. Male CB17 severe combined immunodeficient (SCID) mice of four to six weeks old were used and maintained in laminar flow cabinets under pathogen-free condition. Subcutaneous tumors were first grown by injecting MHCC97L cells (1 × 106) in a 0.2 ml culture medium subcutaneously into the right flank of mice. Once the subcutaneous tumors reached 0.8-1 cm in diameter, mice were sacrificed and tumors were removed and cut into 2 mm3 cubes, which were then implanted into the left liver lobes of another group of SCID mice. Six days after tumor implantation, the animals were randomly divided into five groups: 1) sham operation (Control; n=5), 2) hepatic artery ligation (HAL; n=5), 3) hepatic artery ligation combined with portal vein injection of cisplatin (3 mg/kg) (HAL + C; n=5), 4) hepatic artery ligation combined with oral feeding of everolimus daily (10 mg/kg) (HAL + E), and 5) HAL combined with portal vein injection of cisplatin (3 mg/kg) and oral feeding of everolimus daily (10 mg/kg) (HAL + C + E; n=5). Mice were sacrificed on day 25. Under anesthesia, D-luciferin (Life Technologies) was injected i.p. and the bioluminescence signal was detected by the IVIS imaging system 100 (Xenogen). An elliptical region of interest (ROI) was placed over the tumors and the total signal in the ROI (photons per second) was quantified using the Living Image software (Xenogen). Liver were dissected, fixed in 10% formalin, and paraffin-embedded for further analysis. This animal model was repeated and overall survival was monitor for 80 days for the toxicity test of each treatment.
Microvessel density (MVD) measurement
After antigen retrieval of the liver sections, slides were incubated with anti-CD31 antibody (Thermo Scientific, Waltham, MA) overnight at 4°C and signal was detected by the LSAB + System-HRP kits (Dako) according to the manufacturer’s instruction. Sections were then counterstained with hematoxylin and dehydrated through a series of ethanol and xylenes. Slides were then examined with light microscope (100 ×) and single endothelial cells or clusters of endothelial cells positive for CD31 staining were considered as a vessel. MVD was measured based on the Weidner’s method [21] by two independent investigators who were blinded to the study groups.
Statistical analysis
Data were presented as means ± SD from three independent experiments for the in vitro study and from n=5 for the in vivo study. Data were statistically analyzed with one-way ANOVA. Survival analysis was done by Mantel-Cox (Log-Rank) test. All analysis was performed by Prism 5.0 and data were considered statistically significant at P<0.05.
Results
Anti-proliferative effect of combined treatment with Hypoxia + C + E
The effect of different treatments on cell proliferation was first measured by the MTT cell proliferation assay (Figure 1). Cell proliferation decreased with increasing concentrations of everolimus under all the treatment condition. Without the addition of everolimus, treatment of cells under hypoxic condition and under hypoxic condition with 6 μM cisplatin reduced the cell proliferation by 59.7% and 65.1%, respectively, when compared with that treated under normoxic condition. The IC50 under normoxia, hypoxia and hypoxia with cisplatin were 11.2 nM, 11.6 nM and 12.2 nM, respectively. With the addition of 10 nM everolimus, treatment of cell under hypoxic condition with 6 μM cisplatin significantly reduced the cell proliferation by 64.0% and 39.2% when compared with that treated under normoxic condition and hypoxic condition, respectively. This suggested that 10 nM everolimus is the optimized concentration in enhancing the anti-proliferating effect of single treatment.
Figure 1.
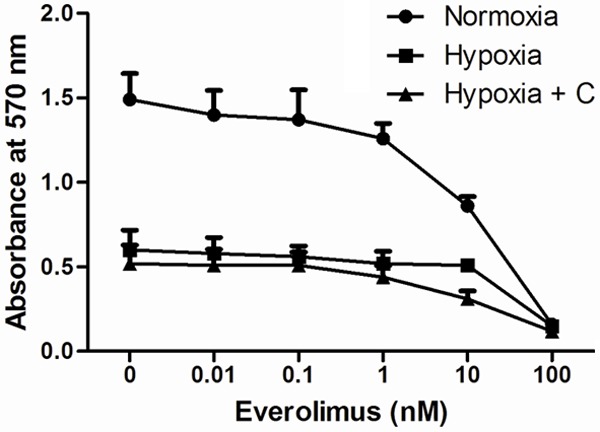
Cell proliferation of MHCC-97L cells. Cells were treated with increasing concentrations of everolimus under normoxic, hypoxic or hypoxic condition with 6 μM cisplatin. Cell proliferation was analyzed by MTT assay. Data were presented as means ± SD from three independent experiments.
Apoptotic effect of the combined treatment with Hypoxia + C + E
We then determined the apoptotic effect of different treatments with the annexin V assay (Figure 2). Treatment of cells with Hypoxia, Hypoxia + C and Hypoxia + E significantly increased the percentage of apoptotic cells by 3.01 folds, 3.89 folds and 2.33 folds, respectively, when compared with the Control. For the combined treatment with Hypoxia + C + E, the percentage of apoptotic cells increased by 11.4 folds when compared with the Control. In addition, treatment with Hypoxia + C + E further increased the percentage of apoptotic cells by 3.78 folds when compared with the treatment with Hypoxia, by 2.93 folds when compared with the treatment with Hypoxia + C, and by 4.89 folds when compared with the treatment with Hypoxia + E treatment. This suggested that combined treatment with Hypoxia + C + E was significant in enhancing cellular apoptosis when compared with the single treatment.
Figure 2.

Cellular apoptosis of MHCC-97L cells. Cells were treated with Control: in normoxic condition (20% O2, 5% CO2 and 75% N2) without any drug treatment, Hypoxia: in hypoxic condition (1% O2, 5% CO2 and 94% N2), Hypoxia + C: in hypoxic condition and 6 μM cisplatin, Hypoxia + E: in hypoxic condition and 10 nM everolimus, and Hypoxia + C + E: in hypoxic condition, 6 μM cisplatin and 10 nM everolimus. The percentage of apoptotic cells were the percentage of annexin V positive cells analyzed by flow cytometry. Data were presented as means ± SD from three independent experiments and statistical analysis were performed by one-way ANOVA. *P<0.05 versus Control. #P<0.05 of Hypoxia + C + E versus Hypoxia, Hypoxia + C or Hypoxia + E.
Inhibiting effect of the combined treatment with Hypoxia + C + E on tube formation ability of HUVEC cells
Treatment with Hypoxia significantly enhanced the tube formation ability of HUVEC cells by 40.0% when compared with the Control (Figure 3). For the combined treatment with Hypoxia + C + E, the tube formation ability of HUVEC cells decreased by 52.0% when compared with the treatment with Hypoxia, and by 46.7% when compared with the treatment with Hypoxia + C. This suggested that combined treatment with Hypoxia + C + E was significant in reducing tube formation ability of HUVEC cells when compared with the single treatments.
Figure 3.
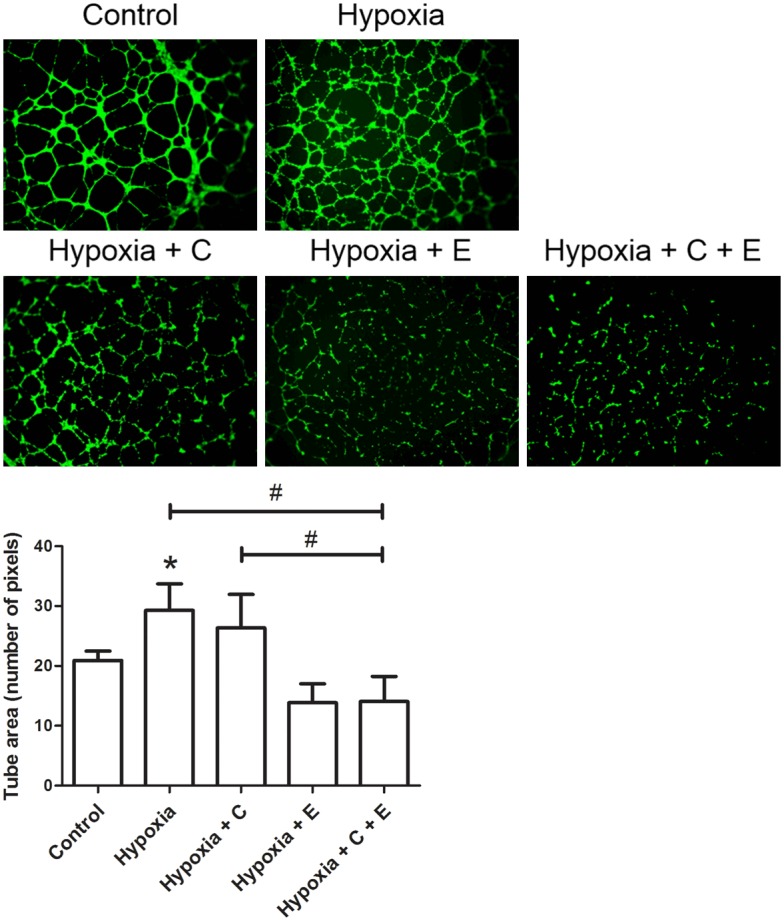
Tube formation ability of HUVEC cells. Cells were treated with Control: in normoxic condition (20% O2, 5% CO2 and 75% N2) without any drug treatment, Hypoxia: in hypoxic condition (1% O2, 5% CO2 and 94% N2), Hypoxia + C: in hypoxic condition and 6 μM cisplatin, Hypoxia + E: in hypoxic condition and 10 nM everolimus, and Hypoxia + C + E: in hypoxic condition, 6 μM cisplatin and 10 nM everolimus. Upper panel: Representative fluorescence images (magnification: 400 ×) of tube formation of cells in Geltrex™ Reduced Growth Factor Basement Membrane Matrix. Lower panel: Bar chart presenting the tube area (number of pixels) of HUVEC cells under different treatment. Data were presented as means ± SD from three independent experiments and statistical analysis were performed by one-way ANOVA. *P<0.05 versus Control. #P<0.05 of Hypoxia + C + E versus Hypoxia or Hypoxia + C.
Inhibiting effect of the combined treatment with Hypoxia + C + E on Akt/mTOR signaling pathway
Phosphoprotein alteration was analyzed using the MILLIPLEX MAP 8-Plex Multi-Pathway Signaling Phosphoprotein Kit (Figure 4). Phosphorylation of different targets/effectors of the Akt/mTOR pathway including p70S6, RPS6, Akt, TSC2, GSK3α and GSK3β was studied and each single treatment caused a significant down-regulation of the Akt/mTOR signaling pathway when compared with the Control. In addition, the combined treatment with Hypoxia + C + E further reduced the levels of these phosphorylated proteins. These results suggested a more potent inhibitory effect on the Akt/mTOR signaling pathway was observed in the combined treatment with Hypoxia + C + E than that of the single treatments.
Figure 4.

Expression of phosphorylated proteins in the Akt/mTOR signaling pathway. MHCC-97L cells were treated with Control: in normoxic condition (20% O2, 5% CO2 and 75% N2) without any drug treatment, Hypoxia: in hypoxic condition (1% O2, 5% CO2 and 94% N2), Hypoxia + C: in hypoxic condition and 6 μM cisplatin, Hypoxia + E: in hypoxic condition and 10 nM everolimus, and Hypoxia + C + E: in hypoxic condition, 6 μM cisplatin and 10 nM everolimus. The expressions of phosphorylated mTOR, p70S6, RPS6, Akt, TSC2, GSK3α and GSK3β were measured by the multiplex bead assay. The expression of GAPDH was used as internal control. Data were presented as means ± SD from three independent experiments and statistical analysis were performed by one-way ANOVA. *P<0.05 versus Control. #P<0.05 of Hypoxia + C + E versus Hypoxia, Hypoxia + C or Hypoxia + E.
Combined treatment with Hypoxia + C + E was effective in degrading hypoxia-induced HIF-1α expression
When compared with the Control, induced expression of HIF-1α was found in the treatment groups of Hypoxia by 2.34 folds and Hypoxia + C by 88.0% (Figure 5). Combined treatment with Hypoxia + C + E was found to reduce HIF-1α expression significantly by 2.66 folds when compared with the treatment with Hypoxia, and by 2.14 folds when compared with the treatment with Hypoxia + C. This change in expression pattern was also found in VEGF expression. When compared with the Control, induced expression of VEGF was found in the treatment groups of Hypoxia by 16.2% and Hypoxia + C by 19.5%. Combined treatment with Hypoxia + C + E was found to reduce HIF-1α expression significantly by 29.5% when compared with the treatment with Hypoxia, and by 13.9% when compared with the treatment with Hypoxia + C. This suggested that combined treatment with Hypoxia + C + E was effective in degrading HIF-1α, which in turn reduced VEGF expression.
Figure 5.
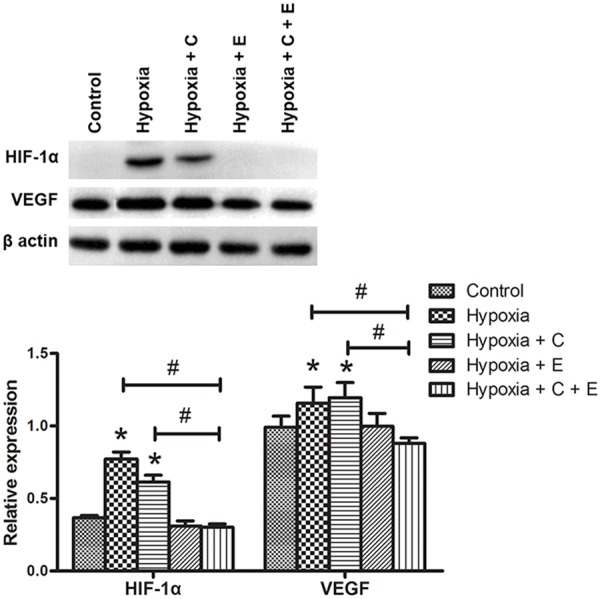
Western blotting analysis of the expression of HIF-1α and VEGF. MHCC-97L cells were treated with Control: in normoxic condition (20% O2, 5% CO2 and 75% N2) without any drug treatment, Hypoxia: in hypoxic condition (1% O2, 5% CO2 and 94% N2), Hypoxia + C: in hypoxic condition and 6 μM cisplatin, Hypoxia + E: in hypoxic condition and 10 nM everolimus, and Hypoxia + C + E: in hypoxic condition, 6 μM cisplatin and 10 nM everolimus. Upper panel: Representative blots of the expression of HIF-1α and VEGF were shown. The expression level of β-actin was used as the loading control. Lower panel: Bar chart presenting the ratio of HIF-1α vs. β-actin and VEGF vs. β-actin was shown. Data were presented as means ± SD from three independent experiments and statistical analysis were performed by one-way ANOVA. *P<0.05 versus Control. #P<0.05 of Hypoxia + C + E versus Hypoxia or Hypoxia + C.
Anti-tumor effect of the combined treatment with HAL + C + E
An in vivo orthotopic mouse model was used to demonstrate the anti-tumor effect of different treatments. Measurement of bioluminescence signal was done on day 6, day 10, day 15 and day 25 after orthotopic tumor implantation (Figure 6A-C). Longitudinal monitoring of tumor growth showed that significant inhibition of tumor growth and progression occurred in all the treatment groups starting on Day 15. In addition, a more pronounced anti-tumor effect was shown in the group with combined treatment with HAL + C + E when compared with the treatment group with HAL and HAL + C. The survival analysis was also performed to study the toxicity effect of different treatments (Figure 6D). Combined treatment with HAL + C + E significantly enhanced the overall survival from 40 days in the control group to 67.5 days in the HAL + C + E group.
Figure 6.
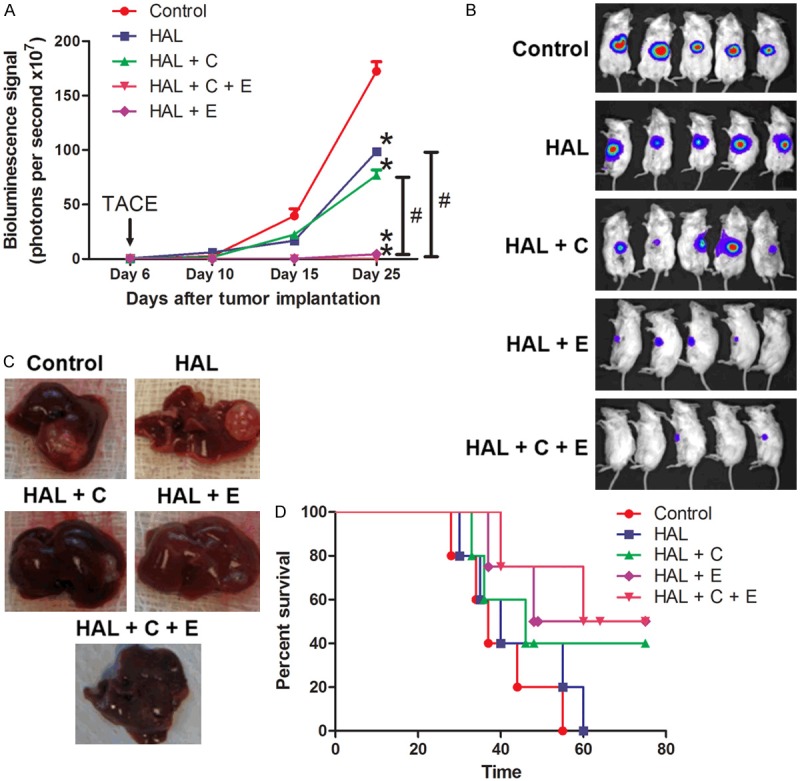
Tumor growth and progression of the mouse orthotopic model. Six days after tumor implantation, the animals were randomly divided into five groups: (1) sham operation (Control; n=5), (2) hepatic artery ligation alone (HAL; n=5), (3) hepatic artery ligation combined with portal vein injection of cisplatin (3 mg/kg) (HAL + C; n=5), (4) hepatic artery ligation combined with oral feeding of everolimus daily (10 mg/kg) (HAL + E) and (5) hepatic artery ligation combined with portal vein injection of cisplatin (3 mg/kg) and oral feeding of everolimus daily (10 mg/kg) (HAL + C + E; n=5). A. Mice were sacrificed on day 25. Tumor growth was monitor and the average bioluminescence signals detected against days after tumor implantation were plotted. Data were presented as means ± SEM and statistical analysis were performed by one-way ANOVA. *P<0.05 versus Control. #P<0.05 of HAL + C + E versus HAL or HAL + C. B. Mice with tumors from MHCC97L cells were shown. C. Representing gross images of the liver from each group were shown. D. Animal survival of each group was analyzed by log-rank test.
To examine the effect of different treatments on microvascular density (MVD) in tumors, blood vessels expressing CD31 were counted (Figure 7). Treatment with HAL and HAL + C significantly enhanced angiogenesis with an increase in MVD by 2.63 folds and 2.78 folds, respectively, when compared with the Control group. For the group with combined treatment with HAL + C + E, MVD reduced by 8.27 folds when compared with the HAL group, and 8.73 folds when compared with the HAL + C group. These results suggested that combined treatment with HAL + C + E was more effective in inhibiting tumor growth and progression when compared with single treatments. In addition, treatment with HAL + C + E was effective in inhibiting hypoxia induced microvascular growth which makes the combined treatment a more potent treatment in reducing tumor size in this in vivo study.
Figure 7.

Immunohistochemistry of CD-31 expression and the MVD analysis. The MVD measurement in the tumor of each liver section by CD31 expression. Upper panel: Representing images of the CD-31 staining (magnification: 100 ×) of tumor in the liver section from each group were shown. Lower panel: the average MVD measurements were plotted. Data were presented as means ± SD and statistical analysis were performed by one-way ANOVA. *P<0.05 versus Control. #P<0.05 of HAL + C + E versus HAL or HAL + C.
Discussion
TACE is one of the effective treatments in advanced HCC with the simultaneous actions of blocking blood supply and administration of a high dose of chemotherapeutic agent to the tumor. However, one of the side effects of TACE is the introduction of hypoxic condition, which in turn activates hypoxia-induced survival pathways and enhances VEGF-induced neovascularization by stabilizing HIF-1α expression. A clinical study demonstrated that increases in neovascularization factors, VEGF and basic fibroblast growth factor (b-FGF), were found in non-responders of TACE treatment and VEGF was even singled out as an independent predictor of survival [22]. Another study on TACE also demonstrated that the residual surviving cancerous tissue in HCC after TACE was highly vascularized and TACE increased VEGF expression in the residual surviving cancerous tissue [23]. In addition, enhanced invasion of tumor and distant metastasis after hepatic artery ligation in orthotopic HCC mouse models were reported [24,25]. These studies suggested that neovascularization is one of the most crucial obstacles affecting the response of TACE in HCC patients.
Everolimus is a potent mTOR inhibitor. Previous studies have provided evidence that the Akt/mTOR pathway is not only important for the survival of tumor cells but also for angiogenesis of tumor under hypoxia [26]. Studies demonstrated that mTOR is a positive regulator of HIF-1 dependent gene transcription in cells exposed to hypoxia [27]. HIF1-α plays an important role in the up-regulation of certain hypoxia-related proteins involved in angiogenesis, such as VEGF, and in the inhibition of apoptosis [28]. HIF1-α has also been reported to interact with Akt to mediate hypoxia-induced chemoresistance of cisplatin [29]. Therefore, blocking the Akt/mTOR/HIF-1 pathway by everolimus was expected to enhance the TACE efficacy in this study.
Our previous study showed that single treatment with everolimus inhibited HCC tumor growth and enhanced the sensitivity of HCC tumor cells to cisplatin-induced cytotoxicity [30]. In this study, we demonstrated that everolimus significantly enhanced the therapeutic efficacy of TACE using the in vitro and in vivo orthotopic mouse model. We showed that everolimus in combined with TACE significantly suppressed cell proliferation and enhanced cellular apoptosis in MHCC97L cells when compared with single TACE treatment or hypoxia treatment. In addition, the combined treatment was effective in inhibiting tube formation of HUVEC cells. This is supported by the inactivation of the Akt/mTOR signaling pathway with the decrease in phosphorylated p70S6, RPS6, Akt, TSC2, GSK3α and GSK3β expressions and the reduction in HIF-1α and VEGF expression.
In conclusion, by blocking the Akt/mTOR signaling pathway, everolimus enhanced the therapeutic efficacy of TACE. Based on this preclinical study, the potential of combining everolimus with TACE was guaranteed which suggested the use of the combination therapy in the clinical treatment of advanced HCC patients.
Acknowledgements
This study was supported by the Central Allocation Group Research Grant ‘Molecular Pathology of Liver Cancer-A Multidisciplinary Study’ from the Research Grant Council of Hong Kong. The animal imaging data were acquired using equipment maintained by the University of Hong Kong Li Ka Shing Faculty of Medicine Faculty Core Facility. We would also like to thank the Center for Cancer Research, The University of Hong Kong Li Ka Shing Faculty of Medicine, for all their technical supports.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: A daunting challenge. Hepatology. 2011;54:757–9. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, Lopez JP, Poon RT, Fan ST. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology. 2010;52:528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- 6.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, Rodes J, Bruix J. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 8.Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295–301. [PubMed] [Google Scholar]
- 9.Sun HC, Tang ZY. Preventive treatments for recurrence after curative resection of hepatocellular carcinoma--a literature review of randomized control trials. World J Gastroenterol. 2003;9:635–640. doi: 10.3748/wjg.v9.i4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 11.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 12.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–442. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Tan D, Zhang Z, Liang JJ, Brown RE. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol Rep. 2008;20:713–719. [PubMed] [Google Scholar]
- 16.Budde K, Fritsche L, Waiser J, Glander P, Slowinski T, Neumayer HH RADW 102 Renal Transplant Study Group. Pharmacokinetics of the immunosuppressant everolimus in maintenance renal transplant patients. Eur J Med Res. 2005;10:169–174. [PubMed] [Google Scholar]
- 17.Imamura T, Kinugawa K, Ono M, Kagami Y, Endo M, Minatsuki S, Muraoka H, Kato N, Inaba T, Maki H, Hatano M, Yao A, Kyo S, Komuro I. Everolimus-incorporated immunosuppressant strategy improves renal dysfunction while maintaining low rejection rates after heart transplantation in Japanese patients. Int Heart J. 2013;54:222–227. doi: 10.1536/ihj.54.222. [DOI] [PubMed] [Google Scholar]
- 18.Levy G, Schmidli H, Punch J, Tuttle-Newhall E, Mayer D, Neuhaus P, Samuel D, Nashan B, Klempnauer J, Langnas A, Calmus Y, Rogiers X, Abecassis M, Freeman R, Sloof M, Roberts J, Fischer L. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 2006;12:1640–1648. doi: 10.1002/lt.20707. [DOI] [PubMed] [Google Scholar]
- 19.Tam KH, Yang ZF, Lau CK, Lam CT, Pang RW, Poon RT. Inhibition of mTOR enhances chemosensitivity in hepatocellular carcinoma. Cancer Lett. 2009;273:201–209. doi: 10.1016/j.canlet.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Chow AK, Ng L, Sing Li H, Cheng CW, Lam CS, Yau TC, Cheng PN, Fan ST, Poon RT, Pang RW. Anti-tumor efficacy of a recombinant human arginase in human hepatocellular carcinoma. Curr Cancer Drug Targets. 2012;12:1233–1243. doi: 10.2174/156800912803988002. [DOI] [PubMed] [Google Scholar]
- 21.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 22.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–529. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Ren ZG, Shen Y, Zhu XD, Zhang W, Xiong W, Qin Y, Tang ZY. Influence of hepatic artery occlusion on tumor growth and metastatic potential in a human orthotopic hepatoma nude mouse model: relevance of epithelialmesenchymal transition. Cancer Sci. 2010;101:120–128. doi: 10.1111/j.1349-7006.2009.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Kobayashi S, Phongkitkarun S, Broemeling LD, Kan Z. Effect of transcatheter hepatic arterial embolization on angiogenesis in an animal model. Invest Radiol. 2006;41:516–521. doi: 10.1097/01.rli.0000209663.00629.8a. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZF, Poon RT, Liu Y, Lau CK, Ho DW, Tam KH, Lam CT, Fan ST. High doses of tyrosine kinase inhibitor PTK787 enhance the efficacy of ischemic hypoxia for the treatment of hepatocellular carcinoma: dual effects on cancer cell and angiogenesis. Mol Cancer Ther. 2006;5:2261–2270. doi: 10.1158/1535-7163.MCT-06-0149. [DOI] [PubMed] [Google Scholar]
- 27.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Molecul Med. 2002;8:S62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 29.Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GC, Poon RT, Fan ST. An Akt/hypoxia-inducible factor-1alpha/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res. 2009;15:3462–3471. doi: 10.1158/1078-0432.CCR-08-2127. [DOI] [PubMed] [Google Scholar]
- 30.Tam KH, Yang ZF, Lau CK, Lam CT, Pang RW, Poon RT. Inhibition of mTOR enhances chemosensitivity in hepatocellular carcinoma. Cancer Lett. 2009;273:201–209. doi: 10.1016/j.canlet.2008.08.018. [DOI] [PubMed] [Google Scholar]


