Abstract
Visfatin, a newly discovered adipocytokine, is a pro-inflammatory cytokine. This study aimed to evaluate the predictive value of visfatin on prognosis of patients with upper tract urothelial carcinoma. One-hundred and five patients (median age=64, range=24-84 years) were included in this study. Visfatin expression in upper tract urothelial carcinoma tissues was analyzed by immunohistochemistry. Visfatin expression was correlated with clinicopathologic variables using the χ2 test. The prognostic value of visfatin for recurrence-free and cancer-specific survival was evaluated by Kaplan-Meier estimates, and the significance of differences between curves was evaluated by the log-rank test. Cox regression model was also used to evaluate the hazard ratios of visfatin on survival. High visfatin expression in upper tract urothelial carcinoma tissues was significantly correlated with tumor stage (P=0.001), grade (P=0.007) and p53 expression (P=0.07). High visfatin expression was associated with poor recurrence-free and cancer-specific survival. Cox regression analysis also revealed that visfatin is an independent predictor of recurrence-free (HR=3.22, P=0.009) and cancer-specific survival (HR=5.74, P=0.023). Our findings indicated that higher visfatin expression is a potential biomarker to predict patient survival. Further study is necessary to investigate the role of visfatin in the carcinogenesis of upper tract urothelial carcinoma.
Keywords: Nampt/PBEF/visfatin, upper tract urothelial carcinoma, immunohistochemistry, prognosis, survival
Introduction
Urothelial carcinoma (UC) is the most common malignancy in urinary tract. The incidence of renal pelvic and ureteral cancer is very rare, accounting for only 4% of all urothelial cancers [1]. In United States, renal pelvic cancer accounted for about only 8% of all renal cancers, and ureter cancer about 5% of all urothelial cancers. However, an unusually high incidence of upper tract urothelial carcinomas (UTUC) had been reported [2,3], showing that there may be some unknown genetic and environment factors for UTUC in Taiwan.
Nephroureterectomy with excision of bladder cuff is the standard treatment for UTUC. Pathological characteristics, such as stage and grade, are the strongest factors to predict prognosis, but even with the same stage or grade, patients still may have different cancer behaviors. Our previous immunochemistry reports showed p53 [4], COX-2 [5], osteopontin [6], hypoxia-induced factor-1α [7], nuclear factor-κB [8] are the prognostic factors for UTUC. However, the exact molecular mechanisms of tumor invasion, recurrence and prognosis of this disease are not clear. Indeed, there is still no conclusive biomarker for cancer detection, prognosis prediction or treatment effect monitoring for UTUC.
Visfatin [pre-B-cell colony-enhancing factor (PBEF), nicotinamide mononucleotide adenylyltransferase (NAMPT)], a 52-kDa protein, is the rate-limiting enzyme of NAD biosynthesis from nicotinamide that regulates growth, apoptosis, DNA replication, repair and angiogenesis of mammalian cells [9,10]. Cancer cells have a high rate of NAD+ turnover compared to normal cells [11], and therefore, visfatin is essential for the survival of tumor cells. Accumulated evidences suggested that increased expression of visfatin is closely associated with the pathogenesis of colon [12], pancreas [13], gastric [14], prostate [15], malignant astrocytoma/glioblastoma [16] and breast cancers [17]. These reports indicate that visfatin can be considered a rational target in cancer. Consequently, several small molecule inhibitors of the enzyme are being investigated and developing [11,18]. Recent evidence shown that pharmacological blockade of visfatin reduces viability in multiple types of cancer cells and can inhibit the growth of tumor xenografts in vivo [19,20].
However, the expression of visfatin and its prognostic significance in UTUC is still unknown. In this study, we evaluated the expression levels of visfatin and P53 in tumor tissues and correlated them with clinicopathologic features in UTUC patients.
Materials and methods
Surgical specimens and clinicopathological data
One-hundred and five formalin-fixed UTUC samples were obtained from the Department of Urology, Kaohsiung Medical University Hospital from 1997-2006. The data were retracted from medical records retrospectively. All the patients received nephroureterectomy and excision of bladder cuff. The data were retracted from medical records retrospectively. Follow-Up protocol was decided according to NCCN guideline. Patients received cystoscopy by 3-month interval within 2 years after surgery and then increasing intervals thereafter. Bladder recurrence was defined as UC proved pathologically. Recurrence-free survival was defined as the time from the date of surgery to the date of bladder recurrence. This study protocol was reviewed and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-20120069).
Immunohistochemistry
Five-μm-thick sections from representative tissue blocks were cut, deparaffinized with xylene rinse, rehydrated with a graded alcohol series for 5 min each, and then rinsed with distilled water. Antigen retrieval was enhanced by autoclaving slides in sodium citrate buffer (10 mM, pH 6.0) for 30 min. Endogenous peroxidase activity was quenched by incubation in 3% hydrogen peroxide/methanol buffer for 10 min. The slides were incubated with a monoclonal antibody against visfatin (sc-166946, Santa Cruz Biotechnology, INC.) at a dilution of 1:500 overnight at 4°C in humidified chambers. The slides were washed three times in phosphate-buffered solution and further incubated with a biotinylated secondary antibody for 30 min at room temperature. Antigen-antibody complexes were detected by the avidin-biotin-peroxidase method using 3,3’-diaminobenzidine as a chromogenic substrate (Dako, Glostrup, Denmark). Finally, the slides were counterstained with hematoxylin and then examined by light microscopy.
Evaluation of immunohistochemical staining
Tumor immunostaining was examined by two qualified pathologists who were blinded to the patients’ clinical status. Discrepancies in scoring between pathologists were reviewed jointly and a consensus was reached. Visfatin expression was determined by the percentage of positive stained cells. Specimen with stained cells less than 50% was marked as low visfatin expression, and those with stained cells more than 50% was marked as high visfatin expression.
Statistical analysis
All statistical analyses were performed using the SPSS 14.0 statistical package for PC (SPSS, Inc.). Chi-square test was applied to study the correlation of visfatin expression with tumor stage, tumor grade, gander, age at diagnosis, body mass index (BMI), tumor distant metastasis, hemodialysis, and serum creatinine level. Survival curves were generated using Kaplan-Meier estimates, and the significance of differences between curves was evaluated by the log-rank test. Furthermore, hazard ratios (HRs) and 95% confidence intervals (CIs) computed from univariate and multivariable Cox regression models were used for investigating the relationship between clinicopathological characteristics and survival. P values less than 0.05 were considered statistically significant.
Results
Figure 1 showed that low (A) and high (B) nuclear staining of tumor cells in UTUC. Negative control was showed in panel (C). (scale bar=50 μm).
Figure 1.
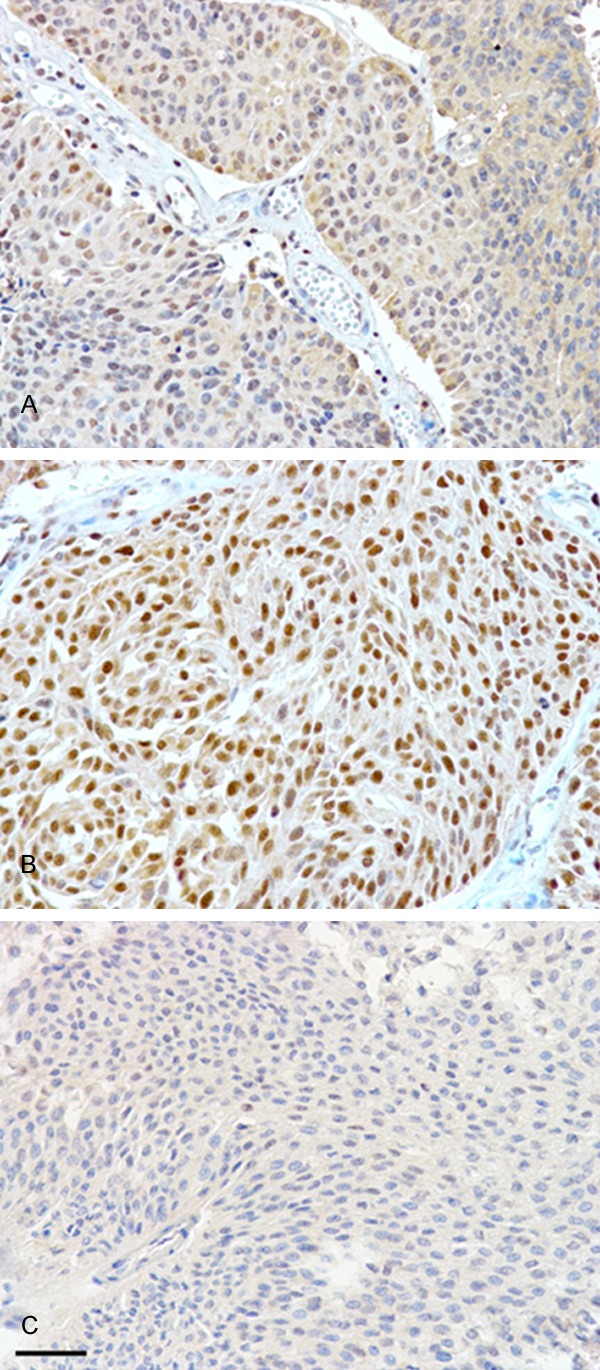
The expression of visfatin, as determined by immunohistochemistry, was divided into low (A) and high (B) nuclear staining of tumor cells in upper tract urothelial carcinomas. Negative control was showed in panel (C). (scale bar=50 μm).
The patients were regularly followed-up for median 45 months (ranged from 6 to 145 months). The demographic distribution of characteristics such stage, grade, gender, age, BMI, in 105 patients with UTUC recruited in this study are demonstrated in Table 1. As shown in Table 1, sixty (57.1%) cancer specimens expressed high visfatin staining. According to their visfatin expression status, there was a significant correlation in stage (P=0.001), grade (P=0.007) and p53 expression (P=0.007). But, visfatin expression status was not associated with gender, age, or BMI.
Table 1.
Clinicopathological characteristics of patients with upper tract urothelial carcinoma and association with visfatin expression
| Visfatin | ||||
|---|---|---|---|---|
|
|
||||
| Low | High | |||
|
|
|
|||
| Variablea | Patient, no. (%) | n (%) | n (%) | P-Valueb |
| No. | 105 (100) | 45 (42.9) | 60 (57.1) | |
| Stage | ||||
| I/II | 64 (61.0) | 36 (80.0) | 28 (46.7) | 0.001 |
| III/IV | 41 (39.0) | 9 (20.0) | 32 (53.3) | |
| Grade | ||||
| Low | 34 (32.4) | 21 (46.7) | 13 (21.7) | 0.007 |
| High | 71(67.6) | 24 (53.3) | 47 (78.3) | |
| Gender | ||||
| Male | 50 (47.6) | 20 (44.4) | 30 (50.0) | 0.573 |
| Female | 55 (52.4) | 25 (55.6) | 30 (50.0) | |
| Age (years) | ||||
| <65 | 37 (35.2) | 17 (37.8) | 20 (33.3) | 0.637 |
| ≥65 | 68 (64.8) | 28 (62.2) | 40 (66.7) | |
| BMI (kg/m2) | ||||
| <25 | 67 (63.8) | 27 (60.0) | 40 (66.7) | 0.482 |
| ≥25 | 38 (36.2) | 18 (40.0) | 20 (33.3) | |
| p53 | ||||
| Low | 37 (36.6) | 23 (51.1) | 14 (25.0) | 0.007 |
| High | 64 (63.4) | 22 (48.9) | 42 (75.0) | |
Undetermined in small cases;
P by the chi-square test.
BMI: body mass index.
In Table 2, we showed the influence of clinicopathological parameters on UTUC tumor recurrence after surgery. We found that tumor grade and stage were significantly correlated with recurrence-free survival in univariate analysis. Visfatin expression was importantly correlated with UTUC recurrence (P<0.001). But, in multivariable analysis of recurrence-free survival of UTUC patients, only visfatin expression showed it is an independent risk factor of cancer recurrence (P=0.009).
Table 2.
Univariate and multivariable analysis of recurrence-free survival for patients with upper tract urothelial carcinoma
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard ratio | 95% Confidence interval | P-Value | Hazard ratio | 95% Confidence interval | P-Value | |
| Stage | ||||||
| III/IV | 2.71 | (1.40-5.25) | 0.003 | 1.62 | (0.80-3.25) | 0.178 |
| I/II | 1.00 | 1.00 | ||||
| Grade | ||||||
| High | 4.05 | (1.68-9.76) | 0.002 | 2.47 | (0.99-6.12) | 0.052 |
| Low | 1.00 | 1.00 | ||||
| Gender | ||||||
| Male | 1.63 | (0.85-3.11) | 0.141 | - | - | - |
| Female | 1.00 | - | ||||
| Age (years) | ||||||
| ≥65 | 0.85 | (0.44-1.65) | 0.637 | - | - | - |
| <65 | 1.00 | - | ||||
| BMI (kg/m2) | ||||||
| ≥25 | 0.82 | (0.42-1.61) | 0.567 | - | - | - |
| <25 | 1.00 | - | ||||
| Visfatin | ||||||
| High | 4.70 | (2.06-10.76) | <0.001 | 3.22 | (1.34-7.76) | 0.009 |
| Low | 1.00 | 1.00 | ||||
BMI: body mass index.
In Table 3, we examined the impact of clinicopathological parameters on UTUC patients’ survival. We discovered that tumor stage and visfatin expression were both correlated significantly with worse cancer-specific survival in univariate and multivariable analysis.
Table 3.
Univariate and multivariable analysis of cancer-specific survival for patients with upper tract urothelial carcinoma
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard ratio | 95% Confidence interval | P-Value | Hazard ratio | 95% Confidence interval | P-Value | |
| Stage | ||||||
| III/IV | 6.80 | (2.48-18.67) | <0.001 | 4.53 | (1.58-12.95) | 0.005 |
| I/II | 1.00 | 1.00 | ||||
| Grade | ||||||
| High | 2.21 | (0.81-5.99) | 0.121 | 0.89 | (0.31-2.55) | 0.831 |
| Low | 1.00 | 1.00 | ||||
| Gender | ||||||
| Male | 0.79 | (0.34-1.86) | 0.595 | - | - | - |
| Female | 1.00 | - | ||||
| Age (years) | ||||||
| ≥65 | 0.75 | (0.32-1.76) | 0.502 | - | - | - |
| <65 | 1.00 | - | ||||
| BMI (kg/m2) | ||||||
| ≥25 | 0.75 | (0.30-1.84) | 0.523 | - | - | - |
| <25 | 1.00 | - | ||||
| Visfatin | ||||||
| High | 9.04 | (2.10-38.88) | 0.003 | 5.74 | (1.27-25.98) | 0.023 |
| Low | 1.00 | 1.00 | ||||
BMI: body mass index.
Kaplan-Meier survival curves for recurrence-free (A) and cancer-specific (B) survival of patients with low and high visfatin expression in UTUC were indicated in Figure 2. Figure 2A showed high visfatin expression was a significant predictor for UTUC recurrence (P<0.001). Figure 2B showed high visfatin expression in UTUC predicted poor survival (P<0.001).
Figure 2.
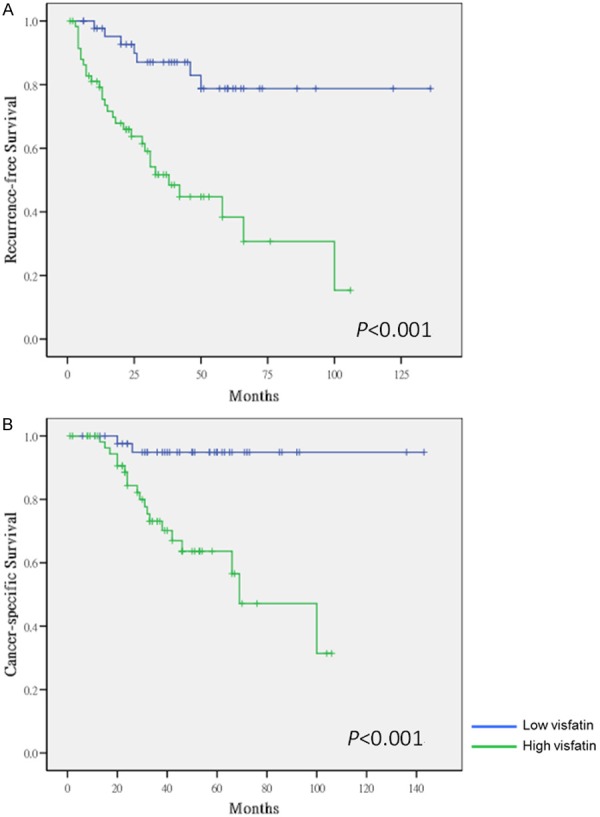
Kaplan-Meier survival curves for recurrence-free (A) and cancer-specific (B) survival of patients with low and high visfatin expression in upper tract urothelial carcinoma.
Discussion
Previous studies showed that high serum levels or tissue expression of visfatin were correlated with various cancers including breast cancer [21], colorectal cancer [22], prostate cancer [23], gastric cancer [14], endometrial cancer [24], melanoma [25], astrocytoma [16] and lymphomas [26]. In urothelial carcinoma, serum visfatin level was higher in patient with bladder cancer than in the control group. Besides, serum visfatin level predicted earlier recurrence in non-muscle invasive bladder cancer [27]. Another study suggested that visfatin is important in the pathogenesis of bladder cancer and its SNPs of visfatin gene might be a novel genetic biomarker for the prognosis of bladder cancer [28]. Accordingly, visfatin is thought to be a useful biomarker for tumorigenesis and for the prediction of cancer survival. But, there are no evidence that demonstrate the significant correlation between visfatin expression in tumor and clinico-pathological characteristics in urothelial carcinoma.
To the best of our knowledge, this is the first study to evaluate the prognostic significance of visfatin expression in UTUC. We found increased visfatin expression in UTUC correlated with advanced stages, higher tumor grade, and or higher p53 expression. We also demonstrated that the higher expression of visfatin in tumor tissues could predict poor prognosis of patients with UTUC.
Aside from the visfatin, we examined the p53 expression status in UTUC tissues. We found the positive correlation between visfatin and p53 expression. Corresponding to our previous work in UTUC, we had demonstrated that p53 was an independent predictor of poor progression-free (HR=3.74, P=0.025) and cancer-specific (HR=5.87, P=0.030) survival [13]. In bladder cancer molecular expression studies, most high grade invasive tumors had p53 mutation [14]. Similar result had been illustrated by Reddy et al. who showed that visfatin overexpressed and its co-expression with p53 was associated with poor survival in glioblastoma [9]. Using HEK293T cells as a model, Thakur et al. reported that visfatin inhibition could elevate p53 activity and lead to carcinogenesis suppression. They concluded that apoptosis induced by visfatin inhibitors was associated with acetylation of p53, which is required for the functional activity of p53 [15].
In this study, we demonstrated that visfatin was identified as independent prognostic predictor for UTUC. Visfatin has recently gathered attention as an important role in carcinogenesis and a potential therapeutic target in cancer and other metabolic diseases [18]. In addition, the increased levels of visfatin observed in malignant versus benign tissues are associated with alterations in tumorigenic activity. There are several issues regarding the role of visfatin and its inhibitors as a therapeutic agent in control of human cancers. These inhibitors efficiently suppress NAD production in a time dependent manner and sustained reduction of NAD levels leads to loss of ATP and ultimately cell death [29]. Inhibition of visfatin activity using the specific inhibitors could be further evaluated for cancer treatment or as a sensitizer for chemotherapy [30].
Visfatin overexpression has been shown to promote acquired resistance to chemotherapeutic agents, including fluorouracil, doxorubicin, paclitaxel, etoposide, and phenylethyl isothiocyanate [15,31]. Folgueira et al. also found visfatin expression in cancer tissue was higher in doxorubicin-resistant breast cancer [17]. In UTUC, the effect of cisplatin-based adjuvant chemotherapy after nephroureterectomy is still questionable [32]. Only part of the patients could have benefit from chemotherapy. Visfatin may play an important role to distinguish those who should receive chemotherapy from those who should not.
Limitations of this study were listed as following as: 1) we did not detect visfatin expression in serum of UTUC patients to demonstrate whether it can be used as a serological prognostic biomarker for UTUC patients; 2) we did not observe the differences of visfatin expression between normal urothelium and tumor tissue of UTUC patients. Thus, we cannot exam whether visfatin could be a risk factor of UTUC or not; 3) since this is a retrospective study, comprehensive data about the survival benefit of systemic chemotherapy cannot be collected thoroughly. We were unable to exam the effect of visfatin on survival of UTUC patients with chemotherapy.
In conclusion, we observed that visfatin expression in tumors was positively correlated with malignant behavior of UTUC. High visfatin expression predicted poor recurrence-free and cancer-specific survivals. Comprehensive studies are needed to clarify the detailed mechanisms of visfatin in UTUC development and progression.
Acknowledgements
This study was supported by grants from Kaohsiung Medical University “Aim for the Top Universities” (KMU-TP103E19, KMU-TP103G00, KMU-TP103G01, KMU-TP103G04, KMUTP103G05), the health and welfare surcharge of tobacco products, Ministry of Health and Welfare (MOHW104-TDU-B-212-124-003), Ministry of Science and Technology (MOST103-2314-B-037-059-MY3), and Kaohsiung Medical University Hospital (KMUH100-0R41, KMUH101-1R46).
Disclosure of conflict of interest
None.
References
- 1.Williams CB, Mitchell JP. Carcinoma of the ureter--a review of 54 cases. Br J Urol. 1973;45:377–387. doi: 10.1111/j.1464-410x.1973.tb12175.x. [DOI] [PubMed] [Google Scholar]
- 2.Li CC, Chang TH, Wu WJ, Ke HL, Huang SP, Tsai PC, Chang SJ, Shen JT, Chou YH, Huang CH. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur Urol. 2008;54:1127–1134. doi: 10.1016/j.eururo.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 3.Li WM, Li CC, Ke HL, Wu WJ, Huang CN, Huang CH. The prognostic predictors of primary ureteral transitional cell carcinoma after radical nephroureterectomy. J Urol. 2009;182:451–458. doi: 10.1016/j.juro.2009.04.026. discussion 458. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Wu WJ, Li WM, Lin HH, Huang CN, Chai CY, Chang LL, Lin HL, Ke HL. Prognostic value of p53 protein overexpression in upper tract urothelial carcinomas in Taiwan. Anticancer Res. 2013;33:1091–1098. [PubMed] [Google Scholar]
- 5.Ke HL, Tu HP, Lin HH, Chai CY, Chang LL, Li WM, Li CC, Lee YC, Yeh HC, Wu WJ, Bau DT. Cyclooxygenase-2 (COX-2) up-regulation is a prognostic marker for poor clinical outcome of upper tract urothelial cancer. Anticancer Res. 2012;32:4111–4116. [PubMed] [Google Scholar]
- 6.Ke HL, Chang LL, Yang SF, Lin HH, Li CC, Wu DC, Wu WJ. Osteopontin overexpression predicts poor prognosis of upper urinary tract urothelial carcinoma. Urol Oncol. 2011;29:703–709. doi: 10.1016/j.urolonc.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Ke HL, Wei YC, Yang SF, Li CC, Wu DC, Huang CH, Wu WJ. Overexpression of hypoxia-inducible factor-1alpha predicts an unfavorable outcome in urothelial carcinoma of the upper urinary tract. Int J Urol. 2008;15:200–205. doi: 10.1111/j.1442-2042.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 8.Yeh HC, Huang CH, Yang SF, Li CC, Chang LL, Lin HH, Ke HL, Wei YC, Wu WJ. Nuclear factor-kappaB activation predicts an unfavourable outcome in human upper urinary tract urothelial carcinoma. BJU Int. 2010;106:1223–1229. doi: 10.1111/j.1464-410X.2010.09210.x. [DOI] [PubMed] [Google Scholar]
- 9.Jieyu H, Chao T, Mengjun L, Shalong W, Xiaomei G, Jianfeng L, Zhihong L. Nampt/Visfatin/PBEF: a functionally multi-faceted protein with a pivotal role in malignant tumors. Curr Pharm Des. 2012;18:6123–6132. doi: 10.2174/138161212803582531. [DOI] [PubMed] [Google Scholar]
- 10.Bi TQ, Che XM. Nampt/PBEF/visfatin and cancer. Cancer Biol Ther. 2010;10:119–125. doi: 10.4161/cbt.10.2.12581. [DOI] [PubMed] [Google Scholar]
- 11.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 12.Van Beijnum JR, Moerkerk PT, Gerbers AJ, De Bruine AP, Arends JW, Hoogenboom HR, Hufton SE. Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer. Int J Cancer. 2002;101:118–127. doi: 10.1002/ijc.10584. [DOI] [PubMed] [Google Scholar]
- 13.Bauer L, Venz S, Junker H, Brandt R, Radons J. Nicotinamide phosphoribosyltransferase and prostaglandin H2 synthase 2 are up-regulated in human pancreatic adenocarcinoma cells after stimulation with interleukin-1. Int J Oncol. 2009;35:97–107. doi: 10.3892/ijo_00000317. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima TE, Yamada Y, Hamano T, Furuta K, Gotoda T, Katai H, Kato K, Hamaguchi T, Shimada Y. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol. 2009;44:685–690. doi: 10.1007/s00535-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Hasan MK, Alvarado E, Yuan H, Wu H, Chen WY. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene. 2011;30:907–921. doi: 10.1038/onc.2010.468. [DOI] [PubMed] [Google Scholar]
- 16.Reddy PS, Umesh S, Thota B, Tandon A, Pandey P, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Rao MR, Kondaiah P, Somasundaram K. PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol Ther. 2008;7:663–668. doi: 10.4161/cbt.7.5.5663. [DOI] [PubMed] [Google Scholar]
- 17.Folgueira MA, Carraro DM, Brentani H, Patrao DF, Barbosa EM, Netto MM, Caldeira JR, Katayama ML, Soares FA, Oliveira CT, Reis LF, Kaiano JH, Camargo LP, Vencio RZ, Snitcovsky IM, Makdissi FB, e Silva PJ, Goes JC, Brentani MM. Gene expression profile associated with response to doxorubicin-based therapy in breast cancer. Clin Cancer Res. 2005;11:7434–7443. doi: 10.1158/1078-0432.CCR-04-0548. [DOI] [PubMed] [Google Scholar]
- 18.Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalamaga M, Archondakis S, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, Lekka A. Could serum visfatin be a potential biomarker for postmenopausal breast cancer? Maturitas. 2012;71:301–308. doi: 10.1016/j.maturitas.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima TE, Yamada Y, Hamano T, Furuta K, Matsuda T, Fujita S, Kato K, Hamaguchi T, Shimada Y. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010;101:1286–1291. doi: 10.1111/j.1349-7006.2010.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel ST, Mistry T, Brown JE, Digby JE, Adya R, Desai KM, Randeva HS. A novel role for the adipokine visfatin/pre-B cell colony-enhancing factor 1 in prostate carcinogenesis. Peptides. 2010;31:51–57. doi: 10.1016/j.peptides.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Tian W, Zhu Y, Wang Y, Teng F, Zhang H, Liu G, Ma X, Sun D, Rohan T, Xue F. Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol. 2013;129:505–512. doi: 10.1016/j.ygyno.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Maldi E, Travelli C, Caldarelli A, Agazzone N, Cintura S, Galli U, Scatolini M, Ostano P, Miglino B, Chiorino G, Boldorini R, Genazzani AA. Nicotinamide phosphoribosyltransferase (NAMPT) is over-expressed in melanoma lesions. Pigment Cell Melanoma Res. 2013;26:144–146. doi: 10.1111/pcmr.12037. [DOI] [PubMed] [Google Scholar]
- 26.Olesen UH, Hastrup N, Sehested M. Expression patterns of nicotinamide phosphoribosyltransferase and nicotinic acid phosphoribosyltransferase in human malignant lymphomas. APMIS. 2011;119:296–303. doi: 10.1111/j.1600-0463.2011.02733.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Zhou B, Zhang P, Zhang Z, Chen P, Pu Y, Song Y, Zhang L. Prognostic value of serum nicotinamide phosphoribosyltransferase in patients with bladder cancer. Croat Med J. 2014;55:507–513. doi: 10.3325/cmj.2014.55.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Zhou B, Zhang P, Zhang Z, Chen P, Pu Y, Song Y, Zhang L. Genetic variants in NAMPT predict bladder cancer risk and prognosis in individuals from southwest Chinese Han group. Tumour Biol. 2014;35:4031–4040. doi: 10.1007/s13277-013-1527-z. [DOI] [PubMed] [Google Scholar]
- 29.Sampath D, Zabka TS, Misner DL, O’Brien T, Dragovich PS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther. 2015;151:16–31. doi: 10.1016/j.pharmthera.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV, Tjornelund J, Dawson KM, Dupuis M, Duchosal MA. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood. 2009;113:3276–3286. doi: 10.1182/blood-2008-08-173369. [DOI] [PubMed] [Google Scholar]
- 31.Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL, Li HJ, Zhao W. Overexpression of Nampt in gastric cancer and chemopotentiating effects of the Nampt inhibitor FK866 in combination with fluorouracil. Oncol Rep. 2011;26:1251–1257. doi: 10.3892/or.2011.1378. [DOI] [PubMed] [Google Scholar]
- 32.Yafi FA, Tanguay S, Rendon R, Jacobsen N, Fairey A, Izawa J, Kapoor A, Black P, Lacombe L, Chin J, So A, Lattouf JB, Bell D, Fradet Y, Saad F, Matsumoto E, Drachenberg D, Cagiannos I, Kassouf W. Adjuvant chemotherapy for upper-tract urothelial carcinoma treated with nephroureterectomy: assessment of adequate renal function and influence on outcome. Urol Oncol. 2014;32:31, e17–24. doi: 10.1016/j.urolonc.2012.11.014. [DOI] [PubMed] [Google Scholar]


