Abstract
Vav1 has been reported to be involved in human cancers, however, the expression and clinical significance of Vav1 in NSCLC are not fully understood. In the present study, we examined the expression of Vav1 in 170 NSCLC patients who underwent radical resection by the immunohistochemical analyses. The association between the Vav1 expression and clinicopathological variables was analyzed. The multivariate Cox proportional hazards model was conducted to determine the prognostic value of Vav1 on the long-term survival. The results showed that the elevated Vav1 expression was correlated positively with lymph node metastasis (P<0.001), T stage (P<0.001) and poor histological differentiation (P<0.001). Patients with negative or low Vav1 expression had better prognoses than those with high Vav1 expression (P<0.001). Multivariate analysis indicated that Vav1 was independent prognostic factor for overall survival (OS) (HR 2.079, 95% CI 1.564 to 2.762, P<0.001) and disease-free survival (DFS) (HR 1.810, 95% CI 1.391 to 2.356, P<0.001). Our findings showed that overexpressed Vav1 was correlated with aggressive tumor behavior. Val1 was an independent factor for NSCLC prognosis, which may serve as a novel prognostic factor and potential target to improve the long-term outcome of NSCLC.
Keywords: Vav1, non-small cell lung cancer, invasion, prognosis
Introduction
As many Non-small cell lung cancer (NSCLC) patients are in the advanced stage at the time of initial diagnosis, they have the aggressive and early metastatic character, which cause poor long-term prognosis. The 5-year survival rate of NSCLC is only lower than 15%, though the diagnosis and treatment had improvements in recent years [1]. Therefore, it is still necessary to explore the novel predictive makers and ideal treatment to improve the out-come of NSCLC.
The Vav family (Vav1-3) of Rho-guanine nucleotide exchange factors (GEFs) was supposed to be able to control a diverse array of signaling pathways, while Vav proteins are modular and contain the Dbl-homology domain [2-4]. Recently, the expression of Vav1 was mainly found in hematopoietic system, and was involved in normal development and homeostasis [5]. Vav1 plays a crucial role in the maturation and function of both myeloid and lymphoid cells [6], and the loss of Vav1 may result in impaired immune system activity [7,8]. Intriguingly, despite the oncogenic form of Vav1 has not been detected in clinical human tumors, its wild-type form has recently been implicated in several human malignancies, such as neuroblastoma [9], breast cancer [10], pancreatic ductal adenocarcinomas (PDA) and ovarian cancer [11,12]. Furthermore, Vav1-positive pancreatic tumors are associated with decreased survival in patients [11]. Notably, even in the presence of oncogenic K-Ras, Vav1 RNAi abrogates neoplastic cellular proliferation in vitro and in vivo [11]. These findings suggest that ectopic Vav1 expression may play an important role in human cancer [13].
However, the expression of Vav1 in NSCLC primary tissues, and its clinical significance in NSCLC has not been reported. In the light of these considerations, the aims of the present study were to explore the clinical value and prognosis factors of Vav1 in NSCLC patients after resection.
Materials and methods
Ethics statement
Our study protocol was approved by the Institutional Ethics Review Board of Tianjin First Center Hospital, Tianjin, China.
Patients and paraffin-embedded tissue samples
Tissue samples were obtained from 170 patients with Ι-ΙΙΙA NSCLC who underwent surgical resection at the Department of Lung Cancer, Cancer Institute and Hospital of Tianjin Medical University between 2006 and 2007. All histology sections and paraffin blocks were obtained from the Department of Diagnostic Pathology, Cancer Institute and Hospital of Tianjin Medical University. All the patients were Chinese. The stage of each patient was diagnosed by two certified pathologists according to the procedures outlined by the International Association for the Study of Lung Cancer (IASLC) TNM 7th [14]. The grade of differentiation was based on World Health Organization criteria (Version 2004) and separated into three subgroups as follows: well, moderate and poor [15]. The clinicopathologic characteristics of patients are summarized in Table 1. Patients were followed until 29 February 2012. The follow-up period ranged from 2 to 86 months (average: 47.1 months; median: 51.5 months). The end point was cancer-related death.
Table 1.
Correlations between Vav1 expression and clinicopathological parameters in Ι-ΙΙΙA NSCLC patients (n=170)
| Vav1 | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Negative | Low | High | P value |
| Age (years) | ||||
| ≤60 | 34 | 36 | 22 | |
| >60 | 24 | 30 | 24 | 0.546 |
| Gender | ||||
| female | 18 | 20 | 22 | |
| male | 40 | 46 | 24 | 0.114 |
| Smoking status | ||||
| non-smoker | 22 | 20 | 22 | |
| smoker | 36 | 46 | 24 | 0.170 |
| Histology | ||||
| suqamous | 30 | 40 | 24 | |
| non-suqamous | 28 | 26 | 22 | 0.540 |
| Tumor location | ||||
| left | 20 | 16 | 19 | |
| right | 38 | 50 | 27 | 0.150 |
| Lesion | ||||
| peripheral | 44 | 40 | 32 | |
| central | 14 | 26 | 14 | 0.186 |
| Histological differentiation | ||||
| well | 22 | 14 | 4 | |
| moderate | 32 | 30 | 22 | |
| poor | 4 | 22 | 20 | <0.001 |
| T stage | ||||
| T1 | 26 | 20 | 10 | |
| T2 | 32 | 28 | 26 | |
| T3-4 | 0 | 18 | 10 | <0.001 |
| Lymph node metastasis | ||||
| yes | 24 | 36 | 38 | |
| no | 34 | 30 | 8 | <0.001 |
P Values <0.05 were considered to be significant.
Immunohistochemistry
Paraffin blocks of tumors were cut into 5-mm slices and mounted on saline-coated slides. Sections were deparaffinized in xylene and rehydrated in graded alcohols. Antigen retrieval was performed by immersing the sections in 10 mM sodium citrate buffer (citric acid and sodium citrate, pH 6.0) for 20 minutes at 98°C in a water bath. Endogenous peroxidase activity was blocked in 3% hydrogen peroxide in water. Sections were incubated with rabbit anti-Vav1 (Acris, Germany) used at a dilution of 1:50 at 4°C overnight. The sections were then washed three times with phosphate-buffered saline (PBS) and incubated with the corresponding secondary antibodies for 30 min at 37°C, after which point the sections were washed with PBS and incubated for 1 min with 3,30-diaminobenzidine (DAB). The sections were then counterstained with haematoxylin, dehydrated, cleared and permanently mounted with resinous mounting medium. All the procedures were carried out at room temperature. Additionally, tissues that stained positive for Vav1 were used as positive controls. Sections that were processed by replacing the primary antibody with PBS were used as negative controls.
Evaluation of immunohistochemically staining
The degree of immunoreactivity for the proteins was evaluated semi quantitatively on the basis of staining intensity and the proportion of positive tumor cells [16]. Under a microscope at ×400 magnification, five fields of vision were randomly selected (with no fewer than 200 cells per field). The staining intensity was graded as follows: 0 (no staining), 1 (light yellow), 2 (yellowish brown) and 3 (brown). The positive cells were graded according to the percentage of positive cells as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (11-50% positive tumor cells), 3 (51-80% positive tumor cells) and 4 (>80% positive tumor cells). The percentage of positive cells and the staining intensity were then multiplied to generate the immunoreactivity score. Based on this score, the immunoreactivity was divided into three groups: negative immunoreactivity (a total score of 0), low immunoreactivity (a total score of 1-4), and high immunoreactivity (a total score of >4). Immunohistochemically staining was evaluated by two independent pathologists who were blinded to the cases.
Statistical analysis
All statistical analyses were carried out using SPSS13.0 software (Chicago, USA). Overall survival (OS) was defined as the interval between the date of surgery and the date of death or last follow-up. Disease-free survival (DFS) was defined as the duration of time between the date of surgery and the date of first recurrence or last follow-up. The χ2 test was used to analyses the correlation between the expression of Vav1 and clinicopathologic parameters. The Kaplan-Meier method and the log-rank test were used to calculate OS and DFS. A prognostic analysis was carried out using univariate and multivariate Cox regressions models. P Values <0.05 were considered to be significant.
Results
Vav1 expression in NSCLC
We evaluated the expression of Vav1 in 170 human lung cancer specimens, primarily NSCLC. Consequently, Vav1 expression was not observed in normal lung tissues, but was present in 112 of 170 (65.9%) of all tumor specimens. 58/170 specimens (34.1%) were considered not stained (intensity score 0), 38.8% had low-intensity cytoplasmic staining (1-4), and 27.1% were highly stained in the cytoplasm (>4) (Table 1; Figure 1).
Figure 1.
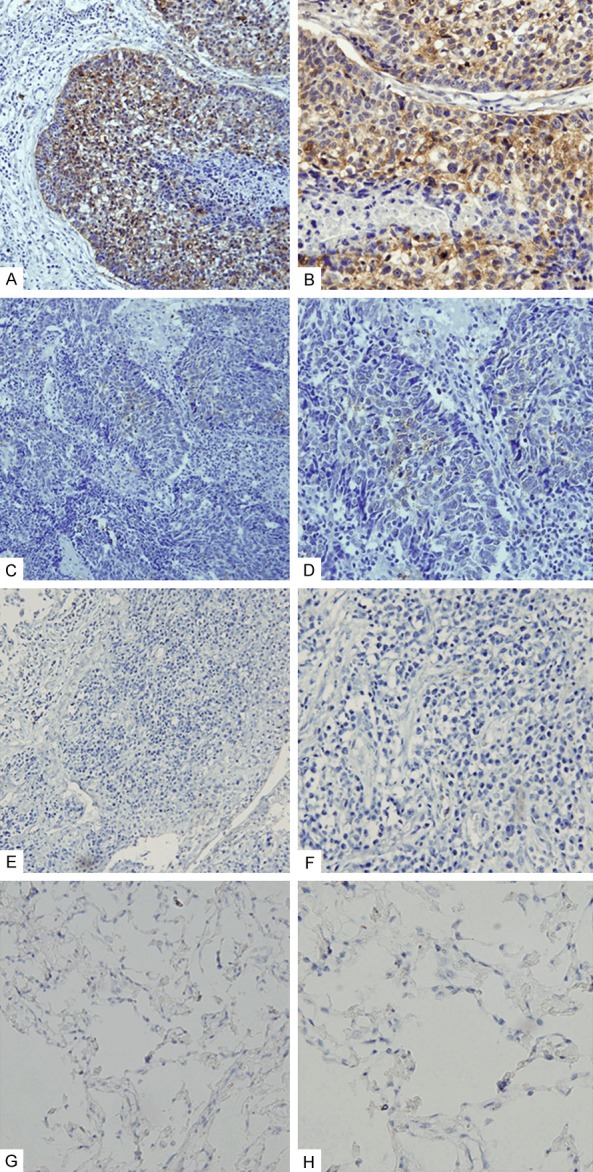
Expression of Vav1 in human NSCLC specimens. A, B. High-intensity cytoplasmic staining of Vav1 in NSCLC specimens. C, D. Low-intensity cytoplasmic staining of Vav1 in NSCLC specimens. E, F. Negative immunoreactivity of Vav1 in NSCLC specimens. G, H. Vav1 expression was not observed in normal lung tissues. A, C, E, G ×200; B, D, F, H ×400.
Correlations between Vav1 expression and clinicopathological parameters
As shown in Table 1, significant associations between Vav1 expression and histological differentiation (P<0.001), lymph node metastasis (P<0.001) and T stage (P<0.001) were identified. Our results indicated that elevated Vav1 expression was correlated positively with lymph node metastasis and T stage, and associated with poor histological differentiation. However, there was no significant correlations between Vav1 expression and age, gender, histology, smoking status, tumor location and lesion (P>0.05).
Survival analysis
We sought to determine whether Vav1 expression in NSCLC affected the clinical outcomes. According to the univariate analysis (Table 2), histological differentiation (P<0.001 and P<0.001), T stage (P<0.001 and P<0.001) and lymph node metastasis (P<0.001 and P<0.001), Vav1 expression (P<0.001 and P<0.001, Figure 2) were significantly associated with OS and DFS, respectively. Patients with negative or low Vav1 expression had better prognoses than those with high Vav1 expression. Ectopic expression of Vav1 was associated with decreased survival in patients with NSCLC. In the multivariate analysis (Table 3), Vav1 expression was an independent prognostic factor for OS (HR 2.079, 95% CI 1.564 to 2.762, P<0.001) and DFS (HR 1.810, 95% CI 1.391 to 2.356, P<0.001). Thus, these data suggested that high level of Vav1 may lead to a decreased survival benefit in NSCLC patients.
Table 2.
Univariate analysis of DFS and OS for 170 NSCLC patients
| DFS | OS | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Age (≤60, >60 years) | 0.815 | 1.043 | 0.732 to 1.487 | 0.264 | 1.226 | 0.858 to 1.751 |
| Gender (female, male) | 0.109 | 0.862 | 0.719 to 1.033 | 0.064 | 0.842 | 0.702 to 1.010 |
| Smoking status (non-smoker, smoker) | 0.798 | 1.049 | 0.726 to 1.517 | 0.714 | 1.072 | 0.740 to 1.551 |
| Histology (suqamous, non- suqamous) | 0.945 | 0.983 | 0.603 to 1.603 | 0.969 | 0.991 | 0.607 to 1.615 |
| Tumor location (left, right) | 0.171 | 0.769 | 0.529 to 1.120 | 0.240 | 0.799 | 0.549 to 1.162 |
| Lesion (peripheral, central) | 0.734 | 1.067 | 0.735 to 1.548 | 0.838 | 1.140 | 0.716 to 1.510 |
| Histological differentiation (well, moderate, poor) | <0.001 | 2.213 | 1.702 to 2.879 | <0.001 | 2.200 | 1.696 to 2.855 |
| T stage (T1, T2, T3-4) | <0.001 | 1.758 | 1.331 to 2.321 | <0.001 | 1.667 | 1.273 to 2.181 |
| Lymph node metastasis (no, yes) | <0.001 | 4.753 | 3.098 to 7.291 | <0.001 | 4.652 | 3.053 to 7.088 |
| Vav1 (negative, low, high) | <0.001 | 2.369 | 1.836 to 3.058 | <0.001 | 2.648 | 2.024 to 3.466 |
P Values <0.05 were considered to be significant. DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Figure 2.
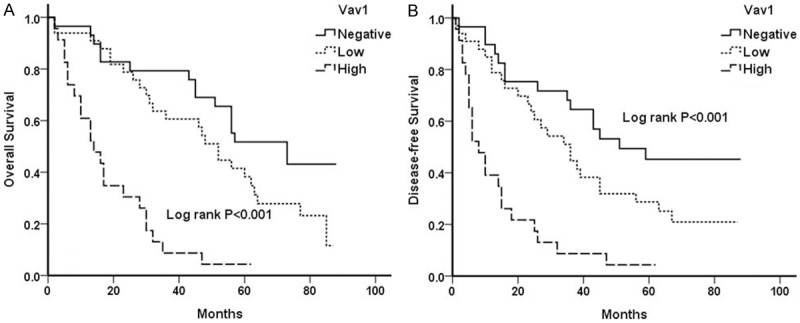
Survival curves of 170 patients with NSCLC. Overall survival (A) and Disease-free survival (B) curves of NSCLC patients with negative, low and high levels of Vav1 expression (log-rank test, P<0.001 and P<0.001), respectively.
Table 3.
Multivariate analysis of DFS and OS for 170 NSCLC patients
| DFS | OS | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Histological differentiation (well, moderate, poor) | 0.031 | 1.434 | 1.033 to 1.992 | 0.017 | 1.520 | 1.077 to 2.145 |
| T stage (T1, T2, T3-4) | 0.909 | 0.981 | 0.702 to 1.370 | 0.290 | 0.833 | 0.594 to 1.168 |
| Lymph node metastasis (no, yes) | <0.001 | 3.261 | 2.044 to 5.204 | <0.001 | 3.035 | 1.910 to 4.823 |
| Vav1 (negative, low, high) | <0.001 | 1.810 | 1.391 to 2.356 | <0.001 | 2.079 | 1.564 to 2.762 |
P Values <0.05 were considered to be significant. DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Discussion
In this study, we examined the expression of Vav1 and its value in predicting clinical outcomes in postoperative patients with NSCLC. The correlations between the expression of Vav1 and clinicopathological parameters were also analyzed.
Vav1 is a hematopoietic-specific signal transducer, which plays an important role in actin cytoskeleton reorganization, gene transcription, development and activation of immune cells [17-19]. The catalytic guanine nucleotide exchange factors (GEFs) function of Vav1 has been shown to be required in these cellular responses, an activity strictly controlled by tyrosine phosphorylation [17,20,21]. The wild-type form of Vav1 is normally expressed exclusively in the hematopoietic system [22]. Accumulating data now indicate that Vav1 is detected in several human malignant tumors and cell lines, including neuroblastoma, breast cancer, ovarian cancer, prostate cancer and uveal melanoma [23], which were all originating from tissues that normally do not express this protein. Its ectopic expression in a wide variety of human cancer tissues suggests it may play a role in human malignancies.
Recent studies revealed that Vav1-depletion in pancreatic and lung cancer cell lines results in the reduction of colony formation in soft agar in vitro and reduction of tumor size in immunocompromised mice [11,13]. Surprisingly, this influence of Vav1 expression was observed despite the presence of an oncogenic K-Ras allele in these cells, demonstrating the cardinal role of Vav1 in tumor development [13]. Blocking Vav1 expression by RNAi in melanoma cells led to impaired activation of the Jak/Vav1/RhoGTPases (Rac1 and RhoA) pathway, inhibiting up-regulation of membrane-type matrix metalloproteinase (MT1-MMP) by CXCL12, a mechanism that contributes to melanoma cell invasion [23]. Moreover, Vav1 was shown via its GEF activity to promote the matrix-degrading processes underlying pancreatic tumor cell migration [24]. Notably, patients with Vav1 positive pancreatic tumors had a lower survival rate than those with Vav1-negative tumors [11]. Another research showed that Vav1 expression was associated significantly with unfavorable prognosis in patients with early-stage epithelial ovarian cancer [12]. These data indicated that Vav1 play a significant role in the invasion and progression of human malignancies.
Recently, Vav1 expression in 42% of 78 lung cancer cell lines and in 46% of 57 human primary lung cancer specimens was analysed [13]. In the present study, we evaluated the expression of Vav1 in 170 NSCLC specimens, and it was present in 65.9% of all tumor specimens. Remarkably, significant associations between Vav1 expression and histological differentiation, lymph node metastasis and T stage were identified. The results are partially in agreement with the findings of Galit Lazer et al, showing that stronger Vav1 staining was associated with larger tumour size [13]. Our findings implicated that ectopic expression of Vav1 may be involved with the invasion and progression of NSCLC. In addition, this work showed that Vav1 expression was associated significantly with unfavorable prognosis in NSCLC patients. Patients with high-intensity Vav1 staining were much more likely to relapse and die than those with negative and low intensity Vav1 staining. Using multivariate analyses, Vav1 was independent prognostic factor for OS and DFS. A newly reported study showed that Vav1 may contribute to the progression of lung cancer by upregulating expression of colony-stimulating-factor-1 (CSF1), which is strongly associated with poor prognosis in early-stage squamous cell carcinoma and its level of expression significantly increases with disease progression in NSCLC patients [25,26]. But the molecular mechanisms that how Vav1 participated in the invasive process in NSCLC remain elusive. Therefore, further studies are needed to explore.
Conclusion
Taken together, our results indicated that the elevated Vav1 expression was correlated with aggressive tumor behavior. And it was an independent factor for NSCLC prognosis, which may serve as a novel prognostic factor and potential target to improve NSCLC long-term outcome.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81273937 and No. 81403220).
Disclosure of conflict of interest
None.
Abbreviations
- NSCLC
Non-small Cell Lung Cancer
- OS
Overall Survival
- DFS
Disease-Free Survival
- PDA
Pancreatic Ductal Adenocarcinomas
- IASLC
Association for the Study of Lung Cancer
- PBS
Phosphate-Buffered Saline
- DAB
3,30-diaminobenzidine
- HR
hazard ratio
- CI
confidence interval
- GEFs
Guanine nucleotide Exchange Factors
- CSF1
Colony-Stimulating-Factor-1
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazer G, Katzav S. Guanine nucleotide exchange factors for RhoGTPases: Good therapeutic targets for cancer therapy? Cell Signal. 2011;23:969–979. doi: 10.1016/j.cellsig.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immunerecognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 5.Coppola J, Bryant S, Koda T, Conway D, Barbacid M. Mechanism of activation of the vav protooncogene. Cell Growth Differ. 1991;2:95–105. [PubMed] [Google Scholar]
- 6.Bertagnolo V, Brugnoli F, Grassilli S, Nika E, Capitani S. Vav1 in differentiation of tumoral promyelocytes. Cell Signal. 2012;24:612–620. doi: 10.1016/j.cellsig.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Janssen EM, Duncan AW, Altman A, Billadeau DD, Abraham RT. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 2002;21:4809–4819. doi: 10.1093/emboj/cdf499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan-Ramu N, Katzav S. The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J Pathol. 2003;199:526–533. doi: 10.1002/path.1314. [DOI] [PubMed] [Google Scholar]
- 10.Lane J, Martin TA, Mansel RE, Jiang WG. The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast cancer. Int Semin Surg Oncol. 2008;5:23. doi: 10.1186/1477-7800-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Wakahashi S, Sudo T, Oka N, Ueno S, Yamaguchi S, Fujiwara K, Ohbayashi C, Nishimura R. VAV1 represses E-cadherin expression through the transactivation of Snail and Slug: a potential mechanism for aberrant epithelial to mesenchymal transition in human epithelial ovarian cancer. Transl Res. 2013;162:181–190. doi: 10.1016/j.trsl.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Lazer G, Idelchuk Y, Schapira V, Pikarsky E, Katzav S. The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis. J Pathol. 2009;219:25–34. doi: 10.1002/path.2579. [DOI] [PubMed] [Google Scholar]
- 14.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 15.Lantuejoul S, Brambilla E. [What’s new in the 2004 WHO classification of the lung tumors?] . Rev Pneumol Clin. 2008;64:187–194. doi: 10.1016/j.pneumo.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010;23:213–224. doi: 10.1038/modpathol.2009.160. [DOI] [PubMed] [Google Scholar]
- 17.Palmby TR, Abe K, Der CJ. Critical role of the pleckstrin homology and cysteine-rich domains in Vav signaling and transforming activity. J Biol Chem. 2002;277:39350–39359. doi: 10.1074/jbc.M202641200. [DOI] [PubMed] [Google Scholar]
- 18.Heo J, Thapar R, Campbell SL. Recognition and activation of Rho GTPases by Vav1 and Vav2 guanine nucleotide exchange factors. Biochemistry. 2005;44:6573–6585. doi: 10.1021/bi047443q. [DOI] [PubMed] [Google Scholar]
- 19.Dustin ML, Shaw AS. Costimulation: building an immunological synapse. Science. 1999;283:649–650. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- 20.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Das B, Wei W, Van Aelst L, Mosteller RD, Khosravi-Far R, Westwick JK, Der CJ, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–258. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razidlo GL, Schroeder B, Chen J, Billadeau DD, McNiven MA. Vav1 as a central regulator of invadopodia assembly. Curr Biol. 2014;24:86–93. doi: 10.1016/j.cub.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebban S, Farago M, Rabinovich S, Lazer G, Idelchuck Y, Ilan L, Pikarsky E, Katzav S. Vav1 promotes lung cancer growth by instigating tumor-microenvironment cross-talk via growth factor secretion. Oncotarget. 2014;5:9214–9226. doi: 10.18632/oncotarget.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrzypski M, Jassem E, Taron M, Sanchez JJ, Mendez P, Rzyman W, Gulida G, Raz D, Jablons D, Provencio M, Massuti B, Chaib I, Perez-Roca L, Jassem J, Rosell R. Three-gene expression signature predicts survival in early-stage squamous cell carcinoma of the lung. Clin Cancer Res. 2008;14:4794–4799. doi: 10.1158/1078-0432.CCR-08-0576. [DOI] [PubMed] [Google Scholar]


